Abstract
Background
The aims of this preliminary study were to evaluate contrast-enhanced ultrasound (CEUS) imaging and the therapeutic response of enlarged superficial lymph nodes in patients with lymphoma before and after chemotherapy and to determine the most useful CEUS response parameters.
Material/Methods
Forty-three patients with lymphoma, with 43 enlarged superficial lymph nodes, underwent CEUS and conventional ultrasound (US), before treatment and after the first three cycles of chemotherapy. Clinical responses included overall response (OR) and no response (NR). Imaging parameters by time-intensity curve (TIC) included basic intensity (B), wash-out slope and/or decent slope (K), wash-in slope or rise slope (C), time to peak (TTP), area under the gamma curve (Area), arrive time(ATM), peak intensity (PI), change of peak intensity (I) were compared. And receiver operating characteristic (ROC) curve analysis was operated.
Results
Quantitative parameters of CEUS before and after the first three cycles of chemotherapy showed a significant difference in the AreaΔ, PIΔ, and IΔ in the OR group compared with NR group (P<0.05). There was a significant difference in the Cpre, Areain, PIin, Iin, AreaΔ, PIΔ, and IΔ in the OR group compared with NR group (P<0.05). The effectiveness of the therapeutic response was predicted by the CEUS parameters of IΔ (P<0.05). And ΔArea has the highest diagnostic performance of ineffectiveness.
Conclusions
The findings of this study have shown that quantitative analysis by CEUS may be a useful, and objective, imaging method for the evaluation of the therapeutic response of enlarged superficial lymph nodes in lymphoma before and after chemotherapy.
MeSH Keywords: Lymph Nodes, Lymphoma, Ultrasonography
Background
Worldwide, the incidence of lymphoma continues to increase. According to the 2015 Chinese cancer statistics, for all forms of malignancy, the incidence of lymphoma and mortality from lymphoma ranked 11th and 10th, respectively [1]. The presence of painless, progressive lymphadenopathy, or a local mass, are common clinical presentations of lymphoma, and different methods for the early assessment include computed tomography (CT), magnetic resonance imaging (MRI) and combined 18F-fludeoxyglucose (FDG) positron emission tomography (PET) and CT, or PET-CT. In the evaluation of treatment response, 18F-FDG PET-CT is not affected by the tumor location, and can accurately show functional changes, but because of the patient exposure to radiation and the cost of this imaging technique, 18F-FDG PET-CT is not used routinely in the evaluation of lymphoma.
Following chemotherapy, conventional ultrasound (US) examination can be performed to examine the changes in superficial lymph nodes changes and is an imaging technique that is simple, inexpensive, and nonradioactive. Compared with other US methods, contrast-enhanced ultrasound (CEUS) has the advantages of convenience, and ease of operability. CEUS can demonstrate changes perfusion of the microcirculation in real time. However, at this time, there have been few reports on the use of CEUS for the evaluation of superficial enlarged lymph nodes and therapeutic response in lymphoma.
The aims of this preliminary study were to evaluate the parameters for CEUS imaging and the therapeutic response of enlarged superficial lymph nodes in patients with lymphoma before and after chemotherapy and to determine the most useful CEUS response parameters.
Material and Methods
Patients studied
This study was approved by the Local Ethics Committee of the Affiliated Hospital of Qingdao University, and all procedures were followed in accordance with the Declaration of Helsinki. All patients who participated in this study provided informed consent.
Between January 2016 and June 2017, 43 patients from the Affiliated Hospital of Qingdao University were enrolled in the study. Before treatment, all study participants had histologically-confirmed lymphoma by ultrasound-guided biopsy or surgery. There were 43 superficial enlarged lymph nodes evaluated in the study. All patients underwent bone marrow biopsy to confirm that there was no bone marrow involvement by lymphoma, and all patients were clinically staged according to the modified Ann Arbor staging system for Hodgkin’s lymphoma and non-Hodgkin’s lymphoma (NHL), prior to chemotherapy.
The clinical prognostic scoring systems used for each patient were based on the International Prognostic Score (IPS) for Hodgkin’s lymphoma, the International Prognostic Index (IPI) for non-Hodgkin’s lymphoma (NHL), and the Follicular Lymphoma International Prognostic Index (FLIPI) for follicular lymphoma. Patients with Hodgkin’s lymphoma underwent an ABVD chemotherapy regimen (doxorubicin, bleomycin, vinblastine, and dacarbazine); patients with non-Hodgkin’s lymphoma underwent R-CHOP (rituximab, cyclophosphamide, doxorubicin, vincristine, and prednisone) treatment.
