Abstract
Background
Caries and periodontal diseases are caused by the biofilm formed by caries- and periodontal disease-related bacteria. Specific biofilms could be formed on different filling materials in oral cavity. Thus, to explore the inhibition effect of restorative filling materials on biofilm formation is of great significance in the treatment of caries and periodontal disease.
Material/Methods
The supernatants of S. mutans, S. sanguinis, and P. gingivalis suspension were combined with BHI broth. After 24 h, the live P. gingivalis number was calculated by colony counting and the biofilm was monitored by fluorescence microscopy. To test the adhesive ability of S. mutans and S. sanguinis on different dental materials, the biofilm was formed on different dental materials and then the bacterial number was calculated by using a Spectramax 250 microplate reader at OD 550, and the adhesive ability of S. mutans and S. sanguinis on different dental materials was analyzed by scanning electron microscopy.
Results
The growth and biofilm formation of P. gingivalis was significantly inhibited by S. mutans and S. sanguinis supernatants (P<0.05). All groups except the zinc phosphate cement group (B) exerted a strong inhibitory effect on the biofilm formation of S. mutans and S. sanguinis (P<0.05).
Conclusions
The supernatants of S. mutans and S. sanguinis significantly inhibited the growth and biofilm formation of P. gingivalis, and the adhesive ability of S. mutans and S. sanguinis are different on different dental materials. These results provide useful information on dental caries, periodontal disease, and dental materials.
MeSH Keywords: Biofilms, Biomedical and Dental Materials, Dental Caries, Periodontal Diseases
Background
Caries and periodontal diseases are the most common bacterial diseases of humans and are caused by changes in the oral microflora. Dental plaque biofilms, which can be formed on enamel, filling material surfaces, and implants, are one of the key etiological factors of dental caries and periodontal diseases, and is also a popular research topic in oral microbiology and ecology [1].
With increasing aesthetic requirements, restorative filling materials that match with the color of natural teeth have been developed. The filling materials include nanoparticle resin composites, zinc phosphate cement, and glass ionomer cement [2]. However, because of their improved mechanical properties, there are at present certain disparities regarding the ideal material for tooth repair [3]. Secondary caries occur on the edge of the restoration when dental plaque biofilms are formed [4]. Different filling materials in the mouth may form specific biofilms [5]. Thus, the inhibitory efficiency of restorative filling materials has attracted more and more research attention.
S. mutans and S. sanguinis are the main bacteria that form biofilms and cause caries [6], and P. gingivalis is one of the main bacteria that forms biofilms and causes periodontal disease [7]. Therefore, control of biofilm formation is the key to preventing caries and periodontal disease. The relationship between periodontal disease and caries-causing bacteria has been an important topic in the field of oral microbiological research [8]. In the present study, in order to explore the interaction between S. mutans, S. sanguinis, and P. gingivalis and evaluate the adhesive ability of S. mutans and S. sanguinis on different dental filling materials, we compared the inhibitory effects of supernatants of S. mutans and S. sanguinis on P. gingivalis growth and biofilm formation and tested the adhesive ability of S. mutans and S. sanguinis on different materials.
Material and Methods
Strains and conditions
The strains used in this study were S. mutans ATCC 25175, S. sanguis ATCC 10556, and P. gingivalis ATCC 53977. These 3 strains were incubated on Brian Heart Infusion agar at 37°C under microaerophilic conditions. The bacteriostatic and biofilm experiments were conducted in Brian Heart Infusion broth and Brian Heart Infusion agar (Oxoid, UK).
Preparation of sterile supernatants
The mixtures of 1 ml of S. mutans (Sm) and S. sanguis (Ss) (3×108 CFU/ml) and 4.0 ml of BHI broth were incubated under microaerophilic conditions for 48 h at 37°C. Then, the culture was centrifuged for 15 min at 2100 rpm to remove all cells. After centrifugation, the supernatant was filtered through 0.22-μm cellulose nitrate filters (Millipore, USA) and stored at 4°C [9].
