Abstract
Purpose:
The aim of this study was to evaluate the inhibitory effect of different concentrations of KH902 eye drops on rabbit corneal neovascularization (CNV) induced by alkali burn.
Methods:
Forty-eight adult rabbits were randomized into four groups after alkali burning: Group A (2.5 mg/ml), Group B (5 mg/ml), and Group C (10 mg/ml) by different concentrations of KH902 eye drops and Group D by saline solution as control with three times a day for 2 weeks. At days 7, 14, and 28, the anterior segment photographs, confocal microscopy, and histopathology were performed to evaluate corneal opacity, neovascularization, inflammatory cell density, vessel size, and edema. Immunohistochemistry was applied to analyze the vascular endothelial growth factor (VEGF) level.
Results:
(1) The CNV in the medicine-treated groups showed a reduction without obvious corneal side effects histologically. (2) Compared to the control group, the three medicine-treated groups showed a reduction in the VEGF levels and CNV areas on days 7, 14, and 28 and in the inflammatory cell density on days 14 and 28 (P < 0.01). The difference of inflammatory cell density between the three medicine-treated groups existed on day 14 (P < 0.01). There were differences in the VEGF levels between Groups A, B, and C on days 7, 14, and 28 (P < 0.01), not for Groups B and C on day 28 (P > 0.05).
Conclusion:
KH902 eye drops in lower concentration showed an obvious reduction of the CNV growing for rabbit corneal alkali burn without side effects.
Keywords: Alkali burn, corneal neovascularization, KH902 eye drops, vascular endothelial growth factor
Normal cornea is avascular and transparent physiologically. The cornea uses complicated antiangiogenic mechanisms that actively maintain corneal avascularity, collectively accounting for corneal angiogenic privilege.[1] Although the human cornea is avascular in normal homeostatic conditions, corneal angiogenic privilege loses efficacy in some pathological conditions such as trauma, infection, and burning.
Corneal neovascularization (CNV) is a sight-threatening condition that can develop in response to inflammation, hypoxia, trauma, or limbal stem cell deficiency.[2] Alkali burn trauma is an important cause of CNV. Recently, vascular endothelial growth factor (VEGF) has been proven a major inducer of CNV as well.[3,4,5] In general, corneal angiogenesis is associated with upregulating VEGF which is a key proangiogenic factor in pathologic CNV.[6,7,8] VEGF is a family of proteins comprising VEGF-A, -B, -C, and -D, the viral VEGF homolog VEGF-E, and placental growth factor.[9] VEGF promotes several steps of angiogenesis including proteolytic activities, endothelial cell proliferation, migration, and capillary tube formation.[6,10] Extensive evidence indicates that VEGF plays a major role in inflammatory angiogenesis, and its production is elevated significantly in inflamed vascularized corneas.[6,11,12,13,14,15] Numerous studies showed that anti-VEGF agents, used either alone or in combination with steroids, verteporfin, or anti-tumor necrosis factor-α microantibody, are effective in the treatment of CNV.[16] Anti-VEGF agents reported for the treatment of CNV nowadays include bevacizumab, pegaptanib, ranibizumab, and trastuzumab, all of which have the ability to inhibit CNV.[17]
Compared to bevacizumab, a typical monoclonal antibody that binds to VEGF-A and comprises both human immunoglobulin G (IgG) (93%) and murine antibody (7%), KH902 is a recombinant human-soluble vascular endothelial growth factor receptor (VEGFR) fusion protein with a 100% human protein sequence. The new drug contains several ligand-binding domains of human VEGFR 1 and VEGFR 2 and the fragment crystallizable portion of human IgG1. In vitro studies indicate that the receptor portion of the molecule has a very high affinity not only for VEGF-A isoforms but also for VEGF-B and placenta growth factor (PIGF) 1 and 2. It has a stronger antiangiogenesis effect than bevacizumab in theory.[18]
The objective of the present paper is to evaluate the inhibitory effect of the different concentrations of KH902 eye drops in treating the CNV induced by alkali burn and to find the lowest effective concentration.
Methods
Animals
Forty-eight New Zealand white rabbits weighing 2–3 kg were collected. Food and water was provided and a 12 h’ light/dark cycle maintained. This study has been approved by the Institutional Animal Care and Use Committee of Jinling Hospital, Jiangsu, China. All experimental procedures on animals were conducted in accordance with the Association for Research in Vision and Ophthalmology Resolution on the use of animals in research.
KH902 (Chengdu Kanghong Biotechnology Limited, China) was used in the study. The rabbits were randomized into four groups: Group A (2.5 mg/ml), Group B (5 mg/ml), and Group C (10 mg/ml) by different concentrations of KH902 eye drops and Group D by saline solution as control. KH902 was preserved by cold chain technology and stored in a special refrigerator at 2°C–8°C.
Twelve eyes were collected in each group: six eyes were used for examining confocal microscope in vivo and measuring CNV area, while the others were prepared for histological examination and VEGF quantification.
