Abstract
Purpose:
Conventionally, keratoconus (KC) has been considered a noninflammatory corneal ectatic disorder. Recent evidence suggests a possible role of inflammation in the pathogenesis of KC. Hence, we analyzed the levels of inflammatory factors in the tear fluid of Indian KC patients.
Methods:
Tear fluid samples were collected from age- and sex-matched healthy controls and KC patients (with different grades). The levels of the inflammatory factors in tears were analyzed using cytometric bead array (Human Soluble Protein Flex Set System, BD Biosciences) for levels of interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p70, IL-23p40, IL-13, IL-17A, IL-17F, IL-21, interferon-α (IFNα), IFNγ, tumor necrosis factor-α, CCL2/monocyte chemotactic protein-1, CCL4/macrophage inflammatory protein-1β (MIP-1β), MIP-1α, CCL5/RANTES, CXCL10/IP10, ICAM1, CD62E, vascular endothelial growth factor and transforming growth factor β.
Results:
An increase in Kmax and Kmean, and a decrease in central corneal thickness was observed with increasing grades of KC. Tear analysis showed that most of the tear soluble factors, including cytokines, chemokines, growth factors and cell adhesion molecules were significantly elevated in the KC patients compared to the controls.
Conclusion:
Our findings suggest that inflammatory factors associated with KC may play a role in its pathogenesis. This opens the potential to explore anti-inflammatory strategies to either halt or delay the progression of KC.
Keywords: Inflammation, keratoconus, tear fluid
Keratoconus (KC) is a progressive corneal ectatic disorder characterized by stromal thinning, protrusion and the resultant irregular astigmatism and myopia.[1,2] Conventionally, it has been classified as a noninflammatory disease, as the classical signs of inflammation (redness, heat, swelling, and pain) are not apparent in KC.[2] Recently, a combination of oxidative stress, genetic and environmental risk factors, and atopic eye disease has been suggested to play a role in KC disease pathology.[3] Despite the absence of obvious inflammation, studies have demonstrated inflammatory factors such as matrix metalloproteinases (MMPs) in tears of patients with clinical and subclinical KC.[4,5,6] Furthermore, information regarding the tear inflammatory factors profile in Indian KC patients is sparse. Although knowledge about the etiopathogenesis of KC is evolving, the molecular factors that drive the loss of corneal structure integrity are poorly understood. Altered collagen structure leading to biomechanical weakening as well as deregulated epithelial and stromal proteins have been demonstrated in KC.[7,8] It is well known from the biology of extracellular matrix modeling that inflammatory factors do play a key role in the remodeling process. Therefore, to determine the possible inflammatory status in KC, we evaluated the levels of inflammatory factors in tears of KC patients with different grades, compared to controls.
Methods
Cohort details
This study was performed in accordance with the tenets of the Declaration of Helsinki with prior approval from the Narayana Nethralaya Institutional Review Board, as per Indian Council of Medical Research (ICMR) and institutional human ethics guidelines. Patients’ and control volunteers’ tear samples were collected after obtaining informed written consent for its research use. The study group was selected from KC patients who reported to the cornea clinic at a tertiary eye hospital in Bangalore, India. A total of 11 KC patients and six healthy control volunteers were enrolled in the study. Patients using contact lenses or systemic anti-inflammatory medications, or systemic inflammatory and autoimmune diseases were excluded from the study. Individuals with a history of ocular surgical intervention (e.g., cataract surgery, vitreoretinal surgery, penetrating keratoplasty/corneal collagen cross-linking) for either eye were also excluded from the study. Patients with recent infection or allergic history were also excluded from the study. All individuals underwent a dry eye evaluation and those patients with signs or symptoms of dry eye disease (as per DEWS classification) were excluded from the study. KC diagnosis was performed by retinoscopy, corneal refraction, and slit lamp biomicroscopy. All patients underwent corneal topography acquired with Pentacam (OCULUS Optikgeräte GmbH, Germany) which was used for grading of the KC patients. Keratometry values for the flat axis (K1), steep axis (K2), mean keratometry (K mean), maximum keratometry (K max), and central corneal thickness (CCT) were established by Scheimpflug image analysis (Pentacam, Oculus Optikgeräte GmbH, Wetzlar, Germany). KC grades were determined by Amsler–Krumeich classification.