Inhibitory effect of sterile supernatants on P. gingivalis growth
P. gingivalis was first grown in BHI broth at 37°C for 24 h and then was suspended in saline to form a concentration of 0.5 MCF (1.5×108 CFU/ml) suspension. Then, 250 μl of the Sm and Ss supernatants and 250 μl of the P. gingivalis suspension were combined with 0.35 ml BHI broth. The cultures were incubated under microaerophilic conditions at 37°C for 24 h. The BHI broth was used in the control instead of Sm and Ss supernatants. After 24 h, the cultures were vortexed for 1 min, diluted to 10−4, and the colony number was determined.
Biofilm tests
Inhibitory effects of sterile supernatants of S. mutans and S. sanguis on P. gingivalis
For the biofilm formation of P. gingivalis, the Pg culture was incubated in BHI broth under aerophilic conditions [10] (10% CO2, 5% H2, 85% N2), for 24 h at 37°C. Then, the concentration of P. gingivalis suspension was adjusted with saline to 0.5 MCF (1.5×108 CFU/ml). Individually, 10 ml of BHI broth and a sterile coverglass (18 mm diameter) were added in the sterile Petri dishes. Then, 100 ml of the Pg suspension and 500 μl of the Sm and Ss supernatants were mixed in the dishes. The BHI broth was used as the control groups. All dishes were incubated under aerophilic conditions at 37°C for 24 h.
After 24 h, the coverglasses in dishes were washed with phosphate-buffered saline (PBS) 3 times and fixed with 2.5% glutaraldehyde for 1 h. After fixation, the coverglasses were stained using the BacLight Live/Dead staining kit (Invitrogen). The images of the biofilms were acquired using a Nikon 80i fluorescence microscope at an excitation wavelength of 488 nm (green fluorescence). Eight images per field per sample of the biofilm were acquired randomly and the experiment was independently repeated 3 times. The biofilm images were analyzed by using Image-Pro Plus 6.0 software and expressed as the integrated optical density (IOD) [11].
Adhesive ability of S. mutans and S. sanguis on different dental materials
Sixty specimens containing 4 repair materials and three 3-root canal sealers were divided into 7 groups according to the type of composite used, as follows:
Group A: Nanoparticle resin composite Filtek Z350 (3M ESPE, USA), shade A3;
Group B: Zinc phosphate cement (Ultradent, South Jordan, UT, USA), shade A3;
Group C: Glass ionomer cement (3M ESPE, St. Paul, MN, USA), shade A3.
Group D: Infiltrant resin (ICON®-Infiltrant; DMG) shade A3.
Group E: Mineral Trioxide Aggregate (MTA) (ProRootMTA; Dentsply).
Group F: iRoot SP root canal sealer (Innovative BioCreamixInc, Canada).
Group G: iRoot BP root canal sealer (Innovative BioCreamixInc, Canada).
Group H: iRoot FS root canal sealer (Innovative BioCreamixInc, Canada).
Group I: AH Plus sealer (Dentsply).
The composites were inserted at a single increment into a cylindrical metal matrix of 1-mm height with 10-mm diameter, and placed on a glass plate. After curing, the material was removed with the aid of a spatula. The composite surfaces were ground with 1200-grit silicon carbide abrasive paper (3M ESPE, St. Paul, MN, USA) with water-cooling for surface standardization and then disinfected using ultraviolet illumination for 2 h [12].
Biofilm formation was conducted using the methods of Stoodle et al. [13]. In brief, the material from the 3 groups was placed in 16-well plates, and 2-ml BHI and 100-μl MS suspension (1.5×108 cfu/ml) was added to each hole. The holes were incubated under anaerobic conditions for 24 h.
Assessment of bacterial adhesion
After 24 h, the coverglass was washed with PBS 3 times to remove unattached cells and were then sonicated in 10 ml PBS for 6 min to detach adherent bacteria on the biomaterial surfaces. After sonication, the suspensions were added in the tube and the OD at 550 nm (OD 550) was measured using a Spectramax 250 microplate reader [14].
Scanning electron microscopy (SEM) of biofilms
The biofilms were fixed with 2.5% glutaraldehyde, dried at 37°C for 24 h, and then the biofilms were coated with gold and examined under a scanning electron microscope (Hitachi SU-70) in high-vacuum mode at 3 kV. The SEM images were analyzed using Image J software to measure the biofilm area (in pixels) [15,16].