Alkali burning and drug administration
The left eye of each rabbit was exposed to defined alkali injury under general anesthesia with intramuscular injections of 35 mg/kg ketamine, 5 mg/kg xylazine, and local anesthesia with oxybuprocaine drops on the corneal surface. A round, 4-mm diameter filter paper was soaked in 1 mol/L sodium hydroxide and placed onto the limbal cornea immediately for 30 s. The surface was then carefully rinsed with 100 ml physiological saline solution.[19] The rabbits received topical administration of 50 μl of the different solutions on the cornea immediately after the alkali burning while no CNV existed in, with three times per day for 14 days. Besides the solutions, all samples in the four groups received chloramphenicol and dexamethasone eye drops for a common treating. The procedure was conducted by one operator.
Quantification of corneal neovascularization
The chemical cauterization modeling day was considered day 0. We examined all eyes daily and reported the development of CNV at 7, 14, and 28 days after chemical cauterization by slit-lamp examination (SL-120; Zeiss, Jena, Germany). Digital photographs were obtained with a Nikon megapixels digital camera (TOPCON, TRC NW200). The area of CNV was determined by the following formula: AC = C/12 × 3.1416× [r2−(r−L)2] where C represents the clock hours of CNV, r represents the radius of the animal cornea, and L represents the length of the longest new vessel. The length of vessel was measured with a reticule from the limbus to the tip of the vessels.[20,21] The observation was carried out by one person who knew nothing about the grouping.
Confocal microscopy
A laser confocal microscope (Heidelberg Retina Tomo-graph II Rostock Cornea Module, HRT II, Heidelberg, Germany) was used to examine the animals in vivo at days 7, 14, and 28 after the alkali burning. When used to view one of the coin-shaped lesions located at the CNV of the cornea, we captured the morphological pictures of the corneal epithelium, Bowman's layer, stroma, descemet membrane, and endothelium by the camera. The visual field was 400 μm × 400 μm, the resolving power was 1 μm, and the amplifying power was ×800. Five different zones of the stroma of each specimen were examined and three limpid pictures of each zone were chosen. We used the Rostock software (carried by HRT II) to analyze the photographs and calculate the inflammatory cell density. The examination was carried out by another person who had no information about the grouping.
Histology
Six animals from each group were killed for enucleating the left globes on days 7, 14, and 28 after the alkaline burning (two globes each time). Enucleation was performed after the animals had been euthanized. The eyes were prepared for histological and immunological examination using 10% formaldehyde. After fixation for 24 h, they were removed from the fixative and corneas were dehydrated and embedded in paraffin. Tissue sections of 5 mm thickness were prepared and one-half were stained with hematoxylin and eosin for light microscopy.
Immunohistochemistry of vascular endothelial growth factor
VEGF expression was evaluated in the other half of the paraffin sections, using the anti-VEGF monoclonal antibody (Mouse Ab-3, Clone JH121, Neomarker). Before staining, sections were hydrated, washed in phosphate-buffered saline (PBS), pretreated in 10 mm citric acid (95°C) for 20 min, and blocked using 1% BSA/0.5% Tween at room temperature for 30 min. Sections were then incubated with the primary antibody to VEGF (1:50) overnight at 4°C, followed by routine biotin/streptavidin methodology, according to the manufacturer's protocols. After washing with PBS, the sections were counterstained with hematoxylin, dehydrated, and mounted for light microscopy observations.[22] Cytoplasmic or nuclear envelope showed brown was positive. The software Image-pro 6.0 (Media Cybernetics, USA) selected the same brown color as the uniform standard for judging all positive which were analyzed to obtain the respective integrated optical density (IOD) values. The high IOD value demonstrated the strong expression of the positive sheet. Light microscopic and immunological examination was performed on every section by the same examiner who was blinded to the groups.
Statistical analysis
Statistical analyses were performed using the Statistical Package for Social Sciences software (SPSS, Version 18.0, Chicago, IL, USA). All data were presented with means ± standard deviations. We used repeated measures analysis of covariance to compare the results. After finding differences among the groups, the Student–Newman–Keuls-q test was used to compare between groups. P < 0.05 was considered statistically significant.
Results
Morphological structure of corneal neovascularization
In the corneal alkali burning model, all samples (six eyes for each group) appeared edema in the central zone. CNV grew up in the 1st week, and mild peripheral neovascularization was apparent on the 7th day. Small vessels grew into the corneas in Groups A and B, while some micro-CNV appeared in the limbus of Group C. The vessels in Group D emerged from the adjacent limbus and were tiny and dense.
On day 14, there was clear evidence of neovascularization with major visible vessels. The CNV in Groups A, B, and C increased rapidly and showed centripetal growth. All samples in Group D had gross vessels in the central zone, with ulcers in three samples (50%). The perforation occurred in one sample (16.7%) of Group D, covered with yellow-white secretions on the surface. No ulcer existed in the three medicine-treated groups.
