Abstract
Purpose:
Overexpression of the inhibitors of apoptosis proteins have been demonstrated in a variety and of solid tumors including melanomas and nonmelanomas skin cancers. X-linked inhibitor of apoptosis protein (XIAP) is an inhibitor of apoptosis which prevents apoptosis by inhibiting caspases 9, 7, and 3. The prognostic value of XIAP in sebaceous gland carcinoma (SGC) remains unexplored.
Methods:
The immunohistochemical expression of XIAP was evaluated in 29 SGC cases.
Results:
The cytoplasmic overexpression of XIAP was detected in 62% SGC cases. XIAP expression was found to be significantly associated with advanced age, large tumor size, and with reduced disease-free survival (P = 0.0174). XIAP expression and advance tumor Grade III emerged as significant risk factors on univariate analysis. On stepwise multivariate analysis, both increased cytoplasmic XIAP expression and high tumor grade were found to be significantly associated with recurrence. Patients with low XIAP immunoexpression had a longer disease-specific survival than those with high expression in the 5-year follow-up.
Conclusion:
The present study demonstrates at the immunohistochemical level that XIAP is overexpressed in SGC and that high expression could be of biological significance in the development of eyelid SGC. Our finding suggests that up-regulation of XIAP may aggravate tumor metastasis in SGC.
Keywords: Eyelid, sebaceous gland carcinoma, X-linked inhibitor of apoptosis
Sebaceous gland carcinoma (SGC) is the most aggressive type of eyelid skin cancer, with high recurrence rate and metastatic potential. It is one of the most common eyelid malignancies in Southeast Asia where it accounts for 28%–32.7% of all eyelid malignancies.[1] Although Eyelid sebaceous carcinoma can be cured by surgical resection in its early stages; it often exhibits an aggressive local behavior leading to a regional lymph node. The risk of recurrence and distant organ metastasis remains high as compared to other nonmelanoma skin cancers.[1,2]
The molecular pathogenesis of SGC is still being determined. Alterations in p53 signaling, Wnt/β-catenin signaling, COX signaling, and Indian hedgehog signaling pathways have been associated, along with mutations in p53 and LEF-1 gene.[3,4,5]
Promoter hypermethylation of cell adhesion molecule such as CHD1 and DNA mismatch repair gene have also been associated with poor prognosis of SGC.[6,7] However, the presence of apoptotic-resistant cell has not been demonstrated in SGC. Therefore, the identification of antiapoptotic markers that provide an insight into the potential behavior or aggressiveness of SGC is a necessary step for the improvement of SGC treatment.
X-linked inhibitor of apoptosis protein (XIAP) is a powerful potential regulator of apoptosis. First discovered as 237 bp site on chromosome and XIAP is a member of the endogenous caspase inhibitor family the inhibitor of apoptosis (IAP).[8] The IAPs were first described in baculovirus and were shown to protect virally infected cells by adhering to the activated caspases and inhibiting their proapoptotic function.[9]
Eight IAP-encoding genes have been identified in human genome, namely, XIAP, Survivin, neuronal apoptosis inhibitory protein, Apollon, Livin, testis-specific IAP, cellular IAP1, and cellular IAP2 of these proteins XIAP has been shown to be more potent caspase inhibitor.[10] Elevated XIAP expression has been shown related with malignant cancer progression and aggressive nature in several cancers including, melanoma, prostate cancer, and lung cancer.[11]
Among the various IAPs, only survivin has been assessed for its diagnostic and prognostic utility periocular and extraocular SGC.[12] However, the expression of XIAP which is a potent modulator of programmed cell death (PCD) has not been assessed in eyelid SGC. The purpose of this study was to analyze the expression of XIAP in eyelid SGC and correlate with high-risk features of eyelid SGC.
