Abstract
Background
Accurate measurement of blood lipids is crucial in cardiovascular disease risk management. The Centers for Disease Control and Prevention (CDC) Cholesterol Reference Method Laboratory Network (CRMLN) has assured the accuracy of these measurements forover 20 years using beta quantification (BQ) method as reference measurement procedure (RMP) for high- and low-density lipoprotein cholesterol (HDL-C, LDL-C). Only limited data exist about the performance of the BQ RMP.
Methods
Bottom fraction cholesterol (BFC), HDL-C, and LDL-C results after ultracentrifugation from the CDC lipid reference laboratory and the Japanese CRMLN laboratory were compared using 280 serum samples measured over the past 15 years. Data were compared statistically using method comparison and bias estimation analysis.
Results
Regression analysis between CDC (x) and Osaka (y) for BFC, HDL-C, and LDL-C were y = 0.988x + 1.794 (R2 = 0.997), y = 0.980x + 1.118 (R2 = 0.994), and y = 0.987x + 1.200 (R2 = 0.997), respectively. The Osaka laboratory met performance goals for 90% to 95% of the CDC reference values.
Conclusions
The BQ method by the Osaka CRMLN laboratory is highly accurate and has been stable for over 15 years. Accurate measurement of BFC is critical for the determination of LDL-C.
Keywords: Beta quantification, Bottom fraction cholesterol, HDL cholesterol, LDL cholesterol
1. Introduction
Increased concentrations of low-density lipoprotein cholesterol (LDL-C) are associated with an increased risk for the development of cardiovascular diseases (CVDs), especially coronary heart disease (CHD) [1,2]. Other major risk factors include hypertension, diabetes mellitus, smoking, and chronic kidney diseases [3,4]. Interventions to decrease LDL-C levels can improve the risk of CVD and result in reductions in atherosclerotic lesions [5–8]. Because of the strong and positive association between LDL-C and CVD, 2013 ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults [9], the Third Report of the U.S. National Cholesterol Education Program (NCEP) [10,11], the European Atherosclerosis Society [12], and Japan Atherosclerosis Society Guidelines for the Prevention of Atherosclerotic Cardiovascular Diseases 2012 [13] focused primarily on LDL-C for the categorization and treatment of dyslipidemia. Thus, measuring LDL-C has been the cornerstone of cardiovascular risk assessment and prevention for the past decades.
The precise and accurate measurement of LDL-C is of particular importance for correctly and consistently classifying individuals at risk for CVD as outlined in clinical guidelines for subsequent treatment of patients. The precision and accuracy of LDL-C measurements needed to assure that appropriate patient care was established by the NCEP [14]. The beta quantification (BQ) procedure, which relies on ultracentrifugation (UC) to separate apo B lipoprotein (apo B) particles according to the hydrated density at d = 1.006, has been the established reference measurement procedure (RMP) for HDL-C and LDL-C [15,16]. BQ RMP performed at the U.S. Centers for Disease Control and Prevention (CDC) and Cholesterol Reference Method Laboratory Network (CRMLN) is considered the highest order RMP for this analyte. For over 15 years, the National Cerebral and Cardiovascular Center at Osaka, Japan has standardized their LDL-C BQ RMP through participation in the CRMLN. Members of the CRMLN are required to meet stringent performance criteria for precision and accuracy to allow both calibration and calibration verification of routine assays. Few reports are available on the performance of BQ RMP.
Using data obtained between May 1997 and October 2012, the precision and accuracy for HDL-C and LDL-C as measured at the Osaka laboratory were determined. We determined the fixed and/or proportional bias and correlations between the CDC and Osaka laboratories, and assessed factors that may affect results obtained with the BQ method by verifying relationships among bottom fraction cholesterol (BFC) —one major component of the BQ procedure, HDL-C, and LDL-C.
2. Material and methods
2.1. Materials
All materials were prepared according to Clinical Laboratory Standards Institute (CLSI) document C37-A. This implies that no preservatives or no additives were added. In this study, 67 different pool concentrations (lots) were used among the 280 survey samples provided by the CDC as part of the CRMLN monitoring surveys. One lot (bq47) was used 8 times over 2.5 years, which represented the longest period any lot was used. All CDC survey pools were blinded to the CRMLN participants. The pools were shipped frozen and stored at −70 °C before BQ analysis, and they were analyzed between May 1997 and October 2012 in 70 survey runs, with each survey run consisting of 3 to 5 different pools.
Measurements were conducted in the Osaka Medical Center for Cancer and Cardiovascular Diseases between July 1997 and June 2001, in the Osaka Medical Center for Health Science and Promotion between July 2001 and March 2012, and in the National Cerebral and Cardiovascular Center at Osaka continuously since April 2012 (all laboratories are referred to as ‘Osaka laboratory’).