On day 28, we observed no ulcer in Groups B and C, whereas two ulcers (33.3%) and one perforation (16.7%) in Group D with gross CNV grew centripetally in all samples [Fig. 1]. One sample in Group A showed superficial ulcer without any perforation (16.7%). A few number of tiny CNV grew into the central corneas in three medicine-treated groups. The CNV in Group C was minimal.
Figure 1.
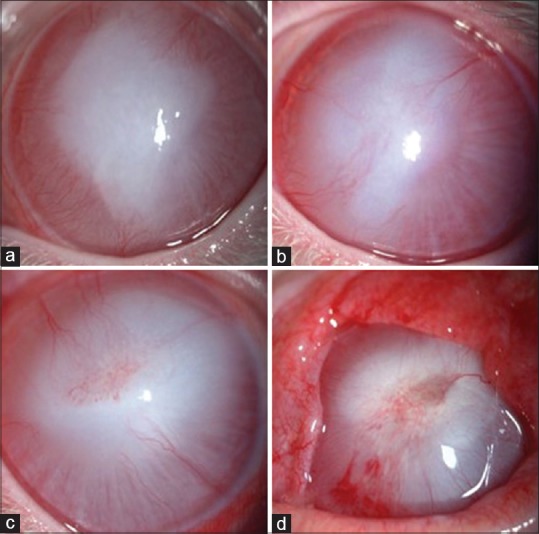
The anterior segment photographs of each group 28 days after modeling: (a) Group A, (b) Group B, (c) Group C, (d) Group D; the corneal neovascularization of the three medicine-treated groups was significantly lower than that of Group D. A large number of corneal neovascularization, corneal ulcers, and symblepharon appeared in Group D
Hematoxylin and eosin staining
On day 7, the epithelial cells were edema and localized exfoliation existed in all samples of Group D with an amount of inflammatory cells infiltrated. The other three groups had little epithelial cells exfoliation and inflammatory cell infiltration.
On day 14, the samples in all groups had the corneal epithelial cell swelling and intercellular space expanding more obviously. The number of swelling fibers in stroma increased and a large number of inflammatory cells were infiltrated into the stroma. CNV appeared in the stroma, much more in Group D than other groups. The samples in Group D showed tissue necrosis, while none existed in other groups [Fig. 2].
Figure 2.
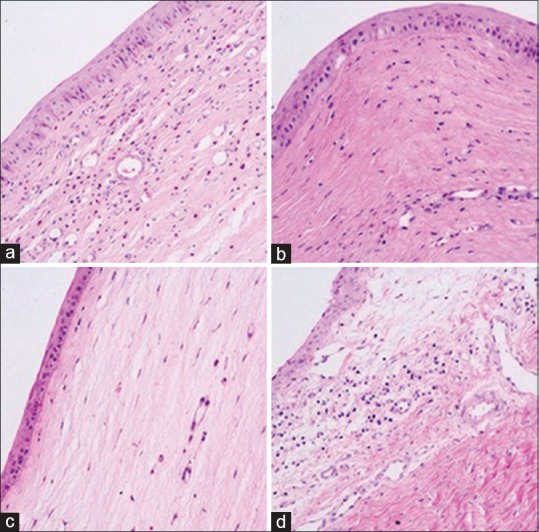
The keratin - eosin staining of corneal tissue 14 days after modeling (×200): (a) Group A, (b) Group B, (c) Group C, (d) Group D. Intravascular wall formation, stromal cell edema, and inflammatory cell infiltration appeared in all groups. The degree of inflammatory cell density and stromal cell edema was D > A > B > C
On day 28, the epithelial cells exfoliation only existed in Group D and a few number of sections of Group A. The epithelial and matrical edema and the infiltration of the inflammatory decreased in Groups B and C. The CNV density in the three KH902 treated groups was less than that in Group D. Meanwhile, no specific toxicity was seen regarding epithelium, keratocytes, or endothelium in the three medicine-treated groups.
Confocal microscopy
On day 7, the confocal microscopy showed that the deficient epithelial cells, the swelling stroma fibers, and high-reflective nuclei existed in all groups, with little inflammatory cell infiltrated. We identified some tiny CNV only in Group D. On day 14, the edema of the epithelial cells and matrix fibers decreased, while the CNV in all groups grew rapidly. The central CNV in Groups A, B, and D grew in different densities, whereas Group C showed peripheral CNV. The numerous inflammatory cell infiltration was found only in Group D. On day 28, the three medicine-treated groups had intact epithelial lines and the CNV densities decreased, while the samples in Group D had epithelial cells defect and a great number of inflammatory cells infiltrated [Fig. 3].
Figure 3.
