Abstract
Objective
To examine associations between inflammatory marker C-reactive protein (CRP) measured preoperatively (PREOP) and on postoperative day 2 (POD2) with delirium incidence, duration, and feature severity.
Design
Prospective cohort study.
Setting
Two academic medical centers.
Participants
Adults aged ≥70 undergoing major non-cardiac surgery (N=560).
Measurements
Plasma CRP was measured using ELISA. Delirium was assessed from Confusion Assessment Method (CAM) interviews and chart review. Delirium duration was measured by number of hospital days with delirium. Delirium feature severity was defined as the sum of CAM-Severity (CAM-S) scores across all postoperative hospital days. We used generalized linear models to examine independent associations between CRP (PREOP and POD2, separately) and delirium incidence, duration, feature severity; prolonged hospital length of stay (LOS, >5 days); and discharge disposition.
Results
Postoperative delirium occurred in 24% of participants, 12% had ≥2 delirium days, and the mean(standard deviation) sum CAM-S was 9.3(11.4). After adjusting for age, sex, surgery type, anesthesia route, medical comorbidities, and postoperative infectious complications, participants with PREOP CRP ≥3mg/L had a 1.5 greater risk of delirium (95% confidence interval[CI] 1.1–2.1), 0.4 more delirium days (p<.001), more severe delirium (3.6 CAM-S points higher, p<.001), and a 1.4 greater risk of prolonged LOS (95 % CI 1.1–1.8) relative to patients with CRP <3mg/L. Using POD2 CRP, participants in the highest quartile (≥235.73mg/L) were 1.5 times as likely to develop delirium (95% CI 1.0–2.4), had 0.2 more delirium days (p<.05), and had more severe delirium (4.5 CAM-S points higher, p<.001) than participants in the lowest quartile (≤127.53mg/L).
Conclusions
High PREOP and POD2 CRP were independently associated with delirium incidence, duration, and feature severity. CRP may be useful to identify patients at-risk for delirium.
Keywords: Delirium, Postoperative, Delirium feature severity, C-reactive protein, Inflammation
INTRODUCTION
Delirium is characterized by acute change and fluctuations in attention, thinking, and consciousness. It occurs in 12–51% of surgical patients,1 and delirium following major surgery has been associated with longer hospitalization,2 higher rates of discharge to nursing homes,3 greater postoperative complications,4 and higher inhospital mortality.5,6 Despite our knowledge of its epidemiology, delirium remains a purely clinical diagnosis with no biomarkers to inform risk, diagnosis, or monitoring.
Delirium is frequently present in conditions where a systemic inflammatory response occurs, and there is growing evidence supporting a link between inflammation markers and delirium in varied settings. For instance, delirium has been associated with higher levels of pro-inflammatory cytokines in both surgical and medical patients.7,8 Additionally, delirium has been associated with proteins involved in the stress response, including one of the most commonly examined markers of systemic inflammation, the acute phase reactant C-reactive protein (CRP).9–16 However, these studies have been limited in that they used relatively small samples of patients who had either been admitted to medical wards, had experienced a cerebrovascular accident, or had undergone hip and vascular surgery. Furthermore, most studies examined CRP only at a single time point after patients became ill, and thus, lacked a true ‘pre-morbid’ baseline measure of CRP. In addition, most studies focused solely on the association of CRP with delirium incidence. No study has addressed whether additional delirium-related outcomes, such as delirium duration and feature severity, and important clinical outcomes previously associated with delirium (including hospital length of stay [LOS)] and post-acute facility discharge), are linked to CRP.
Our previous work demonstrated in a nested, matched case-control subset of the Successful Aging after Elective Surgery (SAGES) cohort that higher CRP preoperatively (PREOP) and on postoperative day 2 (POD2) levels could predict postoperative delirium in older patients.17 We now extend this research by examining the associations between CRP and postoperative delirium incidence, duration, feature severity; LOS; and discharge disposition across the entire SAGES study cohort.
METHODS
Study Sample
The SAGES Study is a prospective observational study focused on elucidating novel risk factors, including biomarkers, of delirium and its associated long-term cognitive and functional decline.18,19 Between 2010–2013, SAGES enrolled 560 dementia-free adults aged ≥70 who were scheduled for elective major non-cardiac surgery. Major inclusion and exclusion criteria have been previously published.18 Patients with dementia were excluded from SAGES using a previously reported detailed, sequential screening process.18 Informed consent for study participation was obtained from all subjects according to procedures approved by the institutional review boards of Beth Israel Deaconess Medical Center and Brigham and Women’s Hospital, the two surgical sites, and Hebrew SeniorLife, the study coordinating center, all located in Boston, MA.
Specimen Collection
All patients underwent serial blood collection, including PREOP and on POD2. When possible, the sampling was incorporated into clinical blood draws taken in the pre-admitting testing center prior to operation (PREOP) and on the surgical wards (POD2). During phlebotomy, mechanical disruption was minimized to prevent hemolysis or platelet activation. Blood was stored on ice in heparinized tubes until processing. During processing, low speed centrifugation was used to separate plasma from cellular material, and plasma was stored at −80°C until laboratory analysis. Nearly all samples satisfied rigorous quality control standards (99%), including ≤4 hours between blood draw and processing. Phlebotomy was performed on average 2 weeks prior to the index surgery (mean 13 ± 15 days).19
CRP Enzyme-Linked Immunosorbant Assay
CRP at PREOP and POD2 were measured in the entire SAGES sample using a high-sensitivity enzyme linked immunosorbent assay (ELISA) kit from R&D, with all standards and samples run in duplicate. Each 96-well plate contained the standard curve and cases and controls across both timepoints. Coefficient of variations (CVs) of duplicate measures were generally ≤5%. If any CV was >10%, that plasma sample was repeated. An internal calibrator sample for both PREOP and POD2 time points was present on every 96-well ELISA plate. The cross plate variation was consistently below the range of 5–10% based on the internal calibrator value. ELISA plates were read using a BioTek MX plate reader at Optical Density (OD)=450. A 4-parameter logistic curve was used with final calculations determined in an Excel template containing built-in macros for optimizing the best-fit model (http://www.rheumatologie-neuss.net/index-Dateien/RheumatologieNeuss13.htm).