2.2. Ultracentrifugation
BQ employs preparative ultracentrifuge (Beckman Coulter, Optima L-70K) to remove the chylomicrons and very-low-density lipoproteins (VLDL) of apo B-containing lipoproteins [17]. The methods at CDC and Osaka used 5 ml of serum per sample at a density of d = 1.006 kg/L (0.195 mol/L NaCL solution) and a 50.4 Ti rotor (Beckman Coulter) for UC. UC was carried out at CDC for 16.2 hours at 120,000 ×g, and 18 °C, and at Osaka for 18.5 hours, 105,000 ×g, and 18 °C. After UC, chylomicrons and VLDL in the top fraction (d < 1.006 kg/L) were removed and the remaining bottom fraction (d > 1.006 kg/L) including high-density lipoprotein (HDL), low-density lipoprotein (LDL), intermediate-density lipoprotein (IDL), and lipoprotein(a) (Lp(a)) was quantitatively transferred to a 5.00 mL volumetric flask and adjusted for volume with 0.15 mol/L NaCL solution [14,15]. The total cholesterol in this bottom fraction (BFC) was determined from one aliquot.
2.3. HDL-C precipitation
One mL aliquots of the apo B-containing lipoproteins in the bottom fraction were precipitated with 40 μL heparin (sodium injection, 5000 USP units/mL, Baxter Healthcare Corp.) and 50 μL manganese reagents (manganese(II) chloride solution, 1.00 mol/L ± 0.01 mol/L, SIGMA). The precipitate was removed by centrifugation for 30 min at 1500 ×g, 4 °C [18]. HDL-C was determined in the supernatant in duplicate measurements by the Abell–Kendall RMP [19]. LDL-C was calculated as the difference between BFC and HDL-C. A total of 8 replicate values per sample were obtained, and the mean of these replicates is used for comparison of assay performance.
2.4. Performance criteria
Performance criteria applied to the CRMLN lipid reference laboratories are summarized in Table 1. Because the LDL-C is the difference between BFC and HDL-C, the bias criterion for BFC was determined by the allowable bias for LDL-C and HDL-C and was considered to be ± the sum of the allowable HDL-C and LDL-C bias.
Table 1.
Performance criteria applied to CRMLN lipid reference laboratory using BQ RMP.
| Lipid | Precision | Accuracy |
|---|---|---|
| BFC | CV ≤ 1.5% | ±(CDC LDL-C reference value × 0.02 + HDL-C bias vs. CDC) [max = ±2 mg/dL or 0.04 (HDL-C reference value) if smaller] |
| HDL-C | SD ≤ 1 mg/dL | ±CDC HDL-C reference value × 0.04 |
| LDL-C | CV ≤ 1.5% | ±CDC LDL-C reference value × 0.02 |
CRMLN: Cholesterol Reference Method Laboratory Network. BQ RMP: Beta quantification reference measurement procedure.
CDC: US Centers for Disease Control and Prevention.
BFC: Bottom fraction cholesterol.
2.5. Statistical analysis
We used protocol EP9-A from the Clinical and Laboratory Standards Institute [20–22] for bias estimation and STATA12 analysis program for all other calculations.
3. Results
The concentration ranges of the 67 lots used in the CRMLN surveys were 122.3–223.7 mg/dL, 27.0–72.4 mg/dL, and 71.5–173.3 mg/dL for BFC, HDL-C, and LDL-C, respectively. For 15 years, the reference laboratory at Osaka meets CRMLN accuracy and precision performance goals for BFC, HDL-C and LDL-C (Table 2).
Table 2.
Measurement performance of the CRMLN laboratory at Osaka determined with 280 pooled sera measured between May 1997 and October 2012 in 70 survey runs.
| Statistical item | BFC | HDL-C | LDL-C |
|---|---|---|---|
| Mean precision as %CV (SD) | 0.60 (0.342) | 1.01 (0.605) | 0.85 (0.461) |
| Mean bias as % (SD) | −0.12 (0.853) | 0.45 (1.708) | −0.34 (1.148) |
| Pass rate for imprecision (N) | 95.4% (267) | 95.4% (267) | 91.8% (257) |
| Pass rate for bias (N) | 91.4% (256) | 94.6% (256) | 89.6% (251) |
| Absolute bias (%) | 0.63 ± 0.589 | 1.23 ± 1.270 | 0.86 ± 0.830 |
| Bias in mg/dL (95% CI) | 0.34 (0.14, 0.53) | −0.16 (−0.26, −0.07) | 0.49 (0.32, 0.66) |
| Limits of agreement in mg/dL | −2.87 to 3.54 | −1.76 to 1.43 | 0.31 to 0.66 |
| Slope (95% CI) | 0.988 (0.981, 0.995) | 0.980 (0.971, 0.989) | 0.987 (0.980, 0.993) |
| Intercept (95% CI) | 1.794 (0.581, 3.006) | 1.118 (0.676, 1.560) | 1.200 (0.388, 2.011) |
| Correlation coefficient as R2 | 0.997 | 0.994 | 0.997 |
CRMLN: Cholesterol Reference Method Laboratory Network.