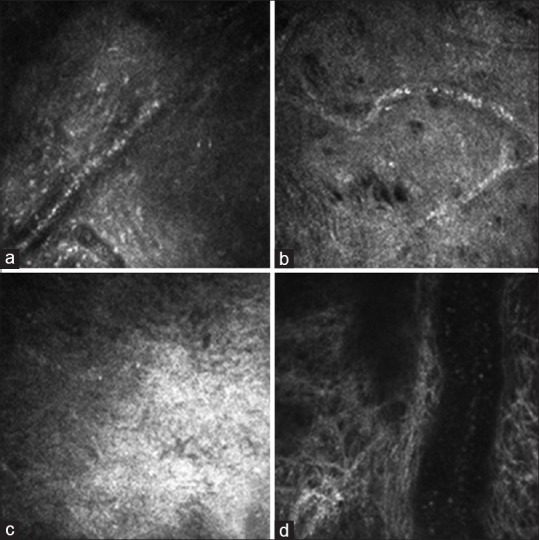
Corneal structure under confocal microscope at 28 days after modeling (400 μm × 400 μm): (a) Group A; (b) Group B; (c) Group C; (d) Group D. In the four groups, the matrix scar was observed, inflammatory cell infiltration was significantly reduced, and the corneal neovascularization formation was obvious. Blood cells flow within large vessels, especially common in Group D
The software carried by the HRT-II confocal microscopy measured the inflammatory cell density of the four groups [Fig. 4]. All results were observed from three typical photographs, calculating the mean value for the statistical analysis. The homogeneity of variance was tested for all groups, and the results of each group were all in line. Statistical analysis revealed that the inflammatory cells decreased, but the density had no statistical difference on day 7 (F = 0.42, P = 0.7411). The density in three KH902-treated groups was significantly reduced compared with Group D on days 14 and 28, showing statistically significant (F14 =1495.92, P14 =0.0000; F28 =137.85, P < 0.001). The pairwise comparison between Groups A, B, and C, respectively, demonstrated the statistical differences on day 14 (QAB= 15.0054, QAC= 23.3797, QBC= 8.3743; P < 0.01), whereas no differences on day 28 (QAB= 1.7338, QAC= 2.4793, QBC= 0.7455; P > 0.05).
Figure 4.
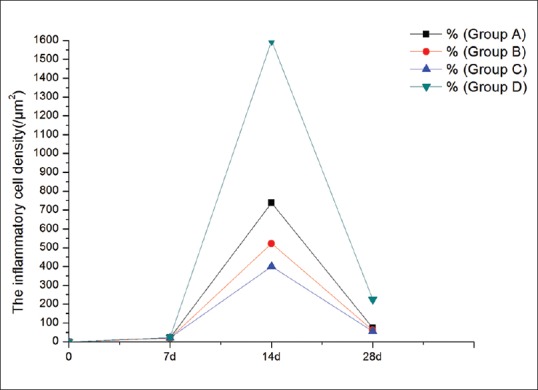
The inflammatory cell density of the four groups
Vascular endothelial growth factor
We measured the VEGF levels on days 7, 14, and 28 [Fig. 5]. The VEGF peak appeared on day 14 [Fig. 6], and the VEGF level was lowest in Group C. After the withdrawal of drugs on day 14, VEGF levels decreased further. The homogeneity of variance was tested on days 7, 14, and 28, and the results were all consistent in. Repeated measures of variance analysis showed that, at each time point, the overall mean of each group was not equal (F7 =4450.73, F14 =7167.03, F28 =1470.60, P < 0.001). Each group was compared with other groups, respectively, at each time point, showing that the difference between the groups, respectively, was statistically significant (Q7AB = 37.5407, Q7AC = 53.8611, Q7AD = 95.1068, Q7BC = 16.3204, Q7BD = 132.6475, Q7CD = 148.9679; Q14AB = 306.9255, Q14AC = 402.3567, Q14AD = 321.0678, Q14BC = 95.4313, Q14BD = 627.9932, Q14CD = 723.4245; Q28AB = 19.4616, Q28AC = 20.0552, Q28AD = 61.2264, Q28BD = 80.6880, Q28CD = 81.2816; P < 0.01), except for Groups B and C on day 28 (Q28BC = 0.5936, P = 0.23).
Figure 5.
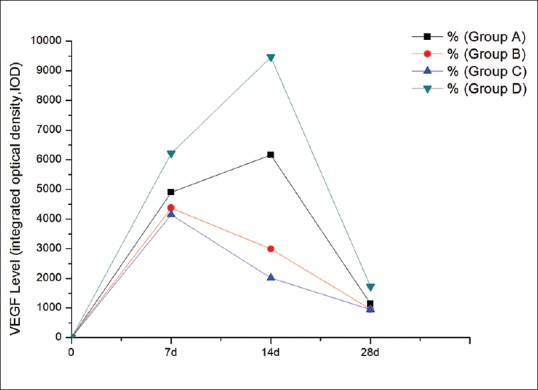
The vascular endothelial growth factor level of the four groups
Figure 6.