Statistical analysis
SPSS 14.0 software was used for statistical analysis by a one-way analysis of variance (ANOVA) and Dunnett’s two-sided t test with the level of significance set at P<0.05.
Results
Inhibitory effect of supernatants on P. gingivalis growth and biofilm formation
The growth of P. gingivalis was significantly inhibited by S. mutans and S. sanguinis supernatants (P<0.05; Table 1). In the fluorescence images, the IOD of the supernatants of Sm and Ss showed a significant reduction as compared with controls, which indicated that the supernatants of Sm and Ss significantly inhibit the biofilm formation by P. gingivalis (P<0.05; Figure 1). The images of the biofilms captured by fluorescence biofilms are shown in Figure 2.
Table 1.
Viable Pg cells in mixed culture with the supernatants of Sm, Ss. Viable Pg, 108 CFU/ml (means ±SD).
| Pg concentrations | Sm supernatants | Ss supernatants | Control |
|---|---|---|---|
| 1.5 | 3.33±1.32* | 3.65±0.74* | 7.01±2.45 |
| 3 | 2.55±0.65* | 1.84±0.64** | 5.20±1.44 |
P<0.05;
P<0.01compared with controls (Dunnett’s two-sided t-test).
Figure 1.
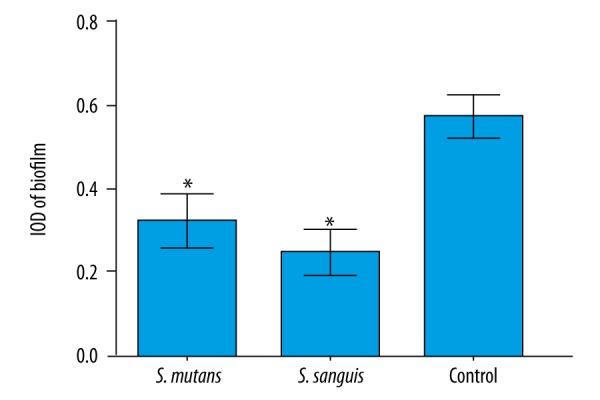
The adhesion/biofilm formation (IOD) of Pg grown in the presence of Sm and Ss supernatants. Data are expressed as the mean ±SD, * P<0.05.
Figure 2.
The effect of supernatants on Pg biofilm formation. Bacteria are stained with BacLight Live/Dead staining kit: (A) S. mutans, (B) S. sanguis, (C) control.
Adhesive ability of S. mutans and S. sanguis on different dental materials