BFC: Bottom fraction cholesterol.
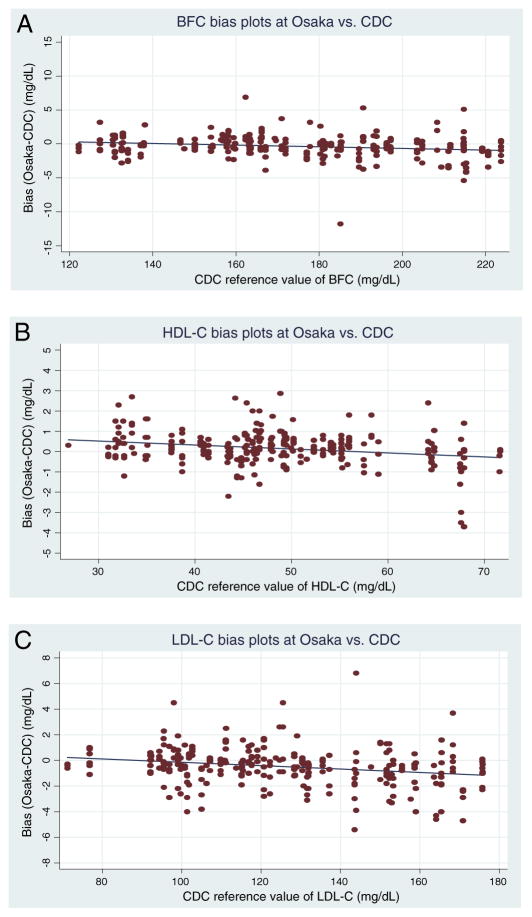
The mean percent bias between the Osaka laboratory and the CDC reference laboratory was <0.5% for all analytes, with limits of agreement being very narrow. Bias and regression analyses show that the bias, though small, is significant. The observed bias is well-below the allowable bias for CRMLN laboratories. The individual sample biases at low analyte concentrations tend to be positive, and at high concentration the biases are negative for all analytes (Fig. 1A–C).
Fig. 1.
Scatter plots of bias at Osaka vs. CDC for BFC (A), HDL-C (B) and LDL-C (C). (A) CDC: US Centers for Disease Control and Prevention. BFC: Bottom fraction cholesterol. x-axis indicates CDC reference value of BFC (unit: mg/dL) in the concentration range from 122.3 to 223.7 mg/dL and y-axis indicates the BFC bias between Osaka and CDC (unit: mg/dL). y (bias (Osaka–CDC)) = −0.012 × (CDC reference value) + 1.759 [n: 280, R2 = 0.042 (p-value: 0.001)]. p-value and 95% CI are 0.001 and (−0.019, −0.005) for slope, respectively. p-value and 95% CI are 0.004 and (0.551, 2.968) for intercept, respectively. (B) CDC: US Centers for Disease Control and Prevention. HDL-C: High-density lipoprotein cholesterol. x-axis indicates CDC reference value of HDL-C (unit: mg/dL) in the concentration range from 27.0 to 72.4 mg/dL and y-axis indicates the HDL-C bias between Osaka and CDC (unit: mg/dL). y (bias (Osaka–CDC)) = −0.020 × (CDC reference value) + 1.112 [n: 280, R2 = 0.063 (p-value: <0.001)]. p-value and 95% CI are <0.001 and (−0.029, −0.011) for slope, respectively. p-value and 95% CI are <0.001 and (0.671, 1.553) for intercept, respectively. (C) CDC: US Centers for Disease Control and Prevention. LDL-C: Low-density lipoprotein cholesterol. x-axis indicates CDC reference value of LDL-C (unit: mg/dL) in the concentration range from 71.5 to 173.3 mg/dL and y-axis indicates the LDL-C bias between Osaka and CDC (unit: mg/dL). y (bias (Osaka–CDC)) = −0.013 × (CDC reference value) + 1.186 [n: 280, R2 = 0.059 (p-value: <0.001)]. p-value and 95% CI are <0.001 and (−0.020, −0.007) for slope, respectively. p-value and 95% CI are 0.004 and (0.376, 1.996) for intercept, respectively.
From the estimation by regression line, the absolute bias between CDC and Osaka in the clinical decision levels was estimated as 0.40 mg/dL for BFC at 180 mg/dL, 0.32 mg/dL for HDL-C at 40 mg/dL and 0.62 mg/dL for LDL-C at 140 mg/dL. The bias was small, but the mean value of absolute bias in upper 10% and lower 10% concentration of reference value was larger than that in middle 80% for BFC (1.45 mg/dL vs. 0.98 mg/dL: p = 0.01). There was no difference of bias related to concentration for HDL-C (0.69 mg/dL vs. 0.54 mg/dL: p = 0.19) and LDL-C (1.04 mg/dL vs. 1.10 mg/dL: p = 0.70) (Table 3).
