Abstract
Objective: This study reports the prevalence of Nonconvulsive Status Epilepticus (NCSE) in patients with altered mental status (AMS), and describes the clinical presentation, etiology, neurophysiological findings, neuroimaging, treatment, and outcome of NCSE in Qatar. Recording duration of continuous EEG monitoring was also discussed.
Methods: This was a 3-year, prospective, hospital-based study involving patients with AMS and continuous EEG monitoring admitted to the Emergency and ICUs of Hamad Hospital, Qatar. Patients with confirmed diagnosis of NCSE were compared to the patients who did not show EEG and clinical features compatible with NCSE. Descriptive statistics in terms of mean with standard deviation, as well as frequency and percentages for categorical variables, were calculated; Student’s t test as well as Chi-square tests or Fisher’s exact tests were applied. Logistic regressions NSCE was performed using significance level 0.05 for independent variables at univariate analysis.
Results: Number of patients with AMS and continuous EEG monitoring was 250. Number of patients with EEG compatible with NCSE: 65 (age range, 12–79 ys; m, 37; f, 28). Number of controls (defined as patients with EEG not compatible with NCSE): 185 (age range, 12–80 ys; m, 101; f, 84). Rate of occurrence of NCSE in patients with AMS: 26%. NCSE group was younger than controls (p < .001). Twenty patients with NCSE (31%) and 35 patients in the control group (19%) died. Death was more frequent in comatose NCSE compared to controls (p < .0007). NCSE proper and comatose NCSE had longer hospital stays than controls (p < .02 and p < .03, respectively). Complete recovery occurred in 26 NCSE patients (40%) and in 98 controls (53%) (p < .08). Twenty-one patients (31%) presented with refractory NCSE: 12 patients survived, 9 died.
Conclusion: This was the first prospective study reporting a high number of NCSE in Qatar, a small country in the MENA region. This prevalence (26%) was in the middle range. NCSE patients did not perform better than controls, outcome being worse with comatose NCSE. NCSE is an emergent condition warranting expedited diagnosis and management. Three days of continuous EEG monitoring were able to diagnose most cases of NCSE.
Keywords: cEEG monitoring, NCSE, epidemiology, treatment, outcome
Introduction
Non-convulsive status epilepticus (NCSE) is an electroclinical state associated with an altered mental status (AMS), but lacking convulsive motor activity [1].
It is difficult to diagnose in the obtunded/comatose patient. Such patients have often other serious medical conditions, and the diagnosis of NCSE is frequently delayed in these patients. Diagnosing NCSE demands a high degree of clinical suspicion [2] and for that reason likely remains under recognized.
The estimated incidence of status epilepticus (SE) in the United States is 15–20/100,000 cases per year [3], 63% of SE being NCSE [4]. Nonconvulsive seizures (NCSs)/NCSE occur with high frequency in the Intensive Care Unit (ICU) and the Emergency Department (ED): 8–48% of ICU patients may have NCSs/NCSE [5–8], many fatal [9–11].
Several recent studies from different geographic areas in the world, report the prevalence of NCSE in patients with AMS [12–16]; however, little is known of the burden of NCSE in some developing countries and particularly the Middle East and North African region (MENA). The only studies dealing with such incidence/prevalence of NCSE, come from MENA neighboring countries (Pakistan, India, Turkey and Israel) [17–21]. In addition, there is a lack of consensus regarding the optimal length of continuous EEG (cEEG) monitoring when looking for NCSE in patients with AMS. The duration of cEEG monitoring varies widely [22–25].
The objectives of this study are:
Calculate the rate of occurrence of NCSE among patients with AMS and coma admitted to the ED and ICUs of Hamad General Hospital (HGH), Doha, Qatar, using cEEG monitoring. There is a need for studies regarding the prevalence and morbidity of NCSE in MENA countries [26].
Describe the clinical presentation, etiology, neurophysiological and neuroimaging findings in patients with AMS, and determine the treatment and outcome in NCSE patients, comparing these to a matched control group with similar clinical presentations of AMS and coma.
Discuss the lack of consensus regarding cEEG monitoring duration in patients with AMS and propose our suggestions.
Methods
The study was conducted in accordance with Good Clinical Practice (GCP) guidelines and the Declaration of Helsinki. The Institutional Review Board (IRB) of Hamad Medical Hospital as well as the ethical committee of the same Institution reviewed and approved the study protocol. Written Informed consent was obtained from all subjects or relative(s) (caregivers)
Definition and selection of NCSE
NCSE traditionally involves the clinical picture of an AMS with diminished responsiveness, a diagnostic EEG and often a response to Antiseizure Drug (ASD) therapy; NCSE is traditionally considered to be present after 30 minutes of continuous non-convulsive seizure activity or after repeated seizures without recovery of consciousness between events [1], although more recent investigators suggest shorter durations.
To diagnose NCSE, Young’s criteria [27] of electrographic SE and modified criteria of Chong and Hirsch [28] were used; Also more recent researches like the new ILAE Definition and classification of Status Epilepticus [29] and EEG Salzburg Consensus Criteria for NCSE [30] have been applied for clinical and EEG diagnosis of NCSE. Additionally, frequent or continuous generalized spike wave discharges on EEG with changes in intensity or frequency, intermittent/recurrent epileptiform activity with ictal patterns that wax and wane, rhythmic and periodic discharges, subtle and discrete electrographic seizures, when lasting at least for 30 minutes, were categorized as NCSE [10,13,15]. In comatose patients epileptiform discharges faster than 2.5 Hz or Generalized Periodic Discharges (GPDs) (previously called Generalized periodic epileptiform discharges (GPEDs), Lateralized Periodic Discharges (LPDs) (previously Periodic lateralized epileptiform discharges (PLEDs) and Continuous 2/s GPDs with triphasic morphology (Previously Triphasic Waves (TWs) [31] of less than 2.5 Hz as well as Rhythmic Discharges (RDs) faster than 0.5 Hz, were also considered NCSE if they showed a characteristic EEG pattern and responded to a rapidly-acting ASD treatment with improvement in the EEG or in patient mental status, as reported by Bottaro et al. [13], Naeije et al. [15], Chong et al. [28], Trinka et al. [29], Bauer et al. [32]. These EEG patterns correspond to the Chong and Hirsch primary criteria # 3 [28].
In each patient suspected of presenting NCSE, at least two EEG specialists agreed independently that the EEG patterns were NCSE. When an “ictal” EEG pattern did not meet above criteria, a benzodiazepine trial was undertaken: the patient was considered a case of NCSE if dramatic improvement in EEG and/or level of consciousness occurred.
Patients selection for cEEG monitoring
cEEG monitoring was performed in all patients with AMS, aged 12 years or above, from the Emergency and ICUs [2,33]. Criteria for AMS were as follows: unexplained confusional state or change in behavior; mild to moderate obtundation or alteration in cognition and behavior from baseline; unexplained decrease in level of consciousness including after convulsive status epilepticus treatment [2,33]. Elderly patients with altered level of consciousness, inattention, disorganized thinking and a fluctuating course were also included as cases of delirium [15].
No cEEG monitoring was done: (1) in patients with open head injury (ies) (EEG recording not possible), (2) in patients prematurely discharged from the ED before the start of cEEG monitoring. Excluded were also, (3) Treated patients with Convulsive SE who did not develop NCSs/NCSE during 3 days of cEEG Monitoring, (4) patients whose relatives did not agree to sign consent form for cEEG monitoring and (5) ICU patients with an isoelectric EEG waiting for an apnea test to confirm brain death.
