Abstract
Backgound
The identification of potential sources of error is a crucial step for any new assessment technique. This is the case for transcutaneous variables, such as flow and arterial gases, which have been applied as functional indicators of various aspects of human health. Regarding gender, a particular subject-related determinant, it is often claimed that women present higher transcutaneous oxygen pressure (tcpO2) values than men. However, the statistical significance of this finding is still uncertain.
Methods
The haemodynamical-vascular response to a local reactive hyperaemia procedure (the tourniquet cuff manoeuvre) was studied in two previously selected group of volunteers (n = 16; 8 women and 8 men). The effect of gender was assessed under standardised experimental conditions, using the transcutaneous flow-related variables tcpO2-tcpCO2 and Laser-doppler Flowmetry (LDF).
Results
Regarding tcpO2, statistically significant differences between genders were not found, although higher values were consistently found for the gases in the female group. Regarding LDF, high statistically significant differences (p < 0.005) were found, with the men's group presenting the highest values and variability. Other derived parameters used to characterise the vascular response following the cuff-deflation (t-peak) were similar in both groups.
Conclusions
The relative influence of gender was not clearly demonstrated using these experimental conditions. However the gender-related LDF differences suggest that further investigation should be done on this issue. Perhaps in the presence of certain pathological disparities involving peripheral vascular regulation, other relationships may be found between these variables.
Background
Transcutaneous measurement of flow related variables such as blood flow velocity obtained by Laser Doppler "flowmetry" (LDF) and arterial gases (tcPO2 and tcPCO2) obtained by transcutaneous "gasometry" have proven to be very useful from both clinical and basic science perspectives [1-4]. Relevant research produced over the last 15–20 years has demonstrated a wide interest involving these variables, far beyond the primary areas of interest such as dermatology and vascular medicine [4-7]. It also contributed to identify most of the determinants affecting this variables when obtained in the adult, therefore contributing to define its full applicability. And this seems to be particularly important to the tcPO2 assessment, through which several inferences involving the local arterial blood flow and tissue oxygenation [7-9] and the skin metabolic activity [10,11] are drawn.
It is known that the LDF signal is particularly dependent upon tissue light scattering which is complex at the skin's surface due to local variations and the structural specificity of the skin's vascular architecture [4,12] Artefacts such as the pulsatile profile of the signal [4,13] and some degree of laser instability (probably as a consequence of the reduced spatial resolution of most unidimensional flowmeters [14,15]) are systematically present. However it can be extremely useful to assess local microvascular perfusion as long as its depence on the focal point evaluated is considered [4,12,14-16]. As far as the arterial gases (tcpO2 is and tcpCO2) are concerned, further considerations should be taken into account. The assessment is usually made using an electrochemical technique based on a modified clark electrode coupled to a heat-source [17,18]. Once applied on the skin's surface the gas diffusion is set and detected by the gas-sensitive electrode. However (a) gas diffusion through the skin is specially dependent from the cardio-respiratory function [1,3,19] (b) a previous pre-heating of the skin (43 to 45°C) is necessary since at 37°C recorded values are near zero; in turn, some thermal instability is evoked at the skin surface leading to additional oxygen consumption [3,20](c) epidermal (cellular) consumption should also be considered as a factor contributing to decrease oxygen partial pressure detected at skin surface [1,3,19,20]; (d) Stratum corneum thickness and cohesion also affect the tcpO2 diffusion [1]. Regarding tcPCO2 it is accepted that, compared with tcPO2, (a) it is faster eliminated, (b) it presents higher solubility in water, (c) it is not consumed by the evaluation electrode and, (d) it is relatively constant due to the high buffering capacity of the extracellular fluids [2,3]. However, there are limitations to its utility; its dependence on respiratory control, and relative difficulty in correlation with tissue metabolic activity, are among these.
Other person linked limitations and therefore, main potential sources of error specially affecting all flow dependent variables includes:
- regular (or even occasional) consumption of vasoactive substances such as alcohol and/or nicotine (cigarette smoking), specially capable of affecting the cutaneous microcirculation [21,22];
- posture of the individuals, specially when evaluated near the extremities, due to the special characteristics of microcirculatory dynamics at this regions [23-25]
- anatomical variation of tcPO2 measurements, almost negligible in healthy individuals for various anatomical regions with the exception of the face (probably related with the pilosebaceous units) and the palm and sole (as a result of the particular epidermal thickness at this points)[3,26,27]
- age – values obtained in young children greatly differ from adults not only in terms of the respective cardiorespiratory indicators which influence this variables. The oxygen consumption rate of the skin was also found to be age dependent[1,26,28];
- gender – regarding tcPO2, sex related differences have been reported [26]; in particular, it was suggested that women have significantly higher values than men [3,26,29].