Delirium
Presence of postoperative delirium was determined from daily interviews from POD1 until hospital discharge, supplemented by a validated chart review method.20 Trained study staff conducted structured cognitive assessments of attention, orientation, and memory. Delirium, assessed using the Confusion Assessment Method (CAM) diagnostic algorithm, was defined as having an acute onset of change or fluctuating mental status, inattention, and either disorganized thinking or altered level of consciousness.21,22 Presence of delirium by chart review was adjudicated by at least two delirium experts, and discordance was resolved by consensus.23 Patients were considered delirious if delirium was present on either the CAM (score based on the daily interview) or the chart review method on any postoperative day; otherwise, patients were considered non-delirious.19,20
Delirium Duration and Feature Severity
We also examined the association of CRP with delirium duration and feature severity. Delirium duration was determined by summing the total number of postoperative days the patient was considered delirious by either the CAM or chart review methods from the day after surgery until hospital discharge. Delirium feature severity was quantified using the CAM-Severity long form (CAM-S LF) score.24 The CAM-S LF represents the sum of severity ratings of 10 CAM features (range 0–19, 19 most severe); each scored as 0 (absent), 1 (present, mild), and 2 (present, marked) – except for fluctuating course, which is scored 0 (absent) and 1 (present). Our primary outcome for delirium feature severity was defined as the sum of all CAM-S scores (sum CAM-S), which considers both intensity and duration and thus reflects the total ‘burden’ of delirium features across the entire hospitalization. We previously described sum CAM-S and found it to be the delirium feature severity measure most strongly associated with clinical outcomes24. In supplementary analyses, we examined peak CAM-S score, defined as the highest CAM-S score on any postoperative day, an alternate delirium feature severity measure previously associated with clinical outcomes.25
Length of Stay (LOS) and Discharge Disposition
To examine the predictive validity of CRP for clinical outcomes related to delirium, we examined its relationship with hospital LOS and post-acute facility discharge. Prolonged LOS (defined as >5 days; average LOS=5.2 days) and post-acute facility discharge (defined as discharge to a nursing home, rehabilitation or sub-acute facility) were determined from medical record review. No patients resided in a nursing home prior to surgery.
Baseline Sample Characteristics
Baseline information on education and cognitive performance was collected. Total years of education was measured by patient self-report. Preoperative cognitive functioning was measured using the General Cognitive Performance (GCP), a composite variable of commonly used neuropsychological measures.26 Medical record review was used to gather information on the Charlson comorbidity index.27
Potential Confounders
We adjusted for potential confounders including age, sex, surgical procedure, anesthesia route, Charlson index (which includes preoperative connective tissue disease), and postoperative infectious complications. Surgical procedures were categorized into three types: orthopedic, vascular, and colorectal.18, 19 We reviewed medical records to obtain information on preoperative comorbidities (e.g., connective tissue disease), anesthesia route (general or spinal), and postoperative complications. Postoperative infectious complications (urinary tract infection, pneumonia, wound infection) were further verified and adjudicated by three physicians. We did not control for postoperative medications because of the risk of over-controlling for variables potentially along the causal pathway to delirium.36,37
Statistical Analysis
We used generalized linear models (GLM) with a log-link and normal error distribution to determine the association between CRP (PREOP and POD2) and delirium incidence, prolonged LOS, and post-acute facility discharge. For delirium duration and feature severity, we used GLM with an identity-link and normal error distribution. For all outcomes, separate analytic models examined postoperative delirium risk, duration, feature severity; prolonged LOS; or post-acute facility discharge by considering CRP quartiles at PREOP and POD2 based on the SAGES sample distribution. For CRP at PREOP, we also examined a CRP cutpoint previously validated to indicate high-risk for cardiovascular disease (≥3 mg/L).28 A high-risk cutpoint analysis could not be completed for CRP at POD2 because postoperative CRP levels were much higher than those in community based samples. All reported analytic models adjusted for age, sex, surgery type, anesthesia route, preoperative Charlson comorbidity index, and postoperative infectious complications.
Consideration of Subsyndromal Delirium (SSD)
We further considered the association between CRP (PREOP and POD2) and a three-level outcome: delirium, SSD, and no delirium (Supplementary Materials include detailed methods).
Added Value of CRP in Predicting Delirium Risk
We investigated the added value of CRP at PREOP and POD2 to an existing delirium risk prediction tool, the Inouye delirium risk score29 (Supplementary Materials include detailed methods).
All analyses were conducted in SAS version 9.3 (SAS Institute, Cary, NC).
RESULTS
Table 1 reports the clinical characteristics of our study sample, which included no missing data. On average, our sample was 76.7 years old (standard deviation [SD] 5.2), highly educated (15.0[SD 2.9] years total), and had a higher than average US preoperative general cognitive performance (GCP) score (57.6[SD 7.3]). More than half of the study sample was female (58%). Most patients underwent orthopedic surgery (81%), 87% had general anesthesia, 12% had a Charlson comorbidity index of ≥3, 8% had preoperative connective tissue disease, and 11% had a postoperative infectious complication. Twenty-four percent of the study sample developed postoperative delirium, 12% had ≥2 delirium days, and the average sum CAM-S was 9.3[SD 11.4].
Table 1.
