Abstract
Background and aims
Impulsivity is a core feature of gambling disorder (GD) and is related to the treatment response. Thus, it is of interest to determine objective neurobiological markers associated with impulsivity in GD. We explored resting-state electroencephalographic (EEG) activity in patients with GD according to the degree of impulsivity.
Methods
In total, 109 GD subjects were divided into three groups according to Barratt impulsiveness scale-11 (BIS-11) scores: high (HI; 25th percentile of BIS-11 scores, n = 29), middle (MI; 26th–74th percentile, n = 57), and low-impulsivity (LI) groups (75th percentile, n = 23). We used generalized estimating equations to analyze differences in EEG absolute power considering group (HI, MI, and LI), brain region (frontal, central, and posterior), and hemisphere (left, midline, and right) for each frequency band (delta, theta, alpha, beta, and gamma).
Results
The results indicated that GD patients in the HI group showed decreased theta absolute power, and decreased alpha and beta absolute power in the left, right, particularly midline frontocentral regions.
Discussion and conclusions
This study is a novel attempt to reveal impulsive features in GD by neurophysiological methods. The results suggest different EEG patterns among GD patients according to the degree of impulsivity, raising the possibility of neurophysiological objective features in GD and helping clinicians in treating GD patients with impulsive features.
Keywords: gambling disorder, impulsivity, resting-state electroencephalography
Introduction
Gambling disorder (GD) is a psychiatric condition that includes persistent and recurrent maladaptive patterns of gambling behavior (Hodgins, Stea, & Grant, 2011). In the fifth edition of the Diagnostic and Statistical Manual (DSM-5; American Psychiatric Association, 2013), GD was classified as a “Substance-related and Addictive Disorder,” a change from the diagnosis of pathological gambling (PG) in DSM-IV. This change was due to similarities between substance addictions and problematic gambling behavior (Grant, Potenza, Weinstein, & Gorelick, 2010).
GD is related to various comorbid psychiatric conditions and underlying maladaptive personality traits, such as problematic substance use, mood disorders, and anxiety disorders (Milosevic & Ledgerwood, 2010). Among the characteristics of GD that are similar to substance addiction, one of the most dominants is impulsivity (Leeman & Potenza, 2012). Impulsivity, a core feature of human personality, can be described as spontaneous or unintentional behavior where one acts without thought or self-control (Raylu & Oei, 2002). Impulsivity can also be defined by the following elements: “decreased sensitivity to negative consequences of behavior,” “rapid, unplanned reactions to stimuli before complete processing of information,” and a “lack of regard for long-term consequences” (Moeller, Barratt, Dougherty, Schmitz, & Swann, 2001). A relationship between impulsivity and GD (or PG) has been revealed in previous studies (Chiu & Storm, 2010; Marmurek, Switzer, & D’Alvise, 2015; Verdejo-García, Lawrence, & Clark, 2008).
Comparisons of GD with other addictive disorders have shown that impulsivity is a core feature shared in those disorders. MacLaren, Fugelsang, Harrigan, and Dixon (2011) argued that the personality profiles of pathological gamblers, such as unconscientious and disagreeable disinhibition, were similar to that of patients with substance-use disorder (SUD). In addition, a previous study that compared impulsivity features among GD, alcohol-use disorder (AUD), and Internet gaming disorder (IGD) showed that the GD group had levels of trait impulsivity in cognitive domains similar to those of the AUD and IGD groups (Choi et al., 2014).
Moreover, impulsivity is a significant predictor of relapse and dropout among patients exhibiting GD. Ramos-Grille, Gomà-i-Freixanet, Aragay, Valero, and Vallès (2015) confirmed that impulsivity may be a prominent trait in predicting the risk of relapse or dropout in PG. High impulsivity (HI) may also make it difficult for the individual to benefit from treatment because the excitement of gambling is an immediate reward, whereas the benefits of treatment are more long term (Ledgerwood & Petry, 2006). Consequently, impulsive PG patients were less likely to complete treatment and to benefit from psychotherapy (Leblond, Ladouceur, & Blaszczynski, 2003). Thus, it is clear that studying impulsive traits in GD and exploring objective neurobiological markers related to impulsivity may be important in providing beneficial treatment and preventing relapse among GD patients.
Electroencephalography (EEG) is an electrophysiological recording method that shows electrical activity of the brain and provides measure of baseline or underlying brain states before information processing. This method has several advantages: high temporal resolution, non-invasive method, and significantly lower costs than those of most other techniques (Wang et al., 2013). Spontaneous brain activity in a resting state has been used to identify the brain activity correlates of cognition and behavior (Barry et al., 2010). A network of brain regions known as the “default mode network” shows increased activity even during the resting state, reflecting spontaneous cognitive processes (Andrews-Hanna, Reidler, Huang, & Buckner, 2010). In addition, resting-state brain activity has also been associated with event-related cognitive processes involved in attention, memory, and thinking (Kounios et al., 2008). This method shows high test–retest reliability over time, and is thought to reflect stable, trait-like indices of brain function (Massar, Kenemans, & Schutter, 2014). Thus, the examination of resting-state EEG data may enhance our understanding of basic brain function.
Several resting-state EEG studies related to impulsivity have also been reported. In the studies with healthy controls, Stenberg (1992) revealed that impulsive individuals showed increased activities in the theta and alpha bands. Lansbergen, Schutter, and Kenemans (2007) stated the associations between subjective impulsivity measured by self-report measures, theta/beta EEG ratio, and inhibitory control measured by cognitive tasks. They suggested that individual with increased theta/beta ratio tends to be more motivated to maximize inhibition-related performance. In terms of studies with attention-deficit hyperactivity disorder (ADHD) patients, one of the disorders related to impulse control, adult with ADHD showed elevated absolute and relative theta power (Bresnahan & Barry, 2002). In addition, van Dongen-Boomsma et al. (2010) suggested that deviant patterns of increased slow power and decreased fast power in adult ADHD patients may be a biomarker of impulse control disorders.
