Abstract
Liver possesses many critical functions such as synthesis, detoxification, and metabolism. It continually receives nutrient-rich blood from gut, which incidentally is also toxin-rich. That may be why liver is uniquely bestowed with a capacity to regenerate. A commonly studied procedure to understand the cellular and molecular basis of liver regeneration is that of surgical resection. Removal of two-thirds of the liver in rodents or patients instigates alterations in hepatic homeostasis, which are sensed by the deficient organ to drive the restoration process. Although the exact mechanisms that initiate regeneration are unknown, alterations in hemodynamics and metabolism have been suspected as important effectors. Key signaling pathways are activated that drive cell proliferation in various hepatic cell types through autocrine and paracrine mechanisms. Once the prehepatectomy mass is regained, the process of regeneration is adequately terminated. This review highlights recent discoveries in the cellular and molecular basis of liver regeneration.
Keywords: partial hepatectomy, cellular and molecular basis, hepatocyte proliferation, inflammation, stellate cells, endothelial cells, initiation, signaling pathways, hepatocytes, nonparenchymal cells
Liver regeneration (LR) remains an extensively studied topic, as the liver is the only organ capable of complete regrowth after 70% removal. The first report of surgically induced liver regeneration dates back to 1931: Higgins and Anderson describe a compensatory hyperplasia after removal of two-thirds of a liver mass via a partial hepatectomy (PHx) in rats.1 Our understanding of LR has increased over the past several decades, yet hepatocyte death and a lack of regeneration remain the most frequent causes of liver disease-related deaths.2,3 Liver transplant is the only curative treatment for end-stage liver disease, which is a sequela of many chronic hepatic diseases.4 However, the lack of donor organs makes liver transplantation an unlikely option for most patients supporting the need for innovative therapies that may be able to stimulate regeneration in the diseased liver. Living-donor transplant is another treatment option, which relies on the successful regeneration of the healthy liver in the donor and recipient alike. However, recipients can experience small-for-size syndrome,5 where the transplanted liver fails to regenerate to full size, causing hepatocyte dysfunction and liver failure. Lastly, a subset of hepatocellular carcinoma patients undergo surgical resection6; however, the remnant liver may exhibit poor regenerative capacity resulting from underlying pathologies including cirrhosis.7 These clinical examples of defective LR provide a motive to better understand the kinetics and molecular machinery involved in successful LR to develop improved therapeutic options for liver disease patients. In this review, we will summarize novel insights into the initiation and progression of LR, with an emphasis on recent discoveries in the field.
Signaling Mechanisms
Optimal LR is a function of various signaling pathways being turned on and off at specific times during the process. Many studies have revealed these pathways to function in tandem such that genetic deletion of a single pathway is compensated quite adequately by redundant signaling mechanisms, leading to a delay rather than abolition of the regenerative process. Hepatic progenitors or oval cells can help with liver repair if signaling mechanisms inadequate, but will not be discussed in the current review. A comprehensive list of known factors with a role in LR is included in Table 1. A broad overview of recent discoveries in some signaling molecules with key roles in LR is presented herein.
Table 1.
Signaling mechanisms important in liver regeneration
| Mitogen | Cellular source | Primary or auxiliary | Function | Citation(s) |
|---|---|---|---|---|
| Hepatocyte growth factor (HGF) | Stellate cells, Kupffer cells, resident and bone marrow-derived endothelial cells, platelets | Primary | Hepatocyte proliferation, DNA synthesis | 1,8,38,a |
| Epidermal growth factor (EGF) | Brunner’s glands of duodenum, platelets | Primary | Hepatocyte proliferation, DNA synthesis | 1,8,38,a |
| Transforming growth factor α (TGFα) | Hepatocytes | Primary | Hepatocyte proliferation, perhaps at later stages, endothelial cell proliferation | 1,8,a |
| Interleukin 6 (IL6) | Kupffer cells | Auxiliary | Hepatocyte DNA/ protein synthesis, biliary epithelial cell proliferation | 1,8,a |
| Tumor necrosis factor α (TNFα) | Kupffer cells | Auxiliary | Regulates IL6 secretion | 1,8,a |
| Urokinase plasminogen activator (uPA) & metalloproteinases (MMPs) | Endothelial cells, stellate cells | Auxiliary | Remodel extracellular matrix prior to hepatocyte proliferation | 9,46,47,a |
| Wnts | Endothelial cells (Wnt2, others), Kupffer cells (unknown Wnts), Hepatocytes | Unknown | Induces β-catenin activation and in turn cyclin-D1 expression to promote hepatocyte cell cycle entry | 10–13 |
| Vascular endothelial growth factor (VEGF) | Kupffer cells | Auxiliary | Induces endothelial cell proliferation and angiogenesis | 22 |
| Interleukin 22 (IL22) & Purinergic receptor P2X1 (P2X1) | Lymphocytes | Primary and Auxiliary | Promote IL6 pathway. Also has direct mitogenic effect on hepatocytes | 29–31 |
| Interleukin 17 (IL17) | Lymphocytes | Primary and Auxiliary | Promotes hepatocyte proliferation directly and through IL6 | 32,34 |
| Interleukin 4 (IL4) | Eosinophils, NKT cells | Primary and Auxiliary | Induces FoxM1 and cyclin-B1 | 35 |
| Serotonin | Platelets | Auxiliary | Induces hepatocyte mitosis through TGFβ inhibition | 40 |
| Platelet-derived growth factor receptor β (PDGFRβ) | Stellate cells | Auxiliary | Protects liver from injury during LR | 49 |
| Angiopoietin-2 (Ang2) | Endothelial cells | Auxiliary | Downregulation during LR enables hepatocyte proliferation | 52 |
| Osteopontin | Biliary epithelial cells | Auxiliary | Activates macrophages | 58 |
| Farnesoid X receptor (FXR) | Hepatocytes | Auxiliary | Protects against bile-toxicity and promotes liver growth | 69 |
| ATP binding cassette subfamily C member 3 (Abcc3) | Hepatocytes | Auxiliary | May activate FXR and regulate hepatic bile content during LR | 71 |
| G-protein-coupled bile acid receptor 1 (TGR5) | Hepatocytes, endothelial cells | Auxiliary | Stimulates nitric oxide production from endothelial cells, protects from bile acid accumulation and stimulate LR after PHx | 72,73 |
| Purinergic receptor P2X4 | Hepatocytes | Auxiliary | Activated by ATP released after PHx, indirectly induces cyclin-D1 | 75 |
| Nitric oxide synthase (NOS) | Endothelial cells | Auxiliary | Stimulate immediate early gene expression | 76 |
| Hypoxia inducible factor 1 (Hif1α) | Hepatocytes | Auxiliary | Activates VEGF to induce angiogenesis | 77,78 |
| Downregulated miRNAs: miR-503, miR-23a, miR-150, miR-663, miR-654, miR-33 | Auxiliary | Promote cell-cycle entry | 15,17 | |
| Up-regulated miRNAs: miR-126, miR-130a, miR-20a, miR-520e, miR-330, miR-21 | Auxiliary | Promote cell-cycle entry | 15,16 | |
| lncRNA-LALR1 | Auxiliary | Regulation of cyclin-D1 via β-catenin | 18 |
Mitogens
Several mitogens, primary or auxiliary, are required for complete LR including hepatocyte growth factor (HGF), epidermal growth factor (EGF), transforming growth factor α (TGFα), interleukin 6 (IL6), and tumor necrosis factor α (TNFα), and have been extensively reviewed elsewhere.1,8 These mitogens are produced by the nonparenchymal cells, platelets (discussed in subsequent sections) and other organs, and stimulate transcription factors to produce an immediate early gene signature evident within the first 30 minutes after PHx. The degradation of extracellular matrix (ECM) products is also a well-known early event after PHx, stimulated by urokinase plasminogen activator and metalloproteinases, also reviewed elsewhere.9 β-Catenin enters the nucleus within 5 minutes in rats,10 within hours in mice and the liver-specific β-catenin knockout mice display a Wnt-dependent lack of regeneration until 72 to 96 hours after PHx.11,12 Further, when liver-specific endothelial cells (LSECs) are unable to secrete HGF and Wnt2, LR is impaired,13 again highlighting the importance of these growth factors and an interplay of hepatocytes and NPCs during the process. A more comprehensive cell-specific signaling is reviewed in forthcoming sections.