Sample characteristics (N=560)
| Characteristic | Total Sample N=560 |
Delirium N=134 |
No Delirium N=426 |
|---|---|---|---|
| Baseline measures: | |||
| Age (M ± SD) | 76.7 ± 5.2 | 77.5 ± 5.0 | 76.4 ± 5.2 |
| Female, N (%) | 326 (58) | 81 (60) | 245 (58) |
| Non-white or Hispanic, N (%) | 42 (8) | ||
| Educational attainment, years, (M ± SD) | 15.0 ± 2.9 | 14.7 ± 3.0 | 15.1 ± 9.9 |
| Surgery Type, N (%) | |||
| Orthopedic | 454 (81) | 105 (79) | 349 (82) |
| Vascular | 35 (6) | 11 (8) | 24 (6) |
| Gastrointestinal | 71 (13) | 18 (13) | 53 (12) |
| Preoperative GCP (externally scaled, M ± SD) | 57.6 ± 7.3 | 54.7 ± 6.5 | 58.5 ± 7.3 |
| Charlson Comorbidity Index, N (%) | |||
| 0 | 257 (46) | 54 (40) | 203 (48) |
| 1 | 139 (25) | 23 (17) | 116 (27) |
| 2 | 97 (17) | 38 (29) | 59 (14) |
| 3+ | 67 (12) | 19 (14) | 48 (11) |
| Vascular comorbidity, N (%) | 214 (38) | 60 (45) | 154 (36) |
| Preoperative connective tissue disease, N (%) | 44 (8) | 10 (7) | 34 (8) |
| Anesthesia route, N (%) | |||
| General | 485 (87) | 122 (91) | 363 (85) |
| Spinal | 75 (13) | 12 (9) | 63 (15) |
| Postoperative infectious complications, N (%) | 62 (11) | 20 (15) | 42 (10) |
| Outcome measures: | |||
| Postoperative delirium, N (%) | |||
| No delirium | 370 (66) | 0 (0) | 426 (100) |
| Delirium | 134 (24) | 134 (100) | 0 (0) |
| Total days with delirium, N (%) | |||
| 0 | 426 (76) | 19 (14) | 426 (100) |
| 1 | 68 (12) | 64 (48) | 0 (0) |
| 2 | 38 (7) | 29 (22) | 0 (0) |
| 3+ | 28 (5) | 22 (16) | 0 (0) |
| Delirium severitya | |||
| Peak CAM-S score (long form, M ± SD) | 3.9 ± 3.2 | 8.1 ± 3.6 | 2.7 ± 1.6 |
| Sum of all CAM-S scores (long form, M ± SD) | 9.3 ± 11.4 | 21.6 ± 17.0 | 5.4 ± 4.4 |
Abbreviations: M=mean, SD=standard deviation; CAM-S=Confusion Assessment Method-Severity (score range 0–19, 19 most severe); GCP=general cognitive performance (a composite measure of neuropsychological measures reflecting cognitive domains vulnerable to delirium,26 where population mean=50 and SD=10, externally scaled to the Health and Retirement Survey ADAMS
Delirium feature severity measured in all patients (including patients without delirium)
Figure 1 plots the distribution of CRP at PREOP and POD2. There was a strong effect of surgery on CRP, with POD2 CRP levels nearly 100 times higher than PREOP levels. Figures 2a and 2b illustrate the median levels and inter-quartile range for PREOP and POD2 CRP levels (respectively) by postoperative delirium status (no delirium, SSD, delirium). Figure 2a suggests a threshold effect, with elevated PREOP CRP only in the delirium group. Figure 2b shows increasing POD2 CRP levels across the delirium categories, suggesting a dose-response relationship.
Figure 1. Distributions of C-reactive protein at PREOP and POD2.
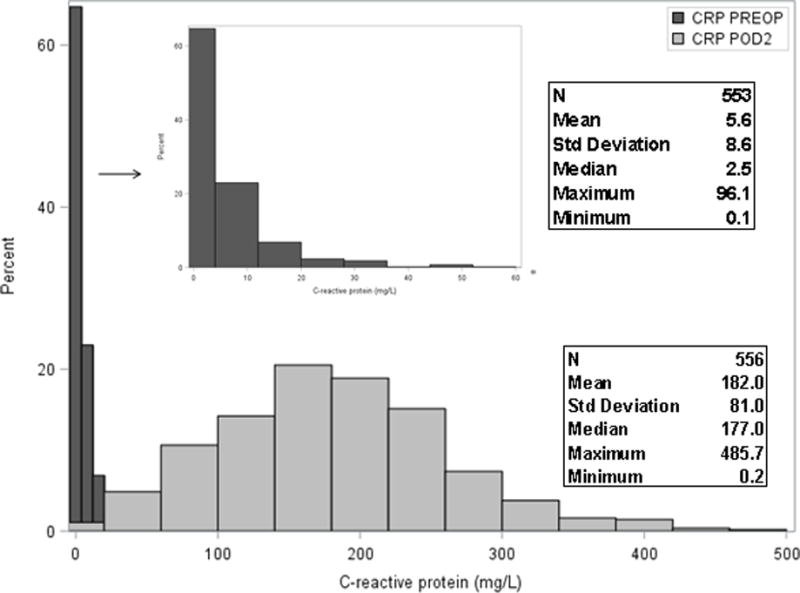
Abbreviations: PREOP=preoperative, POD2=postoperative day 2
*Insert: Expanded plot of PREOP CRP with X-axis plotted for values up to 60 mg/L
Figure 2. Median levels of C-reactive protein by delirium status at (A) PREOP and (B) POD2.