To our knowledge, most of the reported neuroimaging studies related to GD and impulsivity have employed task-based methods, such as Go/NoGo and stop-signal tasks (Potenza et al., 2003; Yip et al., 2013). Furthermore, no resting-state EEG study investigating the neurophysiological features of individuals with GD has been reported. Thus, in this study, we sought to explore the features of resting-state EEG activity in GD patients according to the degree of impulsivity and to identify any neurophysiological markers associated with impulsivity in GD patients. Based on previous resting-state EEG studies, we hypothesized that GD patients who had a higher degree of impulsivity would show increased slow power including delta and theta bands, and decreased fast power including alpha, beta, and gamma bands compared with a lower degree of impulsivity.
Methods
Participants and procedures
In total, 130 male subjects and one female diagnosed with GD participated. Participants were recruited from the outpatient clinics of Gangnam Eulji Hospital. Patients aged 20–50 years were diagnosed with GD, according to DSM-5, by an experienced clinical psychiatrist. They were excluded if they had a history of significant head injury, seizure disorder, intellectual disability, psychotic disorder, or SUD (other than one involving nicotine). In addition, all participants were medication naive during the baseline assessment.
Participants also completed demographic and clinical questionnaires. The Canadian Problem Gambling Index (CPGI; Ferris & Wynne, 2001) was used to assess the severity of GD symptoms. The degree of impulsivity was measured using the Barratt Impulsiveness scale-11 (BIS-11; Patton & Stanford, 1995). BIS-11 includes 23 questions, each scored from 1 to 4, and comprises three factors: cognitive, motor, and non-planning impulsivity. The Beck Depression Inventory (BDI; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) was used to measure depressive symptoms.
To identify any relationship between impulsivity and GD, we separated patients into three groups by degree of impulsivity based on BIS-11 score. In total, 109 participants who completed BIS-11 were included for analysis. To avoid gender differences, we excluded one female participant from further analysis. Participants in the HI group were those with BIS-11 had scores above the 25th percentile (n = 29), the low-impulsivity (LI) group consisted of those with BIS-11 had scores below the 75th percentile (n = 23), and the middle-impulsivity (MI) group had scores between the 26th and 74th percentiles (n = 57).
EEG recording
The participants were seated comfortably in a sound-shielded, dimly lit room for resting-state EEG recordings, which lasted 8 min: 4 min with eyes closed, followed by 2 min with eyes open, and 2 min with eyes closed. Continuous EEG recordings were acquired using a 32-channel HydroCel Geodesic Sensor Net (Electrical Geodesics Inc., Eugene, OR, USA) based on the modified 10–20 international system (Applied Neuroscience, St. Petersburg, FL, USA). The mastoid electrodes served as reference; the impedance at all electrode sites was below 50 kΩ. The EEG data were digitized and amplified at a 500 Hz sampling rate with a Geodesic EEG system 400 (Electrical Geodesics, Inc.) and an online bandpass filter (0.1–100 Hz). Eye-movement artifacts were recorded by vertical and horizontal electrooculogram using electrodes below and on the outer canthus of the left eye. Artifacts were removed using visual inspection and an automatic artifact removal tool in the NeuroGuide software. Eyes-closed conditions were selected for spectral analyses.
Acquired EEG data were processed using the NeuroGuide Deluxe software (version 2.6.1; Applied Neuroscience, St. Petersburg, FL, USA) for spectral analysis in a 32-bit file format, and 19 electrode sites were driven by the following NeuroGuide montage set: FP1, F3, F7, Fz, FP2, F4, F8, T3, C3, Cz, T4, C4, T5, P3, O1, Pz, T6, P4, and O2. Absolute power (μV2) was calculated by fast Fourier transform and averaged in four frequency bands using NeuroGuide’s spectral analysis system: delta (1.0–4.0 Hz), theta (4.0–8.0 Hz), alpha (8.0–12.0 Hz), beta (12.0–25.0 Hz), and gamma (30.0–40.0 Hz).
Statistical analysis
Comparisons of demographic and clinical data among groups were conducted by analysis of variance. For EEG data, activity at the 19 electrodes was divided into nine sites, considering region and hemisphere, and averaged as follows (Barry et al., 2010): left frontal (Fp1, F3, and F7), midline frontal (Fz), right frontal (Fp2, F4, and F8), left central (T3 and C3), midline central (Cz), right central (T4 and C4), left posterior (T5, P3, and O1), midline posterior (Pz), and right posterior (T6, P4, and O2). Nine sites were included in each EEG analysis to reflect region (frontal, central, and posterior) and hemisphere factors (left, midline, and right). Next, three groups, three region factors, three hemisphere factors, and their interactions in each frequency band were analyzed using generalized estimating equations (GEEs; Liang & Zeger, 1986; Zeger & Liang, 1986). GEEs have been used in previous resting-state EEG analyses (e.g., Choi et al., 2013; Claassen et al., 2004; Son et al., 2015). Statistical analyses were performed using IBM SPSS software (version 22; IBM, Inc., NY, USA). p values <.05 were considered to indicate statistical significance. To determine the relationships between EEG absolute power in each band and impulsivity, we conducted Pearson’s correlation analysis between averaged absolute power by regions and BIS-11 score in each band. We used Bonferroni-corrected post hoc comparisons for three groups to determine specific group differences (p < .0167).