Control group and comparison with NCSE
All critically ill patients with a clinical presentation of AMS or coma, and where a cEEG monitoring failed to show NCSE in the first 72 hours, served as a control group.
All NCSE cases as a group, as well as NCSE subgroups and controls were compared: The variables included the clinical characteristics at hospital presentation, AMS etiology, acute medical conditions, head CT and MRI findings, laboratory findings, length of stay, recovery and outcome.
cEEG recording, monitoring and duration
cEEG recording, monitoring
EEGs were recorded using the International 10/20 system with 21 silver/silver chloride cup electrodes. The digital EEG signal was stored electronically and filtered for display. Typical settings for the high-pass filter and a low-pass filter were 0.5–1 and 70 Hz, respectively. An additional 50 Hz notch filter was used for extraneous electrical artifact; impedance was 100 and 5000 ohms. No MRI compatible electrodes were used.
Three well-trained EEG technologists performed EEG recordings; EEG recording was monitored several times a day by a neurologist. Two trained neurophysiologists (B.Mesraoua, D. Deleu) were in charge of Digital EEG reading; they were backed by four EEG experts (B. Uthman, L. Streletz, PW Kaplan, HG Wieser, all PIs in this study).
cEEG duration
The duration of cEEG was based on (1) the available data from the literature, mentioned above in the introduction section, (2) the presence or absence of specific cEEG monitoring features/abnormalities (e.g. NCSs/NCSE, rhythmic and periodic discharges, (3) their responses to treatment and (4) the patient’s condition (e.g. if NCSE/NCSs were treated and the patient recovered, cEEG monitoring was discontinued; if not, cEEG monitoring was continued).
Laboratory and head MRI/CT findings
A complete metabolic panel (including blood count, liver and renal functions, electrolytes) and MRI with diffusion-weighted imaging (MRI-DWI) and/or CT head, were performed in most NCSE cases and controls; imaging was performed either before or after cEEG monitoring, from hours to several days.
Treatment of NCSs/NCSE
NCSs were treated with benzodiazepines (lorazepam or diazepam) on detection. If they persisted, the same treatment as for NCSE was used. For NCSE treatment, we followed EFNS Guidelines and also the recent report by Glauser et al: Initial treatment IV benzodiazepines (lorazepam or diazepam) as bolus, followed by second-line ASDs including valproic acid, levetiracetam and phenytoin. If NCSE persisted, third-line ASD treatment (including topiramate), with or without IV ASDs (continuous infusions of midazolam, propofol and barbiturates) were given [34,35].
Most patients received more than 1 ASD. Anesthetics agents were used in refractory NCSE to induce an EEG burst suppression pattern. Suspected cases of Comatose NCSE were treated as mentioned above (section 2.1. Definition and Selection of NCSE). Control Cases did not receive ASDs treatment.
Outcome parameters
Primary outcomes parameters included seizure control, survival/death. Secondary outcomes parameters were complete recovery, and length of ICU and hospital stay.
Statistical methods
Descriptive statistics in terms of mean with standard deviation have been calculated for continuous variables. Frequency and percentages were calculated for categorical variables. Student’s t test was performed to examine differences between mean levels of NCSE and controls, outcome and morbidity. Chi-square tests or Fisher’s exact tests (wherever applicable) were applied to detect associations between categorical variables and NCSE vs controls, outcome and morbidity. Logistic regressions NCSE were performed using significance level 0.05 for independent variables at univariate analysis. p Value 0.05 (two tailed) was considered as statistical significant level. SPSS 22.0 statistical software was used for statistical analysis.
Results
Occurrence rate of convulsive SE (CSE), NCSs and NCSE
CSE group: 6 patients had CSE; only one of them who evolved to NCSE was included in this study.
NCSs group: 20 patients had NCSs; 6 of them (30%) responded to ASDs and did not develop NCSE; they were not included in this study; The 14 remaining patients with NCSs did not respond to ASDs and developed during the first and second day NCSE. These 14 patients (70%) were included in the study.
NCSE group: During the study period (2012–2014), a total of 250 patients with AMS or coma underwent cEEG monitoring and were included in this study. Sixty-two patients were excluded for the reasons explained above. After expert review, there were 65 confirmed cases of NCSE (Table 1). Hence the occurrence rate of NCSE was 65/250 (26%).
Table 1.
Characteristics of patients with NCSE and controls.
| Variable | NCSE (n, 65) | Controls (n, 185) | p Value |
|---|---|---|---|
| Age | 45.7 ± 19 | 52.3 ± 15.8 | .001 |
| Gender | M = 37/F = 28 | M = 101/F = 84 | .75 |
| Unresponsive/somnolent | 11 (17%) | 46 (25%) | .19 |
| Acute confusion | 7 (11%) | 18 (10%) | .81 |
| Severely decreased level of consciousness | 20 (31%) | 61 (33%) | .74 |
| Stupor/Coma | 27 (42%) | 60 (32%) | .23 |
| Subtle motor phenomena | 12 (18%) | 8 (4%) | .001 |
p Values are calculated using Chi-square tests and student’s t tests wherever appropriate.
Characterization of NCSE and the control group
Control group: 185 patients with AMS or coma in which cEEG monitoring failed to show NCSE served as controls .The main clinical characteristics of NCSE and controls are given in Table 1. Patients’ age and Subtle motor phenomena (twitching of facial muscles or limbs) were the only variable to show statistical difference.
Regarding etiology, previous seizures and cortical dysplasia differed significantly in the two groups (Table 2). Other etiologies had less than 2 entries and therefore were not informative.
Table 2.
Etiology of patients with NCSE and controls.
| Variable | NCSE (n, 65) | Controls (n, 185) | p Value |
|---|---|---|---|
| Stroke (hemorrhagic, ischemic, subarachnoid hemorrhage) | 16 (25%) | 67 (36%) | .09 |
| Status post cardiac arrest | 15 (23%) | 35 (19%) | .59 |
| Head injury | 8 (12%) | 34 (18%) | .34 |
| Previous seizures (Uncontrolled) | 12 (18.4%) | 4 (2%) | .001 |
| Cortical dysplasia | 3 (4.6%) | 0 | .02 |
| Sepsis | 3 (4.6%) | 7 (3.8%) | 1.00 |
| Hepatic encephalopathy | 1 (1.5%) | 3 (1.6%) | 1.00 |
| End stage renal disease, post renal transplant | 2 (3%) | 11 (6%) | .37 |
| Intoxications | 0 | 8 (4.3%) | .12 |
| Hypertensive encephalopathy | 1 (1.5%) | 6 (3.2%) | .68 |
| Personality disorder | 1 (1.5%) | 3 (1.6%) | 1.0 |
| Unknown | 3 (4.6%) | 7 (3.8%) | 1.0 |
p Values are calculated using Chi-square tests or Fisher’s exact test wherever appropriate.
In the NCSE group, Strokes, Status post-cardiac arrest, Head injury and Uncontrolled previous seizures were the most frequent etiologies found among Accident/Emergency compared to the ICU patients.
Results of head CT, performed in 52 patients with NCSE and in 101 of controls, and Head MRI, performed in 41 cases of NCSE and in 97 controls, showed a statistically significant presence of hippocampal sclerosis (CT) and malformations of cortical development and encephalomalacia (MRI) in the NCSE group (Table 3). Other CT and MRI findings had less than 2 entries and therefore were not informative.
Table 3.
