Abstract
Study objectives
Patients with comorbid posttraumatic stress disorder (PTSD) and obstructive sleep apnea (OSA) manifest low adherence to continuous positive airway pressure (CPAP) due to fixed, pressure-induced expiratory pressure intolerance (EPI), a subjective symptom and objective sign aggravated by anxiety sensitivity and somatosensory amplification. As advanced PAP therapy modes (ie, auto-bilevel PAP [ABPAP] or adaptive servo-ventilation [ASV]) may address these side effects, we hypothesized such treatment would be associated with decreased expiratory intolerance and increased adherence in posttraumatic stress patients with co-occurring OSA.
Methods
We reviewed charts of 147 consecutive adult patients with moderately severe posttraumatic stress symptoms and objectively diagnosed OSA. All patients failed or rejected CPAP and were manually titrated on auto-adjusting, dual-pressure ABPAP or ASV modes in the sleep laboratory, a technique to eliminate flow limitation breathing events while resolving EPI. Patients were then prescribed either mode of therapy. Follow-up encounters assessed patient use, and objective data downloads (ODDs) measured adherence.
Results
Of 147 charts reviewed, 130 patients were deemed current PAP users, and 102 provided ODDs: 64 used ASV and 38 used ABPAP. ODDs yielded three groups: 59 adherent per insurance conventions, 19 subthreshold compliant partial users, and 24 noncompliant. Compliance based on available downloads was 58%, notably higher than recently reported rates in PTSD patients with OSA. Among the 19 partial users, 17 patients were minutes of PAP use or small percentages of nights removed from meeting insurance compliance criteria for PAP devices.
Conclusion
Research is warranted on advanced PAP modes in managing CPAP failure in PTSD patients with comorbid OSA. Subthreshold adherence constructs may inform clinical care in a patient-centric model distinct from insurance conventions. Speculatively, clinical application of this transitional zone (“subthreshold” number of hours) may increase PAP use and eventual adherence.
Keywords: obstructive sleep apnea, upper airway resistance syndrome, CPAP, compliance, auto-bilevel, adaptive servo-ventilation
Plain language summary
Obstructive sleep apnea (OSA), a sleep breathing disorder, frequently co-occurs among patients with posttraumatic stress disorder (PTSD), a mental health condition arising after traumatic experiences. Continuous positive airway pressure (CPAP) therapy is the standard OSA treatment that pushes air through the nose or mouth, functioning as a physiological stent to maintain airway patency. CPAP failure is common in PTSD patients, possibly due to expiratory pressure intolerance triggered by fixed pressurized air delivery in which the patient reports difficulty breathing out against the incoming air flow. Advanced positive airway pressure (PAP) devices known as auto-bilevel PAP and adaptive servo-ventilation can be manually tested in the sleep laboratory to decrease expiratory intolerance in order to improve patient comfort and thereby increase use. These objectives are achieved with advanced PAP by diminishing the volume of pressurized air against which the patient breathes during exhalation. Some research suggests PTSD patients using advanced devices will use their PAP machines more consistently. In our study, advanced PAP was associated with PAP adherence rates higher than typically observed in PTSD patients using CPAP.