According to the revised 2007 International Working Group response criteria for malignant lymphoma [2]. The therapeutic efficacy of chemotherapy was evaluated after three cycles of chemotherapy using computed tomography (CT) or 18F-FDG positron emission tomography-computed tomography (PET-CT) imaging findings. The clinical responses evaluated included complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD); CR and PR were collectively referred to as overall response (OR); SD and PD were collectively referred to as no response (NR). All patients underwent conventional ultrasound (US) imaging and contrast-enhanced ultrasound (CEUS) imaging once, before chemotherapy treatment, and again after the first three cycles of chemotherapy.
The inclusion criteria for patients in this study included: adult patients older than 18 years-of-age; patients with superficial abnormal enlarged lymph nodes, including cervical, axillary, or inguinal lymph nodes; patients who agreed to undergo CEUS; patients who signed an informed consent; patients who had an Eastern Cooperative Oncology Group (ECOG) performance status of 0–2, or a Karnofsky Performance Status (KPS) scale >60%.
The exclusion criteria for patients in this study included: an allergic reaction to imaging contrast agents; pregnant or lactating women; patients with coronary heart disease or mental illness; patients with a diagnosis of recurrent lymphoma.
Imaging studies
Conventional ultrasound (US)
Conventional US images were acquired using the Logiq E8 ultrasound machine (GE Medical Systems, Milwaukee, WI, USA), which was equipped with an ML 6–15 linear transducer with a central frequency of 12 MHz (frequency range: 4–15 MHz). All patients were asked to lie in the supine position. The selected lymph node was marked and underwent conventional ultrasound (US) examination to measure the maximum diameter (D), the elasticity index (E), the resistance index (RI), and the peak systolic flow velocity (PS).
Contrast-enhanced ultrasound (CEUS)
The contrast agent, SonoVue (Bracco, Milan, Italy) was used for CEUS, which contained stabilized sulfur hexafluoride microbubbles with a phospholipid shell and a mean size of 2.5 μm. SonoVue was considered to be a second-generation ultrasound contrast agent, and was used with 5 ml of sterile normal saline solution; 2.4 ml of SonoVue with normal saline was injected into the antecubital vein, followed by a flush of 5 ml of normal saline solution.
Lymph node imaging with CEUS was performed with the Logiq E8 ultrasound machine (GE Medical Systems, Milwaukee, WI, USA), which was equipped with a 9L linear transducer with a frequency range of 8.5–11 MHz. The optimum scan position of the selected lymph node for CEUS imaging was, if possible, in the ultrasound backfield and included the largest section of the lymph node, and the mechanical index was set at 0.11. Patients were instructed to breathe slowly and were injected with contrast agents to enable real-time, gray-scale, harmonic ultrasound imaging for scanning the superficial lymph nodes. The CEUS imaging of each lymph node recorded the dynamic image during 90 seconds.
Time-intensity curve (TIC) analysis
The time-intensity curve (TIC) analysis software package was used for quantitative analysis, and stored images were replayed. The region of interest (ROI) was set at size 3×3 mm. Motion tracking was chosen. The TIC was generated with time (s) on the X-axis and signal intensity (dB) on the Y-axis of the curve, and at the same time, smooth and gamma curve fitting were performed.
Parameters provided by TIC analysis were recorded, including basic intensity (B), wash-out slope or descent slope (K), wash-in slope or rise slope (C), time to peak (TTP), area under the gamma curve (Area), arrive time (ATm). Peak intensity (PI) was recorded, and change of peak intensity (I,I=PI (peak intensity) − BI (basic intensity)) was calculated. Measurements were performed in triplicate.
Two physicians, who had more than five years of experience of CEUS imaging, independently evaluated the images and performed the TIC analysis. Any disagreement between the imaging experts was resolved by consensus.
Statistical analysis
Statistical analysis was performed using the SPSS software package, version 23.0 for Windows (Chicago, III, USA). Numerical data were analyzed by the X2 test. Continuous data were expressed as the mean ± standard deviation (SD), and the range was included if a normal distribution was achieved. The paired t-test was performed for comparison before treatment, and after the first three cycles of chemotherapy. The independent t-test was performed for comparison among different groups. All the tests were two-sided. A P-value of <0.05 was considered to indicate statistical significance. Receiver operating characteristic (ROC) curves were used to assess the diagnostic performance (sensitivity and specificity) of quantitative parameters in CEUS imaging. The appropriate cutoff values were determined. The diagnostic performance for the area under the ROC curves (AUROC) were high (AUROC >0.9), moderate (AUROC=0.7–0.9), or low (AUROC=0.5–0.7).