Head CT and MRI findings (Some patients had both CT and MRI)
| Variable | CT (n, pts) |
MRI (n, pts) |
||||
|---|---|---|---|---|---|---|
| NCSE (n, 52) | Controls (n, 101) | p Value | NCSE (n, 41) | Controls (n, 97) | p Value | |
| Abnormal | 32 (62%) | 49 (49%) | .17 | 33 (80%) | 53 (55%) | .01 |
| Ischemia, intracerebral hemorrhage, subarachnoid & subdural hemorrhage | 14 (27%) | 18 (18%) | .21 | 16 (39%) | 32 (33%) | .56 |
| Cortical atrophy | 5 (10%) | 10 (10%) | 1.0 | 3 (7%) | 6 (6%) | 1.0 |
| Polymicrogyria, cortical dysplasia, heterotopia | 3 (7%) | 0 | .02 | |||
| Hippocampal sclerosis | 3 (6%) | 0 | .04 | 3 (7%) | 1 (1%) | .08 |
| Encephalomalacia | 3 (7%) | 10 (10%) | .04 | |||
| Meningeal/Cortical enhancement | 1 (2%) | 2 (2%) | 1.0 | 1 (2%) | 2 (2%) | 1.0 |
p Values are calculated using Chi-square tests or Fisher’s exact test wherever appropriate.
Concerning the laboratory findings, abnormal cholesterol and liver synthesis enzymes were more often abnormal in the NCSE group compared to controls (NCSE 15%, controls 4%, p 0.004).
Time of occurrence of NCSs/NCSE and length of cEEG monitoring
Twenty patients presented with NCSs; 13 of them (65%) had NCSs within the first 40 minutes, in the rest (35%) during the first 48 hours of cEEG monitoring.
In the 65 patients with NCSE, EEG patterns compatible with NCSE were recorded early during the first 3 hours of cEEG in 43 patients (66%), during the first 48 hours in 14 additional patients (22%) and during the third cEEG day in 8 more patients (12%). Among the 22 patients with late NCSE, 17 (77%) were comatose.
NCSE proper and comatose NCSE
As Bauer [32], and Fernandez-Torre [36] suggest, we subdivided the 65 NCSE cases into: NCSE proper without coma (n39) and comatose NCSE (n26). NCSE proper is accompanied by clinical symptoms suggestive of SE and mild impairment of consciousness, such as in absence status or complex focal SE; coma-Lateralized Epileptic Discharges, coma-Generalized Epileptic Discharges represent deep coma of various etiology without any clinical motor signs of SE but with characteristic epileptiform EEG pattern. NCSE proper group was significantly younger than the comatose NCSE (Table 4).
Table 4.
Occurrence and comparison of the listed variables in the NCSE groups and control group.
| Variable | NCSE (n 65) | NCSE proper (= without coma)(n, 39) | NCSE with coma (n, 26) | Control (n, 185) | p Value |
|---|---|---|---|---|---|
| Deaths | 20 (31%) | 8 (21%)* | 12 (46%)*§ | 35 (19%)§ | *.05 §.0007 |
| Gender Male | 37 (57%) | 23 (59%) | 14 (54%) | 101 (55%) | |
| Age (years) | 45.7 ± 19§ | 36.9 ± 24& | 51.3 ± 16.9& | 52.3 ± 15.8§ | §.001 &.006 |
| Hospital stay (days) | 15.2 ± 7.7# | 14.6 ± 7.8 | 16.4 ± 7.7^ | 12.7 ± 5.5#^ | #.02 ^.03 |
| Complete recovery | 26 (40%) | 18 (46%) | 8 (31%)a | 98 (53%)a | a.04 |
p Values are calculated using Chi-square tests, Fisher exact tests and student’s t tests wherever appropriate. S = significant.
More than 24 hours of cEEG were often needed to find NCSE in comatose patients; comatose NCSE was recorded during the first 24 hours in 14/26(54%), within 48 hours in 9/26(35%) additional patients and during the 3rd day in 3/26(11%) more patients; Comparatively, NCSE proper was recorded during the first 24 hours in 30/39 (77%), within 48 hours in 4/39(10%) more and during the 3rd day in 5/39 (13%) additional patients.
Among the comatose NCSE occurring during the first 24 hours, 4 patients suffered from head injury, 4 from stroke, 3 from cardiac arrest and no etiology was found in 3 patients; comparatively, in the NCSE proper, 18 patients were categorized as previous seizures (uncontrolled seizures, ASDs withdrawal…) followed by 5 patients with stroke, 3 with sepsis, 2 with head injury, and 2 with cardiac arrest.
Antiseizure drug (ASD) treatment
NCSs (=20) received: benzodiazepines (=18), valproate IV (=10) and levetiracetam IV plus valproate IV (=8 patients);
NCSE patients (=65): 45 received benzodiazepines (lorazepam 4 to 8 mg IV, diazepam 10 mg IV), 22 levetiracetam IV or PO, 21 phenytoin IV, 18 valproate IV or PO, 5 topiramate PO, 7 phenobarbitone IV, 15 midazolam IV, 7 patients propofol; 2 patients received fentanyl IV and 3 thiopental IV.
Outcome
Primary outcome
NCSE group (=65): 45 patients (69%, m 25, f 20) responded to ASDs within 48 hours; 20 patients (31%, m12, f 8) with NCSE died;
Control group (=185): 35 patients (19%, m 20, f 15) died. Death was more frequent in the NCSE group and statistically significant in the NCSE proper compared to comatose NCSE and in the comatose NCSE compared to controls (Table 4).
The majority of patients with early occurrence of NCSs/NCSE (40 mn to 3 hours) died (13/20 = 65%). Causes of death in NCSE (=20) included cardiac arrest (=6), hemorrhagic and ischemia strokes (=5), sepsis (=3), head injury (=4) subarachnoid hemorrhage (=1) and cerebral abscess (=1).
Secondary outcome
Only 26 patients (m 15, f 11) in the NCSE group achieved complete recovery compared to 98 patients (m 55, f 43) in the control group; this achieved statistical significance when comatose NCSE was compared to controls (Table 4). The same Table shows that NCSE group (NCSE proper plus comatose NCSE) had a longer hospital stay than the controls.
Refractory NCSE
Twenty-one patients (m 13, f 8; 32%) with NCSE had refractory NCSE, i.e. seizures lasting more than 60 minutes with failure of two ASDs [37]; 10 patients received midazolam IV, 5 propofol IV, 4 thiopental IV, and 2 patients fentanyl IV; 12 patients (m 8, f 4; 57%) survived; only 7 patients (m 4, f 3; 33%) recovered completely; 9 patients with Refractory NCSE died (m5, f 4; 43%): they suffered from cardiac arrest (3), sepsis (3), ischemia (1), subarachnoid hemorrhage (1) and cerebral abscess (1)
EEG patterns and location
The following EEG patterns were seen in the 65 patients with NCSE: focal spike/sharp and wave >3/s in 28 patients (43%), generalized spike/sharp and wave >3/s in 18(28%), Generalized Periodic Discharges (GDPs), Lateralized Periodic Discharges (LPDs), Continuous 2/s GPDs with triphasic morphology in 16 patients (25%) and multifocal spikes in 3 patients (4%); Figures 1–5 show recorded NCSE EEGs cases before and after ASDs treatment.