No study has identified a complete failure of regeneration following loss of growth factor/s. Very recently, Dr. Michalopoulos’s group showed that dual inhibition of MET, the HGF receptor, and epidermal growth factor receptor (EGFR) led to complete block of LR with mice dying 2 to 3 weeks after PHx. Death occurred from a lack of basic liver functions including urea and fatty acid metabolism and the lack of cell replication through downregulation of ERK, mTOR, and AKT.14 These findings identify for the first time, limits within the hepatic redundancy program during the LR process, and highlight the importance of HGF and EGF signaling.
Noncoding RNAs
MicroRNAs
In recent years noncoding RNAs have been identified as contributors to LR. MicroRNAs (miRNAs) are noncoding RNAs that play imperative roles in regulating cell cycle entry. Recently, liver biopsies obtained at several stages from acute liver failure patients who received an auxiliary liver transplant, were subjected to miRNA array. Differential miRNA signatures were present in patients with successful LR and patients with failed LR after transplant. Although a comprehensive list of disparately regulated miRNAs is exhaustive, the study showed early downregulation of miR-503, miR-23a, miR-150, miR-663, and miR-654 in patients with successful regeneration, which was evident by the upregulation of cyclin-D1, TNFα, survivin, and other cell-cycle entry genes.15 Further, miR-126, miR-130a, miR-20a, miR-520e, and miR-330 upregulation was associated with angiogenesis and the expression of LR mediators. Patients lacking LR showed the downregulation of miR-152 and upregulation of miRNAs including miR-150 and miR-let-7i.15 Other studies reported miR-21 to be rapidly upregulated following PHx causing increased cyclin-D1 expression.16 miR-33, originally shown to regulate cholesterol metabolism through the regulation of sterol regulatory element binding protein, was recently shown to inhibit the expression of both cyclin-dependent kinase-6 (CDK6) as well as cyclin-D1 (CCND1) via direct interaction with 3’UTR, which contains conserved sequence miR-33 binding sequences. Thus, by regulating expression of CDK6 and CCND1, miR-33 regulated hepatocyte proliferation, which was further validated by suppressing miR-33 after PHx, which promoted LR.17
Long noncoding RNAs
A different class of noncoding RNAs, long noncoding RNAs (lncRNAs) also regulate LR after PHx. Expression profiling from liver samples at different time points after PHx (0–24 hours), revealed 1,231 lncRNAs to be differentially expressed. Intriguingly, assessment using a series test of cluster analysis identified the Wnt/β-catenin pathway to be the most highly regulated pathway via lncRNAs, followed by metabolism, cell cycle, DNA replication, and others. In fact, lncRNA-LALR1 was identified to regulate cyclin-D1 expression through activation of β-catenin signaling during PHx.18 Based on the importance of lncRNAs in regulating key signaling pathways, it is likely that lncRNAs will be highly relevant in modulating the process of LR.
Cellular Mechanisms
Hepatocyte proliferation after PHx is essential to help the lost hepatic mass to function optimally. This requires the cooperation of several cell types including both hepatic and extrahepatic cells. Hepatocytes are typically quiescent and well-differentiated cells, which are critical for delivering hepatic functions including synthesis, detoxication. and metabolism. It is important to realize that to proliferate, hepatocytes require paracrine signals, which are most likely originating from various cell types within the liver, or being brought in to the liver via portal circulation. Only in response to these signals do hepatocytes enter and progress through the cell cycle. When rats were injected with gliotoxin to induce nonparenchymal cell death, mitosis in hepatocytes was absent at 24 hours after PHx.19 In this section, we discuss the major contributions of various cell types in the LR process. The cellular circuitry of LR is complex (Fig. 1); here we present key findings from the last several years.
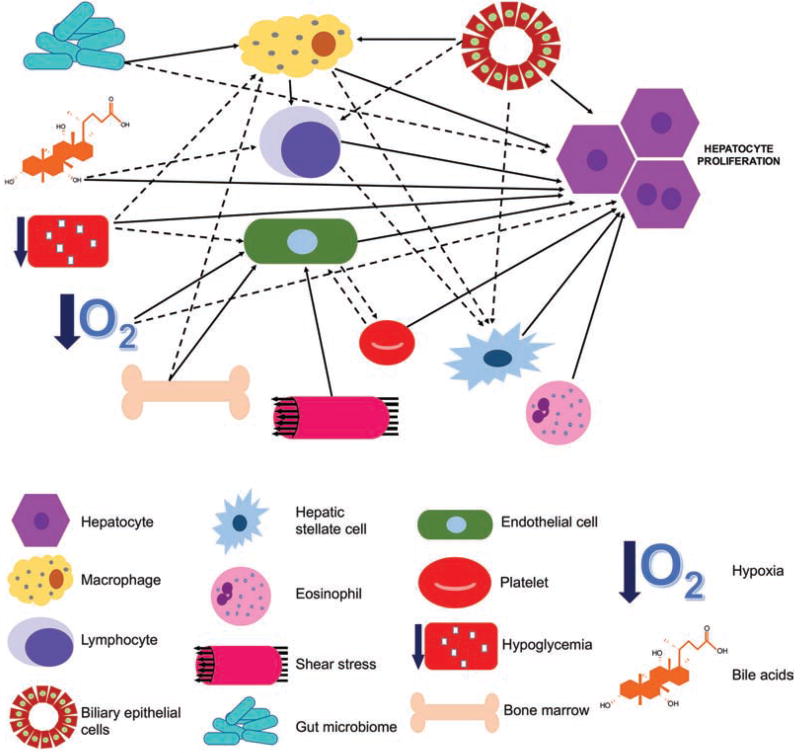
Fig. 1.
Cellular circuitry of liver regeneration. A complex crosstalk between various cellular components of liver cells and innate immune cells has been identified during the liver regeneration process. Similarly, evidence of cellular and tissue processes including altered metabolism, hypoxia, gut microbial products, bile acids, and hypoxia have been shown to be major determinants of regenerative response through effect on either hepatocyte directly or through nonparenchymal cells within the liver or cells recruited from the bone marrow. Solid lines represent previously published studies demonstrating a signaling relationship, dotted lines indicate the existence of preliminary evidence. Arrowheads indicate the directionality of cellular signaling and interactions.