Ethics
The institutional review board of Gangnam Eulji Hospital approved the study protocol, according to the Declaration of Helsinki. All subjects understood the study procedure and provided written informed consent before participation.
Results
Demographic and clinical data
No significant difference was found among groups with regard to age or education (Table 1). The BIS-11 scores were significantly different among groups (F2, 106 = 234.53, p < .001), with mean scores of 39.35 ± 2.08 in the LI group, 46.95 ± 3.15 in the MI group, and 62.24 ± 6.07 in the HI group. Differences in BDI scores were not statistically significant among the three groups (F2, 105 = 2.47, p = .089). CPGI scores did not differ significantly among groups (F2, 101 = 1.00, p = .370), although the correlation between CPGI and BIS-11 scores was significant in our sample (r = .205, p = .037).
Table 1.
Demographic and clinical features
| LI group | MI group | HI group | F | p | Post hoc | ||||
|---|---|---|---|---|---|---|---|---|---|
| n = 23 | n = 57 | n = 29 | |||||||
| Mean | SD | Mean | SD | Mean | SD | ||||
| Demographic data | |||||||||
| Age (years) | 38.52 | 10.98 | 36.98 | 11.30 | 32.14 | 8.53 | 2.84 | .063 | |
| Education (years) | 14.87 | 1.98 | 14.87 | 2.02 | 15.38 | 1.42 | 0.78 | .460 | |
| Clinical data | |||||||||
| BIS-11 | 39.35 | 2.08 | 46.95 | 3.15 | 62.24 | 6.07 | 234.53*** | <.001 | LI < MI < HI |
| CPGI | 16.39 | 7.17 | 18.13 | 6.32 | 18.96 | 5.95 | 1.00 | .370 | |
| BDI | 15.00 | 7.42 | 16.70 | 10.34 | 20.50 | 8.41 | 2.47 | .089 | |
Note. The Bonferroni-corrected post hoc comparison was used (p < .0167). LI: low-impulsivity group; MI: middle-impulsivity group; HI: high-impulsivity group; Mean: estimate mean; SD: standard deviation; BIS-11: Barratt Impulsiveness Scale-11; CPGI: Canadian Problem Gambling Index; BDI: Beck Depression Inventory.
p < .001.
EEG activity
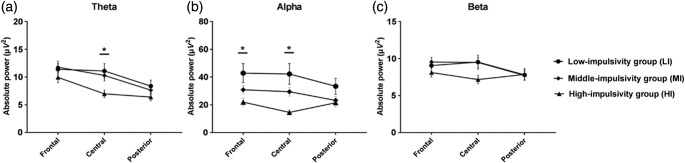
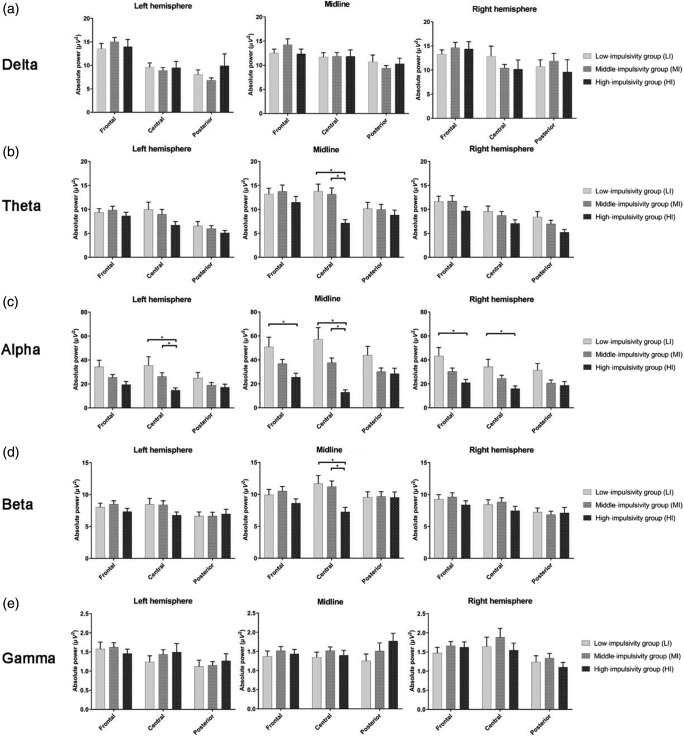
The model effects of absolute power and the group comparisons for each band are presented in Table 2, Figures 1 and 2. In the delta band, there was Group × Region × Hemisphere effect (χ2 = 19.04, p = .015), but group difference was not found in the post hoc test. In the theta band, significant Group × Region (χ2 = 24.67, p < .001) and Group × Hemisphere (χ2 = 13.48, p = .009) effects were found. The HI group showed significantly decreased theta power in the central region versus LI (p = .016), but there was no significant hemispheric difference in the post hoc test with the Bonferroni correction. There was also a Group × Region × Hemisphere effect (χ2 = 40.74, p < .001); the HI group showed decreased theta power in the midline central region compared with the LI (p < .001) and MI groups (p < .001).
Table 2.