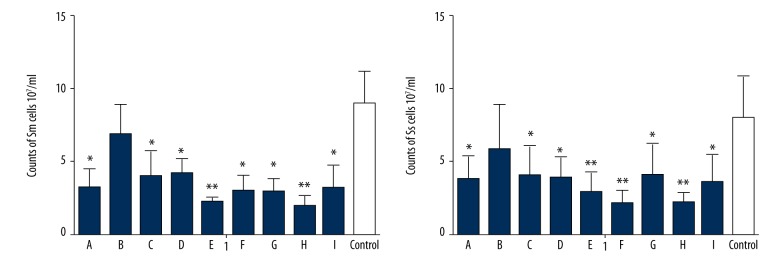
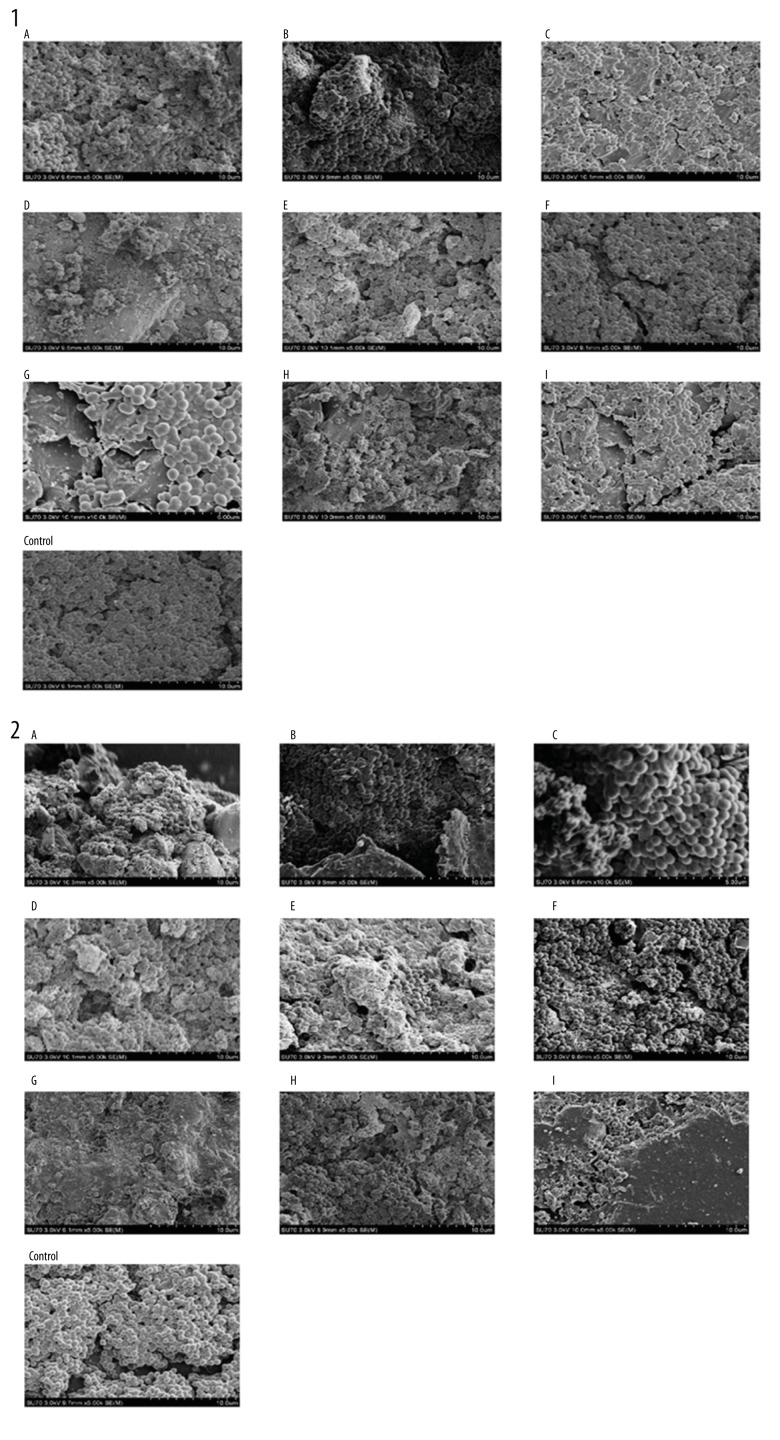
All groups except the zinc phosphate cement group (group B) exerted a strong inhibitory effect on the biofilm formation of S. mutans and S. sanguinis (P<0.05; Figure 3). In the SEM images, the bacterial number on the resin composite (group A), Glass ionomer cement (group C), Infiltrant resin (group D), Mineral Trioxide Aggregate (group E), iRoot SP root canal sealer (group F), iRoot BP root canal sealer (group G), iRoot FS root canal sealer (group H), and AH Plus sealer (group I) was significantly less than in the control group (P<0.05). The SEM images of the biofilm are presented in Figure 4.
Figure 3.
Viable Sm and Ss in the biofilms on different materials (A – nanoparticle resin composite Filtek Z350, B – Zinc phosphate cement, C – glass ionomer cement, D – Infiltrant resin, E – mineral trioxide aggregate (MTA), F – iRoot SP root canal sealer, G – iRoot BP root canal sealer, H – iRoot FS root canal sealer, I – AH Plus sealer). Data are expressed as the mean ±SD, * P<0.05, ** P<0.01.
Figure 4.
SEM images of the biofilm of P. gingivalis co-cultured with supernatants of S. mutans (1) and S. sanguis (2). (A – nanoparticle resin composite Filtek Z350, B – zinc phosphate cement, C – glass ionomer cement, D – infiltrant resin, E – mineral trioxide aggregate (MTA), F – iRoot SP root canal sealer, G – iRoot BP root canal sealer, H – iRoot FS root canal sealer, I – AH Plus sealer).