Tear fluid collection
Capillary micropipette tubes were used to collect tear samples from all individuals included in the study. Tear collection was done from the outer third of the lower fornix and care was taken to avoid touching the conjunctiva or produce any reflex tearing. Tears were extracted from the micropipette tubes into sterile microfuge tubes and stored at −80°C for further analysis.
Cytometric bead array
Tear cytokine and chemokine measurements were done using cytometric bead array (CBA Human Soluble Protein Flex Set System, BD Biosciences) on a flow cytometer (BD FACSCalibur™, BD Biosciences). The CBA was done for interleukin-1α (IL-1α), IL-1β, IL-2, IL-4, IL-5, IL-6, IL-8, IL-9, IL-10, IL-12p70, IL-23p40, IL-13, IL-17A, IL-17F, IL-21, IFNα, IFNγ, CCL2/monocyte chemotactic protein-1, CCL4/MIP-1β, MIP-1α, CCL5/RANTES, CXCL10/IP10, sICAM1, sCD62E/sE-selectin, vascular endothelial growth factor (VEGF), and transforming growth factor β (TGFβ). The experiments were done as per the manufacturer's instruction and analyte signal intensities were calculated with reference to the respective standards and absolute concentrations of individual analytes were calculated using BD FCAP Array Software (BD Biosciences).
Statistical analysis
All statistical analyses were performed with GraphPad Prism 6.0 (GraphPad Software, Inc., La Jolla, CA, USA). Shapiro–Wilk normality test was used to check the distribution of the data set. Mann-Whitney test and Kruskal–Wallis test were used to analyze data sets that were not normally distributed. The data are reported as mean ± standard error of the mean (SEM) and P < 0.05 was considered to be statistically significant.
Results
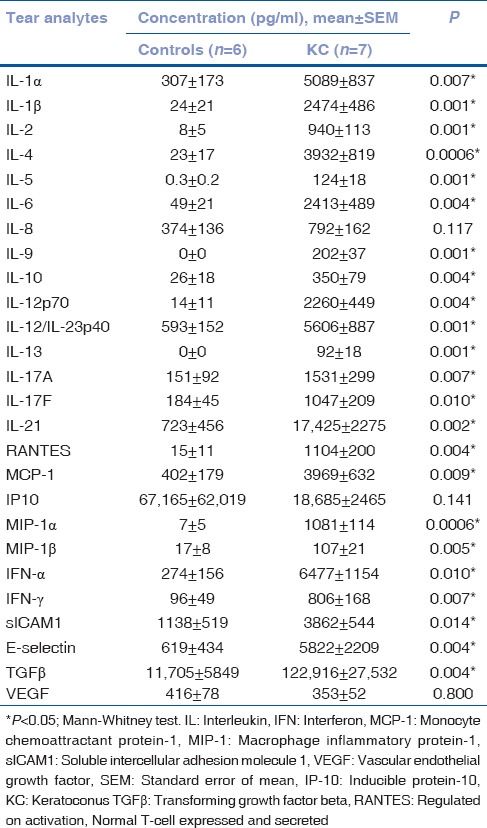
The participants in the study were age (controls – 28.0 ± 3; KC – 28.8 ± 2; mean ± SEM in years)- and sex (M/F; controls – 2/4; KC– 3/8)-matched. The KC patients included in the study had bilateral KC with different grades based on severity. A total of 10 eyes had Grade 1 KC, 7 eyes had Grade 2 KC, and 5 eyes had Grade 3/4 KC. The overall Kmax, Kmean and CCT in KC eyes were 56 ± 2, 50 ± 1, and 430 ± 17, respectively. The Kmax (Grade 1, 49 ± 1; Grade 2, 57 ± 1; Grade 3/4, 69 ± 4) and Kmean (Grade 1, 44 ± 1; Grade 2, 50 ± 1; Grade 3/4, 60 ± 3) increased significantly (P < 0.05, Kruskal–Wallis test) with increasing grades of severity. A significant (P < 0.05, Kruskal–Wallis test) decrease in the CCT (Grade 1, 470 ± 8; Grade 2, 444 ± 12; Grade 3/4, 333 ± 53) was also observed with increasing grades of severity. As shown in Table 1, tear analysis revealed significantly (P < 0.05, Mann–Whitney test) higher levels of inflammation-associated cytokines, chemokines, growth factors, and soluble cell adhesion molecules except for IL-8, IP-10, and VEGF in KC patients. The findings indicate altered inflammatory status or soluble factors milieu in the ocular surface of KC patients compared to controls.