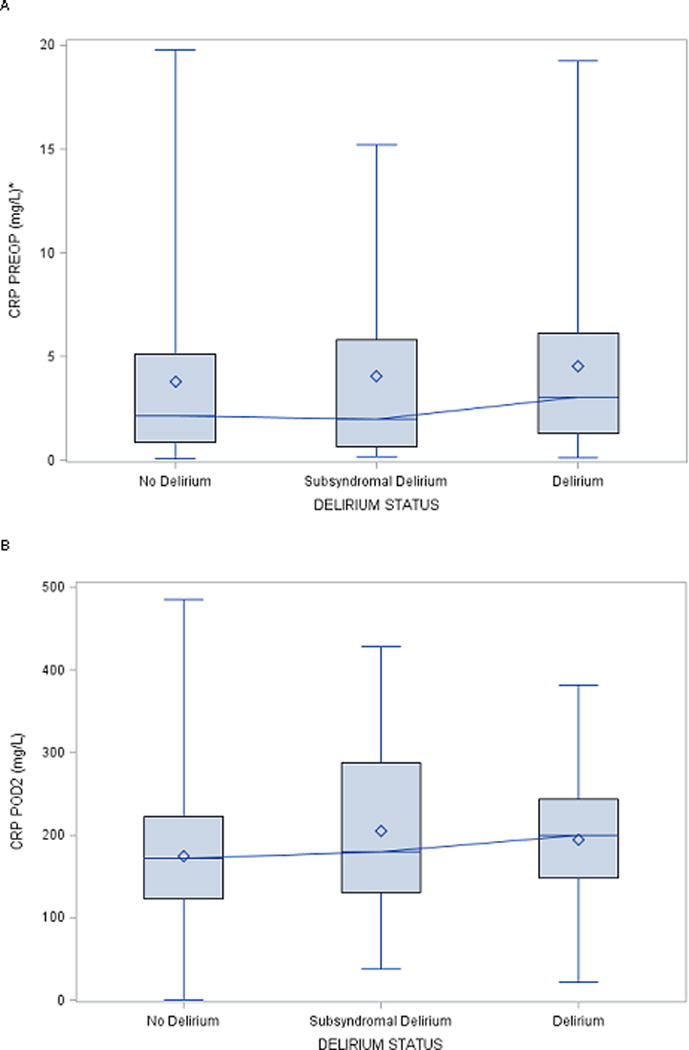
Abbreviations: CRP=C-reactive protein, PREOP=preoperative, POD2=postoperative day 2
*For graphical readability, PREOP CRP values >20 mg/L were not plotted.
Height of the box represents the interquartile range (the distance between the 25th and 75th percentiles).
The diamond symbol in the box interior represents the group mean.
The horizontal line in the box interior represents the group median.
The vertical lines (“whiskers”) extending from the box indicate the group minimum and maximum values.
Table 2 shows the relative risk of postoperative delirium by CRP PREOP and POD2 quartiles examined in separate models. Unadjusted and adjusted models yielded similar results, indicating a significant association between CRP (for both PREOP and POD2) and postoperative delirium (only results for adjusted models shown). After adjusting for age, sex, surgery type, anesthesia route, preoperative Charlson comorbidity index, and postoperative infectious complications, patients with PREOP CRP values in quartile (Q)4 had a significantly increased risk of developing postoperative delirium relative to patients in Q1, with a significant trend of increasing risk across the CRP quartiles. Finally, patients with PREOP ≥3 mg/L were 1.5 times as likely to develop delirium relative to patients with CRP levels <3 mg/L.
Table 2.
Associations of C-reactive protein with postoperative delirium, delirium duration, and delirium feature severity (sum of all CAM-S scores)
| CRP measure (mg/L) | Delirium Incidence
|
Delirium Duration (per day)a
|
Sum CAM-S (per point)a
|
|---|---|---|---|
| RR (95% CI) | Days (95% CI) | Score (95% CI) | |
| CRP PREOP | |||
| Quartiles | |||
| Q1 (≤0.95) | Reference | Reference | Reference |
| Q2 (0.95–2.56) | 1.4 (0.8–2.3) | 0.3 (0.2–0.5)b | 1.5 (0.9–2.1)c |
| Q3 (2.56–6.39) | 1.7 (1.0b–2.7) | 0.3 (0.2–0.5)b | 2.5 (1.8–3.2)c |
| Q4 (≥6.39) | 1.8 (1.2–2.9) | 0.4 (0.2–0.5)b | 3.6 (2.9–4.3)b |
| p-trendd | <.01 | <.01 | <.01 |
| High-risk cutpointe | |||
| ≥3 vs. <3 | 1.5 (1.1–2.1) | 0.2 (0.1–0.4)b | 2.6 (2.1–3.2)b |
| CRP POD2f | |||
| Quartiles | |||
| Q1 (≤127.53) | Reference | Reference | Reference |
| Q2 (127.53–177.05) | 1.1 (0.6–1.7) | 0.1 (−0.1–0.2) | 1.2 (0.6–1.8)c |
| Q3 (177.05–235.73) | 1.5 (1.0g–2.3) | 0.2 (0.0h–0.4)c | 3.5 (2.9–4.2)b |
| Q4 (≥235.73) | 1.5 (1.0i–2.4) | 0.2 (0.0j–0.4)c | 4.5 (3.8–5.2)b |
| p-trendd | 0.02 | 0.02 | <.01 |
All generalized linear log-link models adjusted for age, sex, type of surgery, anesthesia route, Charlson comorbidity index, and postoperative infectious complications (N=553 for CRP PREOP and N=556 for CRP POD2)
Delirium duration and sum of CAM-S was measured in all patients, including those without delirium. Therefore, values <1 are possible.