Discussion
Oral biofilms can be formed on a variety of oral hard tissue surfaces, which leads to the development and occurrence of caries and periodontal disease [17]. The formation of an acquired membrane on the surface of the repair materials promotes bacterial adhesion to surfaces. Streptococcus sanguis is one of the main bacteria present in the human oral cavity, and in vivo studies have shown that Streptococcus sanguis has a negative relationship in dental plaque with bacterial pathogens suspected to be involved in periodontal disease [18]. Our experiments show that the supernatant of Streptococcus sanguis had a strong inhibitory effect on P. gingivalis, which was consistent with previous experimental results reported by Stinson et al. [19]. Previous studies have shown that Streptococcus sanguis can inhibit periodontal pathogens through the secretion of hydrogen peroxide and streptocin [20]. The bacteriostatic activity of the supernatant was observed in our experiments, which indicated that Streptococcus sanguis can produce antimicrobial substance [21]. The application of hydrogen peroxide has begun to make a real impact at the treatment of periodontal disease [22], so the use of S. sanguis, which is able to produce hydrogen peroxide, has become a new way to treat periodontal disease.
In our experiments, the glass ion cement biofilm had fewer bacteria, possibly because the 3M ESPE glass ion cement surface can release fluoride and change the pH, which could reduce the occurrence of caries [23,24]. Although composite resin materials lack effective antibacterial ingredients, to some extent bacterial adhesion can be reduced after polishing [25]. The main effects of zinc phosphate cement, MTA, and root canal sealer are to isolate irritation, rebase the cavity, and seal the root canals. Their surfaces are relatively coarse and lack effective antimicrobial properties, leading to an increased likelihood of bacteria forming biofilms on their surfaces [26].
There may be symbiotic relationships between many of the bacteria associated with caries and periodontal disease, but some bacteria may also inhibit each other. Studies have shown that the detection rate of periodontal pathogens such as P. gingivalis and A. actinomycetem can decrease when certain gram-positive cariogenic bacteria such as S. mutans and S. sanguis are also present [27]. In vitro studies of the relationship between periodontal pathogens and caries have shown that P. gingivalis is antagonistic for S. mutans, which was consistent with our results [28].
Biofilm formation by bacteria on oral material is influenced by many factors, making the pathogenesis of periodontal pathogens and caries pathogenesis perplexing [29,30]. For example, during the early stages of periodontal disease, the etiology is closely associated with some inflammatory biomarkers [31,32]. However, in our experiment, the roughness and other physical parameters of the material surfaces were not evaluated [33] and the results do not show changes of inflammatory biomarkers in vivo; therefore, our results cannot be linked to specific features on the surface of the material and the effects in vivo. We plan to conduct further experiments after the comprehensive consideration of various parameters and to perform in vivo experiments in the future.
Conclusions
Our data indicate that the supernatants of S. mutans and S. sanguinis had significant inhibitory effects on the growth and biofilm formation of P. gingivalis, and the adhesive ability of S. mutans and S. sanguinis are different on different dental materials. The results presented here will be of benefit to further studies related to dental caries, periodontal disease, and dental materials.