Methods
Patients and tissue
Twenty-nine cases of eyelid SGC were selected for study from the pathology records of Dr. R. P. Centre for Ophthalmic Sciences. All India Institute of Medical Sciences (AIIMS), New Delhi. The study was carried out after approval from the Institutional Review Board of AIIMS (Ref No A-58. 2007) and has been carried out in accordance with the Declaration of Helsinki principles. The clinicopathological features; radiological details and gross appearance of the patients were recorded. The tumor stage was determined according to the American Joint Committee on Cancer (7th edition) staging criteria.[13] Hematoxylin and eosin-stained sections were analyzed by light microscope to confirm the diagnosis of SGC. The cases were further as poorly or well differentiated based on the degree of sebaceous differentiation and cytoplasmic vacuolation.[14] The presence of pagetoid involvement was also noted. The patients were followed up at 6-month interval after surgery for a mean period of 54 months (range: 11–88 months). Inclusion criteria for this study: histopathologically, proven cases of eyelid SGC. Exclusion criteria: patients who have received chemotherapy/radiotherapy were excluded from the study.
Immunohistochemistry
Formalin-fixed paraffin-embedded sections of 4–5 μm thickness were cut on poly-L-lysine coated microscope slides. After deparaffinization and rehydration, they were incubated in a solution containing 0.3% H2O2 in methanol for 30 min to inactivate endogenous peroxidases. Microwave antigen retrieval was performed using citrate buffer solution at pH 6.0. The sections were first incubated with the monoclonal antibody against XIAP (A-7 clone, Santa Cruz Biotechnology) at dilution (1:100). Subsequently, the slides were incubated overnight at 4°C in a humidified chamber. Secondary incubations were carried out with Polymer detection method using UltraVision Quanto Detection System HRP DAB (Thermo Scientific, Fremont, USA) and developed by diaminobenzene as chromogen for 2 min. Then, the sections were counterstained with hematoxylin, mounted with DPX, and examined by light microscopy. Granular or nonhomogeneous cytoplasmic staining for XIAP was considered positive. Human normal colon served as positive control for XIAP immunohistochemistry (IHC). The omission of the primary XIAP antibody in simultaneously incubated sections served as a negative control. The immunohistochemical staining results for XIAP was recorded as follows: XIAP staining intensity was recorded as (0: negative; 1+: moderate; and 2+: strong) and the percentage positivity was based on the number of tumor cells showing positivity in 10 high-power fields and was scored as 0 (10%); 1+ (10%–50%); and 2+ (>50%). The scores obtained from the percentage positivity and staining intensity were added to represent a single IHC score. The maximum score in this system is 4 and minimum is 0. The tumors were regarded as immunopositive when the IHC score (percentage positivity + staining intensity) of 3 or 4 was obtained and negative when the IHC score was 0–2. XIAP IHC staining was evaluated by one pathologist (S.S) and two observers simultaneously, and a consensus was reached for each IHC score.
Statistical analysis
The Chi-square test was used to assess the association between immunohistochemical reactivity and clinicopathological characteristics. Kaplan–Meier method was employed for survival analysis and differences in survival were estimated with the log-rank test. P < 0.05 was considered to be statistically significant. The following clinicopathological factors: age, sex, tumor site, tumor size, lymph node metastasis and clinical stage, histopathological differentiation, pagetoid spread, and immune expression of XIAP were included in survival analysis. Univariate and multivariate analysis was performed using Cox proportional hazard model to identify the factors useful in predicting the disease-free survival rates. All of the statistical analysis was performed with MedCalc Software version 14.8.1 (MedCalc Software BVBA, Ostend, Belgium, Europe; http://www.medcalc.org; 2014)
Results
Clinical and light microscopy features
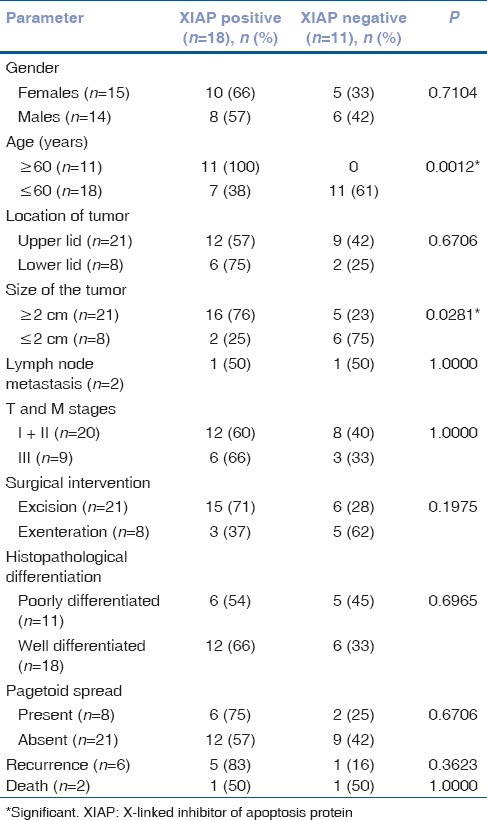
The mean age of SGC patients was 59.9 years (range: 25–88 years). Upper eyelid involvement was observed in (72.4%) with the varied clinical presentation. The size of tumor varied between 0.5 and 5.0 cm with mean of 3 ± 1.2 cm. Tumor size (>2 cm) was seen in 72% cases. On the basis of staging criteria, twenty (68%) cases were in Stage I and II and 9 (31%) in Stage III. Orbital involvement was seen in 27% cases. Light microscopy revealed that 18 (62.1%) well-differentiated SGC (WDSGC) and 11 (37.9%) were poorly differentiated SGC (PDSGC). Pagetoid spread was seen in 27% cases. Lymph node involvement was seen in 6.8% cases [Table 1].
