Abstract
This report demonstrates the blood flow pattern in a case of choroidal hemangioma (CH) using swept-source-optical coherence tomography angiography (SS-OCTA). Fluorescein angiography, SS-OCT, and SS-OCTA images of a patient with CH were obtained using a standard protocol. The internal vascular pattern of the tumor was identified on both OCT and OCTA. Dark areas were identified in the CH. These were interspersed between areas of visible blood flow, as imaged on SS-OCTA. Peripheral vascular arcades were also identified within the tumor. SS-OCTA should be evaluated as an imaging tool to study the blood flow within choroidal tumors.
Keywords: Blood flow in tumors, choroidal hemangioma, optical coherence tomography angiography, Sturge–Weber syndrome
Choroidal hemangioma (CH) is a vascular tumor of the choroid that can cause relentless exudation resulting in irreversible visual loss. Its vascular nature has been previously investigated with dye-based angiography imaging systems.[1] In this report, we describe blood flow patterns in CH utilizing the newly introduced optical coherence tomography (OCT)-based dye-less angiography and discuss the possible impact of variable blood flow patterns on visual prognoses.
Case Report
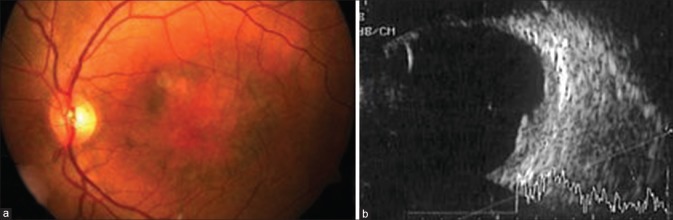
A 40-year-old male presented with a visual acuity of 6/60 OS. OD was completely normal, as was the anterior segment OS. A well-circumscribed, raised, orange subfoveal lesion with overlying areas of depigmentation was noted in OS [Fig. 1a]. This lesion was internally hyperechoic and homogeneous on ultrasound, without associated acoustic shadow [Fig. 1b]. Therefore, a diagnosis of CH was made.
Figure 1.
(a) Fundus photograph of OS depicting the choroidal hemangioma with overlying pigmentary changes. (b) Ultrasound image of OS showing internally hyperechoic and homogeneous choroidal mass
Fluorescein angiography showed a meshwork of dilated and interconnected branching vessels in the atrioventricular phase, with typical mottled appearance and “pushing effect” in the late phase [Fig. 2a]. On OCT [Fig. 2b], subretinal fluid was noted, and the choroidal vasculature appeared to be preserved but for large-dilated vessels. Swept-source-OCT angiography (SS-OCTA) detected a network of variably sized interconnected vessels in the hamartoma, which appeared larger than the surrounding normal choroidal vessels [Fig. 2c]. A distinct peripheral circumferential arcade of vessels could be delineated within the tumor margin, with inward branches in a spoke-wheel fashion [Fig. 2d], corresponding to a previous description on indocyanine green angiography.[1] In addition, prominent dark areas were also seen.
Figure 2.
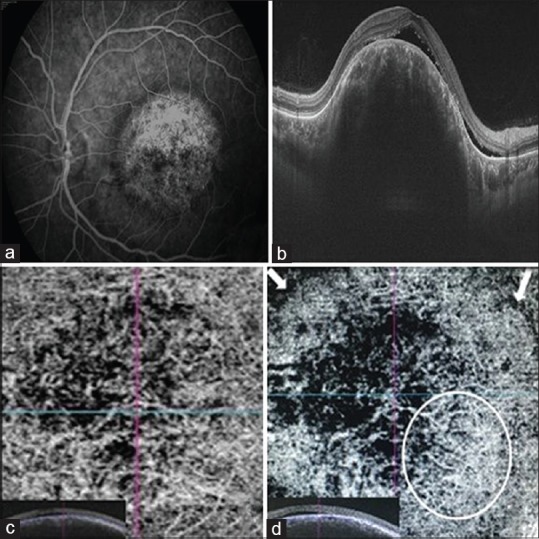
(a) Late phase of fluorescein angiography showing typical mottled appearance with “pushing“ effect. (b) Optical coherence tomography image showing subretinal fluid along with large vessels within the tumor. (c) Swept-source-optical coherence tomography angiography image at the level of choriocapillaris revealing multiple dilated interconnected vessels. (d) Swept-source-optical coherence tomography angiography image at a level deeper to choriocapillaris showing peripheral vascular arcade (white circle) along with inward branches. White arrows indicate borders of the choroidal mass
Discussion
CH consists of capillary or cavernous or mixed type of vascular channels with minimal intervening connective tissue.[2] Enhanced depth imaging-OCT of CH has shown large vessels, diameter nearly 2.5–5 times that of the normal choroidal vessel, as seen in our case.[3] SS-OCTA in this case revealed high-resolution vascular characteristics of the choroidal tumor. This CH could be the “mixed” vascular type, with variable blood flow within it.