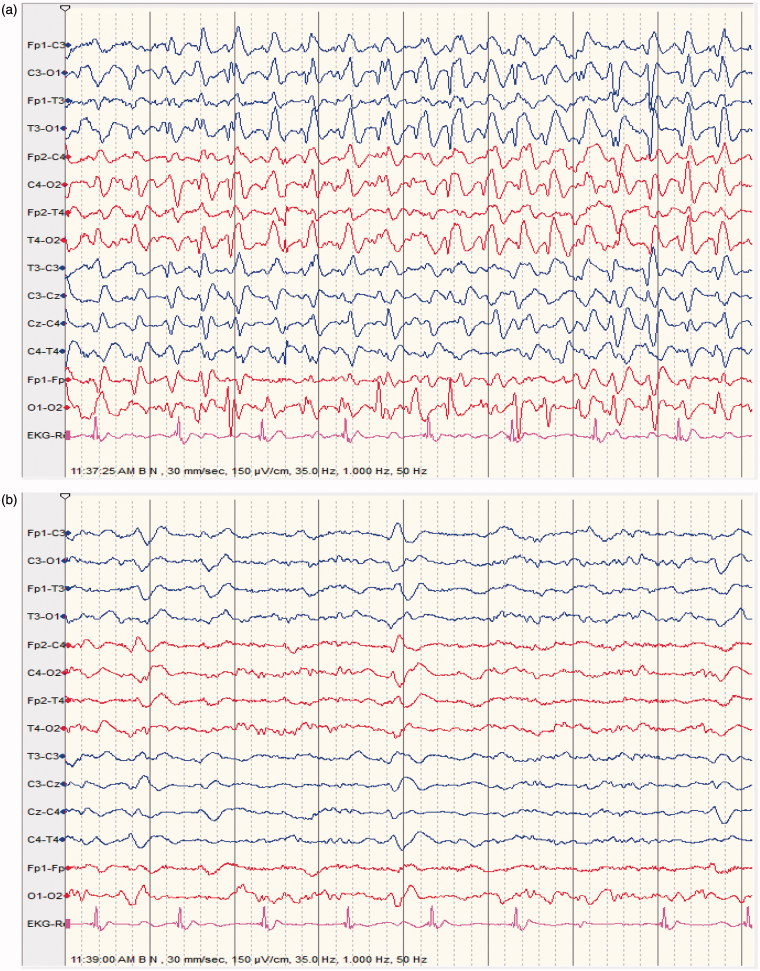
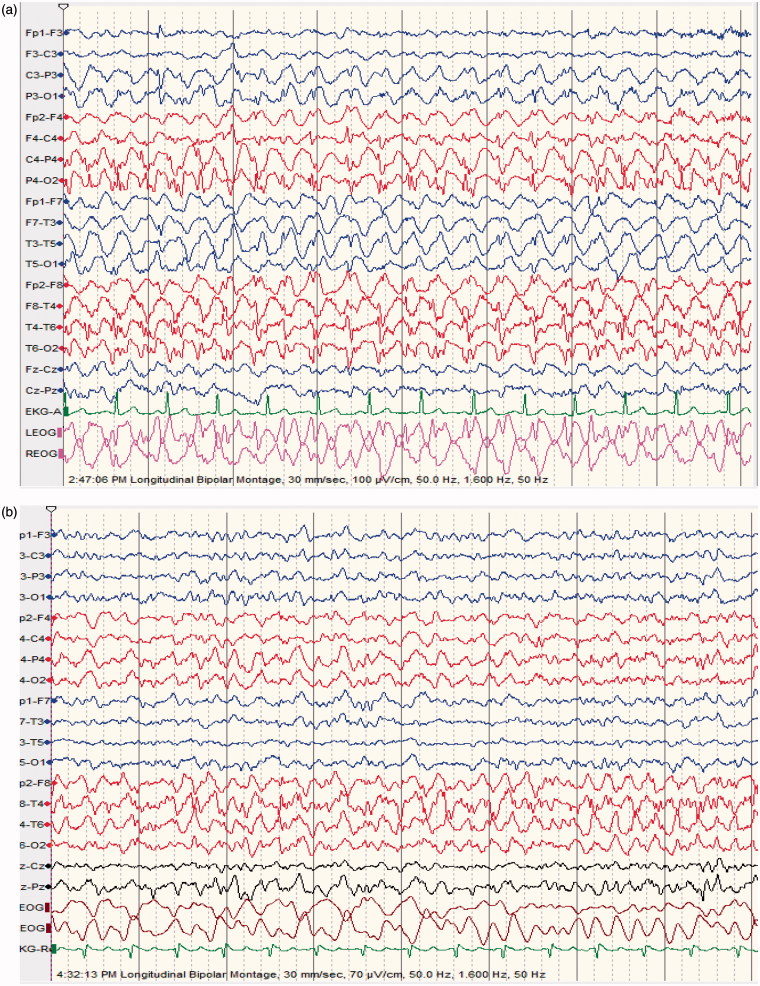
Figure 1.
(a) 64 ys old male patient epileptic with sudden clonazepam withdrawal; semiconscious and confused; no abnormal movements; EEG shows repetitive generalized, >2.5/s sharp wave activity. (b) improvement of level of consciousness and EEG features following diazepam 5 mg IV; made a good recovery discharged home 2 days later on levetiracetam 500 mg BID.
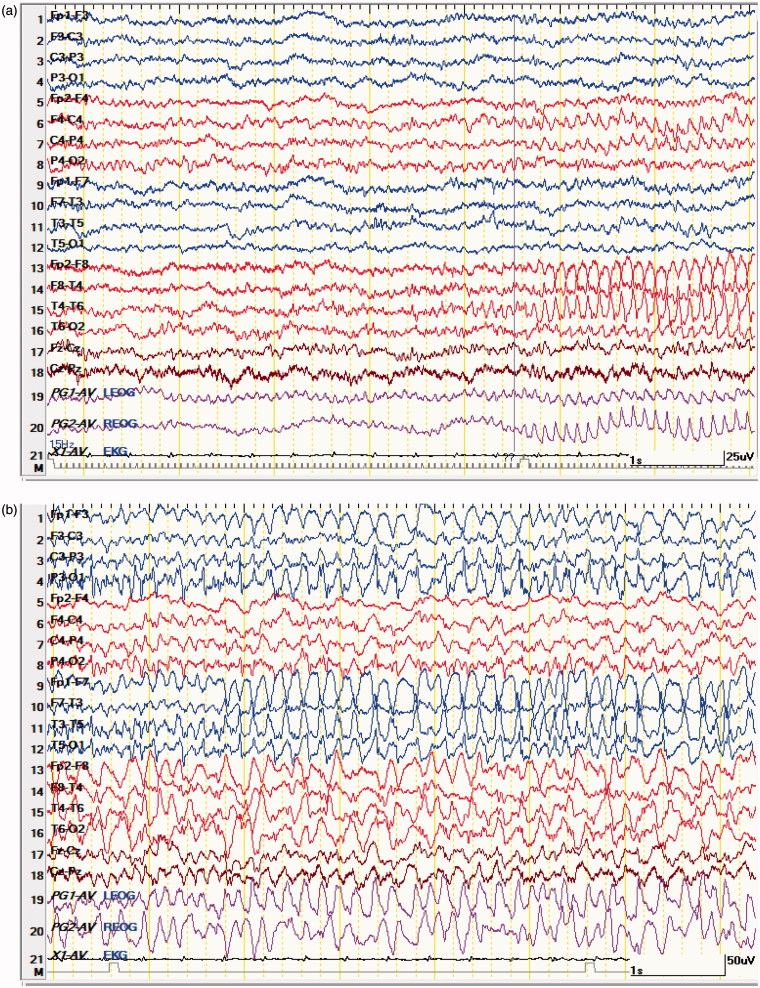
Figure 2.
(a) (comatose focal NCSE) 67 ys old male comatose, following head injury. EEG shows abnormal epileptiform fast activity starting in right fronto-temporal leads accompanied by abnormal eye movements and facial twitching. (b) the ictal fast activity spreads to the controlateral fronto-temporal leads; patient shows same clinical manifestations as in Figure 1 (a); abnormal electrical activity continuous for more than 30 m. (c) 1 minute following 2 mg of Lorazepam IV; patient remains comatose; EEG shows diffuse generalized slowing; no epileptiform activity; no clinical manifestations; survived with memory impairment and hemiplegia.