Although most of this "person-linked" sources of error may be avoided by adequate experimental design, some aspects such as the gender may be critical when defining a control group. Only a few papers approached this particular variable and, in some cases, results have been contradictory [26,29,30]. Thus, recognising the relative importance of gender as an eventual determinant of these flow related variables, we tested hemodinamical response in volunteers from both sexes following a tourniquet cuff occlusion manoeuvre. This methodology allows us to test the local vascular adaptation to controlled stress conditions by the transcutaneous indicators tcPO2 and LDF.
Methods
Data was obtained from healthy volunteers selected after informed written consent, according to previously defined inclusion criteria. Volunteers (n = 16) were equally distributed in two groups according to the gender (females n = 8; and males n = 8). All volunteers were under the age of 25 years (21.9 ± 22.2 years) and presented a normal (non-pathological) profile as defined in the referred inclusion criteria. These included the total absence of (a) any history of skin or cardiovascular diseases; (b) systemic diseases; (c) regular medication 60 days (minimum) prior to selection, or occasional if vasoactive; (d) smoking and/or drinking (alcohol) addiction. An absolute restriction of alcohol and coffee was imposed to all volunteers 6 H before testing.
All measurements occurred in laboratory environment, involving the permanent control of room temperature and humidity, according to the usual recommendations regarding the application of this type of technologies[3,32,35]. Prior to all measurements volunteers were left in the room for at least 20 minutes in order to allow full skin's adaptation to room's (temperature and humidity) condition.
The experimental methodology involved the generation of a loal reactive hyperaemia as a consequence of a localised occlusion manoeuvre at the upper limb extremity following which the different transcutaneous indicators were registered and compared with basal reference values. The sequential steps for this methodology were adapted from previously published protocols [3,19,31] and taken as follows:
1. the cuff application took place, being applied immediately before the flexion surface of the left arm over the forearm ; the anatomical areas selected were the mid portion of the left ventral forearm (along the midline) and hand (3rd finger)
2. all volunteers adopted the seated position in order to avoid any hemodinamical changes influencing measurements [1,24,25]
3. supra-systolic pressure (180–240 mmHg) was insuflated into the pressure cuff in order to ensure full occlusion of the brachial artery and maintained for at least 3 minutes after confirmed stabilisation ;
4. after this period the cuff was deflated determining a local reactive hyperaemia; all variables were recorded for at least 15 minutes after occlusion, until full stabilisation.
Transcutaneous indicators considered adequate for the present study purposes were :
- transcutaneous (tc) pO2 and tcpCO2 (in mmHg) assessed through the TINA TCM3 system (Radiometer, Denmark); the probe consists in a sole combined electrode [3,17,18] which allows simultaneous recordings of tcpO2 and tcpCO2;
- local microcirculation assessed through laser doppler flowmetry (LDF, expressed in arbitrary blood perfusion units or BPU's) with the Oxford Array (Oxford Optronics, UK) system [4].
These variables permitted us to establish the dynamical dependencies between them, expressed in terms of the analysis of tcpO2 and peripheral LDF dynamics modification during the hyperaemic period. Time to reach peak values of tcpO2 and LDF ("t-peak tcpO2" and "t-peak LDF" respectively) were calculated considering the 1st time value (immediately before cuff deflation) as zero until the first LDF curve peak obtained after cuff deflation. Respective slopes were further analysed and correlated (Spearman) to check for additional (functional) parameters and reciprocal relationships between this variables. Slopes obtained during the linear portion of the tcpO2 falling curves during occlusion were used to estimate skin's oxygen consumption rate through the application of the following expression :
VO2 = αΔPO2
where α is the O2 solubility in ml. g-1. atm-1 assumed to be that of water at the same temperature (0.02226 at 43°C) and ΔPO2 the rate of fall of tcpO2 in atm.min-1[10,11]. The oxygen coefficient was fixed at 0.02226 ml O2 (ml water)-1. atm-1 at 43°C.
The non parametric Wilcoxon paired-test and Mann-Withney (for independent variables comparison) were chosen according with a normal or asymmetric data distribution. Spearman non parametric test was also used to study correlation between variables. A significance level of p < 0.05 was adopted.
A "Power Calculation" was also performed to assess the relative power of the analysis.