Results
Baseline characteristics of 43 patients with lymphoma involving superficial lymph nodes
Table 1 shows the baseline characters of 43 patients with lymphoma during chemotherapy treatment. The histopathological diagnosis of these cases of lymphoma included: 26 cases of diffuse large B-cell lymphoma (DLBCL); five cases of follicular lymphoma (FL); three cases of mantle cell lymphoma; one case of peripheral T-cell lymphoma; three cases of anaplastic large cell lymphoma; and five cases of Hodgkin’s lymphoma.
Table 1.
Characteristics of the 43 patients with lymphoma in this study.
| Characteristics | OR | NR |
|---|---|---|
| Total number | 35 | 8 |
| Age | 46.570± 14.671 | 54.500± 11.784 |
| Gender | ||
| Male | 23 | 5 |
| Female | 12 | 3 |
| Ann Arbor stage | ||
| Stage II | 7 | 0 |
| Stage III | 22 | 3 |
| Stage IV | 6 | 5 |
| Histologic diagnosis | ||
| Non-Hodgkin’s lymphoma | ||
| Diffuse large B-cell lymphoma | 22 | 4 |
| Follicular lymphoma | 4 | 1 |
| Mantle cell lymphoma | 2 | 1 |
| Peripheral T-cell lymphoma | 1 | 0 |
| Anaplastic large cell lymphoma | 2 | 1 |
| Hodgkin’s Disease | 4 | 1 |
| Prognostic evaluation | ||
| Low | 4 | 1 |
| Low-intermediate | 8 | 2 |
| High-intermediate | 14 | 2 |
| High | 9 | 3 |
OR – overall response; NR – no response.
The number of cases of complete remission (CR), partial remission (PR), stable disease (SD), and progressive disease (PD) were 27, 8, 4, and 4, respectively, which meant that the number of patients with an overall response (OR) was 35, and the number of patients with no response (NR) was 8.
Features of conventional ultrasound (US)
The parameters of CEUS imaging before chemotherapy treatment and after the first three cycles of chemotherapy were labeled with pre and in, respectively. Tables 2 and 3 show that there was a significant difference in the maximum diameter (D) of the conventional US parameters in both the OR group and the NR group (P<0.05), and there was a significant difference in the peak systolic flow velocity (PS) of the OR group (P<0.05). Other parameters in the two groups were not statistically significant (P>0.05).
Table 2.
Comparison of the overall response (OR) group with the conventional ultrasound (US) parameters before and after the first three cycles of chemotherapy.
| Parameter | Pre-treatment | In-treatment | P-value |
|---|---|---|---|
| D | 3.640±0.981 | 2.049±0.735 | 0.000 |
| E | 4.474±0.439 | 4.411±0.470 | 0.100 |
| RI | 0.523±0.097 | 0.516±0.085 | 0.550 |
| PS | 21.794±7.300 | 19.977±4.459 | 0.030 |
D – maximum diameter; E – the elasticity index; RI – the resistance index; PS – the peak systolic flow velocity.
Table 3.
Comparison of the no response (NR) group with the conventional ultrasound (US) parameters before and after the first three cycles of chemotherapy.
| Parameter | Pre-treatment | In-treatment | P-value |
|---|---|---|---|
| D | 3.063±0.226 | 3.463±0.245 | 0.003 |
| E | 4.486±0.146 | 4.588±0.125 | 0.068 |
| RI | 0.596±0.027 | 0.591±0.030 | 0.649 |
| PS | 24.488±8.883 | 23.875±8.579 | 0.550 |
D – maximum diameter; E – the elasticity index; RI – the resistance index; PS – the peak systolic flow velocity.
Features of contrast-enhanced ultrasound (CEUS)
Table 4 shows that the difference of CEUS characteristics of lymph nodes before treatment and after the first three cycles of chemotherapy, including enhanced mode, homogeneous or inhomogeneous enhancement, and enhancement with or without defect, were not statistically significant (P>0.05). As can be seen in Table 4, the enhanced mode of lymph node CEUS in lymphoma patients was rapid and with homogeneous bolus enhancement without imaging defect.
Table 4.
Comparison of contrast-enhanced ultrasound (CEUS) imaging characteristics before and after the first three cycles of chemotherapy.
| Parameter | Enhanced mode | Homogeneity | Defect | |||
|---|---|---|---|---|---|---|
| Rapid bolus | Non-bolus | Yes | No | Yes | No | |
| Pre-treatment | 30 | 13 | 37 | 6 | 10 | 33 |
| In-treatment | 28 | 15 | 35 | 8 | 17 | 26 |
| χ2 value | 0.212 | 0.341 | 2.645 | |||
| P-value | 0.645 | 0.559 | 0.104 | |||
Contrast-enhanced ultrasound (CEUS) and time-intensity curve (TIC) features
Tables 5 and 6 show that, compared with the quantitative parameters of CEUS before and after the first three cycles of chemotherapy, the difference of Area, PI and I in the OR group were statistically significant (P<0.05); there was no significant difference in the other parameters (P>0.05). The differences in the parameter of the NR group were not statistically significant (P>0.05).