Table 1.
Correlation of X-linked inhibitor of apoptosis protein immunoexpression with clinicopathological parameters
Immunohistochemistry results
Immunohistochemical expression of X-linked inhibitor of apoptosis protein in sebaceous gland carcinoma
IHC revealed cytoplasmic overexpression of XIAP in 62% (18/29) of cases [Figs. 1 and 2]. A significant association was found between increased cytoplasmic expression of XIAP in 16/18 (88.9%) patients with advanced age (≥60 years) (P = 0.0012) and in in tumor size >2 cm (P = 0.0281) [Table 1]. Cytoplasmic overexpression of XIAP was seen in 12/18 (66%) WDSGC cases [Fig. 1c]. Weak cytoplasmic immunostaining was observed in of 54% PDGSC [Fig. 2a]. However, no significant association of XIAP expression was observed with other poor prognostic features of SGC [Table 1].
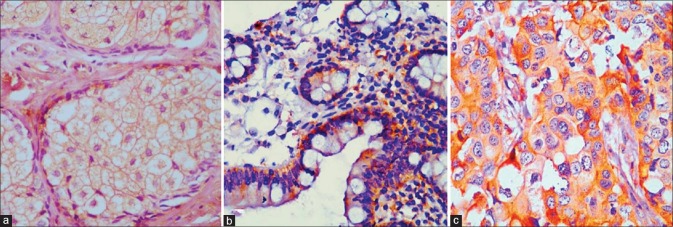
Figure 1.
X-linked inhibitor of apoptosis protein cytoplasmic immunostaining in a normal sebaceous gland ([a] ×400) and in normal colon control ([b] ×400). Strong cytoplasmic immunoexpression in a case of well-differentiated sebaceous gland carcinoma ([c] ×200)
Figure 2.
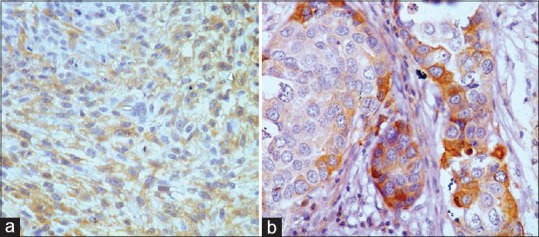
Weak cytoplasmic immunostaining in a case of poorly differentiated sebaceous gland carcinoma ([a] ×200). X-linked inhibitor of apoptosis protein immunopositivity in an exenterated sebaceous gland carcinoma sample ([b] ×400)
X-linked inhibitor of apoptosis protein immunoexpression and clinical outcome
XIAP cytoplasmic expression was observed in a single patient with lymph node metastasis, in five of six patients with tumor recurrence (83%) and in the one patient who died. The prognostic potential of XIAP expression was determined using Kaplan–Meier survival analysis. Significant association of reduced disease-free survival was seen in SGC cases with XIAP overexpression (P = 0.0174, log-rank analysis) [Fig. 3] and in patients with tumor Grade III (P = 0.0125, log-rank analysis).