Table 3.
Comparison of absolute bias between middle 80% and upper/lower 10% of reference values.
| Lipid | Range of middle 80% of reference (CDC) value | Mean of absolute bias in middle 80% of reference (CDC) value | Mean of absolute bias in upper 10% and lower 10% | p-value |
|---|---|---|---|---|
| BFC | 132.80–214.79 mg/dL | 0.98 mg/dL | 1.45 mg/dL | 0.01 |
| HDL-C | 33.50–64.50 mg/dL | 0.54 mg/dL | 0.69 mg/dL | 0.19 |
| LDL-C | 95.50–165.39 mg/dL | 1.10 mg/dL | 1.04 mg/dL | 0.70 |
BFC: Bottom fraction cholesterol.
Assessing measurement bias over time showed no significant trend from May 1997 to October 2012. This is indicated in no significant bias observed with lot bq47, which was analyzed quarterly over 2.5 years. Furthermore, no significant trend in measurement bias was observed for this period (Fig. 2).
Fig. 2.
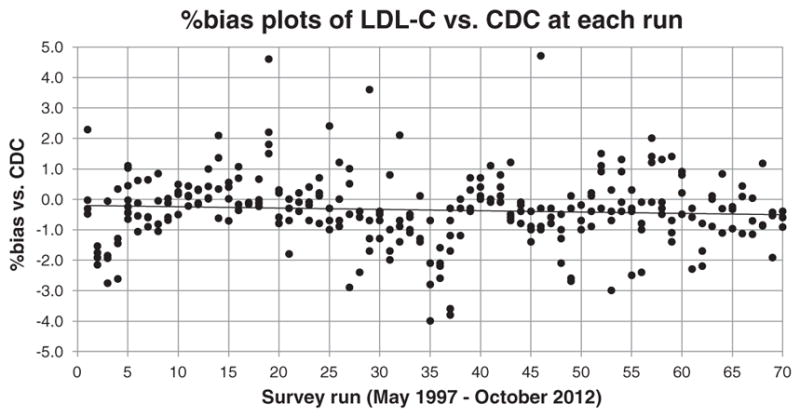
%Bias plots of LDL-C vs. CDC at each survey run. CDC: US Centers for Disease Control and Prevention. LDL-C: Low-density lipoprotein cholesterol. x-axis indicates survey run number during May 1997 and October 2012 with 70 runs and y-axis indicates %bias of LDL-C vs. CDC. The accuracy criteria of %bias plots of LDL-C is ±2% of CDC reference value. Each survey run consists of 3 to 5 CDC pools for beta quantification analysis.
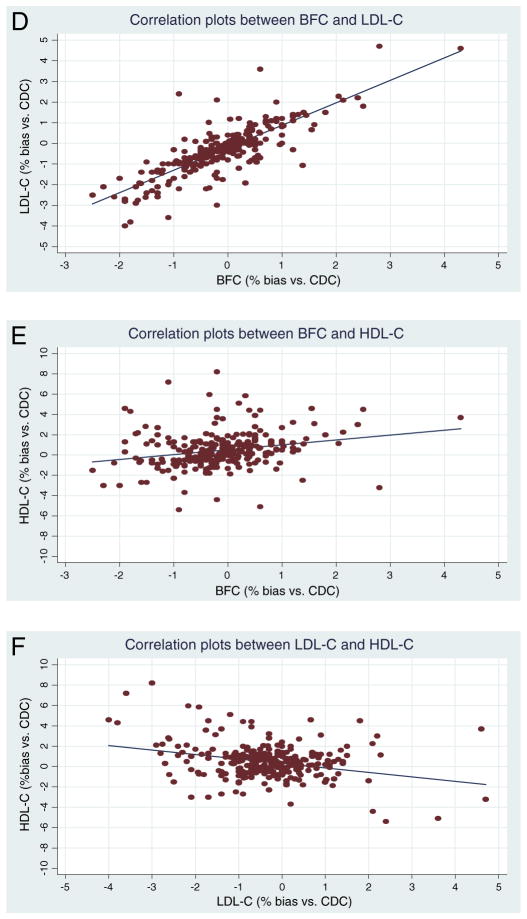
Correlation plots between BFC (x-axis, unit: %bias vs. CDC) and LDL-C (y-axis, unit: %bias vs. CDC) of the Osaka laboratory are positively correlated (y = 1.088x − 0.208, n = 280, R2 = 0.652 (p-value < 0.001), p-value and 95% CI for slope are <0.001 and (0.994, 1.182), respectively, p-value and 95% CI for intercept are <0.001 and (−0.289, −0.128), respectively) (Fig. 3D). In contrast, only weak correlations are observed between the biases from BFC (x-axis, unit: %bias vs. CDC) and HDL-C (y-axis, unit: %bias vs. CDC). (y = 0.480x + 0.513, n = 280, R2 = 0.057 (p-value < 0.001)) (Fig. 3E). Similarly, only weak correlations existed between the biases from LDL-C (x-axis, unit: %bias vs. CDC) and HDL-C (y-axis, unit: %bias vs. CDC). (y = −0.441x + 0.299, n = 280, R2 = 0.087 (p-value < 0.001)) (Fig. 3F).