Table 5.
Comparison of the overall response (OR) parameters in the contrast-enhanced ultrasound (CEUS) groups before and after the first three cycles of chemotherapy.
| Parameter | Pre-treatment | In-treatment | P-value |
|---|---|---|---|
| B | −49.861±4.385 | −48.199±4.807 | 0.123 |
| K | 0.256±0.176 | 0.309±0.192 | 0.157 |
| C | 1.589±0.938 | 1.842±1.289 | 0.265 |
| TTP | 9.277±2.521 | 9.761±4.433 | 0.469 |
| Area | 574.463±123.619 | 244.930±120.784 | 0.000 |
| ATm | 12.808±3.029 | 13.005±5.423 | 0.872 |
| PI | −35.286±3.394 | −40.544±5.182 | 0.000 |
| I | 14.351±4.240 | 7.741±3.026 | 0.000 |
CEUS – contrast-enhanced ultrasound; B – basic intensity; K – wash-out slope and/or decent slope; C – wash-in slope and/or rise slope; TTP – time to peak; Area – area under the gamma curve; ATm – arrive time; PI – peak intensity; I – change of peak intensity.
Table 6.
Comparison of the no response (NR) parameters in the contrast-enhanced ultrasound (CEUS) groups before and after the first three cycles of chemotherapy.
| Parameter | Pre-treatment | In-treatment | P-value |
|---|---|---|---|
| B | −50.719±6.349 | −47.366±4.951 | 0.281 |
| K | 0.315±0.213 | 0.457±0.286 | 0.397 |
| C | 2.664±1.354 | 2.361±1.986 | 0.764 |
| TTP | 9.499±1.833 | 8.920±2.709 | 0.607 |
| Area | 484.854±67.036 | 455.456±135.043 | 0.606 |
| ATm | 12.542±1.983 | 11.677±3.819 | 0.493 |
| PI | −35.858±3.600 | −34.246±2.689 | 0.285 |
| I | 14.861±6.213 | 13.119±5.285 | 0.534 |
CEUS – contrast-enhanced ultrasound; B – basic intensity; K – wash-out slope and/or decent slope; C – wash-in slope and/or rise slope; TTP – time to peak; Area – area under the gamma curve; ATm – arrive time; PI – peak intensity; I – change of peak intensity.
Table 7 shows that for the OR group, compared with NR group, the statistically significant CEUS quantitative parameterswere Cpre, Areain, PIin, Iin (P<0.05); there was no significant difference in the other parameters (P>0.05)
Table 7.
Comparison of the overall response (OR) and no response (NR) parameters using contrast-enhanced ultrasound (CEUS) before and after the first three cycles of chemotherapy.
| Parameter | B | K | C | TTP | Area | ATm | PI | I | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pre | In | Pre | In | Pre | In | Pre | In | Pre | In | Pre | In | Pre | In | Pre | In | |
| P-value | 0.649 | 0.662 | 0.418 | 0.080 | 0.010 | 0.360 | 0.816 | 0.611 | 0.055 | 0.000 | 0.815 | 0.517 | 0.673 | 0.002 | 0.831 | 0.000 |
CEUS – contrast-enhanced ultrasound; B – basic intensity; K – wash-out slope and/or decent slope; C – wash-in slope and/or rise slope; TTP – time to peak; Area – area under the gamma curve; ATm – arrive time; PI – peak intensity; I – change of peak intensity.
Changes before treatment and after the first three cycles of chemotherapy were calculated and marked as Δ. Table 8 shows that, compared with the OR and NR group of CEUS quantitative parameters, there was a statistically significant difference in AreaΔ, PIΔ, and IΔ (P<0.05): there was no significant difference in the other parameters (P>0.05)
Table 8.
Comparison of the overall response (OR) and no response (NR) parameters for contrast-enhanced ultrasound (CEUS).
| Parameter | OR | NR | P-value |
|---|---|---|---|
| BΔ | 1.662±6.211 | 3.353±8.122 | 0.515 |
| KΔ | 0.522±0.213 | 0.142±0.445 | 0.595 |
| CΔ | 0.253±1.321 | −0.302±2.738 | 0.592 |
| TTPΔ | 0.484±3.913 | −0.579±3.046 | 0.477 |
| AreaΔ | −329.533±129.440 | −29.398±153.833 | 0.000 |
| ATmΔ | 0.196±7.134 | −0.865±3.383 | 0.686 |
| PIΔ | −5.258±5.849 | 1.611±3.932 | 0.003 |
| IΔ | 6.610±3.486 | −1.742±7.523 | 0.016 |
CEUS – contrast-enhanced ultrasound; B – basic intensity; K – wash-out slope and/or decent slope; C – wash-in slope and/or rise slope; TTP – time to peak; Area – area under the gamma curve; ATm – arrive time; PI – peak intensity; I – change of peak intensity.