Table 1.
Cytokines, chemokines, and growth factor profile in tears of patients with keratoconus as compared to controls
Discussion
The exact etiopathogenesis of KC is debatable. Although initially described as noninflammatory in nature,[2] eye rubbing, atopy, genetic, and environmental risk factors have been well reported.[3,9] More recently, the role of inflammation in the pathogenesis of KC has been elucidated.[4,10,11,12] The inflammatory process refers to an increase in the vascular permeability and vascular dilatation, which in turn causes exudation of fluid and proteins at the site of inflammation.[13] These processes are not seen in the cornea as it is an avascular structure. However, at the molecular level, the presence of inflammatory cytokines, increased expression of inflammatory proteins and transcription factors point toward the possibility of an active inflammation.[13] Balasubramanian et al. reported an increase in inflammatory factors including IL-6 and MMPs after 60 seconds of eye rubbing.[14] They suggested that habitual eye rubbing for prolonged periods can exacerbate these inflammatory markers and play a role in the progression of KC. The increased shear stress and hydrostatic pressure associated with eye rubbing have also been considered triggers of inflammation.[13] Both shear stress and hydrostatic pressure have a role to play in eye rubbing as well.[15] Another study has reported elevated levels of MMP-9 and IL-6 in patients with KC.[16]
We also found elevated levels of inflammatory cytokines and growth factors in tears of patients with KC when compared to controls. Similar results have been reported earlier.[11] LOX enzyme plays an important role in the formation of fibrillar extracellular matrix by oxidative linkage of collagen in the cornea.[17] Deregulation of this enzyme has been reported in several ocular disorders.[18] Dudakova et al. observed LOX activity to be more than 2.5 times lower in KC corneas when compared to normal corneas.[19] Shetty et al. demonstrated a similar finding in a larger cohort and showed that LOX had a reducing trend with increasing severity of KC grades.[20] COLIA1 and COLIVA1 are the major types of collagen required to maintain the normal corneal structure. While COLIA1 is found all across the corneal stroma,[7] COLIVA1 is found predominantly in the epithelial basement membrane.[21] COLIA1 is reduced in the early stages and COLIVA1 is decreased in the advanced stages of KC.[20] This could be responsible for the stromal thinning seen early KC and the thinning of the Bowman's membrane in advanced KC.[22] KC has also been found to be associated with several autoimmune and allergic eye diseases and several factors that contribute to the risk of progression.[23,24] In the current study, we demonstrate increased inflammatory factors on the ocular surface which would contribute toward KC and its progression. The knowledge regarding the role of inflammatory factors including cytokines, chemokines, cell adhesion molecules and certain growth factors in influencing the extracellular remodeling is well known. Hence, our findings suggest that inflammation has a plausible role to play in the pathogenesis of KC. This allows us to explore the potential of anti-inflammatory treatment to halt the progression of KC. Multiple studies have shown that cyclosporine A (CsA) has an inhibitory effect on MMPs and the expression of MMP-9 in vitro on human epithelial cells.[16,25,26] It has also been shown to reduce the tear levels of MMP-9[16] and other inflammatory factors. However, the potential role of CsA in KC needs to be explored further. In conclusion, our study demonstrates a potential role of inflammation in KC. Future directions may include targeting these inflammatory factors in the management of KC.