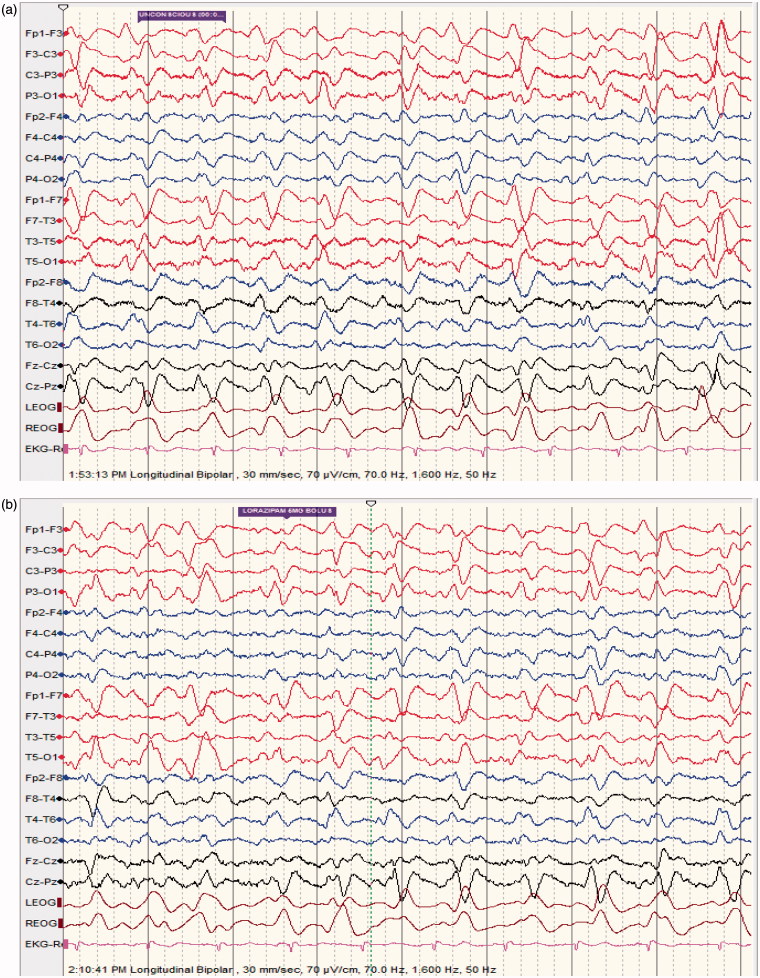
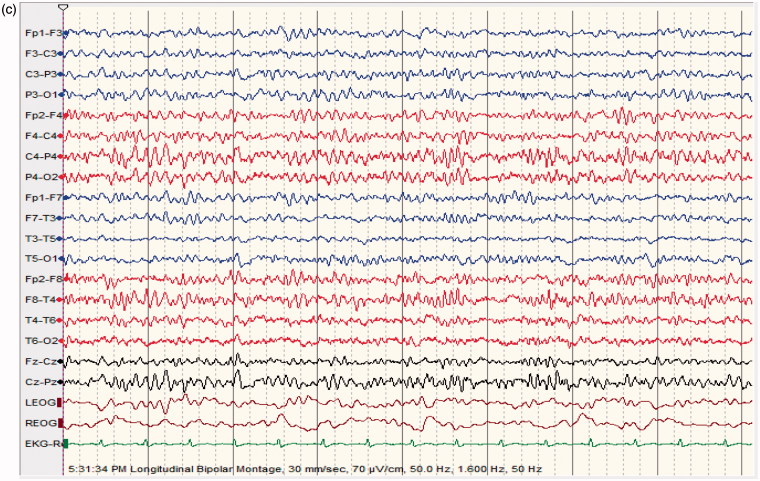
Figure 3.
(a) (NCSE following baclofen intoxication); 40 ys old male patient suffering from uremia; started on baclofen 10 mg TID for lower limb spasticity; found confused and mute; no focal neurological deficit (able to move all limbs); EEG shows quasi-periodic, predominantly left sided continuous 2/s GPDs with triphasic morphology lasting for more than 30 mn. (b) patient given lorazepam 6 mg IV bolus. (c) 1 mn following lorazepam; dramatic improvement in level of consciousness; patient awake, well oriented, able to speak; EEG shows moderately low voltage with moderate generalized slowing; EEG normal the following day; patient made a good recovery
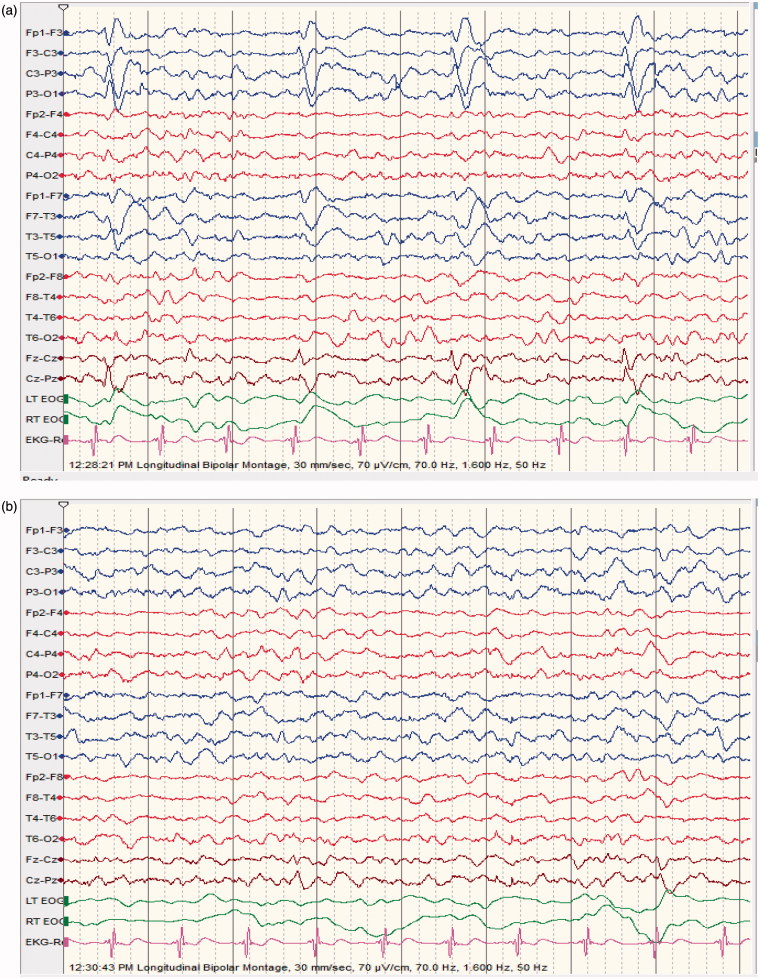
Figure 4.
(a) (NCSE associated with LPDs);65 ys old male; suffered an ischemic stroke; alternating level of consciousness with frequent prolonged confusional state; no focal neurological signs; EEG shows typical left Lateralized Periodic Discharges (LPDs). (b) (NCSE associated with LPDs); 2 mn following 4 mg lorazepam IV: dramatic improvement in level of consciousness (patient not confused any more, fully aware though little slow in answering questions); EEG: no more LPDs, moderate generalized slowing; good recovery before discharge.
Figure 5.