Receiver operating characteristic (ROC) curve
Comparison of the OR and NR group of CEUS imaging quantitative parameters showed that there was a statistical significance in Cpre, Areain, PIin, Iin, AreaΔ, PIΔ, IΔ (P<0.05). A ROC curve was constructed to assess the accuracy of these parameters for the prediction of the therapeutic responses.
Figure 1 shows that the effectiveness of therapeutic response could be predicted by the parameter of IΔ. The IΔ area under the ROC curve (AUC) was 0.889, which can be regarded as a good diagnostic profile. The maximum Youden index (sensitivity + specificity −1) corresponds to the best cutoff point that had best cutoff value. The best cutoff value of the IΔ was 2.858 by coordinating the points of the ROC curve; the predicted sensitivity and specificity for IΔ were 91.4% and 87.5%, respectively.
Figure 1.
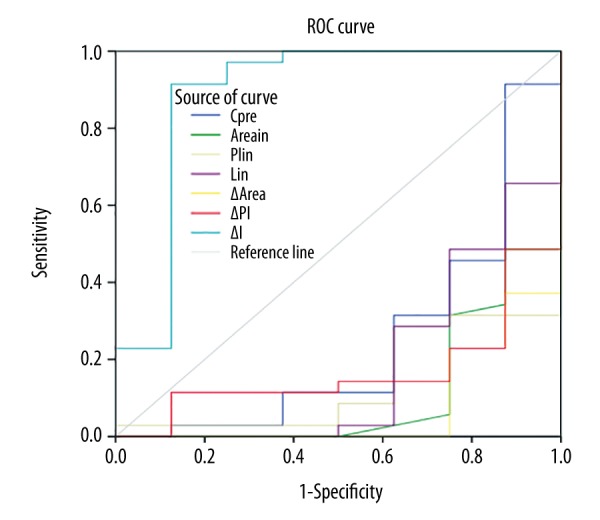
Parameters of the receiver operating characteristic (ROC) curve for effective of therapeutic response.
Figure 2 shows that the lack of efficacy of therapeutic response could be predicted by the parameters of Cpre, Areain, PIin, Iin, AreaΔ, PIΔ, with the AUC of 0.754, 0.891, 0.861, 0.818, 0.925, 0.832, respectively. The CEUS imaging parameter of AreaΔ had the greatest diagnostic performance, with a cutoff value of −60.310, a sensitivity of 75%, and a specificity of 100%.
Figure 2.
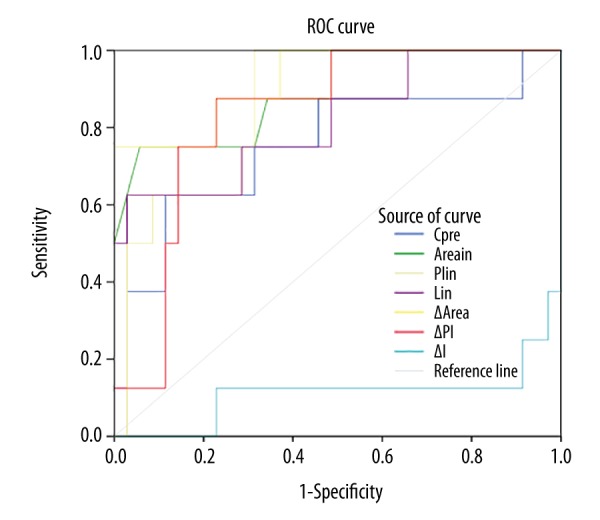
Parameters of the receiver operating characteristic (ROC) curve for lack of effective therapeutic response.
Discussion
The use of ultrasound imaging using targeted micro-bubbles, as in contrast-enhanced ultrasound (CEUS), has recently shown an application in clinical trials to assess the microvasculature in angiogenesis, but imaging features of tumor-associated blood vessels at the molecular level may be better able to diagnose and monitor tumor treatment. Resistance to chemotherapy and tumor recurrence following treatment continue to be a clinical challenge. Therefore, the early evaluation of treatment response is necessary for the development of clinical treatment strategies [3]. However, there have been few reports that have studied CEUS imaging parameters for the evaluation of superficial enlarged lymph nodes in lymphoma and the therapeutic response to treatment.