Results
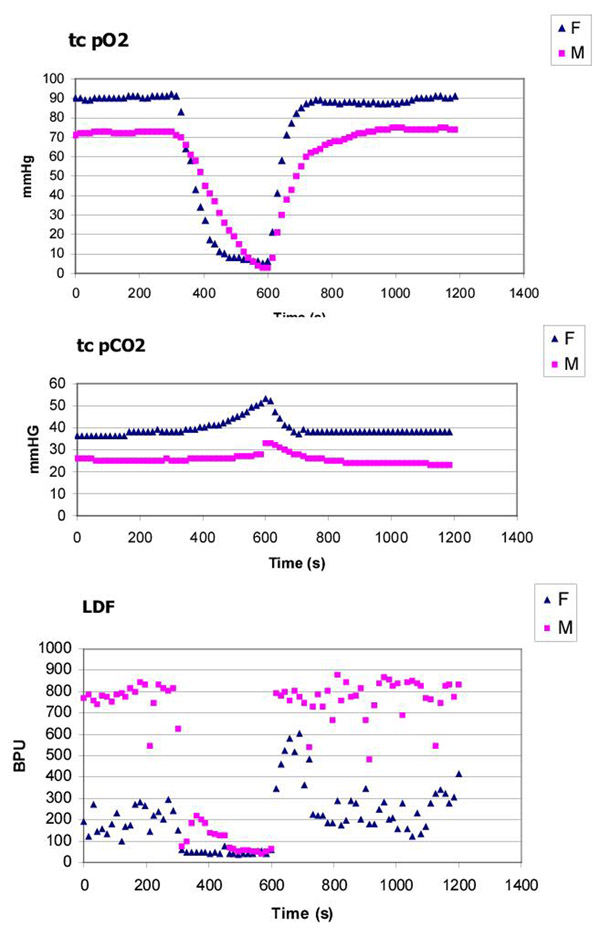
Typical curves obtained under the present experimental conditions are shown in Figure 1 as an illustrative example of the methodology chosen. Recordings of transcutaneous gases and LDF served also to inform about the efficacy of the occlusion process (Table 1 and Table 3) although, for well known reasons a true zero value cannot be obtained for none of this variables [4,12,13].
Figure 1.
Example of transcutaneous recordings during a 300 second tourniquet cuff occlusion (initiated at 300 s and finished at 600 s) obtained in the upper extremity at arm level illustrating the sequential events detected in the variables tcpO2, tcpCO2 and LDF. Gender related differences, as reported in the literature for the transcutaneous gases are apparent. However, striking differences are consistently found for the LDF measurements obtained under the present experimental conditions (see text)
Table 1.
Descriptive statistics for the transcutaneously assessed gases
| Female | ||||
| tcO2 (mmHg) | Basal | Occlusion | Post occlusive | t-peak |
| (T0) | (T1) | (T2) | (s) | |
| Mean | 73,9 | 3,6 | 76,9 | 346,9 |
| St. deviation | 14,6 | 2,1 | 10,8 | 147,1 |
| NS | NS | NS | ||
| p | ||||
| * | * | |||
| toCO2 (mmHg) | Basal | Occlusion | Post occlusive | |
| (T0) | (T1) | (T2) | ||
| Mean | 40,5 | 52,6 | 39,2 | |
| St. deviation | 9,8 | 13,1 | 10,2 | |
| * | * | * | ||
| p | ||||
| * | * | |||
| Male | ||||
| tcO2 (mmHg) | Basal | Occlusion | Post occlusive | t-peak |
| (T0) | (T1) | (T2) | (s) | |
| Mean | 65,7 | 2,8 | 64,1 | 343,1 |
| St. deviation | 11,9 | 2,4 | 14,1 | 160,4 |
| NS | NS | NS | ||
| p | ||||
| * | * | |||
| toCO2 (mmHg) | Basal | Occlusion | Post occlusive | |
| (T0) | (T1) | (T2) | ||
| Mean | 36,0 | 44,9 | 34,5 | |
| St. deviation | 7,4 | 11,5 | 8,4 | |
| NS | NS | NS | ||
| p | ||||
| * | * | |||
NS non significant differences found * p < 0.05
Table 3.