Figure 3.
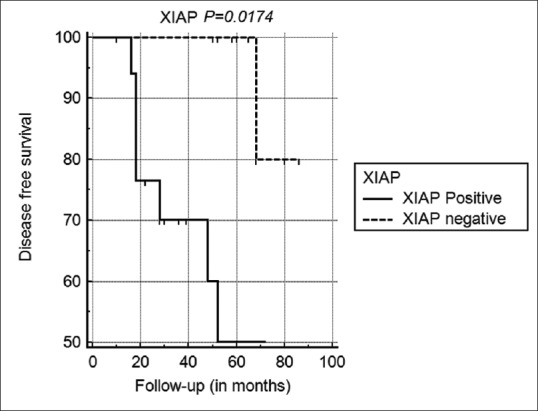
Kaplan–Meier analysis showing reduced disease-free survival rates in sebaceous gland carcinoma patients with X-linked inhibitor of apoptosis protein overexpression
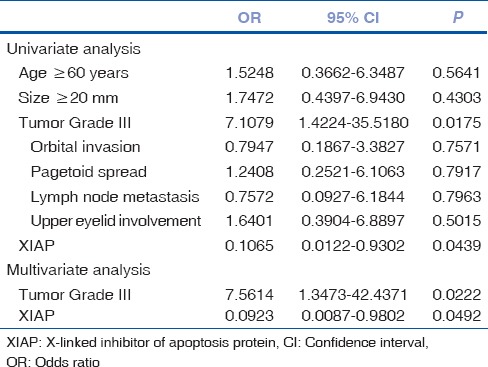
Univariate and multivariate analysis to identify independent prognostic markers
For disease-free survival, XIAP expression (P = 0.0439) and tumor Grade III (P = 0.0175) emerged as significant risk factors in the univariate analysis. When the stepwise multivariate analysis was performed on these factors both cytoplasmic XIAP expression (odds ratio:-0.0923; 95% confidence interval: 0.0087–0.9802) and high tumor grade (odds ratio: 7.5614; 95% confidence interval: 1.3473–42.4371) continued to be significantly associated with SGC recurrence [Table 2].
Table 2.
Risk factor affecting disease-free survival in patients with sebaceous gland carcinoma
Discussion
SGC is often locally aggressive, multifocal and has a tendency to recur after treatment. The mortality rate for SGC of the eyelid is about 5%–10%. Diverse clinical presentation of SGC often ends in delayed diagnosis and tends have higher rate of recurrence and metastasis.[15,16] The aggressiveness of cancer is often characterized by the presence of apoptosis-resistant cancer cells which can be a result of various genetic alterations and increased expression of antiapoptotic factors.[17] PCD pathway requires the activation of cysteine aspartyl proteases termed caspases – the executor of the apoptotic pathway,[18] which are activated proteolytically by the specific apoptogenic agents.[5] XIAP hinders the activation of apoptosis-promoting caspases (caspase 3, 7, and 9) thereby blocking extrinsic and intrinsic apoptotic pathways. Increased expression of XIAP in cell culture has been shown to confer resistance to apoptosis-inducing chemotherapeutic agents and radiations.
XIAP is known to be expressed in normal tissues, however, overexpression of XIAP has been demonstrated in several human cancers including nonsmall lung cancer,[19] myeloid leukemias,[20] prostate cancer, pancreatic cancer,[21] and renal carcinoma.[22] In this study, the pattern of immunoexpression and biological application of XIAP expression was examined for the first time in eyelid SGC patients. This study provides evidence that XIAP expression was up-regulated in SGC. Compared with normal sebaceous glands XIAP overexpression positively correlated with well-differentiated state of SGC and with an advanced grade of SGC.