Fig. 3.
Scatter plots of correlation and regression at Osaka between BFC and LDL-C (D), BFC and HDL-C (E), and LDL-C and HDL-C (F). (D) CDC: US Centers for Disease Control and Prevention. BFC: Bottom fraction cholesterol. LDL-C: Low-density lipoprotein cholesterol. CI: Confidence interval. x-axis indicates Osaka BFC (unit: %bias vs. CDC) and y-axis indicates Osaka LDL-C (unit: %bias vs. CDC). y (Osaka LDL-C) = 1.088 × (Osaka BFC) − 0.208 [n: 280, R2 = 0.652 (p-value: <0.001)]. p-value and 95% CI are <0.001 and (0.994, 1.182) for slope, respectively. p-value and 95% CI are <0.001 and (−0.289, −0.128) for intercept, respectively. (E) CDC: US Centers for Disease Control and Prevention. BFC: Bottom fraction cholesterol. HDL-C: High-density lipoprotein cholesterol. CI: Confidence interval. x-axis indicates Osaka BFC (unit: %bias vs. CDC) and y-axis indicates Osaka HDL-C (unit: %bias vs. CDC). y (Osaka HDL-C) = 0.480 × (Osaka BFC) + 0.513 [n: 280, R2 = 0.057 (p-value: <0.001)]. p-value and 95% CI are <0.001 and (0.250, 0.711) for slope, respectively. p-value and 95% CI are <0.001 and (0.316, 0.710) for intercept, respectively. (F) CDC: US Centers for Disease Control and Prevention. HDL-C: High-density lipoprotein cholesterol. LDL-C: Low-density lipoprotein cholesterol. CI: Confidence interval. x-axis indicates Osaka LDL-C (unit: %bias vs. CDC) and y-axis indicates Osaka HDL-C (unit: %bias vs. CDC). y (Osaka HDL-C) = −0.441 × (Osaka LDL-C) + 0.299 [n: 280, R2 = 0.087 (p-value: <0.001)]. p-value and 95% CI are <0.001 and (−0.609, −0.273) for slope, respectively. p-value and 95% CI are 0.004 and (0.098, 0.499) for intercept, respectively.
4. Discussion
LDL-C is a key biomarker for cardiovascular disease risk assessment, and it is the primary target for treatment. No RMP currently exists for direct measurement of LDL-C. Therefore, the BQ approach was established to assign LDL-C reference values to serum materials. Like all RMPs, it is not intended for use in patient care because of its technical demands (e.g. overnight UC, manual volumetric sampling, and reconstitution of the bottom fractions) [23,24]. However, the technical limitations of this method such as sample throughput or complexity are similar to those of other RMPs [25]. Because measurement results are traceable to an RMP and the International System of Units, it is important to assure that this method is highly reproducible and accurate over time. Efforts by CDC and its partners to assure the accuracy of LDL-C measurements have been ongoing for over 15 years. The CRMLN assures the accuracy of LDL-C measurements by providing reference measurement service to the clinical laboratory community to establish metrological traceability to the CDC RMP. Only a few studies have examined the performance of BQ RMP [26–28]. This study describes the performance of LDL-C value-assignment performed in one CRMLN laboratory over 15 years.
The actual cholesterol measurements are traceable to pure compound certified reference materials and thus are traceable to SI as outlined in ISO 17511. The isolation of the lipid fractions is traceable to a RMP, which is also outlined in ISO 17511. To our knowledge, ISO 17511 does not define nor require a so called “gold standard”. Because cholesterol measurements are traceable to SI, we prefer to use the term “accuracy” in the manuscript. The CDC BQ RMP is classified as a higher order reference measurement procedure used to assign reference values on frozen reference materials. The CDC LDL-C RMP is the reference point for LDL-C recommended by the NCEP Lipoprotein Measurement Working Group. The accuracy reported in the paper refers to the accuracy compared to the CDC LDL reference values. The CRMLN laboratories achieve traceability to CDC RMP through monitoring.
The BQ method combines the removal of triglyceride (TG)-rich VLDL by UC, isolation of HDL from the UC bottom fraction, and cholesterol analysis of the bottom fraction and HDL supernatant. Therefore, the performance of HDL-C and BFC measurements needs to be considered when assessing factors affecting LDL-C target value assignments.