Abbreviations: CAM-S=Confusion Assessment Methods-Severity, CI=confidence interval, CRP=C-reactive protein, Q=quartile, PREOP=preoperative, POD2=postoperative day 2, RR=relative risk
For the quartile CRP variable: The values represent the increase in delirium days (or CAM-S points) for patients in a given quartile relative to patients in quartile 1. For the high-risk cutpoint CRP variable: The values represent the increase in delirium days (or CAM-S points) for patients with CRP ≥3 mg/L relative to patients with CRP <3 mg/L.
p<.001
p<.001
p-trend determined from p-value from the association between the quartile-based CRP variable and delirium status
Based on a previously validated high-risk cutpoint for cardiovascular disease28
No high-risk cutpoint analysis was completed for CRP at POD2 because the scores are much higher than those in prior (community based) samples.
Actual value 0.99 (not statistically significant)
Actual value 0.03
Actual value 1.01 (statistically significant)
Actual value 0.004
Considering POD2 CRP, patients with values in Q4 had an increased risk of postoperative delirium compared to patients in Q1, with a significant trend of increasing risk across CRP quartiles. Exclusion of 131 patients taking drugs that might influence CRP levels, including steroids, non-steroidal anti-inflammatory drugs, and immune modulators, did not change these findings.
Higher CRP quartile was generally associated with a greater risk of developing SSD, but this association did not achieve statistical significance (Supplementary Table SI).
Table 2 also reports the associations between CRP and delirium duration and feature severity. All models yielded consistently strong associations for patients with CRP values in the highest quartile (Q4) for PREOP and POD2 CRP having more delirium days and greater delirium feature severity relative to those in Q1. For instance, relative to patients in Q1 for PREOP CRP, on average patients in Q4 for PREOP CRP experienced almost one half more delirium days and had sum CAM-S scores 3.6 points higher. There was also a significant trend across the CRP quartiles for both delirium days and feature severity. There was also a significant trend across the CRP quartiles for both delirium days and feature severity. Similar to the findings for sum CAM-S, higher CRP at both time points was associated with higher peak CAM-S scores (Supplementary Table S2).
Table 3 shows the relative risk of LOS and post-acute facility discharge by CRP (PREOP and POD2). Patients with PREOP CRP in Q4 were 1.7 times as likely to have a prolonged LOS (>5 days) relative to patents in Q1. Moreover, this increased risk was observed for patients with high-risk PREOP CRP ≥3 mg/L, who were 1.4 times as likely to have a LOS>5 days relative to patients with CRP levels <3 mg/L. Higher CRP on POD2 was associated with an increased risk of having a prolonged LOS (p-trend=0.03) and an increased risk of post-acute facility discharge (p-trend=0.04).
Table 3.
Associations of C-reactive protein with length of stay and post-acute facility discharge
| CRP measure | LOS > 5 days vs ≤5 days
|
Post-acute facility discharge
|
|---|---|---|
| RR (95% CI) | RR (95% CI) | |
| CRP PREOP | ||
| Quartiles | ||
| Q1 (≤0.95) | Reference | Reference |
| Q2 (0.95–2.56) | 1.5 (1.0a–2.5) | 1.0 (0.7–1.4) |
| Q3 (2.56–6.39) | 1.8 (1.2–2.7) | 0.9 (0.7–1.3) |
| Q4 (≥6.39) | 1.7 (1.1–2.7) | 0.9 (0.6–1.2) |
| p-trendc | 0.02 | 0.30 |
| High-risk cutpointd | ||
| ≥3 vs. <3 | 1.4 (1.1–1.8) | 1.1 (0.9–1.4) |
| CRP POD2e | ||
| Quartiles | ||
| Q1 (≤127.53) | Reference | Reference |
| Q2 (127.53–177.05) | 1.0 (0.7–1.6) | 1.1 (0.8–1.5) |
| Q3 (177.05–235.73) | 1.5 (1.0a-2.2) | 1.3 (0.9–1.7) |
| Q4 (≥235.73) | 1.4 (0.9–2.1) | 1.3 (1.0b-1.8) |
| p-trendc | 0.03 | 0.04 |
All generalized linear regression models adjusted for age, sex, type of surgery, anesthesia route, Charlson comorbidity index, and postoperative infectious complications (N=553 for CRP PREOP and N= 556 for CRP POD2). For the LOS outcome, postoperative infectious complications was not included in the model due to collinearity.
Abbreviations: CI=confidence interval, CRP=C-reactive protein, LOS=length of stay, Q=quartile, PREOP=preoperative, POD2=postoperative day 2, RR=relative risk
Actual value 0.97 (not statistically significant)
Actual value 0.99 (not statistically significant)
p-trend determined from p-value from the association between the quartile-based CRP variable and LOS or discharge disposition
Based on a previously validated high-risk cutpoint for cardiovascular disease28
No high-risk cutpoint analysis was completed for CRP at POD2 because the scores are much higher than those in prior (community based) samples
Added Prediction Analyses
Supplementary Table S3 reports the prevalence of the Inouye delirium risk score factors by delirium status, and the Supplemental Text reports results for the prediction analyses.
DISCUSSION
In this study of older adults without dementia undergoing elective major noncardiac surgery, we observed significant associations of PREOP and POD2 CRP with delirium incidence, duration, and feature severity. Patients with high levels of PREOP (and POD2) CRP had greater delirium incidence, longer delirium duration, and more severe delirium relative to patients with lower levels of PREOP (and POD2) CRP. Moreover, a significant dose-response relationship was observed for PREOP and POD2 CRP with all three delirium outcomes, such that higher CRP was associated with worse outcomes. Our results suggest that PREOP CRP may be an important risk marker for delirium incidence and that CRP measured PREOP and on POD2 may aid in predicting and monitoring the severity of postoperative delirium.