Introduction
The prevalence of comorbid PTSD and OSA appears to be much higher than previously recognized.1,2 The gold standard intervention for OSA is CPAP therapy, and therapeutic effects are most apparent among patients achieving adherence to therapy.3 Interestingly, both older research4,5 and very recent studies6,7 also indicate treatment of OSA in PTSD patients is associated with decreased PTSD symptom severity. Unfortunately, trauma survivors demonstrate low adherence rates or partial use for PAP therapy.8–11 Lettieri et al reported PTSD/OSA patients used PAP on 53.3%±35.6% of nights for an average of 3.4±2.8 hours/night, but only 30.2% were adherent per standard insurance compliance metrics compared to 55.1% of OSA patients without PTSD.9 In a recent study examining the effects of PAP therapy on PTSD symptoms, Orr et al assessed 59 patients prescribed a device, yet only 12 (20%) were compliant at 6-month follow-up. Another 15 patients were using prior to being lost to follow-up, and half were probably adherent, yielding an approximate overall compliance rate of 34%.7 In contrast to these low adherence rates, Orr et al demonstrated very high partial use rates in their sample; for example, at 3 months, although 18 patients were lost to follow-up, the remaining 41 patients were all using their devices at least some of the time. At 6 months, after another 9 patients were lost to follow-up, 81% of the remaining 32 patients were still using PAP.7
Low adherence and partial use of PAP are widespread among sleep apnea patients in the general population,12–14 and more so in psychiatric patients with OSA comorbidity.15–17 Poor PAP compliance is typically investigated through frameworks attending to behavioral change models,18 psychoeducation,13,14 and other coaching strategies; and, many clinical practice models have been researched and described to improve adherence12,19–22 though few address the issue of partial use, an outcome incisively explored by Stepnowsky et al.23,24 Though partial use (subthreshold adherence) research studies are sparse, they have consistently demonstrated improved clinical outcomes, most notably daytime sleepiness.25–29 Such exploratory findings in subthreshold adherence suggests a potential value in defining the clinical importance of partial PAP use. However, research on partial use in PTSD patients is even more scarce, perhaps due to the influences of insurance carriers on dispensing PAP devices wherein subjective outcomes are often relegated to objective duration of use.30,31
Our clinic implements a compliance protocol also focusing on psychological attributes, but we differ from conventional approaches by targeting the patient’s experiential and emotional reactions to pressurized air.32 In brief, psychiatric patients exposed to CPAP are susceptible to the problems of anxiety sensitivity33,34 and somatosensory amplification.35–37 In our clinical experience, both of these transdiagnostic vulnerabilities are triggered when attempting to use fixed pressurized air. As CPAP delivers the same pressure setting on inspiration and expiration for any given breath, the patient is always exhaling against a pressure greater than required to maintain a patent airway, an objectively proven observation described in the sleep literature since 1990.38 This fixed pressure on exhalation often produces an uncomfortable sensation sufficient to trigger PAP intolerance,39 and in vulnerable patients, claustrophobic or panic reactions flare up.40–42 Thus, at our sleep medical center, which specializes in the treatment of psychiatric patients with sleep disorders,43 we observe CPAP technology itself as a major cause of low adherence or outright rejection.
We recently reported on this phenomenon in a consecutive series of psychiatric patients with comorbid OSA or UARS.30 Using CMS criteria,44 we measured adherence (>4 hours/night on >70% of nights) and subthreshold compliance (subcompliant regular users, who were PAP users not meeting CMS guidelines but who averaged regular, nightly use >3 hours/night) in 113 patients naïve to treatment who failed CPAP and subsequently filled prescriptions for advanced PAP devices. Among 113 patients, 104 (92%) were current users at 7-month follow-up. ODDs available for 93 patients showed 59 adherent, averaging 42.0 (SD 12.1) hours/week, and 21 subthreshold compliant (partial users), averaging 18.0 (SD 5.6) hours/week.30 Adherence equaled 63% for patients with ODDs; another 10 partial users achieved levels minutes removed from the 4 hours/night criteria or a few percentage points from attaining 70% of nights criteria for adherence.30
The advanced PAP modes applied in the prior and current research were dual-pressure, auto-adjusting devices, manually titrated in the sleep laboratory, that is, ABPAP or ASV, the latter in those qualifying for complex sleep apnea, a condition in which iatrogenic central apneas emerge upon exposure to CPAP.45 As described elsewhere, advanced PAP technology has been associated with reversing CPAP failure,46 improving adherence in chronic insomnia patients with comorbid OSA/UARS,47,48 decreasing residual sleepiness,49 and yielding higher-than-usual rates of compliance in psychiatric patients.30