Model effects for absolute power
| Absolute power (μV2) | χ2 | df | p | Post hoc |
|---|---|---|---|---|
| Delta | ||||
| Group | 0.41 | 2 | .814 | |
| Group × Region | 6.80 | 4 | .147 | |
| Group × Hemisphere | 9.01 | 4 | .061 | |
| Group × Region × Hemisphere | 19.04* | 8 | .015 | N.S. |
| Theta | ||||
| Group | 5.27 | 2 | .072 | |
| Group × Region | 24.67*** | 4 | <.001 | Central: HI < LI |
| Group × Hemisphere | 13.48** | 4 | .009 | N.S. |
| Group × Region × Hemisphere | 40.74*** | 8 | <.001 | Midline central: HI < LI, MI |
| Alpha | ||||
| Group | 10.64** | 2 | .005 | HI < LI |
| Group × Region | 41.68*** | 4 | <.001 | Frontal: HI < LI |
| Central: HI < LI, MI | ||||
| Group × Hemisphere | 24.97*** | 4 | <.001 | Midline: HI < LI, MI |
| Group × Region × Hemisphere | 55.49*** | 8 | <.001 | Left central: HI < MI |
| Midline frontal: HI < LI | ||||
| Midline central: HI < LI, MI | ||||
| Right frontal: HI < LI | ||||
| Beta | ||||
| Group | 2.28 | 2 | .320 | |
| Group × Region | 19.74** | 4 | .001 | N.S. |
| Group × Hemisphere | 16.28** | 4 | .003 | N.S. |
| Group × Region × Hemisphere | 18.95* | 8 | .015 | Midline central: HI < LI, MI |
| Gamma | ||||
| Group | 0.72 | 2 | .698 | |
| Group × Region | 2.15 | 4 | .709 | |
| Group × Hemisphere | 7.10 | 4 | .131 | |
| Group × Region × Hemisphere | 23.68** | 8 | .003 | N.S. |
Note. The Bonferroni-corrected post hoc comparison was used (p < .0167). LI: low-impulsivity group; MI: middle-impulsivity group; HI: high-impulsivity group; N.S.: not significant.
*p < .05. **p < .01. ***p < .001.
Figure 1.
Statistically significant Group (LI, MI, and HI) × Region (frontal, central, and posterior) interaction effects of (a) theta, (b) alpha, and (c) beta bands. HI group showed decreased theta power in the central regions, lower alpha in the frontocentral region. Beta band was not different among groups. The horizontal bars represent standard errors. *Significant difference in the post hoc test with the Bonferroni correction (p < .0167)
Figure 2.
A Group (LI, MI, and HI) × Region (frontal, central, and posterior) × Hemisphere (left, midline, and right) interaction effects of (a) delta, (b) theta, (c) alpha, (d) beta, and (e) gamma bands. HI group showed decreased theta power in the midline central regions, lower alpha in the left central and midline/right frontocentral regions, and lower beta power in the midline central regions compared with LI or MI groups. The horizontal bars represent standard errors. *Significant difference in the post hoc test with the Bonferroni correction (p < .0167)
Regarding the alpha band, GEEs analysis revealed group (χ2 = 10.64, p = .005), Group × Region (χ2 = 41.68, p < .001) and Group × Hemisphere effects (χ2 = 24.97, p < .001). The alpha power in the frontal region among the HI group was lower than that in the LI group (p = .015), and it was lower in the central region compared with the LI and MI groups (LI: p = .001; MI: p < .001). The HI group showed significantly decreased alpha power in the midline area versus LI (p = .004) and MI (p = .016). There was also a Group × Region × Hemisphere effect (χ2 = 55.49, p < .001). In a post hoc test, lower alpha power of the HI group was seen at the left central regions versus the MI groups (p = .006), midline frontal versus the LI group (p = .012), midline central regions versus the LI and MI group (LI: p < .001; MI: p < .001), and the right frontal versus the LI group (p = .009).
We found Group × Region (χ2 = 19.74, p = .001), Group × Hemisphere (χ2 = 16.28, p = .003), and Group × Region × Hemisphere effects (χ2 = 18.95, p = .019) in the beta power. The HI group showed decreased beta power at the midline central compared with the LI (p = .009) and MI groups (p = .001). With respect to the gamma band, there was no statistically significant Group × Region or Group ×Hemisphere effect. A Group × Region × Hemisphere interaction effect was found (χ2 = 23.68, p = .003), but there was no group difference in the post hoc test.
Correlation analysis
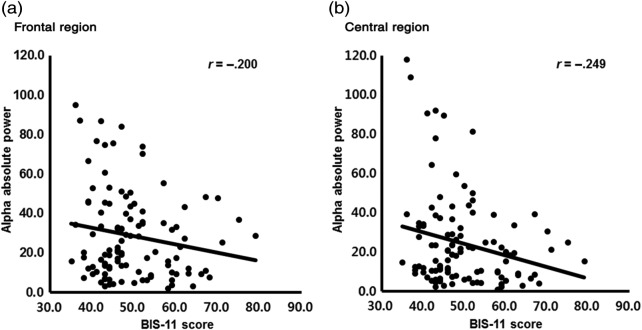
Based on the significant group difference revealed by the GEE analyses, the correlations between the averaged theta, alpha, beta, and gamma absolute power in the frontal or central regions and the degree of impulsivity assessed by the BIS-11 were conducted. In the total group, there were significant negative correlations between the alpha power in the frontal or central regions and the BIS-11 score (frontal: r = −.200, p = .037; central: r = −.249, p = .009; Figure 3). No significant correlation was found in the delta, theta, beta, and gamma band activities.
Figure 3.
Statistically significant correlation between averaged alpha band activity and Barratt Impulsiveness Scale-11 (BIS-11) in total group; Significant p value set at .05; (a) correlation between averaged alpha power activity and BIS-11 in the frontal area (r = −.200, p = .037), (b) correlation between averaged alpha power activity and BIS-11 in the central area (r = −.249, p = .009)
Discussion and Conclusions
We investigated the eyes-closed resting-state EEG patterns of patients with GD according to degree of impulsivity. The findings showed that GD patients who had higher impulsivity scores showed lower theta, alpha, and beta power in the left, right, particularly midline frontocentral regions compared with GD patients with a lower degree of impulsivity. GD patients with higher impulsivity had decreased fast power (alpha and beta bands), as we hypothesized, whereas the decreased absolute power in the slow wave (theta band) was not consistent with our hypothesis. The results of this study are necessary to be considered with neurobiological evidence of GD, which is related to impulsivity (Raylu & Oei, 2002).