Leukocytes
Macrophages
Liver macrophages (Kupffer cells) are required for LR because macrophage depletion via clodronate liposomes led to decreased HGF, IL6, and hepatocyte proliferation.20,21 Macrophages also contribute to angiogenesis, which is required for LR after PHx, in part through expression of vascular endothelial growth factor A (VEGF-A).22 Further, macrophages produce Wnts, which are required for β-catenin-dependent hepatocyte proliferation. We showed that mice with a deletion of Wntless (protein required specifically for Wnt secretion) in monocytes and macrophages, have low cyclin-D1 expression along with decreased numbers of hepatocytes in S-phase at 40 hours after PHx.12
There is also evidence that circulating monocytes are required for complete LR. After PHx, Kupffer cells remain in the space of Disse and are absent from the sinusoids. However, macrophages derived from circulating monocytes interact with the endothelium peaking at 40 hours. This correlated with increases in liver monocyte adhesion molecules including CCR2, VCAM-1, and ICAM-1, supporting increased monocyte recruitment to the liver. In CD11b knockout mice that lack the leukocyte adhesion receptor, there was a marked decrease in monocyte-derived macrophages in the sinusoids, correlating with a lower angiogenesis and hepatocyte proliferation. Further, expression of sprouting factors including Wnt5a and Notch1 were reduced in CD11b knockout mice after PHx.23 Thus, both Kupffer cells and infiltrating macrophages are important for angiogenesis and hepatocyte proliferation after PHx.
Lymphocytes
T lymphocytes have been shown to contribute to LR. Mice lacking T cells show decreased DNA synthesis in hepatocytes after PHx, which is mediated through T-cell-surface lymphotoxin.24 The transfer of splenocytes from WT mice improved LR in T-cell-deficient mice.
Other subsets of lymphocytes such as natural killer (NK) and natural killer T (NKT) cells that constitute the majority of innate immunity in the liver, are also relevant in LR.25 Depleting NK and NKT cells together reduced numbers of BrdU- and proliferating cell nuclear antigen-positive (PCNA+) hepatocytes 48 hours after PHx, which was accompanied by a decrease in cyclin-D1 expression.26 There was a lack of increases in TNFα, IL6, and HGF in NK- and NKT-cell-depleted animals. Interestingly, depleting either NK or NKT cells alone had no impact on LR, suggesting redundant functions of the two cells.26 However, a more profound contribution of NK cells in LR could be implied based on LR studies in Rag2/common gamma-deficient mice that lack T, B, NKT, and NK cells versus Rag1-deficient mice, which lack T, B, and NKT cells, but retain NK cells. Although the former mice lacked hepatocyte proliferation, the latter showed normal LR after PHx.27 One concern with these observations is the Rag2/common gamma-deficient mice may have impaired LR due to the interaction of T, B, and NK cells and not from the loss of NK cells itself, warranting further confirmation.28 In fact, some reports suggest depleting NK cells alone enhances LR.26
A novel mechanism of how lymphocytes contribute to LR is via production of IL22, which is regulated through the adenosine triphosphate (ATP) receptor P2X1. Inhibiting IL22 or upstream P2X1 impaired LR.29 IL22 is a key cytokine shown to promote IL6/Stat3 activation and hepatocyte proliferation, and liver-specific IL22 transgenic mice show accelerated LR after PHx.30,31 Similarly, lymphocyte-specific inhibition of IL17 or IL4 (NKT cells) also impairs LR.32–34
Eosinophils
Additional cells of innate immunity including eosinophils have also recently been shown to play a role in LR. A 2.4-fold increase in the number of eosinophils was observed after PHx, likely due to increased eotaxin-1, a chemotactic factor for eosinophils.35 The functional role of eosinophils in LR was convincingly shown by a blunted hepatocyte proliferation between 36 to 72 hours in ΔdblGATA mice that carry a mutation in the Gata1 promoter and lack eosinophils. Because eosinophils are a known source of IL4, which has been previously shown to play a role in tissue restitution,36 the authors tested IL4 secretion by the infiltrating cells. Using reporter and knock-in mice, IL4 was shown to be secreted by eosinophils and acted on hepatocytes. Based on functional redundancy between IL4 and IL13, double knockout for these two cytokines also showed a defect in LR in the form of a notable decrease in BrdU incorporation and decreased in Ki-67 staining indicating reduced numbers of hepatocytes in S-phase of cell cycle.35 Transcriptomic analysis and gene ontology studies revealed alterations in cell cycle, nuclear division, and mitosis-related genes, suggesting the two cytokines to be critical in promoting entry of quiescent hepatocytes into cell cycle. Specifically, the double knockouts revealed the low expression of FoxM1, a well-known regulator of LR, which in turn regulates cyclin-B1 expression.37 The effect of IL4 on hepatocyte proliferation was due to multiple mechanisms. Although its role in macrophage polarization is well known, the effect on LR was shown to be not indirectly through macrophages.36 Instead, IL4 was shown to be important in optimum immunoglobulin M deposition in livers after PHx, which is critical for complement activation and the regulation of LR. This effect on LR was only partly due to IL6 induction.33 The direct effect of IL4 on hepatocytes was observed in vitro in primary hepatocytes where IL4 treatment led to a significant increase in thymidine incorporation. This effect appeared to be due to the upregulation of FoxM1, a well-known proproliferative transcription factor. A direct mitogenic effect of IL4 was also evident in vivo, and mice treated for 5 days showed a substantial increase in hepatocyte proliferation.35 Further, primary hepatocyte cultures from IL-4Rα knockout and IL4/IL13 double knockout showed decreased proliferation.35 Thus, IL4 is necessary for optimal LR after PHx.
Platelets
After PHx, platelets accumulate in the liver sinusoids and space of Disse by 5 minutes in mice,38 where they interact with endothelial cells and hepatocytes to play critical roles in proliferation.39 Their function is in part mediated by serotonin release to induce mitosis in hepatocytes, as mice lacking platelet serotonin have impaired LR.40 Intriguingly, rats lacking serotonin transporter showed normal LR likely due to some circulating serotonin in the blood.41 Platelets also contain other growth factors required for LR including HGF and EGF.38 More recently, rats transfused with platelets during PHx had faster AKT and ERK activation, and showed increased hepatocyte proliferation42 in addition to an increase in GSK3β phosphorylation and cyclin-D1 expression.38
Additionally, a recent study showed the direct transfer of RNA from platelets to hepatocytes that induced hepatocyte proliferation in vitro.43 This study raises the question if such direct transfer may occur during LR.
Lastly, because platelets are known to impact leukocyte recruitment and function, there may be an indirect contribution of platelets in LR via regulation of hepatic inflammation.44
Hepatic Stellate Cells
During chronic liver injury, hepatic stellate cells (HSCs) transdifferentiate into activated myofibroblasts and are the major source of collagen deposition, which eventually leads to hepatic fibrosis and cirrhosis over time. Although their contribution to a wound-healing response in chronic injury is well established, their role in acute injury is less well known and will be discussed here, especially in the context of LR after PHx. Hepatic stellate cells are quiescent at baseline and become fibrogenic during injury. During LR, HSCs penetrate hepatocyte clumps surrounding capillaries and become activated.1,45 Hepatic stellate cells also regulate extracellular matrix degradation during LR through increases in uroplasminogen activator, matrix metalloproteinases, and several proteoglycans.46,47 Moreover, HSCs contribute to HGF at early stages and to mitoinhibitory TGFβ1 at later times, demonstrating dual roles during LR.48 Nejak-Bowen et al showed that when gliotoxin, which causes HSC death, was administered 24 hours before PHx, hepatocyte proliferation was decreased due to a lack of HGF. However, if gliotoxin was administered 5 days after PHx, hepatocyte proliferation was prolonged due to decreased HSC-specific extracellular matrix proteins including collagen and TGFβ1.19 These studies also suggest that HSCs are required for matrix remodeling, which is of relevance in the termination of LR.