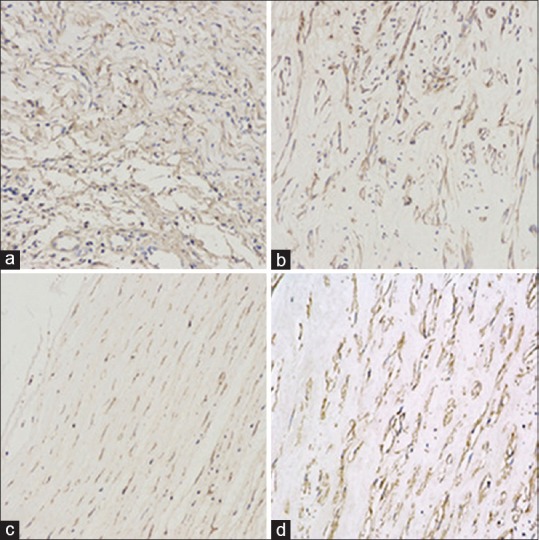
The corneal vascular endothelial growth factor immunohistochemistry 14 days. After modeling (×200), (a) Group A; (b) Group B; (c) Group C; (d) Group D
Corneal neovascularization
The CNV area was calculated on days 7, 14, and 28 [Fig. 7]. The peak of the CNV area appeared on day 14, and the area was lowest in Group C. The homogeneity of variance was tested at each time point, and the results were all consistent in. The results counted by repeated measures of variance analysis showed that the overall mean of each group was not equal at each time point. Each group was compared with another group, respectively, at each time point, showing that the sample means of Groups A, B, and C were statistically different from that of Group D (F7 =37.25, F14 =119.70, F28 =101.38, P < 0.001). No significant difference appeared in the Groups A, B, and C (Q7AB = 2.4354, Q7AC = 2.7002, Q7BC = 0.2648, Q14AB = 2.1490, Q14AC = 3.4980, Q14BC = 1.349, Q28AB = 2.2127, Q28AC = 2.4435, Q28BC = 0.2308, P > 0.05). The corneas in Group D showed a large number of blood vessels.
Figure 7.
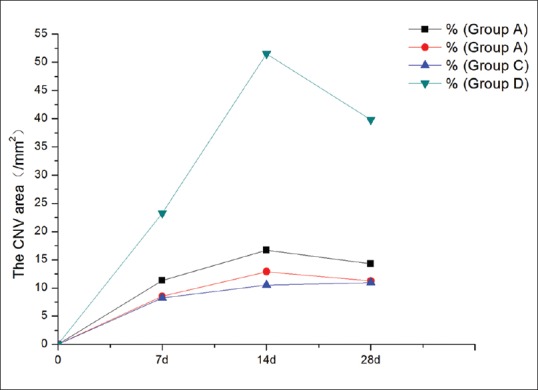
The corneal neovascularization area of the four groups (/mm2)
Discussion
The normal cornea is devoid of both blood and lymphatic vessels and maintains its avascularity actively.[23,24] This so-called corneal “angiogenic privilege” is essential to corneal transparency and vision.[25] CNV occurs as a result of a disequilibrium between angiogenic and antiangiogenic factors of which alkali burn is an important reason.
Many drugs and operations are being used to treat CNV. Nowadays, steroids remain the most popular choice in clinical treatment on CNV by controlling inflammation, whereas with the increased chance of the replication of infectious keratitis, retarded corneal wound healing, secondary glaucoma, and cataract.[26,27,28] Other choices such as laser photocoagulation, diathermy, and photodynamic therapy are also effective,[29,30] but they are not available for extensive CNV and have complicated manipulations.[31]
So far, no specific anti-angiogenic treatment against CNV has been available. Recently, DeStafeno and Kim[32] found that VEGF acts as the key mediator during inflammation and neovascularization, which regulates the vascular endothelium growing and controls the formation of new blood vessels. VEGF is secreted mainly from macrophages, T-cells, retinal pigment epithelial cells, smooth muscle cells, and tumor cells with the stimulation of various environmental factors, especially hypoxia. Binding to its receptors, VEGF triggers a signaling cascade that promotes endothelial cell growth, survival, migration, differentiation, and mobilization of endothelial progenitor cells from the bone marrow into the peripheral circulation.[33] Due to rapid progress made in angiogenesis research in recent years, specific antiangiogenic drugs, such as bevacizumab and ranibizumab, are now used widely in oncology and against neovascular diseases at the retina and choroid.[24,34]
KH902 has been successfully used systemically and intravitreally in humans with exudative age-related macular degeneration, diabetic retinopathy controlling retina, and retinopathy of prematurity with minimal or no adverse effects.[35,36,37] We recently revealed that KH902 eye drops can inhibit corneal angiogenesis and lymphangiogenesis in animal models of inflammatory CNV. Zhou et al.[38] administered KH902 by intraperitoneal injection in the mice suffered corneal injuries by alkali burn. KH902 significantly inhibited new vessel growth and promoted the regression of established vessels in a mouse model of CNV, and it also reduced the levels of VEGF and PIGF in the cornea. Wang et al.[39] used KH902 injected subconjunctivally for treating the rats with CNV, showing the inhibiting effect. However, regarding that repeated subconjunctival injection could damage the ocular surface, we dropped the medicine directly into the conjunctival sac. In our study, KH902 has been proved very effective in preventing neovascularization when administered topically in different concentrations. The length and area of CNV decreased significantly compared with the control group. We measured the VEGF concentration at 7, 14, 28 days after modeling, and the VEGF level decreased significantly in all medicine-treated groups compared with the control group. We compared the VEGF levels, respectively, between the medicine-treated groups, which indicated statistically significant difference between each two groups, except for Groups B and C on day 28 (P > 0.05). The results revealed that the anti-VEGF expression gradually increased by increasing the KH902 concentration. Meanwhile, we measured the density of inflammatory cells after modeling on days 7, 14, and 28, which were significantly reduced in the medicine-treated groups on days 14 and 28. It indicated that KH902 had a certain inhibitory effect on inflammatory cells. Last not the least, the CNV area of the three medicine-treated groups was significantly lower than the control group on days 7, 14, and 28(P < 0.01). The area of CNV decreased gradually with the increased concentration in the three medicine-treated groups without significant difference (P > 0.05).