Over 15 years, the BQ RMP operated at the Osaka laboratory provided highly accurate and precise measurements of HDL-C and LDL-C, as indicated in the high agreement with the CDC reference laboratory. The observed mean bias is well within the allowable bias for CRMLN laboratories. The CRMLN focuses mainly on assuring accuracy of measurements around the clinical decision levels, which would be 40–60 mg/dL for HDL-C and 100–160 mg/dL for LDL-C (Fig. 1B,C); most of the serum pools used in CRMLN cover these ranges. Within these ranges, no significant mean bias and no proportional bias between the 2 methods were observed (Table 3). Considering that the LDL-C value assignments are derived from two separate measurements and that this RMP is technically very demanding, the overall performance and performance over time is remarkable. The data demonstrate that this method can be operated in a highly precise manner over long periods of time.
The CDC BQ method has been accepted as the most reliable RMP for HDL-C and LDL-C measurements, and it was recommended by the NCEP as the RMP method for HDL-C and LDL-C. The BQ method was used to establish the concentrations of the major lipoprotein classes in almost all epidemiological studies and clinical trials on which current guidelines for CVD risk assessment are based. It is used in the assignment of LDL-C reference values to calibrators or standards, patient specimens or bench-level quality control materials, and in the evaluation of direct [29,30] and homogeneous methods [31–33]. In the “Program Recommendations for the Measurement of Low-Density Lipoprotein Cholesterol: Executive Summary” [16], Bachorik et al. encouraged the early development of homogeneous methods and suggested that new methods for measuring LDL-C should be developed that are capable of directly quantifying LDL-C, and which should not be based on calculations of the difference between two or more measured values. The developed homogeneous methods have some advantages, such as the direct measurement of LDL-C by automated analytical instruments and possible use of non-fasting samples. However, they do have limitations [31–33]. Therefore, the BQ method is needed to assure accurate patient data that can be compared to current clinical decision points.
The reference values obtained with the BQ approach are based on the density of lipoprotein particles and their separation using specific UC conditions. LDL is not a unique molecular species; it consists of a group of similar, mixed, and atherogenic lipoproteins that vary to some degree in their chemical composition and physico-chemical particles [34]. The bottom fraction contains minor, but atherogenic lipoprotein classes such as IDL and Lp(a) [17,35,36]. In normal individuals, both lipoprotein classes can be expected to contribute 2–4 mg/dL, on average, to the total cholesterol measurement; however, their concentrations may be higher in patients with CHD and in patients at risk of developing CHD by virtue of dyslipidemia. The alterations of these lipid classes can affect cardiovascular disease risk, which may not be adequately detected by the BQ approach. Therefore, new approaches, such as measurement of LDL particle numbers, have been suggested to better assess cardiovascular risk in patients with such conditions [37]. The limitation of the BQ approach needs to be considered when using this RMP for reference value assignments.
The strong correlation between the BFC bias and the LDL-C bias, as well as the weak correlation between the LDL-C bias and HDL-C bias, suggests that the accuracy of LDL-C performed is directly affected by the accuracy of the BFC measurement and, to a much lesser extent, by the HDL-C measurement. This is expected because the LDL-C is calculated from the BFC, while HDL-C is an independent measurement. Because of the good agreement between CDC RMP and Osaka RMP, the different UC conditions used by these laboratories do not appear to have a profound effect on the mean bias or individual sample biases.
In conclusion, this study demonstrates that accurate measurement of BFC is critical for LDL-C value assignment. The BQ RMP performed at the Osaka laboratory is accurate and consistent over time. This assures that calibrations of assays used in patient care are accurate, and that measurements performed in patient care meet established performance criteria. Thus, the BQ RMP ensures that current guidelines using LDL-C levels for CVD risk assessments can be applied correctly and consistently.
Acknowledgments
The authors thank Dr. W. Greg Miller, Dr. Shinji Yokoyama, Dr. Katsuyuki Nakajima and Dr. Ikunosuke Sakurabayashi for their valuable comments and discussion, and Mr. Christopher Ghattas and Ms. Yukari Ichikawa for their help in providing the references and manuscript.
Footnotes
Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the views of the Centers for Disease Control and Prevention.