A more recent study suggests that HSCs after PHx may prevent hepatic injury. Platelet-derived growth factor β (PDGFRβ) is induced in HSCs during liver injury to promote cell survival. Intriguingly, expression of this profibrotic molecule is also induced after PHx. When PDGFRβ knockout mice were subjected to PHx, hepatic injury markers serum alanine aminotransferase and aspartate aminotransferase were significantly elevated.49 There was also a concomitant reduction in the number of HSCs at 72 hours compared with controls. There was a marked reduction in PCNA and Ki-67 staining in the hepatocytes during LR, in addition to decreases in growth factors HGF, IGF1, and IGFPB1. One limitation of this study is that the authors utilized glial fibrillary acidic protein promoter to drive Cre expression, which is not specific to HSCs.50
Sinusoidal Endothelial Cells
The role of liver sinusoidal endothelial cells (LSECs) is twofold in LR. Liver sinusoidal endothelial cells are a known source of growth factors necessary for hepatocyte proliferation, such as HGF and Wnt2,13 and nitric oxide signaling (discussed later). Second, LSECs are responsible for forming new vasculature to support new liver mass. Typically, hepatocytes and macrophages secrete VEGF between 48 hours and 72 hours after PHx, which interacts with Flt1 receptors on endothelial cells to begin proliferation and angiogenesis.51 In fact, LSEC proliferation is augmented by angiogenesis simulants and suppressed by angiogenesis inhibitors during LR; however, neither impacts hepatocyte proliferation.52 But the dual roles of LSECs in hepatocyte proliferation and angiogenesis are not mutually exclusive. Transcriptomic analysis on LSECs isolated from PHx and sham animals after 24 hours identified angiopoietin-2 (Ang2) to be significantly downregulated. Angiopoietin-2 and its receptor Tie2 are expressed solely in LSECs, and Ang2 knockout mice have enhanced hepatocyte proliferation through the downregulation of TGFβ1. Angiopoietin-2 knockout mice also show decreased LSEC proliferation concomitant with decreased expression of angiocrine factors VEGFR2 and Wnt2, and reduced angiogenesis. Together, this suggests that LSECs downregulate Ang2 to promote hepatocyte proliferation via TGFβ1 suppression, followed by a dynamic switch, leading to an increase in Ang2 expression to induce LSEC proliferation and angiogenesis via VEGFR2 and Wnt2 signaling.53
Bone marrow-derived endothelial cells have been shown to also contribute to LR. Bone marrow cells enter the liver and localize in the sinusoids after PHx, where up to 70% differentiate into LSECs.54 In rat livers, CD133+ LSEC subpopulation was identified to be bone marrow-derived. The number of CD133+ LSECs increased from 20% in sham-operated to 50% in PHx rats. Transplantation of male bone marrow into female rats led to 25% of LSECs being Y chromosome positive by 72 hours after PHx. Further, irradiated rats have impaired LR, which is rescued with infusion of bone marrow-derived LSECs at 24 hours after PHx. When green fluorescent protein-negative (GFP−) rats were transplanted with GFP+ bone marrow, greater HGF expression was evident in GFP+ LSECs compared with GFP- LSECs, supporting the claim that bone marrow-derived LSECs are the major endothelial cell source of HGF after PHx.55 Liver sinusoidal endothelial cells isolated 6 hours and 24 hours after PHx secreted more HGF in the media, which augmented hepatocyte proliferation.56 Hepatocyte growth factor levels increase in plasma within 1 hour after PHx in rats, which may not be from LSECs, but from HSCs and Kupffer cells. Regardless, bone marrow-derived LSECs are recruited to the liver and contribute to HGF and perhaps other factors to initiate LR.
Biliary Epithelial Cells
The contribution of biliary epithelial cells (BECs) to LR is not extensively studied. Biliary epithelial cells have been studied extensively in cholestatic injury and ductular reaction, none of which occur after PHx. However, despite constituting only 3 to 5% of liver mass, BECs are required for successful LR, as they are the main source of osteopontin necessary to recruit and activate macrophages in the liver.57 Osteopontin knockout mice experience impaired LR because of blunted macrophage activity and decreased IL6.58 Biliary epithelial cells have recently been isolated at several time points after PHx in rats to perform gene array.57 Around a thousand genes each were upregulated or downregulated. Many upregulated genes pertained to cell cycle, cellular transport, metabolism, and signal transduction. Intriguingly, genes involved in signal transduction at 2 to 6 hours after PHx primarily involved GTPase-mediated signaling, and by 12 to 24 hours were mainly composed of G-protein coupled receptor signaling and Wnt signaling.57 This transcriptomic analysis suggests proactive change in signaling in BECs after LR, and warrants further investigation. It is likely that BECs not only contribute in the restitution of the intrahepatic biliary tree following PHx, but also are a source of factors that may directly or indirectly through inflammatory cell recruitment help in hepatocyte proliferation, and eventually in regaining hepatic mass.
Biomechanical and Miscellaneous Mechanisms
In addition to cell–molecule circuits that dictate the initiation of LR, increasing evidence suggests unique cellular and tissue processes that may be of utmost relevance in initiating and driving the process (Fig. 1). The impetus for such discoveries has been an intriguing void in our understanding of how liver realizes a loss in its mass for the first time. Here, we discuss some hepatic homeostatic processes, which may be disrupted by hepatic resection; such alterations can be sensed by the remnant liver as triggers for the initiation of LR.
Metabolic Changes
Considering the liver’s major role in metabolic regulation, it is likely that metabolic alterations occur and are sensed immediately by the remnant liver after PHx.
Lipid and Glucose Metabolism
Transient steatosis is evident during LR at early stages and is the result of fatty acid release from adipose tissue, increased fatty acid uptake, and lipogenesis, all of which leads to increased hepatocyte triglycerides.59–61 This allows for ATP production via fatty acid oxidation, thus highlighting a role for steatosis in energy production during LR. Similarly, mice develop hypoglycemia as early as 3 hours after PHx, causing decreased insulin levels and increased fat accumulation preceding hepatocyte proliferation.62,63 To compensate for the loss of glucose, several transcription factors involved in immediate early gene transcription such as CEBPβ and HNF1 upregulate glucose metabolism genes including glucose-6-phosphatase.63 Upon correction of this spontaneous hypoglycemia via dextrose administration for 14 hours after PHx in mice, circulating amino acid levels are altered and free fatty acids and liver triglycerides are reduced. Intriguingly, dextrose supplementation led to decreased activation of Tcf4, a β-catenin binding transcription factor, and subsequent reduction in cyclin-D1 expression, DNA replication, and liver-weight-to-body-weight ratio.64 Collectively, these data support previous findings that the in vitro addition of amino acids to hepatocytes induces cyclin-D1, and protein deprivation prevents cyclin-D1 expression in vitro and in vivo.65 The LR process thus integrates the processes of metabolism and proliferation and may be a chief cellular process initiating LR.