In terms of alpha power, GD patients in the HI group showed relatively low alpha absolute power in the left central, midline frontal, midline central, and right frontal sites as we hypothesized. Alpha power is implicated with inhibitory functions and is involved in cognitive processes associated with attention and memory (Knyazev, 2007). GD patients with HI showed decreased alpha power at the frontocentral region, which may be related to their dysfunction of the prefrontal frontal cortex (PFC). The PFC is important for many cognitive processes, such as attention, working memory, decision-making, and delay discounting. It is repetitively reported that patients with addictive disorders showed dysfunction of the PFC (Goldstein & Volkow, 2011). In the functional magnetic resonance imaging (fMRI) study with GD patients, it is reported that reduction of the ventral striatal and ventromedial PFC activation in the problematic gamblers, which is associated with impulse control (Reuter et al., 2005). In the EEG study, patients with the orbitomedial prefrontal lesions showed inordinate impulsiveness, irritability, hyperactivity that are usually related to decreased alpha power (Knyazev, 2007). In addition, alpha power has positive correlation in the cingulate gyrus and occipital cortex and widespread negative correlation in the lateral frontal and parietal cortices in the study of a simultaneous fMRI and EEG registration at rest. The authors concluded that these results may suggest alpha power indicates a neural baseline with inattention (Laufs et al., 2003). In this study, alpha power in the frontal and central regions significantly correlated with the degree of impulsivity indicating alpha absolute power was especially associated with impulsivity (Figure 3). In summary, decreased alpha power in the frontocentral region in impulsive GD patients may reflect their cognitive dysfunction, such as inattention and impulse control. Another possible explanation is that it may infer different alpha oscillation patterns raising the possibility of neurobiological markers for impulsivity in GD patients. However, this concept should be interpreted with future studies comparing healthy controls and GD patients. In addition, EEG source localization study should be necessary to compare more particular brain regions.
It is necessary to be inferred with the genetic variants to understand decreased alpha power of impulsive GD patients. Previous study suggested that genetic variants at dopamine receptor D2 gene play a role in PG and supported the concept that variants of this gene are risk factors for impulsive and addictive behaviors (Comings et al., 1996). A review study found that dopamine polymorphisms associated with the risk of SUD were also present at elevated levels in PG, and twin data indicated that 12%–20% of the genetic vulnerability to PG was shared with alcohol dependency (Verdejo-García et al., 2008). Therefore, it seems that GD patients are not only influenced by genetic variants but also share genetic commonalities with SUD. Meanwhile, previous studies revealed that reduced alpha power may be associated with genetic variants. Finn and Justus (1999) found that subjects with a family history of alcoholism had reduced relative and absolute alpha power in the occipital and frontal regions compared with the offspring of non-alcoholics. A recent study also indicated that genetic variation in 5-hydroxytryptamine receptors 3B may influence vulnerability to alcoholism with comorbid antisocial personality disorder, and may contribute to the low alpha power (Ducci et al., 2009). Thus, reduced alpha power in GD patients with HI may seem to be influenced by their genetic variants. However, further studies about genetic variants of GD patients and their EEG activities would be required.
Moreover, alpha power is the predominant EEG rhythm in the relaxed, alert person; indeed, it is an index of relaxation (Porjesz & Begleiter, 2003). Ledgerwood and Petry (2010) revealed that antisocial-impulsive gamblers tend to not only have significant emotional vulnerability but also experience elevated psychopathology. They stated that their emotional vulnerabilities may involve more emotional dysregulation, as seen in disorders such as borderline personality disorder and antisocial personality disorder. Thus, decreased alpha power in GD patients with HI is more likely to reflect these unrelaxed psychological state and emotional dysregulations, as affected by problematic gambling.
GD patients with a higher degree of impulsivity had decreased beta absolute power in the midline central sites compared with those with a lower degree of impulsivity, as expected. The beta activity was superior to severity of illness, depression level, and childhood conduct problems, and anterior frontal region was identified as the most likely source of fast beta activity (Bauer, 2001). Moreover, it is reported that beta bands have also been associated with response inhibition (Porjesz & Begleiter, 2003). Previous study with Internet-addiction patients revealed that they showed decreased beta power in the frontal areas, which was related to inhibitory control (Choi et al., 2013). Based on the previous studies, the result of decreased beta power in GD patients with HI may be related to their impaired inhibitory control features.
Lower beta power is consistent with neurological evidence in identifying ADHD patients. Specifically, decreased beta power has been related to inattention and impulsivity, as observed in patients with ADHD (Snyder & Hall, 2006). Regarding the relationship of EEG activity with GD and ADHD, decreased beta power in GD suggests neurophysiological similarities with ADHD. Furthermore, this result may support previous findings that GD may have a candidate neurophysiological biomarker of behavioral addiction. Son et al. (2015) examined resting-state EEG in patients with AUD and IGD, and showed that IGD, a behavioral addiction, was distinguishable from AUD in the resting-state EEG activity of those with IGD was lower in terms of absolute beta power. This difference between AUD and IGD in resting-state EEG activity may be a neurobiological marker for IGD, with lower absolute beta power as a trait marker. Even though this study does not have a comparison with healthy controls, a relative decrease in the beta power of GD patients may indicate an objective neurobiological feature for GD in terms of impulsivity.