Footnotes
Source of support: This investigation was funded by the Education Department of Zhejiang Province (grant number Y201329612 and Y201432178), the National Natural Science Foundation of China (Grant No 81371142), and 2011 China State Key Clinical Department Grants
References
- 1.Marsh P. Dental plaque: Biological significance of a biofilm and community life-style. J Clinl Perio. 2005;32:7–15. doi: 10.1111/j.1600-051X.2005.00790.x. [DOI] [PubMed] [Google Scholar]
- 2.Wang Z, Shen Y, Haapasalo M. Dental materials with antibiofilm properties. Dent Mater. 2014;30:e1–e16. doi: 10.1016/j.dental.2013.12.001. [DOI] [PubMed] [Google Scholar]
- 3.Baur V, Ilie N. Repair of dental resin-based composites. Clin Oral Invest. 2013;17:601–8. doi: 10.1007/s00784-012-0722-4. [DOI] [PubMed] [Google Scholar]
- 4.Peris AR, Mitsui FH, Lobo MM, et al. Adhesive systems and secondary caries formation: Assessment of dentin bond strength, caries lesions depth and fluoride release. Dent Mater. 2007;23:308–16. doi: 10.1016/j.dental.2006.02.001. [DOI] [PubMed] [Google Scholar]
- 5.Auschill TM, Arweiler NB, Brecx M, et al. The effect of dental restorative materials on dental biofilm. Europ J Oral Sci. 2002;110:48–53. doi: 10.1046/j.0909-8836.2001.101160.x. [DOI] [PubMed] [Google Scholar]
- 6.Balakrishnan M, Simmonds RS, Tagg JR. Dental caries is a preventable infectious disease. Austra Dent J. 2000;45:235–45. doi: 10.1111/j.1834-7819.2000.tb00257.x. [DOI] [PubMed] [Google Scholar]
- 7.Hajishengallis G. Periodontitis: From microbial immune subversion to systemic inflammation. Nat Rev Immunol. 2015;15:30–44. doi: 10.1038/nri3785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zhou C, Zhang J, Wu B, et al. Investigation on interaction between Streptococcus sanguis and Porphyromonas gingivalis in specific pathogen free rats. Chin J Dent Res. 1999;3:310–13. [PubMed] [Google Scholar]
- 9.Stutz A, Horvath GL, Monks BG, et al. ASC speck formation as a readout for inflammasome activation. Metho Molec Biol. 2013;1040:91–101. doi: 10.1007/978-1-62703-523-1_8. [DOI] [PubMed] [Google Scholar]
- 10.Eskan MA. Interleukin-1β modulates proinflammatory cytokine production in human epithelial cells. Infect Immun. 2008;76:2080–89. doi: 10.1128/IAI.01428-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin X, Chen X, Chen Y, et al. The effect of five probiotic lactobacilli strains on the growth and biofilm formation of Streptococcus mutans. Oral Dis. 2015;21:e128–34. doi: 10.1111/odi.12257. [DOI] [PubMed] [Google Scholar]
- 12.Li L, Finnegan M, Özkan S, et al. In vitro study of biofilm formation and effectiveness of antimicrobial treatment on various dental material surfaces. Molec Oral Microbiol. 2010;25:384–90. doi: 10.1111/j.2041-1014.2010.00586.x. [DOI] [PubMed] [Google Scholar]
- 13.Stoodley P, Wilson S, Hall-Stoodley L, et al. Growth and detachment of cell clusters from mature mixed-species biofilms. Appl Environ Microbiol. 2001;67:5608–13. doi: 10.1128/AEM.67.12.5608-5613.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pillai SK, Sakoulas G, Eliopoulos GM, et al. Effects of glucose on fsr-mediated biofilm formation in Enterococcus faecalis. J Infect Dis. 2004;190:967–70. doi: 10.1086/423139. [DOI] [PubMed] [Google Scholar]
- 15.Weber K, Delben J, Bromage TG, Duarte S. Comparison of SEM and VPSEM imaging techniques with respect to Streptococcus mutans biofilm topography. FEMS Microbiol Lett. 2014;350:175–79. doi: 10.1111/1574-6968.12334. [DOI] [PubMed] [Google Scholar]
- 16.Garcez AS, Núnez SC, Azambuja N, Jr, et al. Effects of photodynamic therapy on Gram-positive and Gram-negative bacterial biofilms by bioluminescence imaging and scanning electron microscopic analysis. Photomed Laser Surg. 2013;31:519–25. doi: 10.1089/pho.2012.3341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Scannapieco FA. The oral microbiome: Its role in health and in oral and systemic infections. Clin Microbiol Newsl. 2013;35:163–69. [Google Scholar]