References
- 1.Castelli WP. Epidemiology of coronary heart disease: the Framingham study. Am J Med. 1984;27:4–12. doi: 10.1016/0002-9343(84)90952-5. [DOI] [PubMed] [Google Scholar]
- 2.Castelli WP. Lipids, risk factors and ischaemic heart disease. Atherosclerosis. 1996;124(Suppl):S1–9. doi: 10.1016/0021-9150(96)05851-0. [DOI] [PubMed] [Google Scholar]
- 3.Teramoto T, Sasaki J, Ueshima H, et al. Risk factors of atherosclerotic diseases. Executive summary of Japan Atherosclerosis Society (JAS) guideline for diagnosis and prevention of atherosclerosis cardiovascular diseases for Japanese. J Atheroscler Thromb. 2007;14:267–77. doi: 10.5551/jat.e578. [DOI] [PubMed] [Google Scholar]
- 4.Teramoto T, Sasaki J, Ueshima H, et al. Primary hyperlipidemia, committee for epidemiology and clinical management of atherosclerosis. J Atheroscler Thromb. 2008;15:49–51. doi: 10.5551/jat.e590. [DOI] [PubMed] [Google Scholar]
- 5.The lipid research clinics coronary primary prevention trial results. II. The relationship of reduction in incidence of coronary heart disease to cholesterol lowering. JAMA. 1984;251:365–74. [PubMed] [Google Scholar]
- 6.Grundy SM. HMG-CoA reductase inhibitors for treatment of hypercholesterolemia. N Engl J Med. 1988;319:24–33. doi: 10.1056/NEJM198807073190105. [DOI] [PubMed] [Google Scholar]
- 7.Teramoto T, Nakaya N, Yokoyama S, et al. Association between lowering low-density lipoprotein cholesterol with pravastatin and primary prevention of cardiovascular disease in mild to moderate hypercholesterolemic Japanese. J Atheroscler Thromb. 2010;17:879–87. doi: 10.5551/jat.4176. [DOI] [PubMed] [Google Scholar]
- 8.Imano H, Noda H, Kitamura A, et al. Low-density lipoprotein cholesterol and risk of coronary heart disease among Japanese men and women: the Circulatory Risk in Communities Study (CIRCS) Prev Med. 2011;52:381–6. doi: 10.1016/j.ypmed.2011.02.019. [DOI] [PubMed] [Google Scholar]
- 9.Stone NJ, Robinson J, Lichtenstein AH, et al. ACC/AHA Guideline on the Treatment of Blood Cholesterol to Reduce Atherosclerotic Cardiovascular Risk in Adults: a report of the American College of Cardiology/American Heart Association Task Force on practice guidelines. J Am Coll Cardiol. 2013;62:1–84. doi: 10.1016/j.jacc.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 10.National Cholesterol Education Program: executive summary of the third report of the National Cholesterol Education Program (NCEP) expert panel on detection, evaluation, and treatment of high blood cholesterol in adults (Adult Treatment Panel III) JAMA. 2001;285:2486–97. doi: 10.1001/jama.285.19.2486. [DOI] [PubMed] [Google Scholar]
- 11.Warnick GR, Myers GL, Cooper GR, et al. Impact of the third cholesterol report from the adult treatment panel of the national cholesterol education program on the clinical laboratory. Clin Chem. 2002;48:11–7. [PubMed] [Google Scholar]
- 12.European guidelines on cardiovascular disease prevention in clinical practice (version 2012) Eur Heart J. 2012;33:1635–701. doi: 10.1093/eurheartj/ehs092. [DOI] [PubMed] [Google Scholar]
- 13.Japan Atherosclerosis Society. Japan Atherosclerosis Society (JAS) guidelines for prevention of atherosclerotic cardiovascular diseases. 2012. [PubMed] [Google Scholar]
- 14.Part one: recommendations for measurement of low density lipoprotein cholesterol, National Cholesterol Education Program Working group on lipoprotein measurement. National Institutes of Health, National Heart, Lung, and Blood Institute; Sep, 1995. NIH Publication No. 95-3044. [Google Scholar]
- 15.Hainline A, Jr, Karon J, Lippel K. Manual of laboratory operations: lipid and lipoprotein analysis. Bethesda: MD: National Heart, Lung and Blood Institute, Lipid Research Clinics Program; 1982. [HEW Pub. No. (NIH) 75–628 (rev.), US Government Printing Office Publication No. 1982-361-132:678] [Google Scholar]
- 16.Bachorik PS, Ross JW for the National Cholesterol Education Program Working Group on Lipoprotein Measurement. National Cholesterol Education Program Recommendations for measurement of low-density lipoprotein cholesterol: executive summary. Clin Chem. 1995;41:1414–20. [PubMed] [Google Scholar]
- 17.Bachorik PS. Measurement of low-density-lipoprotein cholesterol. In: Rifai N, Warnick GR, Dominiczak MH, editors. Handbook of lipoprotein testing. 2. AACC Press; 2000. pp. 245–63. [Google Scholar]
- 18.Clinical chemistry standardization activity. Atlanta, Georgia: Division of Environmental Health Laboratory Sciences, National Center for Environmental Health and Injury Control, Centers for Disease Control; 1991. High-density lipoprotein cholesterol reference method; p. 30333. [Google Scholar]