Bile Acid Metabolism
Increased bile acid (BA) concentration leads to liver toxicity. However, after PHx there is twice the amount of BA per liver mass in addition to increased blood BA while no hepatic toxicity is evident.66,67 In fact, bile flow is stimulated by PHx, which activates farnesoid X receptor (FXR), the master regulator of BA signaling to promote liver growth and prevent bile-induced toxicity.68,69 After PHx, mice on a cholic acid diet have enhanced LR; this is reversed by a resin diet, which sequesters intestinal BA. These findings were supported in FXR knockout mice, which had less liver growth and increased mortality after PHx.70
In recent years this mechanism has been further characterized. In mice without BA transporter Abcc3, neither cholic acid nor PHx elicited sufficient liver growth. Further, peak DNA synthesis occurred later in Abcc3 knockout mice after PHx. FXR activation was reduced after a cholic acid diet in these animals. Intriguingly, hepatic BA content was reduced in Abcc3 knockout mice after PHx, until late stages when levels surpassed controls, highlighting the effects of aberrant BA signaling in the liver.71
In addition to FXR, G-protein-coupled BA receptor 1 (TGR5) has recently been identified as a major BA receptor during LR. Intriguingly, activation of TGR5 by BA stimulates nitric oxide production via endothelial cells, discussed later as an important initiator of LR.72 TGR5 knockout mice have increased necrosis, cholestasis, inflammation, and liver injury after PHx, resulting from impaired LR and increased BA accumulation.73 Another receptor, the purinergic P2X4, plays a unique role in BA regulation during LR. Adenosine triphosphate is released immediately after PHx into the blood and bile as a result of increased portal pressure where it activates P2X4.74 P2X4 knockout mice had reduced cyclin-D1 and PCNA expression after PHx, and displayed jaundice and increased bilirubin and BA concentration. Further, areas of hepatocyte necrosis resembling bile infarcts were evident after PHx, and bile composition and flow were altered compared with control mice. As P2X4 is expressed mainly in hepatocyte lysosomes, it suggests exocytosis may affect BA regulation during PHx, which has not yet been reported.75
Thus BA signaling via receptors including FXR, TGR5, and P2X4, and the strict requirements to regulate BA levels to prevent toxicity during LR, may be important drivers of LR. Also, altered BA homeostasis may be sensed by remnant liver quite early to initiate the LR process.
Shear Stress
Removal of two-thirds of the liver forces the same prehepatectomy volume of blood through the remnant hepatic mass. This leads to enhanced shear stress immediately after PHx due to increased portal pressure, which precedes humoral factors entering the liver.42 Increased portal pressure alone can induce regeneration, evident in rats that underwent portal vein to restrict portal blood flow to one-third of the liver similarly to PHx. After portal vein ligation, rats exhibited similar proliferative factors and immediate early gene expression as rats after PHx, which was attenuated when given a nitric oxide synthase inhibitor.76 It remains unclear whether shear stress exerts a stimulatory effect directly on LSECs or indirectly impacts macrophages, HSCs, or hepatocytes to initiate LR.
Hypoxia
There is increasing evidence that hypoxia is also a proximal event during LR. Although hepatic oxygen concentrations are difficult to measure in vivo, hypoxia inducible factor 1 increases at early stages of LR and activates VEGF-A through inhibition of miR150.77,78 When hypoxia preconditioned bone marrow mesenchymal stem cells (BMMSCs) were injected into rats during 85% PHx, cyclin-D1 and PCNA positivity increased in hepatocytes, along with liver-weight-to-body-weight ratio and survival compared with rats infused with normoxic BMMSCs. VEGF increased in rats with hypoxic BMMSCs, and antagonizing VEGF blocked the effects of hypoxic BMMSCs on LR.79
Gut Microbiome
Because liver receives 70% of its blood supply through portal circulation, which carries gut-derived products including endotoxins and bacterial components, it is expected that gut microbiome plays an important role in regulating hepatic homeostasis.80 The gut–liver axis has now been implicated in hepatocarcinogenesis, nonalcoholic fatty liver disease, and alcoholic liver disease. A few studies are now beginning to study the link between gut microbiota and LR. Mice treated with oral ampicillin demonstrated impaired LR after PHx, resulting from alterations in commensal bacteria and increased NKT cells in the liver.81 Generally, Kupffer cells secrete IL12 required for NKT cell activation, and antibiotic-treated mice had increased Kupffer cell-dependent IL12 secretion possibly through gut-derived LPS. Overall, these data suggest bacteria from the microbiome modulate Kupffer cells to prevent NKT cell overactivation during LR. This is contradictory to previous findings, which identified depletion of NKT cells via anti-NK1.1 antibody-reduced hepatocyte proliferation after PHx.82 It is possible that physiological NKT cell activation is a bell-shaped curve during LR, and under- or overactivation, both are detrimental. Indeed, Yin et al identified Jα18 knockout mice lacking NKT cells had normal LR, but when NKT cells were activated via α-GalCer treatment, LR was inhibited.83 More studies to directly address the role of gut microbiota in LR are essential.
Conclusions
Liver regeneration remains one of the most curious features of the liver, as the organ regrows without any loss of hepatic function, although long-term studies are missing. There is tight regulation of LR kinetics, as many components cooperate to initiate, sustain, and eventually terminate LR. There is remarkable redundancy in cell-molecule circuitry that ensures the regain of hepatic mass, underscoring the importance of hepatic function to survival. Improved understanding of LR has allowed surgical therapies like split-liver transplantation, living donor transplantation, PHx for hepatic and metastatic tumors, and others. We are lagging in translating medical therapies to promote LR in the setting of end-stage liver disease, living donors, and small-for-size syndromes. We remain hopeful that our continued and superior understanding of cell-molecule signaling will enable innovative therapies to promote LR in a subset of patients. Indeed, agents such as triiodothyronine or thyroid receptor-β agonists like GC-1 may have clinical applications in settings of hepatic insufficiency.84–86
Acknowledgments
Satdarshan P. Monga is a consultant for Abbvie Pharmaceuticals and Dicerna Pharmaceuticals. He also has corporate research agreements with both companies. Dr. Monga has received funding support from NIH grants R01DK62277, R01DK100287, R01DK095498, R01CA204586 and an Endowed Chair for Experimental Pathology.