A current model of delirium pathophysiology proposes that individuals predisposed to a heightened inflammatory response when exposed to an acute stressor (e.g., surgery or infection) are at increased risk for delirium.31 It is postulated that under certain conditions, systemic inflammatory mediators cross the blood-brain barrier, activate brain microglia, and initiate neuroinflammation.29 This model has gained substantial attention in recent years,32 and our findings linking CRP with delirium incidence and feature severity lend support for the role of systemic inflammation in delirium pathophysiology. Moreover, emerging evidence from animal models,32 and from human studies,33 suggests that heightened systemic inflammation may indeed affect the brain. For instance, several studies have demonstrated that animals sacrified after being exposed to systemic inflammatory insults, such as lipopolysaccharide and gram negative bacteria, showed evidence of neuroinflammation on brain autopsy. In humans, increasing evidence of an association between neuroinflammation and dementia has been reported.34,35
Our previous work,17 a proteomics approach designed for discovery of proteins associated with delirium incidence, used a matched case-control study design of patients with and without delirium, which imposed significant constraints on the types of analyses we could perform. For instance, the case-control study did not consider patients with SSD and required delirium cases to have delirium on POD2 to correspond with the time of blood draw. While the inclusion of only carefully selected delirium cases and delirium-free controls allows for maximal contrast to discover biomarkers, it is not representative of the full clinical cohort. The current study substantially extends this previous work by: 1) examining the association between PREOP CRP and delirium incidence in a more clinically representative surgical sample, 2) including patients with SSD, and 3) examining four additional outcomes: delirium duration, delirium feature severity, LOS, and post-acute facility discharge. Additional strengths of our study include the consistent findings across all four outcomes and evidence of a dose-response effect, which underscores the robustness of our results and strengthens our conclusion of an association between CRP (PREOP and POD2) and delirium.
This study adds new, clinically relevant information to the literature on CRP and delirium. CRP might be a useful marker for risk stratification of patients before major surgery given its observed association with delirium incidence, duration, feature severity, and prolonged LOS. In particular, the addition of CRP at PREOP and POD2 to the Inouye delirium risk score29 significantly improved the predictive model, lending further support for the use of CRP to identify patients who may benefit from proactive interventions. Of note, it was not our primary Aim to examine the association of CRP with LOS and discharge disposition; rather, these were added as secondary outcomes to support the association of CRP with delirium. Nonetheless, our findings of the association of CRP with these outcomes are interesting, and warrant further exploration in future studies.
Some limitations about our study warrant mention. First, our analyses were limited to examination of a single marker of inflammation, CRP. Although CRP is the most clinically used summary marker of inflammation, it will be important to examine the associations of other more specific markers of inflammation (e.g., cytokines) on delirium incidence and feature severity, in addition to examining their joint effects. A priority area for future research will be to explore the synergistic effects of multiple inflammatory markers on delirium incidence and feature severity. Second, the availability of blood on POD2 may not align with delirium occurring several days following surgery. Third, a detailed examination of postoperative medication use and its influence on inflammation and delirium is beyond the scope of the current manuscript. In future work, we plan to conduct detailed pharmacoepidemiologic analyses to address this important question. Fourth, our findings may not be generalizable to the overall population of older adults undergoing major scheduled surgery since we enrolled adults without dementia from two academic medical centers in one geographic region and had a low minority representation. Future studies with more diverse samples are required. Contrary to expectations, we did not find an association between CRP and SSD, but our power was limited by the small size of this group within the sample.
In conclusion, we found that high PREOP and POD2 CRP was associated with delirium incidence, duration, and feature severity. If these findings are validated in future studies, CRP may be useful clinically to stratify patients at risk of delirium prior to surgery and to track its severity. Our findings also provide robust support for the role of inflammation in delirium pathophysiology, and may motivate development of new pathophysiologically-based intervention strategies to prevent and/or treat this common, morbid, and costly geriatric syndrome that threatens the independence of older patients.
Supplementary Material
Supplementary Table S1: Associations of C-reactive protein with postoperative delirium
Supplementary Table S2: Associations of C-reactive protein with postoperative delirium feature severity (peak CAM-S scores)
Supplementary Table S3: Inouye Risk Score Factors by Delirium Status
Supplementary Appendix S1: SAGES Study Group
Acknowledgments
Funding sources:
This research was supported by National Institute on Aging grants (T32AG023480, P01AG031720, K07AG041835, R01AG044518, R01AG030618, K24AG035075, R01AG051658) and the Charles A. King Trust Postdoctoral Research Fellowship Program, Bank of America, N.A., Co-Trustee. Dr. Inouye holds the Milton and Shirley F. Levy Family Chair. The authors gratefully acknowledge the contributions of the patients, family members, nurses, physicians, staff members, and members of the Executive Committee who participated in the Successful Aging after Elective Surgery (SAGES) Study (See Appendix).
Sponsor’s Role: None of the sponsors were involved in the design, methods, subject recruitment, data collection, analysis, or preparation of the paper.