- 18.Wilson M. Photolysis of oral bacteria and its potential use in the treatment of caries and periodontal-disease. J Appl Bacteriol. 1993;75:299–306. doi: 10.1111/j.1365-2672.1993.tb02780.x. [DOI] [PubMed] [Google Scholar]
- 19.Stinson MW, Safulko K, Levine MJ. Adherence of porphyromonas (Bacteroides) gingivalis to Streptococcus sanguis in vitro. Infect Immun. 1991;59:102–8. doi: 10.1128/iai.59.1.102-108.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Schlegel R, Slade HD. Alteration of macromolecular synthesis and membrane permeability by a Streptococcus sanguis bacteriocin. J Gen Microbiol. 1974;81:275–77. doi: 10.1099/00221287-81-1-275. [DOI] [PubMed] [Google Scholar]
- 21.Herrero ER, Slomka V, Bernaerts K, et al. Antimicrobial effects of commensal oral species are regulated by environmental factors. J Dent. 2016;47:23–33. doi: 10.1016/j.jdent.2016.02.007. [DOI] [PubMed] [Google Scholar]
- 22.Žekonis G, Žekonis J, Gleiznys A, et al. Effect of supragingival irrigation with aerosolized 0.5% hydrogen peroxide on clinical periodontal parameters, markers of systemic inflammation, and morphology of gingival tissues in patients with periodontitis. Med Sci Monit. 2016;22:3713–21. doi: 10.12659/MSM.900338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Carey CM, Spencer M, Gove RJ, Eichmiller FC. Fluoride release from a resin-modified glass-ionomer cement in a continuous-flow system: Effect of pH. J Dent Res. 2003;82:829–32. doi: 10.1177/154405910308201013. [DOI] [PubMed] [Google Scholar]
- 24.Masoum A, Meratnia N, Dilo A, et al. Fluoride exposure effects and dental fluorosis in children in Mexico City. Med Sci Monit. 2015;21:3664–70. doi: 10.12659/MSM.895351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bürgers R, Schneider-Brachert W, Rosentritt M, et al. Candida albicans adhesion to composite resin materials. Clin Oral Invest. 2009;13:293–99. doi: 10.1007/s00784-008-0226-4. [DOI] [PubMed] [Google Scholar]
- 26.Obeid MF, Nagy MM. Retreatability of different endodontic sealers using chemical solvents. Tanta Dent J. 2015;12:286–91. [Google Scholar]
- 27.Kamaguchi A, Baba H, Hoshi M, Inomata K. Coaggregation between Porphyromonas gingivalis and Mutans streptococci. Microbiol Immun. 1994;38:457–60. doi: 10.1111/j.1348-0421.1994.tb01807.x. [DOI] [PubMed] [Google Scholar]
- 28.Sadeq A, Risk JM, Pender N, et al. Evaluation of the co-existence of the red fluorescent plaque bacteria P. gingivalis with S. gordonii and S. mutans in white spot lesion formation during orthodontic treatment. Photodiag Photodyn Ther. 2015;12:232–37. doi: 10.1016/j.pdpdt.2015.03.001. [DOI] [PubMed] [Google Scholar]
- 29.Yang J, Zhang Q, Chen M, et al. Association between Helicobacter pylori infection and risk of periodontal diseases in Han Chinese: A case-control study. Med Sci Monit. 2015;22:121–26. doi: 10.12659/MSM.894583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hall BE, Zhang L, Sun ZJ, et al. Conditional TNF-α overexpression in the tooth and alveolar bone results in painful pulpitis and osteitis. J Dent Res. 2015;95:188–95. doi: 10.1177/0022034515612022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Matarese G, Currò M, Isola G, et al. Transglutaminase 2 up-regulation is associated with RANKL/OPG pathway in cultured HPDL cells and THP-1-differentiated macrophages. Amino Acids. 2015;47:2447–55. doi: 10.1007/s00726-015-2039-5. [DOI] [PubMed] [Google Scholar]
- 32.Currò M, Matarese G, Isola G, et al. Differential expression of transglutaminase genes in patients with chronic periodontitis. Oral Dis. 2014;20:616–23. doi: 10.1111/odi.12180. [DOI] [PubMed] [Google Scholar]
- 33.Kou W, Molin M, Sjögren G. Surface roughness of five different dental ceramic core materials after grinding and polishing. J Oral Rehab. 2006;33:117–24. doi: 10.1111/j.1365-2842.2006.01546.x. [DOI] [PubMed] [Google Scholar]