The aims of this current study were to compare the use of CEUS with conventional US in the evaluation of chemotherapy response in lymphoma involving superficial enlarged lymph nodes before and after chemotherapy, to determine the most sensitive and specific CEUS imaging parameters, and to investigate the role of the identified CEUS parameters for the evaluation of treatment response in superficial enlarged lymph nodes involved with lymphoma.
The findings of this study showed that with conventional US, the maximum diameter (D) in the overall response (OR) group and no response (NR) group were statistically significant. After three cycles of chemotherapy, D of the OR group decreased, and D of the NR group increased, which was related to the clinical efficacy of the evaluation of malignant lymphoma [2]. There was no significant difference in the elasticity index (E) between the OR group and the NR group, which may be related to the selection of samples and the position of sample frame. In a previously published study, Squillaci et al. [4] showed that patients with relapsed and refractory Hodgkin’s lymphoma, who had undergone brentuximab vedotin treatment, showed no statistically significant difference between the strain ratio of the responder group and the non-responder group. In this previous study, the authors used a Philips IU22 machine to target lymph nodes in relapsed and refractory Hodgkin’s lymphoma, which was different from our study [4].
However, further data from the published literature related to lymph node involvement in lymphoma and imaging are very limited. The results from studies using elastosonography to assist the differential diagnosis of enlarged superficial lymph nodes have been controversial. In most cases, the efficacy of quantitative imaging in the analysis of lymph nodes affected by lymphoma and in the differential diagnosis from other nodal malignancies or even benign disease, such as tuberculous lymphadenitis, have varied [5]. Recently, Chen et al. [6] proposed that the shear wave velocity (SWV) for metastatic carcinoma was significantly greater than that for lymphoma. in a noninvasive study to evaluate benign and malignant superficial lymph nodes using a virtual touch tissue quantification method (Siemens Medical Solutions, Erlangen, Germany).
For several years, quantitative analysis software, for time-intensity curve (TIC) analysis, has been used in the differential diagnosis of superficial lymph node lesions. At present, TIC analysis is becoming more widely used in clinical practice, but the results from the published clinical literature are controversial. In 2012, Cao et al. [7] showed that the CEUS TIC showed quantitative tumor blood perfusion changes, and that following neoadjuvant chemotherapy, blood perfusion was found to be reduced, enhancement intensity decreased, time to peak (TTP) increased, peak intensity (PI) was reduced, and the wash-in slope (C) was reduced. Recently, in 2016, Peng et al. [8] showed that changes in quantitative CEUS parameters indirectly reflect the effectiveness of chemotherapy in cervical tumors. Furthermore, significant changes in maximum intensity, rise time, and TTP were observed in the stable disease (SD) group according to the Response Evaluation Criteria in Solid Tumors (RECIST) criteria and demonstrated that quantitative CEUS analysis could effectively evaluate tumor perfusion even with the stability of the tumor size [8].
Dynamic CEUS imaging has previously been shown to detect changes in vascular volume earlier than the anatomic changes detected by computed tomography (CT) [9]. Quantitative analysis of CEUS imaging offers a reliable way to study the process of angiogenesis in tumors, with a maximum intensity proportional to the concentration of contrast agent and both rise time and time to peak (TTP) to the blood flow rate [10,11]. The study by Wang et al. [12] showed that CEUS imaging demonstrated intratumoral perfusion changes following chemotherapy, which were manifested by decreased contrast agent intake or tumor perfusion defects following chemotherapy. Wei et al. [13] found that the variations of peak intensity and mean intensity before and after the second cycle of R-CHOP treatment showed a significant correlation with the treatment results. However, for arrive time (ATm) and TTP, there were no significant correlations with treatment outcome [13].
There have been few previously reported studies that have evaluated CEUS imaging for superficial enlarged lymph nodes in lymphoma and the evaluation of therapeutic response. There are two types of information that may be obtained from the TIC curve; one is time-related, such as arrival time (ATm) and time to peak (TTP); and the other depends on the intensity, such as peak intensity (PI), area under the gamma curve (Area) and change of peak intensity (I). Since PI is related to blood volume, and signal strength is proportional to micro-bubble concentration, it can be concluded that the blood volume of lymph nodes in the OR group decreases and the number of micro-bubbles entering the lesion decreases after three cycles of chemotherapy. Therefore, compared with the quantitative parameters of CEUS before and after the first three cycles of chemotherapy, the difference of Area, PI, and I in the OR group was statistically significant, and there was no significant difference in the other parameters in this study.
The aim and design of this study were to compare CEUS imaging in lymphoma with superficial enlarged lymph nodes before chemotherapy and after three cycles of chemotherapy, and included 18FFDG positron emission tomography-computed tomography (PET-CT) examination of the clinical efficacy evaluation before the fourth cycle of chemotherapy. The interim 18F-FDG PET-CT results reflected the sensitivity of the patient to the chemotherapy regimen and could help to adjust the treatment strategy. Recently, it has been reported that the 18F-FDG PET-CT imaging should be carried out after one course of chemotherapy [14,15].