Descriptive statistics for the LDF values obtained under the present experimental conditions
| Female | Basal | Occlusion | Post exclusive | t-peak |
| LDF (BPU's) | (T0) | (T1) | (T2) | (s) |
| Mean | 165,4 | 43,0 | 143,6 | 78,75 |
| St. deviation | 126,8 | 18,2 | 108,3 | 57,1 |
| NS | NS | NS | ||
| p | ||||
| * | * | |||
| Male | Basal | Occlusion | Post occlusive | t-peak |
| LDF (BPU's) | (T0) | (T1) | (T2) | (s) |
| Mean | 547,8 | 51,1 | 536,2 | 131,3 |
| St. deviation | 211,5 | 11,1 | 215,4 | 178,7 |
| NS | NS | NS | ||
| p | ||||
| * | * | |||
NS non significant differences found * p < 0.05
The female group have consistently higher mean values of tcpO2 for each period of time considered. For this group tcpO2 varied from 73.9 ± 14.6 mmHg in basal condition, to 3.6 ± 2.1 mmHg during stabilised occlusion, to 76.9 ± 10.8 mmHg in the post-occlusive (stabilised) period. For the male group tcpO2 varied from 65.7 ± 11.9 mmHg in basal condition, to 2.8 ± 2.4 mmHg during stabilised occlusion, to 64.1 ± 14.1 mmHg in the post-occlusive (stabilised) period. Statistically significant differences between reference mean (basal) tcpO2 and those obtained during the post-occlusive period could not be found (p > 0.05) in any of the groups, demonstrating full recovery to reference conditions during the experimental time considered.
The parameter "t-peak tcpO2" values were very similar in both groups, suggesting a similar functional behaviour. In fact, no matter the differences detected between groups during the experiments, statistically significant differences (Mann-Whithey) could not be demonstrated between genders (power 0,8) for any of the considered periods nor for the "t-peak tcpO2" parameter.
Regarding tcpCO2 the same gender-related aspects were found. For the female group, values varied from 40.5 ± 9.8 mmHg in basal conditions, reaching a peak during occlusion at 52.6 ± 13.1 mmHg, to 39.2 ± 10.2 mmHg in the post-occlusive (stabilised) period. For the male group mean values were 36.0 ± 7.4 mmHg in basal conditions, 44.9 ± 11.5 mmHg, during occlusion and 34.5 ± 8.4 mmHg in the post-occlusive (stabilised) period. In both groups post-occlusive values significantly differed from basal values unlike the oxygen changes recorded (power ~1). Statistically significant differences (Mann-Whithney) could not be demonstrated between genders for any of the considered periods.
The initial portion of the tcpO2 occlusion curve was also used to assess the local metabolic oxygen consumption. This parameter was calculated from the linear portion of the decremental rate period of tcpO2 curve during occlusion. As shown in Table 2 similar VO2 values (female:0.008 ± 0.004 ml O2. g-1. min-1 and male: 0.007 ± 0.004 ml O2. g-1. min-1) were obtained in both sexes which reflects the non-significant (p > 0.05) differences detected for the tcpO2 slopes calculated during this period.
Table 2.
Skin metabolic O2 consumption estimation with subjects breathing in atmospheric air
| Female | ΔPO2 | VO2 |
| atm.min-1 | ml O2/g/min | |
| Mean | -0,3500 | |
| St. deviation | -0,1977 | |
| Male | ΔPO2 | VO2 |
| atm.min-1 | ml O2/g/min | |
| Mean | -0,1780 | |
| St. deviation | 0,1204 | |
Evaluation of the peripheral LDF in both groups showed highly significant perfusion differences (power~ 1) between female and male volunteers. Similar profiles but presenting very different magnitudes were obtained in each of the considred periods. For females, LDF varied from 165.4 ± 126.8 BPU's in basal condition, to 43.0 ± 18.2 BPUs during stabilised occlusion, to 143.6 ± 108.3 BPU's in the post-occlusive (stabilised) period (Table 3); statistically significant differences between reference mean (basal) LDF and those obtained during the post-occlusive period could not be found (p > 0.05). For the male group mean LDF values were 547.8 ± 211.5 BPU's in basal condition, 51.1 ± 11.1 mmHg during stabilised occlusion, and 536.2 ± 215.4 BPU's in the post-occlusive (stabilised) period; again, statistically significant differences between basal and post-occlusive values were not found. Regarding the "t-peak LDF", no matter the different signal magnitudes recorded, statistically significant differences could not be found (p = 0.779) comparing "t-peak LDF" parameters in both groups, involving 78.8 ± 57.1 s for the female group and 131.3 ± 178.7 s for the male group, respectively (Table 3).
Discussion
The possibility to quantitatively describe transcutaneous variables such as tcPO2, tcPCO2, and LDF offered the opportunity to non-invasively assess several potential indicators of vascular physiology and pathophysiology from the microcirculatory perspective [5,7,32]. LDF and tcPO2 measurements were advantageous in many different areas of applicability to classical methods such as peripheral pulses and peripheral blood pressure measurements assessed by plethysmography or by doppler segmental pressure analysis [7,11,32,33].