The use of manually titrated, advanced PAP technology derives from our sleep center’s 2005 policies and procedures, emphasizing the need to address the adverse experiences reported by patients while attempting CPAP technology.32,43,50–56 To reiterate, the model was formulated to address “claustrophobic tendencies”41,42,57 in psychiatric or other patients who demonstrate anxiety sensitivity or somatosensory amplification to pressurized air, often appearing as awkward attempts to overcontrol one’s breathing while struggling to adapt to PAP.32 This pressure-related distress manifests as subjective (awake) or objective (asleep) EPI, and usually both problems emerge during titration PSG.30,46,48 As the patient struggles to gain sufficient air or fights smothering sensations from excessive air, claustrophobic tendencies emerge, which for vulnerable patients leads to rapid termination of the titration procedure, culminating in the patient displacing the mask.32 Among patients who continue to use the device, central apneas and eventually complex sleep apnea may emerge.30,45
While some research focuses on the mask as the restrictive element triggering claustrophobic tendencies,41,57 in our experience, most patients with mask difficulties report discomfort or poor fit and request a different mask, whereas only a small proportion report restrictive sensations triggering claustrophobic tendencies. For example, in a recent study on adherence, >50% of patients with claustrophobia requested a full-face mask when filling their PAP prescriptions, a counterintuitive finding.30 In contrast, the distress related to pressurized air is the more common instigating complaint as patients frequently exclaim, “I just cannot breathe with the device” or “the machine is trying to control my breathing”. As one patient recently remarked, “It feels like driving a motorcycle without a wind visor”. Paradoxically, mask issues may signal a covert pressure intolerance problem.32
For PTSD patients already suffering high levels of anxiety and anxiety sensitivity,33,34 both generalized pressure intolerance and EPI-induced claustrophobic tendencies may arise during the process of somatosensory amplification.35–37 Anecdotally, vulnerable PTSD patients’ attempts at PAP may deteriorate to an unambiguous phobic response,40 after which they avoid further trials. Likewise, some patients describe these events as traumatizing, and this conceptualization is supported by subsequent treatment avoidance behavior or long lapses between the initial traumatizing PAP experience and efforts to reattempt PAP therapy.46
The current retrospective chart review examined a consecutive series of OSA/UARS patients with moderately severe posttraumatic stress symptoms, presumptively diagnosed with chronic PTSD and prescribed PAP therapy. We hypothesized the use of advanced PAP technology would be associated with two outcomes: 1) 50% adherence in a sample of patients previously experiencing CPAP failure, intolerance, or rejection; and 2) a substantial subgroup would achieve subthreshold adherence (partial use), leaving them just shy of CMS-derived compliance.
Methods
Informed consent
Patients provided consent during completion of the online intake at MSAS to use their information anonymously for research purposes. All data were de-identified for this case series. The intake questionnaire assessed sleep symptoms based on nosology for sleep disorders in the International Classification of Sleep Disorders.58 The Los Alamos Medical Center Institutional Review Board found the chart review exempt because it was a retrospective analysis of a de-identified dataset, and patients received no experimental procedures or interventions.
Sample and inclusion criteria
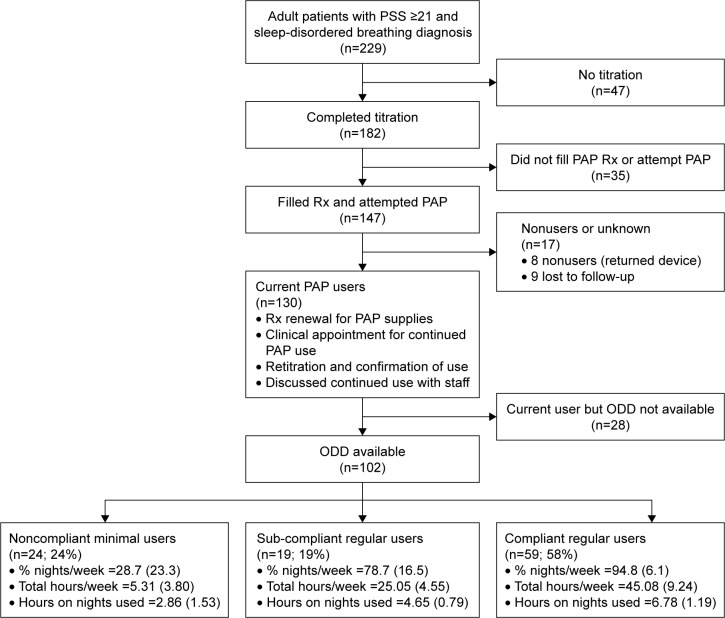
This retrospective chart review included adult patients (≥18 years old), presenting to MSAS between December 2009 and January 2016, who 1) reported a history of traumatic exposure and scored ≥21 on the PSS;59 2) were diagnosed with OSA (AHI ≥5) or UARS (AHI <5 and RDI ≥15) during objective sleep testing; 3) failed CPAP therapy during titration or at home; 4) completed an attended, manual titration (or split-therapy PSG60) of an advanced auto-titrating device (ABPAP or ASV); and 5) filled a prescription for ABPAP or ASV therapy with attempted home use (Figure 1). After starting the chart review with 229 eligible patients with PSS ≥21 and a diagnosis of sleep-disordered breathing, 47 did not complete titration PSG. Of the remaining 182, 35 did not fill their prescription for PAP, leaving 147 patients who attempted PAP. Of the 147 PAP attempters, 130 were current PAP users (Figure 1 provides the use criteria) at follow-up, and 17 were nonusers (8 returned device to DME; 9 lost to follow-up). ODDs were available for 102 of the 130 current users. A significantly larger proportion of users with ODD (76.5%) were anxiety cluster positive compared to users without ODD (53.6%; P=0.02). There was no difference in the remaining psychiatric histories between the two groups. Comparison of sociodemographics, PTSD symptoms scores, subjective intake sleep indices, and objective sleep breathing indices between our final sample of 102 users with ODD and the 28 current users without ODD yielded no significant differences.