Decreased theta absolute oscillation in the midline central region was observed in the impulsive GD patients during the resting state, which was inconsistent with our hypothesis. Theta power is highest in the posterior region when a person is resting and in the front region when the person is actively engaged in mental activity; a normal adult shows relatively little theta power (Porjesz & Begleiter, 2003). In contrast, Rangaswamy et al. (2003) revealed that alcohol-dependent participants had higher theta power in all brain regions tested, and this increase in theta power was a strong feature of the resting-state EEG in chronic alcoholics. In a previous study with ADHD patients, adult ADHD patients showed more absolute and relative theta power than did control subjects (Bresnahan & Barry, 2002). The present result is not in accordance with the previous studies of impulse control disorders or addictive disorders; it seems that the present results are based on the comparisons among the patients with GD. Thus, future studies are needed to confirm this finding with health controls to determine the association between theta absolute power and impulsivity.
This study has several limitations. First, the sample had only male GD patients, with no healthy controls, so any generalization of results may be limited. We suggest that a comparison of resting-state EEG in patients with GD and impulsivity-related disorders and in healthy controls is important to clarify any association with impulsivity. Second, impulsivity was assessed with self-administered questionnaires. Future studies with objective methods to assess the degree of impulsivity including neurocognitive function tests are needed. Third, the comorbidities of GD patients in the sample were restricted. Although it is known that GD is associated with comorbidities, including depression, anxiety disorders, obsessive–compulsive disorder, and ADHD, this study measured only depressive symptoms and impulsivity traits. However, our participants were recruited from an outpatient psychiatric clinic, so we may have failed to distinguish GD symptoms and comorbidities.
Despite these limitations, this study with a large sample of GD patients is the first reported attempt to assess associations in impulsive GD patients with resting-state EEG using a neurophysiological approach. In conclusion, our results showed that GD patients who had stronger impulsive features showed decreased theta, alpha, and beta power in midline/right frontocentral regions compared with GD patients with a lower degree of impulsivity. Impulsivity may be a crucial trait in predicting the risk of relapse or treatment response in GD. These neurophysiological findings with respect to the degree of impulsivity suggest the possibility of an objective feature associated with GD. In future, this EEG finding that has several advantages could help clinicians in treating GD patients with impulsive features.
Acknowledgements
The authors would like to thank all of the participants in this study.
Funding Statement
Funding sources: This study was funded by the Korea Healthcare Technology R&D Project, Ministry for Health and Welfare, Republic of Korea (HI12C-0113), and the National Research Foundation of Korea (2014M3C7A1062894).
Authors’ contribution
S-WC and J-SC contributed to the design of the study. JYL conducted analysis of data and writing of the paper. SMP, YJK, DJK, and JSK provided the reviews of the previous researches and supervised the data collection. All authors contributed to and approved the final manuscript.
Conflict of interest
The authors declare no conflict of interest.
References
- American Psychiatric Association. (2013). Diagnostic and statistical manual of mental disorders (DSM-5®). Arlington, VA: American Psychiatric Association. [Google Scholar]
- Andrews-Hanna J. R., Reidler J. S., Huang C., Buckner R. L. (2010). Evidence for the default network’s role in spontaneous cognition. Journal of Neurophysiology, 104(1), 322–335. doi:10.1152/jn.00830.2009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barry R. J., Clarke A. R., Hajos M., McCarthy R., Selikowitz M., Dupuy F. E. (2010). Resting-state EEG gamma activity in children with attention-deficit/hyperactivity disorder. Clinical Neurophysiology, 121(11), 1871–1877. doi:10.1016/j.clinph.2010.04.022 [DOI] [PubMed] [Google Scholar]
- Bauer L. O. (2001). Predicting relapse to alcohol and drug abuse via quantitative electroencephalography. Neuropsychopharmacology, 25(3), 332–340. doi:10.1016/S0893-133X(01)00236-6 [DOI] [PubMed] [Google Scholar]
- Beck A. T., Ward C. H., Mendelson M., Mock J., Erbaugh J. (1961). An inventory for measuring depression. Archives of General Psychiatry, 4(6), 561–571. doi:10.1001/archpsyc.1961.01710120031004 [DOI] [PubMed] [Google Scholar]
- Bresnahan S. M., Barry R. J. (2002). Specificity of quantitative EEG analysis in adults with attention deficit hyperactivity disorder. Psychiatry Research, 112(2), 133–144. doi:10.1016/S0165-1781(02)00190-7 [DOI] [PubMed] [Google Scholar]
- Chiu J., Storm L. (2010). Personality, perceived luck and gambling attitudes as predictors of gambling involvement. Journal of Gambling Studies, 26(2), 205–227. doi:10.1007/s10899-009-9160-x [DOI] [PubMed] [Google Scholar]