Among the several methodologies proposed, special attention was given to "tourniquet cuff occlusion", a dynamical manoeuvre often used in vascular physiology and pathophysiology to test the regulatory response of the vascular territory to a sudden change of perfusion conditions [3,11,19,34]. This manoeuvre is frequently used to describe dynamical phenomena determining the local oxygen supply and consumption, obtaining parameters such as the Oxygen Reappearance Time (ORT) following occlusion, and the Oxygen Recovery Index (ORI), which is also used to assess the time for O2 molecules to diffuse from capillaries to the tcpO2 sensor[34] or to obtain, from the tcPO2 signal, other hemodinamical representative parameters [20,32,34]. This procedure was also recently suggested to validate the cutaneous blood flow assessment techniques [35].
Therefore, this approach seemed to be particularly suitable to test, under controlled conditions, the influence of several determinants over this variables, including gender. The occlusion methodology, a critical aspect of this procedure, was consistently reproducible as shown from the T1 (stabilised occlusion) values both for tcpO2 and LDF (Table 1 and Table 3) although an absolute "zero" value could not be achieved. It was impossible to obtain a true zero value for the LDF signal. This results from the residual movement capacity of red blood cells still arrested in the occluded vessels, thus producing minor doppler components which are detected by the system [4,12-15]. The same impossibility detected for the tcpO2 seems to result from the slow release of the gas from the blood arrested in the territory under occlusion which cannot be accomplished during the time duration of the experiment.
Even though it is reported as one factor influencing tcPO2 measurements [26], only a few papers refer to the influence of gender on transcutaneous measurements. The most recently published study involving tcpO2 mapping in several anatomical sites could only detect statistically significant differences in the leg [29].
In the present study, consistently higher tcpO2 values were obtained in the female group; however (Table 1 and Figure 1) these values were not significantly different, in statistical terms, from the mean values obtained in the male group. These differences may result from different magnitudes of well known variables affecting measurements in both groups, namely (1) different epidermal thickness between male and female, thus determining different tcpO2 diffusion capacities; (2) different epidermal consumption, apparently lower in the female group, determining lower oxygen consumption levels at skin surface; (3) minor thermal instability at skin's surface as a consequence of the previous factors, determining lower local electrode consumption in the female group. However, it is important to underline that, regarding the "t-peak" parameter which was used as an indicator of the autoregulatory reflex response induced by the occlusion manoeuvre, identical values were obtained in both sexes, suggesting that the vascular response is not affected by the gender in the present experimental conditions. Further analysis involving this property which provides information about local vascular status, may be done in other patient groups with vascular disease meaning that a possible influence of gender should be still investigated [8,31,32,36].
Regarding LDF signals, the influence of gender over the LDF data is not referred to in the literature, where posture [37,40] physical[38] and mental activities [39], anatomical region [40], and age and race [41,42] are claimed to be the main subject-related influencing factors. However, under the present experimental conditions, a clear difference was observed between genders with a clear predominance of the male group (Figure 1) for the absolute means obtained in T1 and T2 (p < 0.005). The "t-peak LDF" assessed in both groups to further characterise the vascular reactive response following occlusion, was not significantly different between groups (Table 3). The evaluation of the slopes obtained by the line defined between cuff deflation and the first 75 seconds following this period significantly differed in both groups (p < 0.005) as a consequence of the basal (T1) differences, thus involving different local blood masses and ejection velocities. Despite these results, conclusions on this matter should further consider (1) the relative unspecificity of this signal, (2) the pulsatile nature of the recordings, complicating the numerical analysis and (3) the reduced number of volunteers involved in the present analysis. Moreover, accepting that, from a physiological point of view LDF and tcpO2 are typically defined by a flow – related character, relationships between the evolution of these variables were investigated using the non-parametric Spearman correlation test. Analysis focusing either the linear portion of the occlusion decremental phase and the hyperaemic response following cuff deflation as well, could not demonstrate any significant relationship regarding these variables evolution, which underlines the high variability of data distribution.
The tcO2 decay curve was also used to estimate the local tissue metabolic activity (Table 2). In order to assess this information the amount of oxygen delivered by haemoglobine (Hb) must be determined by an O2 – Hb binding curve at the evaluation temperature (43°C)[20]. In the present case O2 consumption rate (VO2) was estimated from the oxygen solubility coefficient chosen at 0.02226 ml O2 (ml water)-1. atm-1 at 43°C [38] and no other corrections were made for the patients breathing atmospheric air. So, this information was only estimated to confirm the similar character of the VO2 parameters obtained in both groups, resulting from similar tcO2 curve profiles. Although slightly higher values were obtained for the female group, no statistically significant differences could be found.