Abbreviations
- Ang2
angiopoietin-2
- ATP
adenosine triphosphate
- BA
bile acid
- BECs
biliary epithelial cells
- BMMSCs
bone marrow mesenchymal stem cells
- EGF
epidermal growth factor
- FXR
farnesoid X receptor
- GFP
green fluorescent protein
- HSCs
hepatic stellate cells
- HGF
hepatocyte growth factor
- IL6
interleukin 6
- LR
liver regeneration
- LSECs
liver sinusoidal endothelial cells
- NK
natural killer
- NKT
natural killer T
- PCNA
proliferating cell nuclear antigen
- PDGFRβ
platelet-derived growth factor β
- PHx
partial hepatectomy
- TGFα
transforming growth factor α
- TGR5
G-protein-coupled BA receptor 1
- TNFα
tumor necrosis factor α
- uPA
urokinase plasminogen activator
- VEGF
vascular endothelial growth factor
- VEGF-A
vascular endothelial growth factor A
References
- 1.Michalopoulos GK, DeFrances MC. Liver regeneration. Science. 1997;276(5309):60–66. doi: 10.1126/science.276.5309.60. [DOI] [PubMed] [Google Scholar]
- 2.Kuramitsu K, Sverdlov DY, Liu SB, et al. Failure of fibrotic liver regeneration in mice is linked to a severe fibrogenic response driven by hepatic progenitor cell activation. Am J Pathol. 2013;183(1):182–194. doi: 10.1016/j.ajpath.2013.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Luedde T, Kaplowitz N, Schwabe RF. Cell death and cell death responses in liver disease: mechanisms and clinical relevance. Gastroenterology. 2014;147(4):765–783. doi: 10.1053/j.gastro.2014.07.018. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bernardi M, Gitto S, Biselli M. The MELD score in patients awaiting liver transplant: strengths and weaknesses. J Hepatol. 2011;54(6):1297–1306. doi: 10.1016/j.jhep.2010.11.008. [DOI] [PubMed] [Google Scholar]
- 5.Yagi S, Uemoto S. Small-for-size syndrome in living donor liver transplantation. Hepatobiliary Pancreat Dis Int. 2012;11(6):570–576. doi: 10.1016/s1499-3872(12)60227-6. [DOI] [PubMed] [Google Scholar]
- 6.Ueno S, Sakoda M, Kubo F, et al. Kagoshima Liver Cancer Study Group. Surgical resection versus radiofrequency ablation for small hepatocellular carcinomas within the Milan criteria. J Hepatobiliary Pancreat Surg. 2009;16(3):359–366. doi: 10.1007/s00534-009-0069-7. [DOI] [PubMed] [Google Scholar]
- 7.Golse N, Bucur PO, Adam R, Castaing D, Sa Cunha A, Vibert E. New paradigms in post-hepatectomy liver failure. J Gastrointest Surg. 2013;17(3):593–605. doi: 10.1007/s11605-012-2048-6. [DOI] [PubMed] [Google Scholar]
- 8.Michalopoulos GK. Liver regeneration after partial hepatectomy: critical analysis of mechanistic dilemmas. Am J Pathol. 2010;176(1):2–13. doi: 10.2353/ajpath.2010.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Michalopoulos GK. Liver regeneration. J Cell Physiol. 2007;213(2):286–300. doi: 10.1002/jcp.21172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Monga SP, Pediaditakis P, Mule K, Stolz DB, Michalopoulos GK. Changes in WNT/beta-catenin pathway during regulated growth in rat liver regeneration. Hepatology. 2001;33(5):1098–1109. doi: 10.1053/jhep.2001.23786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tan X, Behari J, Cieply B, Michalopoulos GK, Monga SP. Conditional deletion of beta-catenin reveals its role in liver growth and regeneration. Gastroenterology. 2006;131(5):1561–1572. doi: 10.1053/j.gastro.2006.08.042. [DOI] [PubMed] [Google Scholar]
- 12.Yang J, Mowry LE, Nejak-Bowen KN, et al. β-catenin signaling in murine liver zonation and regeneration: a Wnt-Wnt situation! Hepatology. 2014;60(3):964–976. doi: 10.1002/hep.27082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ding BS, Nolan DJ, Butler JM, et al. Inductive angiocrine signals from sinusoidal endothelium are required for liver regeneration. Nature. 2010;468(7321):310–315. doi: 10.1038/nature09493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Paranjpe S, Bowen WC, Mars WM, et al. Combined systemic elimination of MET and epidermal growth factor receptor signaling completely abolishes liver regeneration and leads to liver decompensation. Hepatology. 2016;64(5):1711–1724. doi: 10.1002/hep.28721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Salehi S, Brereton HC, Arno MJ, et al. Human liver regeneration is characterized by the coordinated expression of distinct microRNA governing cell cycle fate. Am J Transplant. 2013;13(5):1282–1295. doi: 10.1111/ajt.12183. [DOI] [PubMed] [Google Scholar]
- 16.Ng R, Song G, Roll GR, Frandsen NM, Willenbring H. A microRNA-21 surge facilitates rapid cyclin D1 translation and cell cycle progression in mouse liver regeneration. J Clin Invest. 2012;122(3):1097–1108. doi: 10.1172/JCI46039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Cirera-Salinas D, Pauta M, Allen RM, et al. Mir-33 regulates cell proliferation and cell cycle progression. Cell Cycle. 2012;11(5):922–933. doi: 10.4161/cc.11.5.19421. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Xu D, Yang F, Yuan JH, et al. Long noncoding RNAs associated with liver regeneration 1 accelerates hepatocyte proliferation during liver regeneration by activating Wnt/β-catenin signaling. Hepatology. 2013;58(2):739–751. doi: 10.1002/hep.26361. [DOI] [PubMed] [Google Scholar]
- 19.Nejak-Bowen KN, Orr AV, Bowen WC, Jr, Michalopoulos GK. Gliotoxin-induced changes in rat liver regeneration after partial hepatectomy. Liver Int. 2013;33(7):1044–1055. doi: 10.1111/liv.12164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meijer C, Wiezer MJ, Diehl AM, et al. Kupffer cell depletion by CI2MDP-liposomes alters hepatic cytokine expression and delays liver regeneration after partial hepatectomy. Liver. 2000;20(1):66–77. doi: 10.1034/j.1600-0676.2000.020001066.x. [DOI] [PubMed] [Google Scholar]
- 21.Takeishi T, Hirano K, Kobayashi T, Hasegawa G, Hatakeyama K, Naito M. The role of Kupffer cells in liver regeneration. Arch Histol Cytol. 1999;62(5):413–422. doi: 10.1679/aohc.62.413. [DOI] [PubMed] [Google Scholar]
- 22.Nucera S, Biziato D, De Palma M. The interplay between macrophages and angiogenesis in development, tissue injury and regeneration. Int J Dev Biol. 2011;55(4–5):495–503. doi: 10.1387/ijdb.103227sn. [DOI] [PubMed] [Google Scholar]
- 23.Melgar-Lesmes P, Edelman ER. Monocyte-endothelial cell interactions in the regulation of vascular sprouting and liver regeneration in mouse. J Hepatol. 2015;63(4):917–925. doi: 10.1016/j.jhep.2015.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Tumanov AV, Koroleva EP, Christiansen PA, et al. T cell-derived lymphotoxin regulates liver regeneration. Gastroenterology. 2009;136(2):694–704. doi: 10.1053/j.gastro.2008.09.015. e4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dong Z, Wei H, Sun R, Tian Z. The roles of innate immune cells in liver injury and regeneration. Cell Mol Immunol. 2007;4(4):241–252. [PubMed] [Google Scholar]
- 26.Hosoya S, Ikejima K, Takeda K, et al. Innate immune responses involving natural killer and natural killer T cells promote liver regeneration after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2013;304(3):G293–G299. doi: 10.1152/ajpgi.00083.2012. [DOI] [PubMed] [Google Scholar]
- 27.Graubardt N, Fahrner R, Trochsler M, et al. Promotion of liver regeneration by natural killer cells in a murine model is dependent on extracellular adenosine triphosphate phosphohydrolysis. Hepatology. 2013;57(5):1969–1979. doi: 10.1002/hep.26008. [DOI] [PubMed] [Google Scholar]