In vitro analysis have shown XIAP to be important caspase inhibitor than other IAPs including survivin which has been studied extensively in distinct tumors.[23] Few studies have been attempted to delineate the clinical role of survivin in extraocular sebaceous neoplasm.[12] However, its association with tumor recurrence and malignant potential could not be determined in SGC because of smaller cohort size.[24] Although XIAP expression did not correlate with the poor prognostic features of eyelid SGC. Overexpression of XIAP was detected in 66.7% of cases with tumor recurrence. Further, a significant association was observed between cytoplasmic overexpression of XIAP with reduced disease-free survival. XIAP overexpression may serve as an independent prognostic predictor of SGC in multivariate analysis. Our findings are in agreement with those made in other cancers, where XIAP overexpression has been correlated with disease-free survival[22] and has been proposed to serve as a tumor marker.
Conclusion
The present study links SGC defective apoptotic pathway. Our findings suggest that overexpression of XIAP may play a key role in regulating the malignant potential and apoptosis in SGC which may be a prognostic value in SGC. The evaluation of XIAP immunoexpression may be useful in the management of eyelid SGC which may help patients for more intensive surgical approach in combination with XIAP antagonists.
Financial support and sponsorship
This study was financially supported by Delhi university innovation grant.
Conflicts of interest
There are no conflicts of interest.
Acknowledgments
Authors are thankful to Delhi University Innovation Project (SVC-206) for the financial support and to Dr. P. Hemalatha Reddy, Principal, Sri Venkateswara College, Delhi University, for providing the laboratory facilities.
References
- 1.Wali UK, Al-Mujaini A. Sebaceous gland carcinoma of the eyelid. Oman J Ophthalmol. 2010;3:117–21. doi: 10.4103/0974-620X.71885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arathi C, Vijaya C. Scrape cytology in the early diagnosis of eyelid sebaceous carcinoma. J Cytol. 2010;27:140–2. doi: 10.4103/0970-9371.73301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jayaraj P, Sen S, Sharma A, Kashyap S, Rai A, Pushker N, et al. Lymphoid enhancing factor-1(lef-1) gene mutation and its differential mRNA Expression in eyelid sebaceous carcinoma. Invest Ophthalmol Vis Sci. 2012;53:5614. [Google Scholar]
- 4.Jayaraj P, Sen S, Kashyap S, Sharma A, Pushker N, Bajaj MS, et al. Does β-catenin have a role in pathogenesis of sebaceous cell carcinoma of the eyelid? Br J Ophthalmol. 2011;95:284–7. doi: 10.1136/bjo.2009.177204. [DOI] [PubMed] [Google Scholar]
- 5.Kiyosaki K, Nakada C, Hijiya N, Tsukamoto Y, Matsuura K, Nakatsuka K, et al. Analysis of p53 mutations and the expression of p53 and p21WAF1/CIP1 protein in 15 cases of sebaceous carcinoma of the eyelid. Invest Ophthalmol Vis Sci. 2010;51:7–11. doi: 10.1167/iovs.09-4127. [DOI] [PubMed] [Google Scholar]
- 6.Holbach LM, von Moller A, Decker C, Jünemann AG, Rummelt-Hofmann C, Ballhausen WG, et al. Loss of fragile histidine triad (FHIT) expression and microsatellite instability in periocular sebaceous gland carcinoma in patients with Muir-Torre syndrome. Am J Ophthalmol. 2002;134:147–8. doi: 10.1016/s0002-9394(02)01434-4. [DOI] [PubMed] [Google Scholar]
- 7.Jayaraj P, Sen S, Sharma A, Chosdol K, Kashyap S, Rai A, et al. Epigenetic inactivation of the E-cadherin gene in eyelid sebaceous gland carcinoma. Br J Dermatol. 2012;167:583–90. doi: 10.1111/j.1365-2133.2012.10968.x. [DOI] [PubMed] [Google Scholar]