- 19.Abell LL, Levy BB, Brodie BB, et al. A simplified method for the estimation of total cholesterol in serum and demonstration of its specificity. J Biol Chem. 1952;195:357–66. [PubMed] [Google Scholar]
- 20.Method comparison and bias estimation using patient samples; approved guideline. NCCLS EP9-A. 1995 Dec;15(17) [Google Scholar]
- 21.Westgard JO, Hunt MR. Use and interpretation of common statistical tests in method-comparison studies. Clin Chem. 1973;19:49–57. [PubMed] [Google Scholar]
- 22.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;327:307–10. [PubMed] [Google Scholar]
- 23.Cole TG, Ferguson CA, Gibson DW, et al. Optimization of beta-quantification methods for high-throughput applications. Clin Chem. 2001;47:712–21. [PubMed] [Google Scholar]
- 24.Waymack PP, Chen W, Ethridge SF, et al. Accuracy and precision issues in modification of the serum volume requirement of the CDC beta quantification reference method for low-density lipoprotein cholesterol from 5 ml to 2 ml. Clin Chem. 2000;46(Suppl):A102–103. [Google Scholar]
- 25.Weykamp C, John WG, Mosca A, et al. The IFCC reference measurement system for HbA1c: a 6-year progress report. Clin Chem. 2008;54:240–8. doi: 10.1373/clinchem.2007.097402. [DOI] [PubMed] [Google Scholar]
- 26.Waymack PP, Ethridge SF, Chen WX, et al. Beta quantification round robin for low density lipoprotein cholesterol using frozen reference serum. Clin Chem. 1998;44(Suppl):A74. [Google Scholar]
- 27.Waymack PP, Ethridge SF, Miller WG, et al. Use of frozen serum for accuracy transfer from CDC LDL-beta quantification reference method to three homogeneous LDL-cholesterol methods. Clin Chem. 2000;46(Suppl):A103. [Google Scholar]
- 28.Waymack PP, Ethridge SF, Myers GL. The effect of temperature on the beta quantification procedure for low-density lipoprotein cholesterol in serum. Clin Chem. 1999;45(Suppl):A18. [Google Scholar]
- 29.McNamara JR, Cole TG, Contois JH, et al. Immunoseparation method for measuring low-density lipoprotein cholesterol directly from serum evaluated. Clin Chem. 1995;41:232–40. [PubMed] [Google Scholar]
- 30.Pisani T, Pickering S, DeLuca LW, et al. Performance of a direct LDL-cholesterol method compared to beta quantification. Pure Appl Chem. 1996;68:1887–92. [Google Scholar]
- 31.Nakamura M, Koyama I, Iso H, et al. Ten-year evaluation of homogeneous low-density lipoprotein cholesterol methods developed by Japanese manufacturers—application of the Centers for Disease Control and Prevention/Cholesterol Reference Method Laboratory Network lipid standardization protocol. J Atheroscler Thromb. 2010;17:1275–81. doi: 10.5551/jat.5470. [DOI] [PubMed] [Google Scholar]
- 32.Miller WG, Myers GL, Sakurabayashi I, et al. Seven direct methods for measuring HDL and LDL cholesterol compared with ultracentrifugation reference measurement procedures. Clin Chem. 2010;56:977–86. doi: 10.1373/clinchem.2009.142810. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Miida T, Nishimura K, Okamura T, et al. A multicenter study on the precision and accuracy of homogeneous assays for LDL-cholesterol: comparison with a beta-quantification method using fresh serum obtained from non-diseased and diseased subjects. Atherosclerosis. 2012;225:208–15. doi: 10.1016/j.atherosclerosis.2012.08.022. [DOI] [PubMed] [Google Scholar]
- 34.Cole TG, Contois JH, Csako G, et al. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC lipoprotein and vascular diseases division working group on best practices. Clin Chem. 2013;59:752–70. doi: 10.1373/clinchem.2012.196733. [DOI] [PubMed] [Google Scholar]
- 35.Contois JH, Warnick GR, Sniderman AD. Reliability of low-density lipoprotein cholesterol, non-high-density lipoprotein cholesterol, and apolipoprotein B measurement. J Clin Lipid. 2011;5:264–72. doi: 10.1016/j.jacl.2011.05.004. [DOI] [PubMed] [Google Scholar]
- 36.Warnick GR. Lack of agreement of homogeneous assays with the reference method for LDL-cholesterol may not indicate unreliable prediction of risk for cardiovascular disease. Clin Chem. 2002;48:1812–5. [PubMed] [Google Scholar]
- 37.AACC Lipoproteins and Vascular Diseases Division Working Group on Best PracticesCole TG. Contois JH, et al. Association of apolipoprotein B and nuclear magnetic resonance spectroscopy-derived LDL particle number with outcomes in 25 clinical studies: assessment by the AACC Lipoprotein and Vascular Diseases Division Working Group on Best Practices. Clin Chem. 2013;59:752–70. doi: 10.1373/clinchem.2012.196733. [DOI] [PubMed] [Google Scholar]