Finally, it is accepted that tcpCO2 is governed by different factors from those primarily affecting the tcPO2 and LDF. tcpCO2 values basically correspond to the arterial gas content added by the local blood flow CO2, by the temperature effect and by the local tissue production, including the skin (estimated around 2–6 mmHg) [2,3,42]. Good correlation between arterial and transcutaneous pCO2 are described [42,43] but tcpCO2 values are slightly higher mainly due to the surface electrode heating and to the skin's metabolic contribution. As shown in Table 1, the female group presented higher tcpCO2 values in every period but, not significantly different from the male group. It is interesting to note though, that the mean post-occlusion values (T2) were consistently lower than the basal values in both groups (Table 1). This reduction, although not significant from the statistical point of view, may also indicate about the local vascular status, suggesting that the evoked autoregulatory reactive response following occlusion (the respective maximum being reached at the end of the occlusion period) may determine a local "washout" removing the CO2 excess [3,36].
Conclusion
The vascular dynamical changes induced by the present approach suggests, regarding transcutaneous variables, that gender may not represent a main source of error at least from a functional (physiological) point of view. However, considering the preliminary characteristic of the study and the relative importance of a well defined control group specially for clinical purposes, this aspect should be further investigated in order to fully explore the practical relevance of these variables in the transcutaneous assessment of flow related functional parameters.
Competing Interests
None declared
Pre-publication history
The pre-publication history for this paper can be accessed here:
Contributor Information
Luís Monteiro Rodrigues, Email: lrodrigues@ff.ul.pt.
Pedro Contreiras Pinto, Email: pcontreiras@ff.ul.pt.
António Leal, Email: atleal@hotmail.com.
References
- Takiwaki H. Measurement of transcutaneous oxygen tension, in ""Hand Book of Non-Invasive Methods and the Skin" Ed J Serup & GBE Jemec, CRC Press, Boca Raton, 1995. pp. 185–195.
- Nickelsen CN. Measurement of Transcutaneous pCO2, in "Handbook of non-invasive Methods and the Skin", Eds J Serup & G Gemec, CRC Press, Boca Raton, 1995. pp. 197–200.
- Rodrigues L, Ferro IZ, Galego N, Pinto P, Silva N, Rey-Salgueiros Y. Study on the application of transcutaneous pO2 and pCO2 monitoring to the in vivo functional characterisation of the normal human skin, Piel span. 1998;13:380–388. [Google Scholar]
- Berardesca E, Leveque JL, Masson Ph, the EEMCO Group, EEMCO guidance for the measurement of skin microcirculation Skin Pharmacol AppI Skin Physiol. 2001. [DOI] [PubMed]
- Got I. Transcutaneous oxygen pressure (TcPO2): advantages and limitations. Diabetes Metab. 1998;24:379–84. [PubMed] [Google Scholar]
- Hanna GP, Fujise K, Kjellgren O, Feld S, Fife C, Schroth G, Clanton T, Anderson V, Smalling RW. Infra-popliteal transcatheter interventions for limb salvage in diabetic patients: importance of aggressive interventional approach and role of transcutaneous oximetry. J Am Coll Cardiol. 1997;30:664–9. doi: 10.1016/S0735-1097(97)00216-7. [DOI] [PubMed] [Google Scholar]
- Ubbink DT, Spincemaille GH, Reneman RS, Jacobs MJ. Prediction of imminent amputation in patients with non-reconstructible leg ischemia by means of microcirculatory investigations. J Vasc Surg. 1999;30:114–21. doi: 10.1016/s0741-5214(99)70183-7. [DOI] [PubMed] [Google Scholar]
- White RA, Nolan L, Harley D, Long J, Klein S, Tremper K, Nelson R, Trabiski J, Shoemaker W. Non-invasive evaluation of peripheral disease using transcutaneous oxygen tension, Am J Surg. 1982;144:68–75. doi: 10.1016/0002-9610(82)90604-3. [DOI] [PubMed] [Google Scholar]