- Choi J. S., Park S. M., Lee J., Hwang J. Y., Jung H. Y., Choi S. W., Kim D. J., Oh S., Lee J. Y. (2013). Resting-state beta and gamma activity in Internet addiction. International Journal of Psychophysiology, 89(3), 328–333. doi:10.1016/j.ijpsycho.2013.06.007 [DOI] [PubMed] [Google Scholar]
- Choi S. W., Kim H., Kim G. Y., Jeon Y., Park S., Lee J. Y., Jung H. Y., Sohn B. K., Choi J. S., Kim D. J. (2014). Similarities and differences among Internet gaming disorder, gambling disorder and alcohol use disorder: A focus on impulsivity and compulsivity. Journal of Behavioral Addictions, 3(4), 246–253. doi:10.1556/JBA.3.2014.4.6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Claassen J., Hirsch L. J., Kreiter K. T., Du E. Y., Connolly E. S., Emerson R. G., Mayer S. A. (2004). Quantitative continuous EEG for detecting delayed cerebral ischemia in patients with poor-grade subarachnoid hemorrhage. Clinical Neurophysiology, 115(12), 2699–2710. doi:10.1016/j.clinph.2004.06.017 [DOI] [PubMed] [Google Scholar]
- Comings D. E., Rosenthal R. J., Lesieur H. R., Rugle L. J., Muhleman D., Chiu C., Dietz G., Gade R. (1996). A study of the dopamine D2 receptor gene in pathological gambling. Pharmacogenetics and Genomics, 6(3), 223–234. doi:10.1097/00008571-199606000-00004 [DOI] [PubMed] [Google Scholar]
- Ducci F., Enoch M. A., Yuan Q., Shen P. H., White K. V., Hodgkinson C., Albaugh B., Virkkunen M., Goldman D. (2009). HTR3B is associated with alcoholism with antisocial behavior and alpha EEG power – An intermediate phenotype for alcoholism and co-morbid behaviors. Alcohol, 43(1), 73–84. doi:10.1016/j.alcohol.2008.09.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferris J., Wynne H. (2001). The Canadian Problem Gambling Index. Ottawa, ON: Canadian Centre on Substance Abuse. [Google Scholar]
- Finn P. R., Justus A. (1999). Reduced EEG alpha power in the male and female offspring of alcoholics. Alcoholism: Clinical and Experimental Research, 23(2), 256–262. doi:10.1111/j.1530-0277.1999.tb04108.x [PubMed] [Google Scholar]
- Goldstein R. Z., Volkow N. D. (2011). Dysfunction of the prefrontal cortex in addiction: Neuroimaging findings and clinical implications. Nature Reviews Neuroscience, 12(11), 652–669. doi:10.1038/nrn3119 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grant J. E., Potenza M. N., Weinstein A., Gorelick D. A. (2010). Introduction to behavioral addictions. The American Journal of Drug and Alcohol Abuse, 36(5), 233–241. doi:10.3109/00952990.2010.491884 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hodgins D. C., Stea J. N., Grant J. E. (2011). Gambling disorders. The Lancet, 378(9806), 1874–1884. doi:10.1016/S0140-6736(10)62185-X [DOI] [PubMed] [Google Scholar]
- Knyazev G. G. (2007). Motivation, emotion, and their inhibitory control mirrored in brain oscillations. Neuroscience and Biobehavioral Reviews, 31(3), 377–395. doi:10.1016/j.neubiorev.2006.10.004 [DOI] [PubMed] [Google Scholar]
- Kounios J., Fleck J. I., Green D. L., Payne L., Stevenson J. L., Bowden E. M., Jung-Beeman M. (2008). The origins of insight in resting-state brain activity. Neuropsychologia, 46(1), 281–291. doi:10.1016/j.neuropsychologia.2007.07.013 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lansbergen M. M., Schutter D. J., Kenemans J. L. (2007). Subjective impulsivity and baseline EEG in relation to stopping performance. Brain Research, 1148, 161–169. doi:10.1016/j.brainres.2007.02.034 [DOI] [PubMed] [Google Scholar]
- Laufs H., Krakow K., Sterzer P., Eger E., Beyerle A., Salek-Haddadi A., Kleinschmidt A. (2003). Electroencephalographic signatures of attentional and cognitive default modes in spontaneous brain activity fluctuations at rest. Proceedings of the National Academy of Sciences, 100(19), 11053–11058. doi:10.1073/pnas.1831638100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leblond J., Ladouceur R., Blaszczynski A. (2003). Which pathological gamblers will complete treatment? British Journal of Clinical Psychology, 42(2), 205–209. doi:10.1348/014466503321903607 [DOI] [PubMed] [Google Scholar]
- Ledgerwood D. M., Petry N. M. (2006). What do we know about relapse in pathological gambling? Clinical Psychology Review, 26(2), 216–228. doi:10.1016/j.cpr.2005.11.008 [DOI] [PubMed] [Google Scholar]
- Ledgerwood D. M., Petry N. M. (2010). Subtyping pathological gamblers based on impulsivity, depression, and anxiety. Psychology of Addictive Behaviors, 24(4), 680–688. doi:10.1037/a0019906 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leeman R. F., Potenza M. N. (2012). Similarities and differences between pathological gambling and substance use disorders: A focus on impulsivity and compulsivity. Psychopharmacology, 219(2), 469–490. doi:10.1007/s00213-011-2550-7 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang K. Y., Zeger S. L. (1986). Longitudinal data analysis using generalized linear models. Biometrika, 73(1), 13–22. doi:10.1093/biomet/73.1.13 [Google Scholar]