Edelman et al.[40] declared that the neovascular response progresses in three phases: (1) a nonproliferative phase preceding vessel growth; (2) a proliferative phase with maximal growth rate; and (3) a regressive phase with a decrease in vessel density accompanying the completion of vessel elongation. The CNV in our study showed a similar growth pattern. Our study suggested that KH902 could inhibit CNV in the rabbit alkali burning model in all medicine-treated groups. Although our data showed statistical significance among the three treated groups (P < 0.05), the inhibition was far from complete. Some possibilities could explain this incomplete inhibition; other cytokines, such as fibroblast growth factor, might play a role in the CNV growth.
Kim et al.[41] declared that the anti-VEGF drugs might delay the epithelial cells healing. In our study, confocal microscopy showed that the topical use of KH902 had no side effects on the corneal wound healing. On the other hand, the drug could control the inflammatory cells infiltration and decrease the chance of the secondary bacterial corneal ulcer.
Conclusion
Topical administration of KH902 is effective in treating CNV in the rabbit experimental model without adverse effects on the cornea. It is suggested that with the increase of the concentration of anti-VEGF drugs (KH902), the inhibition of VEGF expression was gradually enhanced, but without significant difference between the low and high concentration groups of drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Azar DT. Corneal angiogenic privilege: Angiogenic and antiangiogenic factors in corneal avascularity, vasculogenesis, and wound healing (an American Ophthalmological Society thesis) Trans Am Ophthalmol Soc. 2006;104:264–302. [PMC free article] [PubMed] [Google Scholar]
- 2.Beebe DC. Maintaining transparency: A review of the developmental physiology and pathophysiology of two avascular tissues. Semin Cell Dev Biol. 2008;19:125–33. doi: 10.1016/j.semcdb.2007.08.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Phillips GD, Stone AM, Jones BD, Schultz JC, Whitehead RA, Knighton DR, et al. Vascular endothelial growth factor (rhVEGF165) stimulates direct angiogenesis in the rabbit cornea. In Vivo. 1994;8:961–5. [PubMed] [Google Scholar]
- 4.Gan L, Fagerholm P, Palmblad J. Vascular endothelium growth factor (VEGF) and its receptor VEGFR-2 in the regulation of corneal neovascularization and wound healing. Acta Ophthalmol Scand. 2004;82:557–63. doi: 10.1111/j.1600-0420.2004.00312.x. [DOI] [PubMed] [Google Scholar]
- 5.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–22. [PubMed] [Google Scholar]
- 6.Lim P, Fuchsluger TA, Jurkunas UV. Limbal stem cell deficiency and corneal neovascularization. Semin Ophthalmol. 2009;24:139–48. doi: 10.1080/08820530902801478. [DOI] [PubMed] [Google Scholar]
- 7.van Setten GB. Vascular endothelial growth factor (VEGF) in normal human corneal epithelium: Detection and physiological importance. Acta Ophthalmol Scand. 1997;75:649–52. doi: 10.1111/j.1600-0420.1997.tb00623.x. [DOI] [PubMed] [Google Scholar]
- 8.Witmer AN, Vrensen GF, Van Noorden CJ, Schlingemann RO. Vascular endothelial growth factors and angiogenesis in eye disease. Prog Retin Eye Res. 2003;22:1–29. doi: 10.1016/s1350-9462(02)00043-5. [DOI] [PubMed] [Google Scholar]
- 9.Rodrigues EB, Farah ME, Maia M, Penha FM, Regatieri C, Melo GB, et al. Therapeutic monoclonal antibodies in ophthalmology. Prog Retin Eye Res. 2009;28:117–44. doi: 10.1016/j.preteyeres.2008.11.005. [DOI] [PubMed] [Google Scholar]
- 10.Chang JH, Gabison EE, Kato T, Azar DT. Corneal neovascularization. Curr Opin Ophthalmol. 2001;12:242–9. doi: 10.1097/00055735-200108000-00002. [DOI] [PubMed] [Google Scholar]