Given the prognostic significance of the use of 18F-FDG PET-CT in the evaluation of lymphoma, several global clinical trials are currently underway. The early and effective evaluation of enlarged lymph nodes for different subtypes of lymphoma directly affect the follow-up treatment therapy regimen for lymphoma patients and relates to prognosis. Patients with no response (NR) should be assigned to second-line therapy as soon as possible [16]. Therefore, repeated CEUS, in contrast to the evaluation of clinical efficacy, was performed after several cycles of chemotherapy to obtain sensitivity parameters for TIC analysis, which is an area of this study that should be investigated further.
Diffuse large B-cell lymphoma (DLBCL) is the most common histological type of non-Hodgkin’s lymphoma (NHL) [17], follicular lymphoma is the most common histological type of indolent NHL, and is also the second most common histologic type of NHL [18–21]. Together, Hodgkin’s lymphoma, DLBCL, and follicular lymphoma constitute approximately 80% of adult lymphomas [22]. Most of the cases in this study were DLBCL, follicular lymphoma, and Hodgkin’s lymphoma. Although the number of patients included in this study was small, it was believed to be representative.
This study had several limitations. First, the number of patients was small, and because of this, it was not possible to compare Hodgkin’s lymphoma and NHL in the CEUS imaging of the superficial lymph nodes before and after chemotherapy, and so Hodgkin’s lymphoma and NHL were combined for analysis. The incidence of NHL was greater than that of Hodgkin’s lymphoma so that most of the patients included had NHL. Also, this study did not analyze the differences in CEUS imaging between B-cell lymphoma and T-cell lymphoma, high-grade and low-grade NHL before and after chemotherapy.
Conclusions
This preliminary study has shown that contrast-enhanced ultrasound (CEUS) imaging has a potential role in the evaluation of superficial enlarged lymph nodes in lymphoma. The study has evaluated the therapeutic response by CEUS and has identified parameters for CEUS that can provide the basis for treatment planning or to adjust a more effective clinical treatment plan. The effectiveness of the therapeutic response was predicted by the CEUS parameters of IΔ (P<0.05). And ΔArea has the highest diagnostic performance of ineffectiveness. In this preliminary study, although the number of cases studied was small, but the findings support the value of CEUS imaging in the diagnosis of lymphoma and support the need for future large controlled clinical studies.
Footnotes
Conflict of interest
None.
Source of support: Departmental sources
References
- 1.Chen W, Zheng R, Baade PD, et al. Cancer statistics in China, 2015. Cancer J Clin. 2016;66(2):115–32. doi: 10.3322/caac.21338. [DOI] [PubMed] [Google Scholar]
- 2.Cheson BD, Pfistner B, Juweid ME, et al. Revised response criteria for malignant lymphoma. J Clin Oncol. 2007;25(5):579–86. doi: 10.1200/JCO.2006.09.2403. [DOI] [PubMed] [Google Scholar]
- 3.Plosker GL, Figgitt DP. Rituximab: A review of its use in non-Hodgkin’s lymphoma and chronic lymphocytic leukaemia. Drugs. 2003;63(8):803–43. doi: 10.2165/00003495-200363080-00005. [DOI] [PubMed] [Google Scholar]
- 4.Squillaci E, Antonicoli M, Manenti G, et al. Real-time ultrasound elastography for assessment of response to brentuximab vedotin treatment in relapsed and refractory Hodgkin lymphoma. Eur Rev Med Pharmacol Sci. 2016;20(8):1628–35. [PubMed] [Google Scholar]
- 5.Kocaman O, Ince AT. Endosonography and elastography in the diagnosis of esophageal tuberculosis. Turkish J Gastroenterol. 2013;24(3):290–91. doi: 10.4318/tjg.2013.0488. [DOI] [PubMed] [Google Scholar]
- 6.Chen S, Lin X, Chen X, et al. Noninvasive evaluation of benign and malignant superficial lymph nodes by virtual touch tissue quantification: A pilot study. J Ultrasound Med. 2016;35(3):571–75. doi: 10.7863/ultra.15.05053. [DOI] [PubMed] [Google Scholar]