(a) 62 ys old male patient post anoxic coma. Subtle myoclonic jerks involving facial and all 4 extremities; EEG shows bilateral predominantly posterior >2.5 cps continuous rhythmic delta activity lasting more than 45 min. (b) 2 hours later following midazolam IV bolus; no more myoclonic jerks, attenuation of the abnormal EEG features; patient still stuporous. (c) next day, patient conscious; normalization of the EEG; good clinical recovery.
Dead patients with NCSE (=20) showed: periodic patterns (n = 8, 40%), continuous generalized spike/sharp and waves (n = 6, 30%) and focal spike/sharp and waves (n = 6, 30%).
34 patients with NCSE (52%) showed a continuous ictal pattern, 28 (43%) had an intermittent/recurrent ictal pattern; 3(5%) were not classified; 30 patients (46%) showed a focal onset while 19 (29%) had a generalized onset; the rest, 16 (25%) a periodic pattern; focal seizures originated from the temporal areas (55%) and from the frontal areas (31%).
In the control group (=185), none had EEGs of NCSE. Marked focal/generalized slowing was seen in 80 patients (43%) and slowing with some spike/sharp wave activity in 4 patients (2%).
Discussion
NCSE: Prevalence
This is the first prospective, hospital-based, 3 year-study from Qatar, a small country in the MENA region, using cEEG monitoring in patients with AMS, and reporting a high number of NCSE cases (=65) with a prevalence of 26%. The findings of the NCSE group are compared with a matched, parallel control group.
Table 5 summarizes the most important studies reporting on NCSE prevalence; these researchers used a similar design as in the present study, with a matched, parallel control group; however, half of them were retrospective and the EEG recording duration was often shorter or not mentioned. The NCSE prevalence (16%–37%) given in the four referenced studies (Table 5), are similar to our prevalence rate.
Table 5.
Current and previous studies on NCSE prevalence and outcome.
| Author (year) | Methods | Duration of EEGrecording | Patients withAMS (n) | Patients withNCSE (n) (%) | Outcome |
|---|---|---|---|---|---|
| Mesraoua et al. (2017) Current study | Prospective | 72 h | (250) | 65 (26) | Response to ASDs: NCSE 45/65 (69%); Death: NCSE 20/65 (31%); Death in Controls: 35/185 (19%); Complete recovery: NCSE 26/65 (40%);Controls 98/185 (53%); NCSE longer hospital stay than Controls p < .02 (Table 4) |
| Laccheo et al. [38] (2015) | Prospective | >24 h | (170) | 36 (21) | Mortality 31% NCSE vs 14% in Controls |
| Kurtz et al. [12] (2014) | Retrospective | ? | (154) | NCSE/NCSs 24 (16) PEDs 45 (29) |
NCSs/NCSE independently associated with poor outcome 20% vs 3% controls, p = 0.039 |
| Bottaro et al. [13] (2007) | Retrospective | 20 m | (124) | 22 (18) | NCSE significant association with mortality, longer hospitalization and poor outcome |
| Privitera et al. [9] (1994) | Prospective | 30 m | (198) | 74 (37) | Death was more common in NCSE (37%) compared to controls (23%) |
Our prevalence rate is probably influenced by the NCSE clinical awareness resulting from the intensive in-house education training program on NCSE.
Five studies from MENA’s neighboring countries (enumerated above in section 1. Introduction) examined the prevalence of NCSE in patients with AMS. However, these studies cannot be readily compared to our study because of different study designs, some without a control group, some retrospective, and some without description of the EEG monitoring duration.
NCSE: outcome
Several authors report that NCSE is associated with a high mortality and poor outcome [9,12,13,38]. The figures given by these researchers are even worse than in our study (Table 5). Only Privitera [9] reports similar outcomes in NCSE and controls, but death was more common in NCSE (37%) than in controls (23%) (Table 5). The present study confirms that NCSE carries a bad prognosis (mortality =31%). Statistical analysis demonstrates that length of stay and to a lesser degree age were the only variables found statistically significant associated with mortality in the NCSE group (Table 6), as previously reported by Young et al. [27]
Table 6.
Multivariate logistic regression for mortality in NSCE.
| Variable | OR | 95% C.I. | p Value |
|---|---|---|---|
| Age | 1.16 | 1.0–1.34 | .05 |
| Length of stay | 2.03 | 1.29–3.20 | .002 |
| Cardiac arrest | 3.27 | 0.07–153 | .55 |
| Stroke | 35.0 | 0.33–3629 | .14 |
| Head injury | 30.1 | 0.02–56392 | .38 |
Variables significant at univariate analysis and having adequate numbers were used for multivariate analysis.
Regarding morbidity, head injury and stroke were the only variables associated with bad outcome in this study (Table 7); Actually, 19 patients with NCSE (29%) did not achieve complete recovery; 10 of them were seen 3–6 months after the study was completed; 2 were in vegetative state (following head trauma), 8 had various neurological sequelae (4 following cardiac arrest, 2 after strokes and 2 after head trauma).
Table 7.
Multivariate logistic regression for morbidity in NSCE.
| Variable | OR | 95% C.I. | p Value |
|---|---|---|---|
| Age | 1.0 | 0.96–1.05 | .74 |
| Length of stay | 1.10 | 0.90–1.34 | .36 |
| Cardiac arrest | 4.22 | 0.64–27.9 | .14 |
| Stroke | 26.30 | 3.24–213 | .03 |
| Head injury | 19.5 | 1.30–293 | .002 |
Variables significant at univariate analysis and having adequate numbers were used for multivariate analysis.
In agreement with Bottaro [13] and Naeije [15], our study found longer hospitalizations for NCSE vs Controls (p < .02) and (p < .03) (Table 4).
In agreement with Claassen [14], we found that the majority of patients with typical NCSs/NCSE EEG patterns appearing at an early stage (n = 13, =65%) had a poor recovery and bad outcome. We did not find an association between acute symptomatology and poor outcome as reported by Kang [39].
Sixteen patients with PDs (GPDs =7, LPDs = 5) and Continuous 2/s GPDs with triphasic morphology (=4) did not strictly meet the EEG criteria for NCSE described by in section 2.1 (Definition and selection of NCSE); these EEG patterns have been referred to as lying along an ictal–Interictal continuum in the ICU; however, there are convincing reports that GPDs and LPDs are strongly associated with NCSE and may be ictal in certain situations as shown by Bottaro et al. [13], Naeije et al. [15], Trinka et al. [40], Bauer et al. [32] and Jemeen Sreedharan et al. [41]; on the other hand, Continuous 2/s GDPs with triphasic morphology may not be clearly distinguishable from NCSE in certain cases [40–45]; in our study, most of these EEG patterns were seen in patient with comatose NCSE, were accompanied by subtle clinical ictal phenomena, had typical spatiotemporal evolution and responded to ASD, as illustrated in Figures 3 and 4.
Several authors noted that these periodic EEG patterns forebode a bad prognosis and that NCSE mortality is largely determined by the underlying etiology [8,18–20,39]. We found that 8/16 (50%) of patients with PDs or, Continuous 2/s GPDs with triphasic morphology died, mostly following cardiac arrest, strokes, head injury and sepsis. However, multivariate logistic regression analysis did not associate etiology, especially cardiac arrest, stroke and head injury with mortality in NSCE group (Table 6).
Our results are in agreement with Dericioglu [20] and Foreman [42] who noted that prognosis in NCSE cannot be based on one variable (such as the EEG pattern) alone, but that age, etiology and level of consciousness, play also an important role.