- 11.Amano S, Rohan R, Kuroki M, Tolentino M, Adamis AP. Requirement for vascular endothelial growth factor in wound-and inflammation-related corneal neovascularization. Invest Ophthalmol Vis Sci. 1998;39:18–22. [PubMed] [Google Scholar]
- 12.Philipp W, Speicher L, Humpel C. Expression of vascular endothelial growth factor and its receptors in inflamed and vascularized human corneas. Invest Ophthalmol Vis Sci. 2000;41:2514–22. [PubMed] [Google Scholar]
- 13.Cursiefen C, Rummelt C, Küchle M. Immunohistochemical localization of vascular endothelial growth factor, transforming growth factor alpha, and transforming growth factor beta1 in human corneas with neovascularization. Cornea. 2000;19:526–33. doi: 10.1097/00003226-200007000-00025. [DOI] [PubMed] [Google Scholar]
- 14.Cursiefen C, Chen L, Borges LP, Jackson D, Cao J, Radziejewski C, et al. VEGF-A stimulates lymphangiogenesis and hemangiogenesis in inflammatory neovascularization via macrophage recruitment. J Clin Invest. 2004;113:1040–50. doi: 10.1172/JCI20465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kvanta A. Ocular angiogenesis: The role of growth factors. Acta Ophthalmol Scand. 2006;84:282–8. doi: 10.1111/j.1600-0420.2006.00659.x. [DOI] [PubMed] [Google Scholar]
- 16.Özdemir Ö, Altıntaş Ö, Altıntaş L, Yildiz DK, Sener E, Yüksel N. The comparison of efficacy of topical bevacizumab, etanercept and the combination of both drugs on experimental corneal neovascularization. Turk Klin J Ophthalmol. 2012;21:211–9. [Google Scholar]
- 17.Ozdemir O, Altintas O, Altintas L, Ozkan B, Akdag C, Yüksel N. Comparison of the effects of subconjunctival and topical anti-VEGF therapy (bevacizumab) on experimental corneal neovascularization. Arq Bras Oftalmol. 2014;77:209–13. doi: 10.5935/0004-2749.20140054. [DOI] [PubMed] [Google Scholar]
- 18.Zhang M, Zhang J, Yan M, Luo D, Zhu W, Kaiser PK, et al. Aphase 1 study of KH902, a vascular endothelial growth factor receptor decoy, for exudative age-related macular degeneration. Ophthalmology. 2011;118:672–8. doi: 10.1016/j.ophtha.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 19.Ormerod LD, Garsd A, Reddy CV, Gomes SA, Abelson MB, Kenyon KR, et al. Dynamics of corneal epithelial healing after an alkali burn. A statistical analysis. Invest Ophthalmol Vis Sci. 1989;30:1784–93. [PubMed] [Google Scholar]
- 20.D’Amato RJ, Loughnan MS, Flynn E, Folkman J. Thalidomide is an inhibitor of angiogenesis. Proc Natl Acad Sci U S A. 1994;91:4082–5. doi: 10.1073/pnas.91.9.4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bhattacharjee PS, Huq TS, Mandal TK, Graves RA, Muniruzzaman S, Clement C, et al. A novel peptide derived from human apolipoprotein E is an inhibitor of tumor growth and ocular angiogenesis. PLoS One. 2011;6:e15905. doi: 10.1371/journal.pone.0015905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kadar T, Amir A, Cohen L, Cohen M, Sahar R, Gutman H, et al. Anti-VEGF therapy (bevacizumab) for sulfur mustard-induced corneal neovascularization associated with delayed limbal stem cell deficiency in rabbits. Curr Eye Res. 2014;39:439–50. doi: 10.3109/02713683.2013.850098. [DOI] [PubMed] [Google Scholar]
- 23.Chang JH, Garg NK, Lunde E, Han KY, Jain S, Azar DT. Corneal neovascularization: An anti-VEGF therapy review. Surv Ophthalmol. 2012;57:415–29. doi: 10.1016/j.survophthal.2012.01.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bock F, König Y, Dietrich T, Zimmermann P, Baier M, Cursiefen C. Inhibition of angiogenesis in the anterior chamber of the eye. Ophthalmologe. 2007;104:336–44. doi: 10.1007/s00347-007-1512-2. [DOI] [PubMed] [Google Scholar]