Conclusion
Increased tear fluid inflammatory factors in KC suggests the role of dysregulated inflammation in KC pathogenesis. This opens the potential to explore anti-inflammatory strategies to either halt or delay the progression of KC.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Chan TC, Wang YM, Yu M, Jhanji V. Comparison of corneal dynamic parameters and tomographic measurements using Scheimpflug imaging in keratoconus. Br J Ophthalmol. 2017 doi: 10.1136/bjophthalmol-2017-310355. pii:bjophthalmol-2017-310355. [DOI] [PubMed] [Google Scholar]
- 2.McMonnies CW. Abnormal rubbing and keratectasia. Eye Contact Lens. 2007;33:265–71. doi: 10.1097/ICL.0b013e31814fb64b. [DOI] [PubMed] [Google Scholar]
- 3.Lema I, Durán JA. Inflammatory molecules in the tears of patients with keratoconus. Ophthalmology. 2005;112:654–9. doi: 10.1016/j.ophtha.2004.11.050. [DOI] [PubMed] [Google Scholar]
- 4.Lema I, Sobrino T, Durán JA, Brea D, Díez-Feijoo E. Subclinical keratoconus and inflammatory molecules from tears. Br J Ophthalmol. 2009;93:820–4. doi: 10.1136/bjo.2008.144253. [DOI] [PubMed] [Google Scholar]
- 5.Balasubramanian SA, Mohan S, Pye DC, Willcox MD. Proteases, proteolysis and inflammatory molecules in the tears of people with keratoconus. Acta Ophthalmol. 2012;90:e303–9. doi: 10.1111/j.1755-3768.2011.02369.x. [DOI] [PubMed] [Google Scholar]
- 6.Nakayasu K, Tanaka M, Konomi H, Hayashi T. Distribution of types I, II, III, IV and V collagen in normal and keratoconus corneas. Ophthalmic Res. 1986;18:1–0. doi: 10.1159/000265406. [DOI] [PubMed] [Google Scholar]
- 7.Joseph R, Srivastava OP, Pfister RR. Differential epithelial and stromal protein profiles in keratoconus and normal human corneas. Exp Eye Res. 2011;92:282–98. doi: 10.1016/j.exer.2011.01.008. [DOI] [PubMed] [Google Scholar]
- 8.Krachmer JH, Feder RS, Belin MW. Keratoconus and related noninflammatory corneal thinning disorders. Surv Ophthalmol. 1984;28:293–322. doi: 10.1016/0039-6257(84)90094-8. [DOI] [PubMed] [Google Scholar]
- 9.Naderan M, Shoar S, Rezagholizadeh F, Zolfaghari M, Naderan M. Characteristics and associations of keratoconus patients. Cont Lens Anterior Eye. 2015;38:199–205. doi: 10.1016/j.clae.2015.01.008. [DOI] [PubMed] [Google Scholar]
- 10.Ionescu C, Corbu CG, Tanase C, Jonescu-Cuypers C, Nicula C, Dascalescu D, et al. Inflammatory biomarkers profile as microenvironmental expression in keratoconus. Dis Markers. 2016;2016:1243819. doi: 10.1155/2016/1243819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jun AS, Cope L, Speck C, Feng X, Lee S, Meng H, et al. Subnormal cytokine profile in the tear fluid of keratoconus patients. PLoS One. 2011;6:e16437. doi: 10.1371/journal.pone.0016437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Collier SA. Is the corneal degradation in keratoconus caused by matrix-metalloproteinases? Clin Exp Ophthalmol. 2001;29:340–4. doi: 10.1046/j.1442-9071.2001.d01-17.x. [DOI] [PubMed] [Google Scholar]
- 13.Kushner I. Semantics, inflammation, cytokines and common sense. Cytokine Growth Factor Rev. 1998;9:191–6. doi: 10.1016/s1359-6101(98)00016-1. [DOI] [PubMed] [Google Scholar]
- 14.Balasubramanian SA, Pye DC, Willcox MD. Effects of eye rubbing on the levels of protease, protease activity and cytokines in tears: Relevance in keratoconus. Clin Exp Optom. 2013;96:214–8. doi: 10.1111/cxo.12038. [DOI] [PubMed] [Google Scholar]