Footnotes
|
| ||||||||
| Elements of Financial/Personal Conflicts | *Author 1 Sarinnapha Vasunilashorn |
Author 2 Simon Dillon |
Author 3 Sharon Inouye |
Authors 4–11 Long Ngo, Tamara Fong, Richard Jones, Thomas Travison, Eva Schmitt, David Alsop, Steven Freedman, Steven Arnold, Eran Metzger, Towia Libermann, Edward Marcantonio |
||||
|
| ||||||||
| Yes | No | Yes | No | Yes | No | Yes | No | |
|
| ||||||||
| Employment or Affiliation | X | X | X | X | ||||
|
| ||||||||
| Grants/Funds | X | X | X | X | ||||
|
| ||||||||
| Honoraria | X | X | X | X | ||||
|
| ||||||||
| Speaker Forum | X | X | X | X | ||||
|
| ||||||||
| Consultant | X | X | X | X | ||||
|
| ||||||||
| Stocks | X | X | X | X | ||||
|
| ||||||||
| Royalties | X | X | X | X | ||||
|
| ||||||||
| Expert Testimony | X | X | X | X | ||||
|
| ||||||||
| Board Member | X | X | X | X | ||||
|
| ||||||||
| Patents | X | X | X | X | ||||
|
| ||||||||
| Personal Relationship | X | X | X | X | ||||
|
| ||||||||
| *Authors can be listed by abbreviations of their names. | ||||||||
Author Contributions:
Study concept and design: Vasunilashorn, Dillon, Inouye, Ngo, Fong, Jones, Travison, Schmitt, Alsop, Freedman, Arnold, Metzger, Libermann, Marcantonio
Acquisition of subjects and/or data: Ngo, Jones, Travison, Schmitt, Inouye, Marcantonio
Analysis and interpretation of data: Vasunilashorn, Dillon, Inouye, Ngo, Fong, Jones, Travison, Schmitt, Alsop, Freedman, Arnold, Metzger, Libermann, Marcantonio
Preparation of manuscript: Vasunilashorn, Dillon, Inouye, Ngo, Fong, Jones, Travison, Schmitt, Alsop, Freedman, Arnold, Metzger, Libermann, Marcantonio
References
- 1.Inouye SK, Westendorp RG, Saczynski JS. Delirium in elderly people. Lancet. 2014;383:911–922. doi: 10.1016/S0140-6736(13)60688-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martin BJ, Buth KJ, Arora RC, Baskett RJ. Delirium as a predictor of sepsis in post-coronary artery bypass grafting patients: a retrospective cohort study. Crit Care. 2010;14:171. doi: 10.1186/cc9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Witlox J, Eurelings LS, de Jonhe JF, Kalisvaart KJ, Eikelenboom P, van Gool WA. Delirium in elderly patients and the risk of postdischarge mortality, institutionalization, and dementia: a meta-analysis. JAMA. 2010;304:443–451. doi: 10.1001/jama.2010.1013. [DOI] [PubMed] [Google Scholar]
- 4.Marcantonio ER, Goldman L, Mangione CM, et al. A clinical prediction rule for delirium after elective noncardiac surgery. JAMA. 1994;27:134–139. [PubMed] [Google Scholar]
- 5.Martin BJ, Buth KJ, Arora RC, Baskett RJ. Delirium as a predictor of sepsis in post coronary artery bypass grafting patients: A retrospective cohort study. Crit Care. 2010;14:171. doi: 10.1186/cc9273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Rudolph JL, Jones RN, Rasmussen LS, Silverstein JH, Inouye SK, Marcantonio ER. Independent vascular and cognitive risk factors for postoperative delirium. Am J Med. 2007;120:807–813. doi: 10.1016/j.amjmed.2007.02.026. [DOI] [PubMed] [Google Scholar]
- 7.de Rooij SE, van Munster BC, Korevaar JC, Levi M. Cytokines and acute phase response in delirium. J Psychosom Res. 2007;62:521–525. doi: 10.1016/j.jpsychores.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 8.Vasunilashorn SM, Ngo L, Inouye SK, et al. Cytokines and postoperative delirium in older patients undergoing major elective surgery. J Gerontol A Biol Sci Med Sci. 2015;70:1289–1295. doi: 10.1093/gerona/glv083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Beloosesky Y, Grinblat J, Pirotsky A, Weiss A, Hendel D. Different C-reactive protein kinetics in post-operative hip-fractured geriatric patients with and without complications. Gerontology. 2004;50:216–222. doi: 10.1159/000078350. [DOI] [PubMed] [Google Scholar]
- 10.Cerejeira J, Batista P, Nogueira V, Vaz-Serra A, Mukaetova-Ladinska EB. The stress response to surgery and postoperative delirium: evidence of hypothalamic-pituitary-adrenal axis hyperresponsiveness and decreased suppression of the GH/IGF-1 Axis. J Geriatr Psychiatry Neurol. 2013;26:185–194. doi: 10.1177/0891988713495449. [DOI] [PubMed] [Google Scholar]
- 11.Lee HJ, Hwang DS, Wang SK, Chee IS, Baeg S, Kim JL. Early assessment of delirium in elderly patients after hip surgery. Geropsychiatry. 2011;8:340–347. doi: 10.4306/pi.2011.8.4.340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Macdonald A, Adamis D, Treloar A, Martin F. C-reactive protein levels predict the incidence of delirium and recovery from it. Age Ageing. 2007;36:222–225. doi: 10.1093/ageing/afl121. [DOI] [PubMed] [Google Scholar]
- 13.McGrane S, Girard TD, Thompson JL, et al. Procalcitonin and C-reactive protein levels at admission as predictors of duration of acute brain dysfunction in critically ill patients. Crit Care. 2011;15:78. doi: 10.1186/cc10070. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pol RA, van Leeuwen BL, Izaks GJ, et al. C-reactive protein predicts postoperative delirium following vascular surgery. Ann Vasc Surg. 2014;28:1923–1930. doi: 10.1016/j.avsg.2014.07.004. [DOI] [PubMed] [Google Scholar]
- 15.Ritchie CW, Newman TH, Leurent B, Sampson EL. The association between C-reactive protein and delirium in 710 acute elderly hospital admissions. Int Psychogeriatr. 2014;26:717–724. doi: 10.1017/S1041610213002433. [DOI] [PubMed] [Google Scholar]
- 16.Zhang Z, Pan L, Deng H, Ni H, Xu X. Prediction of delirium in critically ill patients with elevated C-reactive protein. J Crit Care. 2014;29:88–92. doi: 10.1016/j.jcrc.2013.09.002. [DOI] [PubMed] [Google Scholar]