- 25.Cursiefen C, Chen L, Saint-Geniez M, Hamrah P, Jin Y, Rashid S, et al. Nonvascular VEGF receptor 3 expression by corneal epithelium maintains avascularity and vision. Proc Natl Acad Sci U S A. 2006;103:11405–10. doi: 10.1073/pnas.0506112103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Chung JH, Kang YG, Kim HJ. Effect of 0.1% dexamethasone on epithelial healing in experimental corneal alkali wounds: Morphological changes during the repair process. Graefes Arch Clin Exp Ophthalmol. 1998;236:537–45. doi: 10.1007/s004170050118. [DOI] [PubMed] [Google Scholar]
- 27.Sarchahi AA, Maimandi A, Tafti AK, Amani M. Effects of acetylcysteine and dexamethasone on experimental corneal wounds in rabbits. Ophthalmic Res. 2008;40:41–8. doi: 10.1159/000111158. [DOI] [PubMed] [Google Scholar]
- 28.Dan L, Shi-long Y, Miao-li L, Yong-ping L, Hong-jie M, Ying Z, et al. Inhibitory effect of oral doxycycline on neovascularization in a rat corneal alkali burn model of angiogenesis. Curr Eye Res. 2008;33:653–60. doi: 10.1080/02713680802245772. [DOI] [PubMed] [Google Scholar]
- 29.Pillai CT, Dua HS, Hossain P. Fine needle diathermy occlusion of corneal vessels. Invest Ophthalmol Vis Sci. 2000;41:2148–53. [PubMed] [Google Scholar]
- 30.Fossarello M, Peiretti E, Zucca I, Serra A. Photodynamic therapy of corneal neovascularization with verteporfin. Cornea. 2003;22:485–8. doi: 10.1097/00003226-200307000-00018. [DOI] [PubMed] [Google Scholar]
- 31.Hurmeric V, Mumcuoglu T, Erdurman C, Kurt B, Dagli O, Durukan AH, et al. Effect of subconjunctival bevacizumab (Avastin) on experimental corneal neovascularization in guinea pigs. Cornea. 2008;27:357–62. doi: 10.1097/ICO.0b013e318160d019. [DOI] [PubMed] [Google Scholar]
- 32.DeStafeno JJ, Kim T. Topical bevacizumab therapy for corneal neovascularization. Arch Ophthalmol. 2007;125:834–6. doi: 10.1001/archopht.125.6.834. [DOI] [PubMed] [Google Scholar]
- 33.Ferrara N. VEGF and the quest for tumour angiogenesis factors. Nat Rev Cancer. 2002;2:795–803. doi: 10.1038/nrc909. [DOI] [PubMed] [Google Scholar]
- 34.Bakri SJ, Cameron JD, McCannel CA, Pulido JS, Marler RJ. Absence of histologic retinal toxicity of intravitreal bevacizumab in a rabbit model. Am J Ophthalmol. 2006;142:162–4. doi: 10.1016/j.ajo.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 35.Wang F, Bai Y, Yu W, Han N, Huang L, Zhao M, et al. Anti-angiogenic effect of KH902 on retinal neovascularization. Graefes Arch Clin Exp Ophthalmol. 2013;251:2131–9. doi: 10.1007/s00417-013-2392-6. [DOI] [PubMed] [Google Scholar]
- 36.Su L, Ren X, Wei H, Zhao L, Zhang X, Liu J, et al. Intravitreal conbercept (KH902) for surgical treatment of severe proliferative diabetic retinopathy. Retina. 2016;36:938–43. doi: 10.1097/IAE.0000000000000900. [DOI] [PubMed] [Google Scholar]
- 37.Li X, Xu G, Wang Y, Xu X, Liu X, Tang S, et al. Safety and efficacy of conbercept in neovascular age-related macular degeneration: Results from a 12-month randomized phase 2 study: AURORA study. Ophthalmology. 2014;121:1740–7. doi: 10.1016/j.ophtha.2014.03.026. [DOI] [PubMed] [Google Scholar]
- 38.Zhou AY, Bai YJ, Zhao M, Yu WZ, Li XX. KH902, a recombinant human VEGF receptor fusion protein, reduced the level of placental growth factor in alkali burn induced-corneal neovascularization. Ophthalmic Res. 2013;50:180–6. doi: 10.1159/000353437. [DOI] [PubMed] [Google Scholar]
- 39.Wang F, Deng YP, Zhang M. Effect of KH902 in inhibiting suture induced corneal neovascularization. Sichuan Da Xue Xue Bao Yi Xue Ban. 2014;45:419–23. [PubMed] [Google Scholar]
- 40.Edelman JL, Castro MR, Wen Y. Correlation of VEGF expression by leukocytes with the growth and regression of blood vessels in the rat cornea. Invest Ophthalmol Vis Sci. 1999;40:1112–23. [PubMed] [Google Scholar]
- 41.Kim TI, Chung JL, Hong JP, Min K, Seo KY, Kim EK, et al. Bevacizumab application delays epithelial healing in rabbit cornea. Invest Ophthalmol Vis Sci. 2009;50:4653–9. doi: 10.1167/iovs.08-2805. [DOI] [PubMed] [Google Scholar]