- 15.McMonnies CW. Mechanisms of rubbing-related corneal trauma in keratoconus. Cornea. 2009;28:607–15. doi: 10.1097/ICO.0b013e318198384f. [DOI] [PubMed] [Google Scholar]
- 16.Shetty R, Ghosh A, Lim RR, Subramani M, Mihir K, Reshma AR, et al. Elevated expression of matrix metalloproteinase-9 and inflammatory cytokines in keratoconus patients is inhibited by cyclosporine A. Invest Ophthalmol Vis Sci. 2015;56:738–50. doi: 10.1167/iovs.14-14831. [DOI] [PubMed] [Google Scholar]
- 17.Kagan HM, Trackman PC. Properties and function of lysyl oxidase. Am J Respir Cell Mol Biol. 1991;5:206–10. doi: 10.1165/ajrcmb/5.3.206. [DOI] [PubMed] [Google Scholar]
- 18.Coral K, Angayarkanni N, Madhavan J, Bharathselvi M, Ramakrishnan S, Nandi K, et al. Lysyl oxidase activity in the ocular tissues and the role of LOX in proliferative diabetic retinopathy and rhegmatogenous retinal detachment. Invest Ophthalmol Vis Sci. 2008;49:4746–52. doi: 10.1167/iovs.07-1550. [DOI] [PubMed] [Google Scholar]
- 19.Dudakova L, Liskova P, Trojek T, Palos M, Kalasova S, Jirsova K, et al. Changes in lysyl oxidase (LOX) distribution and its decreased activity in keratoconus corneas. Exp Eye Res. 2012;104:74–81. doi: 10.1016/j.exer.2012.09.005. [DOI] [PubMed] [Google Scholar]
- 20.Shetty R, Sathyanarayanamoorthy A, Ramachandra RA, Arora V, Ghosh A, Srivatsa PR, et al. Attenuation of lysyl oxidase and collagen gene expression in keratoconus patient corneal epithelium corresponds to disease severity. Mol Vis. 2015;21:12–25. [PMC free article] [PubMed] [Google Scholar]
- 21.Cameron JD, Skubitz AP, Furcht LT. Type IV collagen and corneal epithelial adhesion and migration. Effects of type IV collagen fragments and synthetic peptides on rabbit corneal epithelial cell adhesion and migration in vitro. Invest Ophthalmol Vis Sci. 1991;32:2766–73. [PubMed] [Google Scholar]
- 22.Pahuja N, Kumar NR, Shroff R, Shetty R, Nuijts RM, Ghosh A, et al. Differential molecular expression of extracellular matrix and inflammatory genes at the corneal cone apex drives focal weakening in keratoconus. Invest Ophthalmol Vis Sci. 2016;57:5372–82. doi: 10.1167/iovs.16-19677. [DOI] [PubMed] [Google Scholar]
- 23.Nemet AY, Vinker S, Bahar I, Kaiserman I. The association of keratoconus with immune disorders. Cornea. 2010;29:1261–4. doi: 10.1097/ICO.0b013e3181cb410b. [DOI] [PubMed] [Google Scholar]
- 24.Shetty R, Kaweri L, Pahuja N, Nagaraja H, Wadia K, Jayadev C, et al. Current review and a simplified “five-point management algorithm” for keratoconus. Indian J Ophthalmol. 2015;63:46–53. doi: 10.4103/0301-4738.151468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bolzani G, Della Coletta R, Martelli H, Júnior, Martelli H, Júnior, Graner E. Cyclosporin A inhibits production and activity of matrix metalloproteinases by gingival fibroblasts. J Periodontal Res. 2000;35:51–8. doi: 10.1034/j.1600-0765.2000.035001051.x. [DOI] [PubMed] [Google Scholar]
- 26.Gawronska-Kozak B, Kirk-Ballard H. Cyclosporin A reduces matrix metalloproteinases and collagen expression in dermal fibroblasts from regenerative FOXN1 deficient (nude) mice. Fibrogenesis Tissue Repair. 2013;6:7. doi: 10.1186/1755-1536-6-7. [DOI] [PMC free article] [PubMed] [Google Scholar]