- MacLaren V. V., Fugelsang J. A., Harrigan K. A., Dixon M. J. (2011). The personality of pathological gamblers: A meta-analysis. Clinical Psychology Review, 31(6), 1057–1067. doi:10.1016/j.cpr.2011.02.002 [DOI] [PubMed] [Google Scholar]
- Marmurek H. H., Switzer J., D’Alvise J. (2015). Impulsivity, gambling cognitions, and the gambler’s fallacy in university students. Journal of Gambling Studies, 31(1), 197–210. doi:10.1007/s10899-013-9421-6 [DOI] [PubMed] [Google Scholar]
- Massar S. A., Kenemans J. L., Schutter D. J. (2014). Resting-state EEG theta activity and risk learning: Sensitivity to reward or punishment? International Journal of Psychophysiology, 91(3), 172–177. doi:10.1016/j.ijpsycho.2013.10.013 [DOI] [PubMed] [Google Scholar]
- Milosevic A., Ledgerwood D. M. (2010). The subtyping of pathological gambling: A comprehensive review. Clinical Psychology Review, 30(8), 988–998. doi:10.1016/j.cpr.2010.06.013 [DOI] [PubMed] [Google Scholar]
- Moeller F. G., Barratt E. S., Dougherty D. M., Schmitz J. M., Swann A. C. (2001). Psychiatric aspects of impulsivity. The American Journal of Psychiatry, 158(11), 1783–1793. doi:10.1176/appi.ajp.158.11.1783 [DOI] [PubMed] [Google Scholar]
- Patton J. H., Stanford M. S. (1995). Factor structure of the Barratt Impulsiveness Scale. Journal of Clinical Psychology, 51(6), 768–774. doi:10.1002/1097-4679(199511)51:6<768::AID-JCLP2270510607>3.0.CO;2-1 [DOI] [PubMed] [Google Scholar]
- Porjesz B., Begleiter H. (2003). Alcoholism and human electrophysiology. Alcohol Research and Health, 27(2), 153–160. [PMC free article] [PubMed] [Google Scholar]
- Potenza M. N., Leung H. C., Blumberg H. P., Peterson B. S., Fulbright R. K., Lacadie C. M., Skudlarski P., Gore J. C. (2003). An fMRI Stroop task study of ventromedial prefrontal cortical function in pathological gamblers. The American Journal of Psychiatry, 160(11), 1990–1994. doi:10.1176/appi.ajp.160.11.1990 [DOI] [PubMed] [Google Scholar]
- Ramos-Grille I., Gomà-i-Freixanet M., Aragay N., Valero S., Vallès V. (2015). Predicting treatment failure in pathological gambling: The role of personality traits. Addictive Behaviors, 43, 54–59. doi:10.1016/j.addbeh.2014.12.010 [DOI] [PubMed] [Google Scholar]
- Rangaswamy M., Porjesz B., Chorlian D. B., Choi K., Jones K. A., Wang K., Rohrbaugh J., O’Connor S., Kuperman S., Reich T., Begleiter H. (2003). Theta power in the EEG of alcoholics. Alcoholism: Clinical and Experimental Research, 27(4), 607–615. doi:10.1111/j.1530-0277.2003.tb04397.x [DOI] [PubMed] [Google Scholar]
- Raylu N., Oei T. P. (2002). Pathological gambling: A comprehensive review. Clinical Psychology Review, 22(7), 1009–1061. doi:10.1016/S0272-7358(02)00101-0 [DOI] [PubMed] [Google Scholar]
- Reuter J., Raedler T., Rose M., Hand I., Gläscher J., Büchel C. (2005). Pathological gambling is linked to reduced activation of the mesolimbic reward system. Nature Neuroscience, 8(2), 147–148. doi:10.1038/nn1378 [DOI] [PubMed] [Google Scholar]
- Snyder S. M., Hall J. R. (2006). A meta-analysis of quantitative EEG power associated with attention-deficit hyperactivity disorder. Journal of Clinical Neurophysiology, 23(5), 441–456. doi:10.1097/01.wnp.0000221363.12503.78 [DOI] [PubMed] [Google Scholar]
- Son K. L., Choi J. S., Lee J., Park S. M., Lim J. A., Lee J. Y., Kim S. N., Oh S., Kim D. J., Kwon J. S. (2015). Neurophysiological features of Internet gaming disorder and alcohol use disorder: A resting-state EEG study. Translational Psychiatry, 5, e628. doi:10.1038/tp.2015.124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stenberg G. (1992). Personality and the EEG: Arousal and emotional arousability. Personality and Individual Differences, 13(10), 1097–1113. doi:10.1016/0191-8869(92)90025-K [Google Scholar]
- van Dongen-Boomsma M., Lansbergen M. M., Bekker E. M., Kooij J. S., van der Molen M., Kenemans J. L., Buitelaar J. K. (2010). Relation between resting EEG to cognitive performance and clinical symptoms in adults with attention-deficit/hyperactivity disorder. Neuroscience Letters, 469(1), 102–106. doi:10.1016/j.neulet.2009.11.053 [DOI] [PubMed] [Google Scholar]
- Verdejo-García A., Lawrence A. J., Clark L. (2008). Impulsivity as a vulnerability marker for substance-use disorders: Review of findings from high-risk research, problem gamblers and genetic association studies. Neuroscience and Biobehavioral Reviews, 32(4), 777–810. doi:10.1016/j.neubiorev.2007.11.003 [DOI] [PubMed] [Google Scholar]
- Wang Y., Zhang X., Huang J., Zhu M., Guan Q., Liu C. (2013). Associations between EEG beta power abnormality and diagnosis in cognitive impairment post cerebral infarcts. Journal of Molecular Neuroscience, 49(3), 632–638. doi:10.1007/s12031-012-9918-y [DOI] [PubMed] [Google Scholar]
- Yip S. W., Lacadie C., Xu J., Worhunsky P. D., Fulbright R. K., Constable R. T., Potenza M. N. (2013). Reduced genual corpus callosal white matter integrity in pathological gambling and its relationship to alcohol abuse or dependence. The World Journal of Biological Psychiatry, 14(2), 129–138. doi:10.3109/15622975.2011.568068 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeger S. L., Liang K. Y. (1986). Longitudinal data analysis for discrete and continuous outcomes. Biometrics, 42(1), 121–130. doi:10.2307/2531248 [PubMed] [Google Scholar]