Thirty-one percent (27/87 = 31%) of comatose patients (NCSE and Controls) developed comatose NCSE: figures much higher than the data reported by Towne et al. (8%) [6]; these authors used only routine short EEG recording, missing comatose NCSE which frequently occurs late during cEEG monitoring, as shown by Claassen [14] and confirmed by this study.
Mean age, hospital stay and mortality rate were significantly higher in comatose NCSE, corroborating results of Fernández-Torre [36] (Table 4).
The prognosis of Refractory NCSE is very poor: in this study, nine out of 21 patients (43%) with Refractory NCSE died, a finding worse than in a recent series [46], reporting a death rate of 25%. However, we have to consider that 17% of patients in that study were left in a vegetative state.
This study also confirms that a history of epilepsy or seizures is a risk factor for NCSs/NCSE, as noted previously [12,13,38].
Length of cEEG monitoring
There is a lack of consensus regarding the optimal duration of cEEG monitoring for critically ill patients with AMS.
We found that most cases of NCSE occurred early, i.e. within the first 3 hours (66%) and 90% during the first 48 hour of cEEG monitoring. Researchers report various cEEG monitoring durations, from a minimum of 12–24 hours [8,12,19,22], 72 hours [16,18,47], to as long as 7–10 days [23], with a maximum of 17 days, and a mean duration of 2.9 days. Recently, Altındağ et al. [47] monitoring patients for 72–75 hours, suggested that al least 24 hours of cEEG monitoring might be useful to detect clinical and/or electrographic seizures. In a survey, Abend [24] found that most ICU physicians recorded for as long as 24hs and a minority went beyond 48 h. Westover [25] found that one fifth of patients without early epileptiform abnormalities (within the first 2 hours of cEEG monitoring), later developed them (within 72 hours of cEEG monitoring). Claassen [14] analyzed the time to first seizure in 110 patients monitored by cEEG. In non-comatose patients, 95% had the first seizure detected in the first 24 hours compared to comatose patients (80%); in the first 48 hours, 98% of seizures were detected in non-comatose patients, compared to only 87% in comatose patients.
In agreement with Claassen [14] and Westover [25], our findings suggest that 3 days of cEEG monitoring diagnose most cases of NCSE in patients with AMS.
Limitation of this study
Our study carries several weaknesses: One is the lack of data regarding NCSE onset and duration and when in the course of NCSE, ASD treatment was begun. This, as in other studies, was difficult to ascertain, because onset of NCSE in some cases was outside the hospital or remained initially undetected in the hospital because of a paucity of clinical signs.
Another weakness is that NCSs/NCSE not witnessed on EEG, might have occurred earlier (before the start of cEEG monitoring) and such cases might have been erroneously assigned into the control group.
Conclusion
The prevalence of NCSE in this part of MENA region is in accordance with previous reports (26%); NCSE is heterogeneous with multiple subtypes [32,36]. Both forms, NCSE proper and comatose NCSE carry a bad prognosis, worse for comatose NCSE. Despite an aggressive ASD treatment, almost one third of NCSE cases died. Most cases of NCSE were detected in the first 24–48 hours, suggesting that a total of 72 hours cEEG monitoring would reveal most NCSE patients.
Transparency
Declaration of funding
This study was made possible by a grant (NPRP 09-785-3-203) from the Qatar National Research Fund (a member of Qatar Foundation). The Grant title study was “Continuous EEG Monitoring in the ICU” and included the development of an EEG algorithm to identify NCSs/NCSE in patients with AMS admitted to HGH (this part of the study was not reported here).
Declaration of financial/other relationships
PWK has received honoraria for lectures and publications on EEG and epilepsy and expert testimony on qEEG and seizures, served on the board of the ABCN, and received grant support from the QNRF. None of the other authors has any conflict of interest to disclose. The statements made herein are solely the responsibility of the authors.
Acknowledgments
Mr. Ameer Jan Bul Bul, Mrs Mona Al Mansour, Mr. Rana Babur, and Mr. Fazal Karim were in charge of EEG recording. Mrs. Indira Santosh helped as a highly devoted assistant. We thank all these people who contributed greatly to this study.
Previous presentations
The materiel of this work has been presented, under the same title, at the XXII World Congress of Neurology (WCN 2015), Santiago, Chili, as an oral presentation by Pr Boulenouar Mesraoua on November 5, 2015.
References
- 1. Kinney MO, Craig JJ, Kaplan PW.. Hidden in plain sight: non-convulsive status epilepticus-recognition and management. Acta Neurol Scand. 2017;136(4):280–292. [DOI] [PubMed] [Google Scholar]
- 2. Kaplan PW. Nonconvulsive status epilepticus in the emergency room. Epilepsia. 1996;37(7):643–650. [DOI] [PubMed] [Google Scholar]
- 3. Rüegg S. Non-convulsive status epilepticus in adults: an overview. Schweiz Arch Neurol Psychiatr. 2008;159:53–83. [Google Scholar]
- 4. Knake S, Rosenow F, Vescovi M, et al. Incidence of status epilepticus in adults in Germany: a prospective, population-based study. Epilepsia. 2001;42:714–718. [DOI] [PubMed] [Google Scholar]
- 5. Maganti R, Gerber P, Drees C, et al. Nonconvulsive status epilepticus. Epilepsy Behav. 2008;12:572–586 [DOI] [PubMed] [Google Scholar]
- 6. Towne AR, Waterhouse EJ, Boggs JG, et al. Prevalence of nonconvulsive status epilepticus in comatose patients. Neurology. 2000;54:340–345 [DOI] [PubMed] [Google Scholar]
- 7. Pandian JD, Cascino GD, So EL, et al. Digital video-electroencephalographic monitoring in the neurological–neurosurgical intensive care unit: clinical features and outcome. Arch Neurol. 2004; 61:1090–1094 [DOI] [PubMed] [Google Scholar]
- 8. Claassen J, Jetté N, Chum F, et al. Electrographic seizures and periodic discharges after intracerebral hemorrhage. Neurology. 2007;69:1356–1365. [DOI] [PubMed] [Google Scholar]
- 9. Privitera M, Hoffman M, Moore JL, et al. EEG detection of nontonic–clonic status epilepticus in patients with altered consciousness. Epilepsy Res. 1994;18:155–166. [DOI] [PubMed] [Google Scholar]
- 10. DeLorenzo RJ, Waterhouse EJ, Towne AR, et al. Persistent nonconvulsive status epilepticus after the control of convulsive status epilepticus. Epilepsia. 1998;39:833–840. [DOI] [PubMed] [Google Scholar]
- 11. Kaplan PW. Nonconvulsive status epilepticus. Semin Neurol 1996a;16(1):33–40. [DOI] [PubMed] [Google Scholar]
- 12. Kurtz P, Gaspard N, Wahl AS, et al. Continuous electroencephalography in a surgical intensive care unit. Intensive Care Med. 2014;40:228–234. [DOI] [PubMed] [Google Scholar]
- 13. Bottaro FJ, Martinez OA, Pardal MM, et al. Nonconvulsive status epilepticus in the elderly: a case-control study. Epilepsia. 2007;48:966–972. [DOI] [PubMed] [Google Scholar]
- 14. Claassen J, Mayer SA, Kowalski RG, et al. Detection of electrographic seizures with continuous EEG monitoring in critically ill patients. Neurology. 2004;62:1743–1748. [DOI] [PubMed] [Google Scholar]