- 28.Besnard A, Julien B, Gonzales E, Tordjmann T. Innate immunity, purinergic system, and liver regeneration: a trip in complexity. Hepatology. 2013;57(5):1688–1690. doi: 10.1002/hep.26312. [DOI] [PubMed] [Google Scholar]
- 29.Kudira R, Malinka T, Kohler A, et al. P2X1-regulated IL-22 secretion by innate lymphoid cells is required for efficient liver regeneration. Hepatology. 2016;63(6):2004–2017. doi: 10.1002/hep.28492. [DOI] [PubMed] [Google Scholar]
- 30.Park O, Wang H, Weng H, et al. In vivo consequences of liver-specific interleukin-22 expression in mice: Implications for human liver disease progression. Hepatology. 2011;54(1):252–261. doi: 10.1002/hep.24339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ren X, Hu B, Colletti LM. IL-22 is involved in liver regeneration after hepatectomy. Am J Physiol Gastrointest Liver Physiol. 2010;298(1):G74–G80. doi: 10.1152/ajpgi.00075.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Furuya S, Kono H, Hara M, Hirayama K, Tsuchiya M, Fujii H. Interleukin-17A plays a pivotal role after partial hepatectomy in mice. J Surg Res. 2013;184(2):838–846. doi: 10.1016/j.jss.2013.03.033. [DOI] [PubMed] [Google Scholar]
- 33.DeAngelis RA, Markiewski MM, Kourtzelis I, et al. A complement-IL-4 regulatory circuit controls liver regeneration. J Immunol. 2012;188(2):641–648. doi: 10.4049/jimmunol.1101925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Rao R, Graffeo CS, Gulati R, et al. Interleukin 17-producing γδT cells promote hepatic regeneration in mice. Gastroenterology. 2014;147(2):473–84.e2. doi: 10.1053/j.gastro.2014.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Goh YP, Henderson NC, Heredia JE, et al. Eosinophils secrete IL-4 to facilitate liver regeneration. Proc Natl Acad Sci U S A. 2013;110(24):9914–9919. doi: 10.1073/pnas.1304046110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Loke P, Gallagher I, Nair MG, et al. Alternative activation is an innate response to injury that requires CD4+ T cells to be sustained during chronic infection. J Immunol. 2007;179(6):3926–3936. doi: 10.4049/jimmunol.179.6.3926. [DOI] [PubMed] [Google Scholar]
- 37.Wang X, Kiyokawa H, Dennewitz MB, Costa RH. The Forkhead Box m1b transcription factor is essential for hepatocyte DNA replication and mitosis during mouse liver regeneration. Proc Natl Acad Sci U S A. 2002;99(26):16881–16886. doi: 10.1073/pnas.252570299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Murata S, Ohkohchi N, Matsuo R, Ikeda O, Myronovych A, Hoshi R. Platelets promote liver regeneration in early period after hepatectomy in mice. World J Surg. 2007;31(4):808–816. doi: 10.1007/s00268-006-0772-3. [DOI] [PubMed] [Google Scholar]
- 39.Matsuo R, Nakano Y, Ohkohchi N. Platelet administration via the portal vein promotes liver regeneration in rats after 70% hepatectomy. Ann Surg. 2011;253(4):759–763. doi: 10.1097/SLA.0b013e318211caf8. [DOI] [PubMed] [Google Scholar]
- 40.Lesurtel M, Graf R, Aleil B, et al. Platelet-derived serotonin mediates liver regeneration. Science. 2006;312(5770):104–107. doi: 10.1126/science.1123842. [DOI] [PubMed] [Google Scholar]
- 41.Matondo RB, Punt C, Homberg J, et al. Deletion of the serotonin transporter in rats disturbs serotonin homeostasis without impairing liver regeneration. Am J Physiol Gastrointest Liver Physiol. 2009;296(4):G963–G968. doi: 10.1152/ajpgi.90709.2008. [DOI] [PubMed] [Google Scholar]
- 42.Abshagen K, Eipel C, Vollmar B. A critical appraisal of the hemodynamic signal driving liver regeneration. Langenbecks Arch Surg. 2012;397(4):579–590. doi: 10.1007/s00423-012-0913-0. [DOI] [PubMed] [Google Scholar]
- 43.Kirschbaum M, Karimian G, Adelmeijer J, Giepmans BN, Porte RJ, Lisman T. Horizontal RNA transfer mediates platelet-induced hepatocyte proliferation. Blood. 2015;126(6):798–806. doi: 10.1182/blood-2014-09-600312. [DOI] [PubMed] [Google Scholar]
- 44.Jenne CN, Kubes P. Platelets in inflammation and infection. Platelets. 2015;26(4):286–292. doi: 10.3109/09537104.2015.1010441. [DOI] [PubMed] [Google Scholar]
- 45.Mabuchi A, Mullaney I, Sheard PW, et al. Role of hepatic stellate cell/hepatocyte interaction and activation of hepatic stellate cells in the early phase of liver regeneration in the rat. J Hepatol. 2004;40(6):910–916. doi: 10.1016/j.jhep.2004.02.005. [DOI] [PubMed] [Google Scholar]
- 46.Benyon RC, Arthur MJ. Extracellular matrix degradation and the role of hepatic stellate cells. Semin Liver Dis. 2001;21(3):373–384. doi: 10.1055/s-2001-17552. [DOI] [PubMed] [Google Scholar]
- 47.Gallai M, Sebestyén A, Nagy P, Kovalszky I, Onody T, Thorgeirsson SS. Proteoglycan gene expression in rat liver after partial hepatectomy. Biochem Biophys Res Commun. 1996;228(3):690–694. doi: 10.1006/bbrc.1996.1718. [DOI] [PubMed] [Google Scholar]
- 48.Taub R. Liver regeneration: from myth to mechanism. Nat Rev Mol Cell Biol. 2004;5(10):836–847. doi: 10.1038/nrm1489. [DOI] [PubMed] [Google Scholar]
- 49.Kocabayoglu P, Zhang DY, Kojima K, Hoshida Y, Friedman SL. Induction and contribution of beta platelet-derived growth factor signalling by hepatic stellate cells to liver regeneration after partial hepatectomy in mice. Liver Int. 2016;36(6):874–882. doi: 10.1111/liv.12933. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Mederacke I, Hsu CC, Troeger JS, et al. Fate tracing reveals hepatic stellate cells as dominant contributors to liver fibrosis independent of its aetiology. Nat Commun. 2013;4:2823. doi: 10.1038/ncomms3823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Shimizu H, Miyazaki M, Wakabayashi Y, et al. Vascular endothelial growth factor secreted by replicating hepatocytes induces sinusoidal endothelial cell proliferation during regeneration after partial hepatectomy in rats. J Hepatol. 2001;34(5):683–689. doi: 10.1016/s0168-8278(00)00055-6. [DOI] [PubMed] [Google Scholar]
- 52.Greene AK, Wiener S, Puder M, et al. Endothelial-directed hepatic regeneration after partial hepatectomy. Ann Surg. 2003;237(4):530–535. doi: 10.1097/01.SLA.0000059986.96051.EA. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Hu J, Srivastava K, Wieland M, et al. Endothelial cell-derived angiopoietin-2 controls liver regeneration as a spatiotemporal rheostat. Science. 2014:3436169416–419. doi: 10.1126/science.1244880. [DOI] [PubMed] [Google Scholar]
- 54.Fujii H, Hirose T, Oe S, et al. Contribution of bone marrow cells to liver regeneration after partial hepatectomy in mice. J Hepatol. 2002;36(5):653–659. doi: 10.1016/s0168-8278(02)00043-0. [DOI] [PubMed] [Google Scholar]
- 55.Wang L, Wang X, Xie G, Wang L, Hill CK, DeLeve LD. Liver sinusoidal endothelial cell progenitor cells promote liver regeneration in rats. J Clin Invest. 2012;122(4):1567–1573. doi: 10.1172/JCI58789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ping C, Xiaoling D, Jin Z, Jiahong D, Jiming D, Lin Z. Hepatic sinusoidal endothelial cells promote hepatocyte proliferation early after partial hepatectomy in rats. Arch Med Res. 2006;37(5):576–583. doi: 10.1016/j.arcmed.2005.12.004. [DOI] [PubMed] [Google Scholar]