- Hauser CJ, Klein SR, Mehringer CM, Apple P, Shoemaker WC. Superiority of transcutaneous oximetry in non-invasive vascular diagnosis in patients with diabetes, Arch Surg. 1984;119:690–694. doi: 10.1001/archsurg.1984.01390180054009. [DOI] [PubMed] [Google Scholar]
- Thunstrom AM, Stafford M, Severinghaus JW. A two temperature, two pO2 method of estimating the determinants of tcpO2, Birth Defects Original Article Series. 1979;15:167–182. [PubMed] [Google Scholar]
- Severinghaus JW, Stafford M, Thunstrom AM. Estimation of skin metabolism and blood flow with tcpO2 and tcpCO2 electrodes by cuff occlusion of the circulation, Acta Anaesthesiol Scand. 1978;suppl.68:9–15. doi: 10.1111/j.1399-6576.1978.tb01386.x. [DOI] [PubMed] [Google Scholar]
- Tenland T, Salerud EG, Nilsson GE, Ake P. Spatial and temporal variations in human skin blood flow, Int J Microcir Clin Exp. 1983;2:81–90. [PubMed] [Google Scholar]
- Obeid AN, Barnett NJ, Dougherty G, Ward G. A critical review of laser Doppler flowmetry, J Med Eng Technol. 1990;14:178–181. doi: 10.3109/03091909009009955. [DOI] [PubMed] [Google Scholar]
- Tenland T, Salerud EG, Nilsson GE, Ake P. Spatial and temporal variations in human skin blood flow, Int J Microcirc Clin Exp. 1983;2:81–90. [PubMed] [Google Scholar]
- Braverman IM, Keh A, Goldminz D. Correlation of laser doppler wave patternswith underlying microvascular anatomy, J Invest Dermatol. 1990;6:107–118. doi: 10.1111/1523-1747.ep12484917. [DOI] [PubMed] [Google Scholar]
- Stucker M, Steinberg J, Memmel U, Avermaete A, Hoffmann K. Differences in the two-dimensionally measured laser doppler flow at different skin localisations, Skin Pharmacol AppI Skin Physiol. 2001;14:44–51. doi: 10.1159/000056333. [DOI] [PubMed] [Google Scholar]
- Clark LC. Monitor and control of blood and oxygen in tissue, Trans Am Soc Artif Intern Organs. 1956;2:41–48. [Google Scholar]
- Huch R, Huch A, Arner B, Rooth G. Continuos transcutaneous oxygen measured with a heat electrode, Scabd J CIin Lab Invest. 1973;31:269–273. doi: 10.3109/00365517309082431. [DOI] [PubMed] [Google Scholar]
- Rodrigues L, Leal A, Ferreira R, Leal F, Fernandes M, Pinto P. Non-invasive in vivo evaluation of cutaneous metabolic activity, Europp. J Pharm Sciences. 2000;11 (Supl.1):S200. [Google Scholar]
- Jaszczak P, Poulsen J. tc pO2 dependence on a sufficient blood flow, in Continuos Transcutaneous Blood Gas Monitoring, Huch R & Huch A Eds M Dekker NY. 1983. pp. 35–43.
- Workman WT, Sceffield PJ. Continuos transcutaneous oxygen monitoring in smokers under normobaric and hyperbaric oxygen conditions; in "Continuos Transcutaneous Bloogd Gas Monitoring" Huch R & Huch A Eds, Marcel Dekker Publ, NY, pp. 1983. p. 649.
- Lusiani L, Visona A, Nicolin P, Papesso B, Pagnan A. Transcutaneous oxygen tension measurement as a diagnostic tool in patients with peripheral vascular disease, Angiology. 1988;39; 10:873–880. doi: 10.1177/000331978803901004. [DOI] [PubMed] [Google Scholar]
- Johnson JM. Non-thermoregulatory control of human skin blood flow, J AppI Physiol. 1986;61:1613–1622. doi: 10.1152/jappl.1986.61.5.1613. [DOI] [PubMed] [Google Scholar]
- Svedman P, Holmberg J, Jacobson S, Lindell SE, Ponnert L. On the relation between transcutaneous oxygen tension and skin blood flow, Scand. J Reconstr Surg. 1982;16:133–141. doi: 10.3109/02844318209006581. [DOI] [PubMed] [Google Scholar]
- Eickhoff J, Jacobsen E. Correlation of transcutaneous oxygen tension to blood flow in heated skin, Scand. J Clin Lab Invest. 1980;40:761–767. doi: 10.3109/00365518009095593. [DOI] [PubMed] [Google Scholar]
- Orenstein A, Mazkereth R, Tsur H. Mapping of the human body skin with the transcutaneous oxygen pressure method, Ann Plast Surg. 1988;20:419–432. doi: 10.1097/00000637-198805000-00004. [DOI] [PubMed] [Google Scholar]