- 15. Naeije G, Depondt C, Meeus C, et al. EEG patterns compatible with nonconvulsive status epilepticus are common in elderly patients with delirium: a prospective study with continuous EEG monitoring. Epilepsy Behav. 2014;36:18–21 [DOI] [PubMed] [Google Scholar]
- 16. Mehendale AM, Goldman MP, Mehendale RP, et al. Ambulatory electroencephalograms in neuropsychiatric practice: opening Pandora’s jar. World J Neurosci. 2014;4:125–132. [Google Scholar]
- 17. Siddiqui M, Jamil N, Malik A, et al. Frequency of non convulsive status epilepticus in patients with impaired level of consciousness. J Pak Med Assoc. 2009;59:296–298. [PubMed] [Google Scholar]
- 18. Rai V, Jetli S, Rai N, et al. Continuous EEG predictors of outcome in patients with altered sensorium. Seizure. 2013;22:656–661. [DOI] [PubMed] [Google Scholar]
- 19. Narayanan JT, Murthy JM.. Nonconvulsive status epilepticus in a neurological intensive care unit: profile in a developing country. Epilepsia 2007;48:900–6. [DOI] [PubMed] [Google Scholar]
- 20. Dericioglu N, Arsava EM, Topcuoglu MA.. The clinical features and prognosis of patients with nonconvulsive status epilepticus in the neurological intensive care unit of a tertiary referral center in Turkey. Clin EEG Neurosci. 2014;45:293–298. [DOI] [PubMed] [Google Scholar]
- 21. Shavit L, Grenader T, Galperin I.. Nonconvulsive status epilepticus in elderly, a possible diagnostic pitfall. Eur J Intern Med. 2012;23:701–704. [DOI] [PubMed] [Google Scholar]
- 22. Sutter R, Fuhr P, Grize L, et al. Continuous video-EEG monitoring increases detection rate of nonconvulsive status epilepticus in the ICU. Epilepsia. 2011;52:453–457. [DOI] [PubMed] [Google Scholar]
- 23. Vespa PM, Nuwer MR, Nenov V.. Incidence of nonconvulsive and convulsive seizures in the ICU following traumatic brain injury: increased incidence detected by continuous EEG monitoring. Crit Care Med. 1997;25(1 suppl):A120. [Google Scholar]
- 24. Abend NS, Dlugos DJ, Hahn CD, et al. Use of EEG monitoring and management of non-convulsive seizures in critically ill patients: a survey of neurologists. Neurocrit Care. 2010;12:382–389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Westover MB, Shafi MM, Bianchi MT, et al. The probability of seizures during EEG monitoring in critically ill adults. Clin Neurophysiol. 2015;126:463–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Mesraoua B, Deleu D, Wieser HG.. Long term monitoring: an overview. Intech 1st Edition, edited by Stevanovic Dejan, Croatia Rijeka. Epileptic seizures; 2012:145–172 [Google Scholar]
- 27. Young GB, Jordan KG, Doig GS.. An assessment of nonconvulsive seizures in the intensive care unit using continuous EEG monitoring: an investigation of variables associated with mortality. Neurology. 1996;47:83–89. [DOI] [PubMed] [Google Scholar]
- 28. Chong DJ, Hirsch LJ.. Which EEG patterns warrant treatment in the critically ill? Reviewing the evidence for treatment of periodic epileptiform discharges and related patterns. J Clin Neurophysiol. 2005;22:79–91. [DOI] [PubMed] [Google Scholar]
- 29. Trinka E, Cock H, Hesdorffer D, et al. A definition and classification of status epilepticus-report of the ILAE task force on classification of status epilepticus Epilepsia. 2015;56(10):1515–1523. [DOI] [PubMed] [Google Scholar]
- 30. Leitinger M, Beniczky S, Rohracher A, et al. Salzburg consensus criteria for non-convulsive status epilepticus – approach to clinical application. Epilepsy Behav. 2015;49:158–163 [DOI] [PubMed] [Google Scholar]
- 31. Hirsch LJ, LaRoche SM, Gaspard N, et al. American Clinical Neurophysiology Society’s standardized critical care EEG terminology: 2012 version. J Clin Neurophysiol. 2013;30(1):1–27. [DOI] [PubMed] [Google Scholar]
- 32. Bauer G, Trinka E.. Nonconvulsive status epilepticus and coma. Epilepsia. 2010;51:177–190. [DOI] [PubMed] [Google Scholar]
- 33. Hirsch LJ. Continuous EEG monitoring in the intensive care unit: an overview. J Clin Neurophysiol. 2004;21:332–340. [PubMed] [Google Scholar]
- 34. Meierkord H, Boon P, Engelsen B, et al. EFNS guideline on the management of status epilepticus in adults. Eur J Neurol. 2010;17:348–355. [DOI] [PubMed] [Google Scholar]
- 35. Glauser T, Shinnar S, Gloss D, et al. Evidence-based guideline: treatment of convulsive status epilepticus in children and adults: report of the guideline committee of the American Epilepsy Society. Epilepsy Curr. 2016;16:48–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Fernández-Torre JL, Rebollo M, et al. Nonconvulsive status epilepticus in adults: electroclinical differences between proper and comatose forms. J Clin Neurophysiol. 2012;123:244–251. [DOI] [PubMed] [Google Scholar]
- 37. Liberalesso PB, Garzon E, Yacubian EM, et al. Refractory nonconvulsive status epilepticus in coma: analysis of the evolution of ictal patterns. Arq Neuropsiquiatr. 2012;70:501–515. [DOI] [PubMed] [Google Scholar]
- 38. Laccheo I, Sonmezturk H, Bhatt AB, et al. Non-convulsive status epilepticus and non-convulsive seizures in neurological ICU patients. Neurocrit Care. 2015;22:202–211. [DOI] [PubMed] [Google Scholar]
- 39. Kang BS, Jhang Y, Kim YS, et al. Etiology and prognosis of non-convulsive status epilepticus. J Clin Neurosci. 2014;21:1915–1919. [DOI] [PubMed] [Google Scholar]
- 40. Trinka E, Leitinger M.. Which EEG patterns in coma are nonconvulsive status epilepticus? Epilepsy Behav. 2015;49:203–222. [DOI] [PubMed] [Google Scholar]
- 41. Sreedharan J, Gourlay E, Evans MR, et al. Falsely pessimistic prognosis by EEG in post-anoxic coma after cardiac arrest: the borderland of nonconvulsive status epilepticus. Epileptic Disord. 2012;14(3):340–344 [DOI] [PubMed] [Google Scholar]
- 42. Foreman B, Claassen J, Abou Khaled K, et al. Generalized periodic discharges in the critically ill: a case-control study of 200 patients. Neurology. 2012;79:1951–1960. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Sivaraju A, Gilmore E.. Understanding and managing the ictal interictal continuum in Neurocritical care. Curr Treat Options Neurol. 2016;18:8–13. [DOI] [PubMed] [Google Scholar]
- 44. Braksick SA, Burkholder DB, Tsetsou S, et al. Associated factors and prognostic implications of stimulus-induced rhythmic, periodic, or ictal discharges. JAMA Neurol. 2016;73:585–590. [DOI] [PubMed] [Google Scholar]
- 45. O’Rourke D, Chen PM, Gaspard N, et al. Response rates to anticonvulsant trials in patients with triphasic-wave EEG patterns of uncertain significance. Neurocrit Care. 2016; 24:233–239. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Rohracher A, Höfler J, Kalss G, et al. Perampanel in patients with refractory and super-refractory status epilepticus in a neurological intensive care unit. Epilepsy Behav. 2015;49:354–358 [DOI] [PubMed] [Google Scholar]
- 47. Altındağ E, Okudan ZV, Tavukçu Özkan S, et al. EEG patterns recorded by continuous EEG monitoring in neurological intensive care unit. Arch Neuropsychiatry. 2017;54:168–174 [DOI] [PMC free article] [PubMed] [Google Scholar]