Dark areas on OCTA imaging should be interpreted with caution.[4] The inability of laser light penetration and masking by retinal pigment epithelium have been questioned in this regard before. Further, orientation of flow being scanned, range of detectable blood flow velocity, and shadow artifacts should also be considered while interpreting dark areas on OCTA.[4] In our case, there was decreased retinal pigment in the area of interest, and depth penetration and orientation of vasculature is unlikely to be the reason for dark areas as the blood flow was well visualized in the surrounding areas [Figs. 1 and 2]. The morphology of the dark areas does not appear to be due to a shadow infarct either.
It has been reported that the current models of OCTA can detect blood flow within a range of 0.5–9 mm/s.[4] Therefore, both very slow and very fast blood flow may appear as dark areas, although by modifying scanning algorithms this artifact can be overcome in some cases. Turbulent flow is also of concern as it may result in inaccurate findings.[4] The choroidal blood flow is generally much higher than retinal blood flow, and has been proven in monkey eyes to be nearly ten times that of the gray matter of the brain.[5] However, blood flow in CH, just like hemangiomas of other organs like liver,[6] has been seen to be slower than that of other choroidal tumors.[7] The blood velocities can be variable depending on the type of CH. Cavernous variant of CH is the most common vascular tumor of the choroid.[8] Cavernous form of vascular tumors generally has low blood flow and has been termed passive.[9] Though it is unlikely that the blood flow in our case was too fast for the OCTA machine to image, it cannot be proven with certainty in this case. Comparative analysis with conventional angiography systems and ultrasound Doppler should be done to assess the correct implication of dark areas on OCTA imaging of CH. If consistency in the interpretation of OCTA findings is established, it would be a useful noninvasive tool for assessing blood flow in ocular tumors.
CH may not cause exudation and thus warrants observation only, or it may result in total retinal detachment and intractable neovascular glaucoma.[10] We believe that the exudation of the fluid from the tumor may be linked to the blood flow pattern within it. Our patient has remained stable without any active intervention for over 6 months now, with no change in visual acuity or subretinal fluid. Treatment with photodynamic therapy[11] has been discussed with the patient but withheld due to financial constraints. The stability or slow progress of the disease may be linked to slow blood flow channels. Larger sample-sized studies including patients of CH with different blood flow would be conclusive in this regard.
Conclusion
SS-OCTA may be used to study the blood flow patterns in choroidal tumors. Given the limitations, it should be evaluated and compared to dye-based angiography prospectively for its accuracy in detecting blood flow within choroidal tumors. The blood flow patterns may impact the amount of fluid exudation and help in judging response to treatment.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
References
- 1.Shields CL, Shields JA, De Potter P. Patterns of indocyanine green videoangiography of choroidal tumours. Br J Ophthalmol. 1995;79:237–45. doi: 10.1136/bjo.79.3.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Day S, Mruthyunjaya P. Circumscribed choroidal hemangioma. In: Ryan SJ, editor. Retina Ch 153. China: Elsevier; 2013. pp. 2340–50. [Google Scholar]
- 3.Rojanaporn D, Kaliki S, Ferenczy SR, Shields CL. Enhanced depth imaging optical coherence tomography of circumscribed choroidal hemangioma in 10 consecutive cases. Middle East Afr J Ophthalmol. 2015;22:192–7. doi: 10.4103/0974-9233.150629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hua R, Wang H. Dark signals in the choroidal vasculature on optical coherence tomography angiography: An artefact or not? J Ophthalmol. 2017;2017:5498125. doi: 10.1155/2017/5498125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alm A, Bill A. Ocular circulation. In: Moses RA, Hart WM, editors. Adler's Physiology of the Eye. 8th ed. Ch. 6. India: Jaypee; 1989. pp. 183–203. [Google Scholar]
- 6.Petrovic N, Artiko V, Obradovic V, Kostic K. Study of blood flow in liver hemangiomas using radionuclide angiography. Acta Chir Iugosl. 2001;48:25–9. [PubMed] [Google Scholar]
- 7.Yang W, Hu S, Wang J, Wang L, Zheng B. Color Doppler imaging diagnosis of intra-ocular tumor. Chin Med J (Engl) 1997;110:664–6. [PubMed] [Google Scholar]
- 8.Shanmugam PM, Ramanjulu R. Vascular tumors of the choroid and retina. Indian J Ophthalmol. 2015;63:133–40. doi: 10.4103/0301-4738.154387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Little JR, Awad IA, Jones SC, Ebrahim ZY. Vascular pressures and cortical blood flow in cavernous angioma of the brain. J Neurosurg. 1990;73:555–9. doi: 10.3171/jns.1990.73.4.0555. [DOI] [PubMed] [Google Scholar]
- 10.Karimi S, Nourinia R, Mashayekhi A. Circumscribed choroidal hemangioma. J Ophthalmic Vis Res. 2015;10:320–8. doi: 10.4103/2008-322X.170353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Porrini G, Giovannini A, Amato G, Ioni A, Pantanetti M. Photodynamic therapy of circumscribed choroidal hemangioma. Ophthalmology. 2003;110:674–80. doi: 10.1016/S0161-6420(02)01968-1. [DOI] [PubMed] [Google Scholar]