- 17.Dillon ST, Vasunilashorn SM, Ngo L, et al. Higher C-reactive protein levels predict postoperative delirium in older patients undergoing major elective surgery: A longitudinal nested case-control study. Biol Psychiatry. 2017;81:145–153. doi: 10.1016/j.biopsych.2016.03.2098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Schmitt EM, Marcantonio ER, Alsop DC, et al. Novel risk markers and long-term outcomes of delirium: The Successful Aging after Elective Surgery (SAGES) study design and methods. J Am Med Dir Assoc. 2012;13:1–10. doi: 10.1016/j.jamda.2012.08.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Schmitt EM, Saczynski JS, Kosar CM, et al. The Successful Aging after Elective Surgery Study: Cohort description and data quality procedures. J Am Geriatr Soc. 2015;63:2463–2471. doi: 10.1111/jgs.13793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Saczynski JS, Kosar CM, Xu G, et al. A tale of two methods: Chart and interview methods for identifying delirium. J Am Geriatr Soc. 2014;62:518–524. doi: 10.1111/jgs.12684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Inouye SK, van Dyck CH, Alessi CA, Balkin S, Siegal AP, Hortwitz RI. Clarifying confusion: The Confusion Assessment Method. A new method for detection of delirium. Ann Intern Med. 1990;113:941–948. doi: 10.7326/0003-4819-113-12-941. [DOI] [PubMed] [Google Scholar]
- 22.Wei LA, Fearing MA, Sternberg E, Inouye SK. The Confusion Assessment Method (CAM): A systematic review of current usage. J Am Geriatr Soc. 2008;56:823–830. doi: 10.1111/j.1532-5415.2008.01674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Inouye SK, Leo-Summers L, Zhang Y, Bogardus ST, Jr, Leslie DL, Agostini JV. A chart-based method for identification of delirium: validation compared with interviewer ratings using the Confusion Assessment Method. J Am Geriatr Soc. 2005;53:312–318. doi: 10.1111/j.1532-5415.2005.53120.x. [DOI] [PubMed] [Google Scholar]
- 24.Vasunilashorn SM, Marcantonio ER, Gou Y, et al. Quantifying the severity of a delirium episode throughout hospitalization: The combined importance of intensity and duration. J Gen Int Med. 2016;31:1164. doi: 10.1007/s11606-016-3671-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Inouye SK, Kosar CM, Tommet D, et al. The CAM-S: Development and validation of a new scoring system for delirium severity in 2 cohorts. Ann Intern Med. 2014;160:526–533. doi: 10.7326/M13-1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Jones RN, Rudolph JL, Inouye SK, et al. Development of a unidimensional composite measure of neuropsychological functioning in older cardiac surgery patients with good measurement precision. J Clin Exp Neuropsych. 2010;32:1041–1049. doi: 10.1080/13803391003662728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40:373–383. doi: 10.1016/0021-9681(87)90171-8. [DOI] [PubMed] [Google Scholar]
- 28.Ridker PM. C-reactive protein: A simple test to help predict risk of heart attack and stroke. Circulation. 2003;108:81–85. doi: 10.1161/01.CIR.0000093381.57779.67. [DOI] [PubMed] [Google Scholar]
- 29.Inouye SK, Viscoli CM, Horwitz RI, Hurst LD, Tinetti ME. A predictive model for delirium in hospitalized early medical patients based on admission characteristics. Ann Intern Med. 1993;119:474–481. doi: 10.7326/0003-4819-119-6-199309150-00005. [DOI] [PubMed] [Google Scholar]
- 30.Marcantonio ER. Postoperative delirium: a 76-year-old woman with delirium following surgery. JAMA. 2012;308:73–81. doi: 10.1001/jama.2012.6857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Fricchione GL, Nejad SH, Esses JA, et al. Postoperative delirium. Am J Psychiatry. 2008;165:803–812. doi: 10.1176/appi.ajp.2008.08020181. [DOI] [PubMed] [Google Scholar]
- 32.Hoogland IC, Houbolt C, van Westerloo DJ, van Gool WA, van de Beek D. Systemic inflammation and microglial activation: systematic review of animal experiments. J Neuroinflammation. 2015;12:114. doi: 10.1186/s12974-015-0332-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.van Munster BC, Aronica E, Zwinderman AH, Eikelenboom P, Cunningham C, de Rooij SEJA. Neuroinflammation in delirium: A postmortem case-control study. Rejuv Res. 2011;14:615–622. doi: 10.1089/rej.2011.1185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mesquita SD, Ferreira AC, Sousa JC, Correia-Neves M, Sousa N, Marques F. Insights on the pathophysiology of Alzheimer’s disease: The cross talk between amyloid pathology, neuroinflammation and the peripheral immune system. Neurosci Biobehav Rev. 2016;68:547–562. doi: 10.1016/j.neubiorev.2016.06.014. [DOI] [PubMed] [Google Scholar]
- 35.Aisen PS, Davis KL. Inflammatory mechanisms in Alzheimer’s disease: Implications for therapy. Am J Psychiatry. 1994;151:1105–1113. doi: 10.1176/ajp.151.8.1105. [DOI] [PubMed] [Google Scholar]
- 36.Glymour MM, Weuve J, Chen JT. Methodological challenges in causal research on racial and ethnic patterns of cognitive trajectories: measurement, selection, and bias. Neuropsychol Rev. 2008;18:194–213. doi: 10.1007/s11065-008-9066-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schisterman EF, Cole SR, Platt RW. Overadjustment bias and unnecessary adjustment in epidemiologic studies. Epidemiology. 2009;20:488–495. doi: 10.1097/EDE.0b013e3181a819a1. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table S1: Associations of C-reactive protein with postoperative delirium
Supplementary Table S2: Associations of C-reactive protein with postoperative delirium feature severity (peak CAM-S scores)
Supplementary Table S3: Inouye Risk Score Factors by Delirium Status
Supplementary Appendix S1: SAGES Study Group