- 8.Holcik M, Gibson H, Korneluk RG. XIAP: Apoptotic brake and promising therapeutic target. Apoptosis. 2001;6:253–61. doi: 10.1023/a:1011379307472. [DOI] [PubMed] [Google Scholar]
- 9.Crook NE, Clem RJ, Miller LK. An apoptosis-inhibiting baculovirus gene with a zinc finger-like motif. J Virol. 1993;67:2168–74. doi: 10.1128/jvi.67.4.2168-2174.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Burke SP, Smith JB. Monomerization of cytosolic mature smac attenuates interaction with IAPs and potentiation of caspase activation. PLoS One. 2010;5:pii: e13094. doi: 10.1371/journal.pone.0013094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.LaCasse EC, Mahoney DJ, Cheung HH, Plenchette S, Baird S, Korneluk RG, et al. IAP-targeted therapies for cancer. Oncogene. 2008;27:6252–75. doi: 10.1038/onc.2008.302. [DOI] [PubMed] [Google Scholar]
- 12.Mulay K, Shah SJ, Aggarwal E, White VA, Honavar SG. Periocular sebaceous gland carcinoma: Do androgen receptor (NR3C4) and nuclear survivin (BIRC5) have a prognostic significance? Acta Ophthalmol. 2014;92:e681–7. doi: 10.1111/aos.12466. [DOI] [PubMed] [Google Scholar]
- 13.Ainbinder DJ, Esmaeli B, Groo SC, Finger PT, Brooks JP. Introduction of the 7th edition eyelid carcinoma classification system from the American Joint Committee on Cancer-International Union against Cancer Staging Manual. Arch Pathol Lab Med. 2009;133:1256–61. doi: 10.5858/133.8.1256. [DOI] [PubMed] [Google Scholar]
- 14.Pe’er J. Pathology of eyelid tumors. Indian J Ophthalmol. 2016;64:177–90. doi: 10.4103/0301-4738.181752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Shields JA, Demirci H, Marr BP, Eagle RC, Jr, Shields CL. Sebaceous carcinoma of the eyelids: Personal experience with 60 cases. Ophthalmology. 2004;111:2151–7. doi: 10.1016/j.ophtha.2004.07.031. [DOI] [PubMed] [Google Scholar]
- 16.Boniuk M, Zimmerman LE. Sebaceous carcinoma of the eyelid, eyebrow, caruncle, and orbit. Trans Am Acad Ophthalmol Otolaryngol. 1968;72:619–42. [PubMed] [Google Scholar]
- 17.Thompson CB. Apoptosis in the pathogenesis and treatment of disease. Science. 1995;267:1456–62. doi: 10.1126/science.7878464. [DOI] [PubMed] [Google Scholar]
- 18.Strasser A, O’Connor L, Dixit VM. Apoptosis signaling. Annu Rev Biochem. 2000;69:217–45. doi: 10.1146/annurev.biochem.69.1.217. [DOI] [PubMed] [Google Scholar]
- 19.Checinska A, Hoogeland BS, Rodriguez JA, Giaccone G, Kruyt FA. Role of XIAP in inhibiting cisplatin-induced caspase activation in non-small cell lung cancer cells: A small molecule Smac mimic sensitizes for chemotherapy-induced apoptosis by enhancing caspase-3 activation. Exp Cell Res. 2007;313:1215–24. doi: 10.1016/j.yexcr.2006.12.011. [DOI] [PubMed] [Google Scholar]
- 20.Tamm I, Kornblau SM, Segall H, Krajewski S, Welsh K, Kitada S, et al. Expression and prognostic significance of IAP-family genes in human cancers and myeloid leukemias. Clin Cancer Res. 2000;6:1796–803. [PubMed] [Google Scholar]
- 21.Nomura T, Yamasaki M, Nomura Y, Mimata H. Expression of the inhibitors of apoptosis proteins in cisplatin-resistant prostate cancer cells. Oncol Rep. 2005;14:993–7. [PubMed] [Google Scholar]
- 22.Mizutani Y, Nakanishi H, Li YN, Matsubara H, Yamamoto K, Sato N, et al. Overexpression of XIAP expression in renal cell carcinoma predicts a worse prognosis. Int J Oncol. 2007;30:919–25. [PubMed] [Google Scholar]
- 23.Ekert PG, Silke J, Vaux DL. Caspase inhibitors. Cell Death Differ. 1999;6:1081–6. doi: 10.1038/sj.cdd.4400594. [DOI] [PubMed] [Google Scholar]
- 24.Calder KB, Khalil FK, Schlauder S, Cualing HD, Morgan MB. Immunohistochemical expression of survivin in cutaneous sebaceous lesions. Am J Dermatopathol. 2008;30:545–8. doi: 10.1097/DAD.0b013e31817ec291. [DOI] [PubMed] [Google Scholar]