- Takiwaki H, Nakanishi H, Shono Y, Arase S. The influence of cutaneous factors on the transcutaneous pO2 and pCO2 at various body sites, Br J Dermatol. 1991;125:243–252. doi: 10.1111/j.1365-2133.1991.tb14748.x. [DOI] [PubMed] [Google Scholar]
- Jaszczac P, Sejrsen P, Sorensen PR. The influence of epidermal membrane on percutaneous pO2 and metabolic rate, Scand. J Clin Invest. 1988;48(Suppl.189):17–24. [Google Scholar]
- Dooley J, King G, Slade B. Establishment of reference pressure of transcutaneous oxygen for the comparative evaluation of problem wounds, Undersea Hyperb Med. 1997;24:4:235–44. [PubMed] [Google Scholar]
- Zamborni WA, Wong HP, Stephenson LL, Pfeifer MA. Evaluation of hyperbaric oxygen for diabetic wounds: a prospective study, Undersea Hyperb Med. 1997;24:3:175–9. [PubMed] [Google Scholar]
- Alves J, Pinto P, Fernandes AP, Abrunhosa S, Panarra A, Galego N, Soromenho F, Riscado MV, Rodrigues L. Quantitative assessment of Sistemic Lupus Changes by non-invasive techniques, Skin Res Technol. 1997;3:186. [Google Scholar]
- Kalani M, Brismar K, Fagrell B, Ostergren J, Jorneskog G. Transcutaneous oxygen tension and toe blood pressure as predictors for outcome of diabetic foot ulcers, Diab Care. 1999;22:147–151. doi: 10.2337/diacare.22.1.147. [DOI] [PubMed] [Google Scholar]
- Bone GE, Pomajzl MJ. The blood pressure by photoplethysmography: an index of healing in forefoot amputation, Surgery. 1981;89:569–574. [PubMed] [Google Scholar]
- Slagsvold CE, Stranden E, Rosen L, Kroese AJ. The role ofr blood perfusion and tissue oxygenation in the postischemic transcutaneous pO2 response, Angiology. 1992;43:155–162. doi: 10.1177/000331979204300210. [DOI] [PubMed] [Google Scholar]
- Bircher A, de Boer E, Agner T, Wahlberg JE, Serup J. Guidelines for measurement of cutaneous blood flow by laser doppler flowmetry. A report from the standardisation group of the European Society of Contact dermatitis, Cont Dermatitis. 1994;30:65–72. doi: 10.1111/j.1600-0536.1994.tb00565.x. [DOI] [PubMed] [Google Scholar]
- Leal A, Alves J, Rodrigues L. Usefulness of Transcutaneous Indicators as Predictors of Peripheral Dysfunction, Abst. 13th International Congress of the ISBS, Skin Res & Technol. 2000;6:172–173. [Google Scholar]
- Bernardi L, Rossi M, Fratino P, Finardi G, Mevio E, Orlandi C. Relationship between changes in human skin blood flow and autonomic tone, Microvasc Res. 1989;37:16–27. doi: 10.1016/0026-2862(89)90069-1. [DOI] [PubMed] [Google Scholar]
- Tur E. Cutaneous laser doppler flowmetry in general medicine, in "Bioengineering of the Skin : Cutaneous Blood Flow and Erythema", Berardesca, E, Elsner, P, Maibach H, Eds, CRC Press, Boca Raton, 1995. pp. 133–153.
- Wilkin JK, Troter K. Cognitive activity and cutaneous blood flow, Arch. Dermatol. 1987;123:1503. doi: 10.1001/archderm.123.11.1503. [DOI] [PubMed] [Google Scholar]
- Tur E, Tur M, Maibach H, Guy R. Basal perfusion of the cutaneous microcirculation : measurements as a function of anatomical position, J Inv Dermatol. 1983;81:442–446. doi: 10.1111/1523-1747.ep12522619. [DOI] [PubMed] [Google Scholar]
- Bircher A, Roskos K, Maibach H, Guy R. Laser doppler measured cutaneous blood flow: effects with age, in "Aging Skin : Properties and Functional Changes", Leveque, JL & Agache, P, edst Marcel Dekker ed, NY, 1993. pp. 105–121.
- Berardesca E, Maibach H. Cutaneous reactive hyperemia: recial differences induced by corticoid application, Brit J Dermatol. 1989;120:787–794. doi: 10.1111/j.1365-2133.1989.tb01376.x. [DOI] [PubMed] [Google Scholar]
- Goldman HR, Gribbin RJ, Martin L. Transcutaneous pCO2 in adults, Anaesthesia. 1982;37:944–946. doi: 10.1111/j.1365-2044.1982.tb01859.x. [DOI] [PubMed] [Google Scholar]
- Lofgren O, Anderson D. Simultaneous transcutaneous carbon dioxide and transcutaneous oxygen monitoring in neonatal intensive care, J Perinat Med. 1983;11:121–134. doi: 10.1515/jpme.1983.11.1.51. [DOI] [PubMed] [Google Scholar]