- 7.Cao X, Xue J, Zhao B. Potential application value of contrast-enhanced ultrasound in neoadjuvant chemotherapy of breast cancer. Ultrasound Med Biol. 2012;38(12):2065–71. doi: 10.1016/j.ultrasmedbio.2012.07.027. [DOI] [PubMed] [Google Scholar]
- 8.Peng C, Liu LZ, Zheng W, et al. Can quantitative contrast-enhanced ultrasonography predict cervical tumor response to neoadjuvant chemotherapy? Eur J Radiology. 2016;85(11):2111–18. doi: 10.1016/j.ejrad.2016.09.025. [DOI] [PubMed] [Google Scholar]
- 9.Williams R, Hudson JM, Lloyd BA, et al. Dynamic microbubble contrast-enhanced US to measure tumor response to targeted therapy: A proposed clinical protocol with results from renal cell carcinoma patients receiving antiangiogenic therapy. Int J Med Radiol. 2011;260(2):581–90. doi: 10.1148/radiol.11101893. [DOI] [PubMed] [Google Scholar]
- 10.Strouthos C, Lampaskis M, Sboros V, et al. Indicator dilution models for the quantification of microvascular blood flow with bolus administration of ultrasound contrast agents. IEEE Trans Ultrason Ferroelectr Freq Control. 2010;57(6):1296–310. doi: 10.1109/TUFFC.2010.1550. [DOI] [PubMed] [Google Scholar]
- 11.Pei XQ, Liu LZ, Zheng W, et al. Contrast-enhanced ultrasonography of hepatocellular carcinoma: correlation between quantitative parameters and arteries in neoangiogenesis or sinusoidal capillarization. Eur J Radiol. 2012;81(3):e182–88. doi: 10.1016/j.ejrad.2011.01.083. [DOI] [PubMed] [Google Scholar]
- 12.Wang JW, Zheng W, Liu JB, et al. Assessment of early tumor response to cytotoxic chemotherapy with dynamic contrast-enhanced ultrasound in human breast cancer xenografts. Ultrasound Med Biol. 2013;39(5):S81–82. doi: 10.1371/journal.pone.0058274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wei X, Li Y, Zhang S, et al. The role of contrast-enhanced ultrasound (CEUS) in the early assessment of microvascularization in patients with aggressive B-cell lymphoma treated by rituximab-CHOP: A preliminary study. Clin Hemorheology Microcirc. 2014;58(2):363–76. [PubMed] [Google Scholar]
- 14.Hutchings M, Loft A, Hansen M, et al. FDG-PET after two cycles of chemotherapy predicts treatment failure and progression-free survival in Hodgkin lymphoma. Blood. 2006;107(1):52–59. doi: 10.1182/blood-2005-06-2252. [DOI] [PubMed] [Google Scholar]
- 15.Hutchings M, Mikhaeel NG, Fields PA, et al. Prognostic value of interim FDG-PET after two or three cycles of chemotherapy in Hodgkin lymphoma. Ann Oncol. 2005;16(7):1160–68. doi: 10.1093/annonc/mdi200. [DOI] [PubMed] [Google Scholar]
- 16.Raizer JJ, Rademaker A, Evens AM, et al. Pemetrexed in the treatment of relapsed/refractory primary central nervous system lymphoma. Cancer. 2012;118(15):3743–48. doi: 10.1002/cncr.26709. [DOI] [PubMed] [Google Scholar]
- 17.Krol AD, Le CS, Snijder S, et al. Primary extranodal non-Hodgkin’s lymphoma (NHL): the impact of alternative definitions tested in the Comprehensive Cancer Centre West population-based NHL registry. Ann Oncol. 2003;14(1):131–39. doi: 10.1093/annonc/mdg004. [DOI] [PubMed] [Google Scholar]
- 18.Horn H, Schmelter C, Leich E, et al. Follicular lymphoma grade 3B is a distinct neoplasm according to cytogenetic and immunohistochemical profiles. Haematologica. 2011;96(9):1327–34. doi: 10.3324/haematol.2011.042531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wahlin BE, Yri OE, Kimby E, et al. Clinical significance of the WHO grades of follicular lymphoma in a population-based cohort of 505 patients with long follow-up times. Br J Haematol. 2012;156(2):225–33. doi: 10.1111/j.1365-2141.2011.08942.x. [DOI] [PubMed] [Google Scholar]
- 20.Freedman A. Follicular lymphoma: 2014 update on diagnosis and management. Am J Hematol. 2014;89(4):429–36. doi: 10.1002/ajh.23674. [DOI] [PubMed] [Google Scholar]
- 21.Hiddemann W, Cheson BD. How we manage follicular lymphoma. Leukemia. 2014;28(7):1388–95. doi: 10.1038/leu.2014.91. [DOI] [PubMed] [Google Scholar]
- 22.Hicks RJ, MacManus MP, Seymour JF. Initial staging of lymphoma with positron emission tomography and computed tomography. Semin Nucl Med. 2005;35(3):165–75. doi: 10.1053/j.semnuclmed.2005.02.003. [DOI] [PubMed] [Google Scholar]