- 57.Xu C, Chen X, Chang C, et al. Transcriptional profiles of biliary epithelial cells from rat regenerating liver after partial hepatectomy. Genes Genomics. 2012;34(3):245–256. [Google Scholar]
- 58.Wen Y, Feng D, Wu H, et al. Defective initiation of liver regeneration in osteopontin-deficient mice after partial hepatectomy due to insufficient activation of IL-6/Stat3 pathway. Int J Biol Sci. 2015;11(10):1236–1247. doi: 10.7150/ijbs.12118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Delahunty TJ, Rubinstein D. Accumulation and release of triglycerides by rat liver following partial hepatectomy. J Lipid Res. 1970;11(6):536–543. [PubMed] [Google Scholar]
- 60.Schofield PS, Sugden MC, Corstorphine CG, Zammit VA. Altered interactions between lipogenesis and fatty acid oxidation in regenerating rat liver. Biochem J. 1987;241(2):469–474. doi: 10.1042/bj2410469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tijburg LB, Nyathi CB, Meijer GW, Geelen MJ. Biosynthesis and secretion of triacylglycerol in rat liver after partial hepatectomy. Biochem J. 1991;277(Pt 3):723–728. doi: 10.1042/bj2770723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Weymann A, Hartman E, Gazit V, et al. p21 is required for dextrose-mediated inhibition of mouse liver regeneration. Hepatology. 2009;50(1):207–215. doi: 10.1002/hep.22979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Newberry EP, Kennedy SM, Xie Y, et al. Altered hepatic triglyceride content after partial hepatectomy without impaired liver regeneration in multiple murine genetic models. Hepatology. 2008;48(4):1097–1105. doi: 10.1002/hep.22473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Huang J, Schriefer AE, Cliften PF, et al. Postponing the hypoglycemic response to partial hepatectomy delays mouse liver regeneration. Am J Pathol. 2016;186(3):587–599. doi: 10.1016/j.ajpath.2015.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Nelsen CJ, Rickheim DG, Tucker MM, et al. Amino acids regulate hepatocyte proliferation through modulation of cyclin D1 expression. J Biol Chem. 2003;278(28):25853–25858. doi: 10.1074/jbc.M302360200. [DOI] [PubMed] [Google Scholar]
- 66.Csanaky IL, Aleksunes LM, Tanaka Y, Klaassen CD. Role of hepatic transporters in prevention of bile acid toxicity after partial hepatectomy in mice. Am J Physiol Gastrointest Liver Physiol. 2009;297(3):G419–G433. doi: 10.1152/ajpgi.90728.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Doignon I, Julien B, Serrière-Lanneau V, et al. Immediate neuroendocrine signaling after partial hepatectomy through acute portal hyperpressure and cholestasis. J Hepatol. 2011;54(3):481–488. doi: 10.1016/j.jhep.2010.07.012. [DOI] [PubMed] [Google Scholar]
- 68.Sainz GR, Monte MJ, Barbero ER, Herrera MC, Marin JJ. Bile secretion by the rat liver during synchronized regeneration. Int J Exp Pathol. 1997;78(2):109–116. doi: 10.1046/j.1365-2613.1997.d01-246.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Modica S, Gadaleta RM, Moschetta A. Deciphering the nuclear bile acid receptor FXR paradigm. Nucl Recept Signal. 2010;8:e005. doi: 10.1621/nrs.08005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Huang W, Ma K, Zhang J, et al. Nuclear receptor-dependent bile acid signaling is required for normal liver regeneration. Science. 2006;312(5771):233–236. doi: 10.1126/science.1121435. [DOI] [PubMed] [Google Scholar]
- 71.Fernández-Barrena MG, Monte MJ, Latasa MU, et al. Lack of Abcc3 expression impairs bile-acid induced liver growth and delays hepatic regeneration after partial hepatectomy in mice. J Hepatol. 2012;56(2):367–373. doi: 10.1016/j.jhep.2011.05.031. [DOI] [PubMed] [Google Scholar]
- 72.Keitel V, Reinehr R, Gatsios P, et al. The G-protein coupled bile salt receptor TGR5 is expressed in liver sinusoidal endothelial cells. Hepatology. 2007;45(3):695–704. doi: 10.1002/hep.21458. [DOI] [PubMed] [Google Scholar]
- 73.Péan N, Doignon I, Garcin I, et al. The receptor TGR5 protects the liver from bile acid overload during liver regeneration in mice. Hepatology. 2013;58(4):1451–1460. doi: 10.1002/hep.26463. [DOI] [PubMed] [Google Scholar]
- 74.Gonzales E, Julien B, Serrière-Lanneau V, et al. ATP release after partial hepatectomy regulates liver regeneration in the rat. J Hepatol. 2010;52(1):54–62. doi: 10.1016/j.jhep.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Besnard A, Gautherot J, Julien B, et al. The P2X4 purinergic receptor impacts liver regeneration after partial hepatectomy in mice through the regulation of biliary homeostasis. Hepatology. 2016;64(3):941–953. doi: 10.1002/hep.28675. [DOI] [PubMed] [Google Scholar]
- 76.Schoen JM, Wang HH, Minuk GY, Lautt WW. Shear stress-induced nitric oxide release triggers the liver regeneration cascade. Nitric Oxide. 2001;5(5):453–464. doi: 10.1006/niox.2001.0373. [DOI] [PubMed] [Google Scholar]
- 77.Maeno H, Ono T, Dhar DK, Sato T, Yamanoi A, Nagasue N. Expression of hypoxia inducible factor-1 alpha during liver regeneration induced by partial hepatectomy in rats. Liver Int. 2005;25(5):1002–1009. doi: 10.1111/j.1478-3231.2005.01144.x. [DOI] [PubMed] [Google Scholar]
- 78.Yu ZY, Bai YN, Luo LX, Wu H, Zeng Y. Expression of microRNA-150 targeting vascular endothelial growth factor-A is downregulated under hypoxia during liver regeneration. Mol Med Rep. 2013;8(1):287–293. doi: 10.3892/mmr.2013.1493. [DOI] [PubMed] [Google Scholar]
- 79.Yu J, Yin S, Zhang W, et al. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4(4):83. doi: 10.1186/scrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Liu HX, Keane R, Sheng L, Wan YJ. Implications of microbiota and bile acid in liver injury and regeneration. J Hepatol. 2015;63(6):1502–1510. doi: 10.1016/j.jhep.2015.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu X, Sun R, Chen Y, et al. Oral ampicillin inhibits liver regeneration by breaking hepatic innate immune tolerance normally maintained by gut commensal bacteria. Hepatology. 2015;62(1):253–264. doi: 10.1002/hep.27791. [DOI] [PubMed] [Google Scholar]
- 82.Nakashima H, Inui T, Habu Y, et al. Activation of mouse natural killer T cells accelerates liver regeneration after partial hepatectomy. Gastroenterology. 2006;131(5):1573–1583. doi: 10.1053/j.gastro.2006.08.028. [DOI] [PubMed] [Google Scholar]
- 83.Yin S, Wang H, Bertola A, et al. Activation of invariant natural killer T cells impedes liver regeneration by way of both IFN-γ- and IL-4-dependent mechanisms. Hepatology. 2014;60(4):1356–1366. doi: 10.1002/hep.27128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Alvarado TF, Puliga E, Preziosi M, et al. Thyroid hormone receptor β agonist induces β-catenin-dependent hepatocyte proliferation in mice: implications in hepatic regeneration. Gene Expr. 2016;17(1):19–34. doi: 10.3727/105221616X691631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Fanti M, Singh S, Ledda-Columbano GM, Columbano A, Monga SP. Tri-iodothyronine induces hepatocyte proliferation by protein kinase A-dependent β-catenin activation in rodents. Hepatology. 2014;59(6):2309–2320. doi: 10.1002/hep.26775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gebhardt R. Speeding up hepatocyte proliferation: how triiodothyronine and β-catenin join forces. Hepatology. 2014;59(6):2074–2076. doi: 10.1002/hep.26984. [DOI] [PubMed] [Google Scholar]