Figure 1.
Flowchart showing inclusion and exclusion criteria resulting in the 102 patients comprising our three compliance groups: 1) compliant regular users, 2) subcompliant regular users, and 3) noncompliant minimal users.
Abbreviations: PSS, PTSD Symptom Scale; PAP, positive airway pressure; Rx, prescription; ODD, objective data download.
PAP titration protocol
An overarching premise embedded within our policies and procedures is the well-documented fact that early experiences with PAP are highly predictive of success or failure.20,61–64 When we encounter patients with high probability for CPAP failure, we err on the side of a proactive practice model focusing on the individual’s early subjective experiences with the CPAP mode. Although for insurance purposes any patient must “fail” CPAP to be switched to an advanced form of PAP, there are no formal criteria for CPAP failure. In these circumstances where an individual develops an immediate panicky or frank claustrophobic response when attempting to breathe outward against continuous pressure delivered inward, further attempts at patient persuasion by the physician or sleep technologist to “try a little harder” with CPAP may prove counterproductive. More commonly, pushing this incipient failing strategy often elicits emotional responses from the patient, including frustration, discouragement, anxiety, and fear.43
In these early attempts at PAP initiation, acceptance, and adaptation, discretion has guided us to switch the individual to an expiratory relief mode, which may rapidly enhance the patient’s level of comfort and evoke a more positive attitude. During the course of the titration itself as these patients manifest objective expiratory tolerance, even with APAP, EPR, or BPAP devices, we attempt to smooth out the airflow signal65 with the use of ABPAP or ASV devices, the latter when meeting complex sleep apnea diagnosis (CAI ≥5, CAI/AHI >50%).45 Central apneas are not uncommon at higher elevations (Albuquerque, NM [elev. 1,627.6 meters]) or among anxiety patients prone to hyperventilation30,48 that may trigger CO2 decrements and resultant drop off in breathing drive (loop gain).45,66
When manually titrated, the sleep technologist overrides the aspects of the auto-adjusting algorithms that appear to have responded ineffectually to the dueling problems of residual flow limitation and iatrogenic EPI.46,67 As described elsewhere, this nuanced approach to titrations often requires changes in the 0.2- to 0.4-cm H2O range, while adjusting both inspiratory and expiratory settings.48 Although our experience reflects case series, we have published on this clinical care model, involving 744 OSA/UARS patients,30,46–48,67 and have treated 3,934 clinical patients in this manner at our sleep center from 2008 to the present.
As an aside, as long as patients attempt standard PAP modes during the presleep desensitization or the titration PSG or through home use, subsequent subjective or objective failure of the treatment usually provides sufficient grounds for insurance purposes to escalate the patient’s care to receive a trial with an advanced PAP mode. In our extensive clinical experience, the manual titration of ABPAP or ASV in the sleep laboratory has proven consistently superior to home use of prescribed, default settings for these advanced devices. Thus, the sleep laboratory plays a critical role in this protocol.46,67,68
Adherence metrics
The 102 patients with objective compliance data were divided into three subgroups: C-RU, patients averaging ≥4 hours/night on ≥70% of nights used, thus meeting CMS criteria; SC-RU, patients using PAP regularly with average nightly use ranging from 3 to almost 6 hours per night but not meeting the 4 hours of use on 70% of nights metric for compliance; and NC-MU, patients with minimal PAP use and averaging <2 hours/night. Rare differences were noted in baseline characteristics among the three groups (Table 1). We also calculated compliance using an alternate metric based on total weekly PAP hours derived from Medicare criteria where their minimum of 4 hours/night on 4.9 nights/week totaled 19.6 hours/week. Rounding up to 20 hours/week, we defined two groups: adherent, averaging >20 hours/week and non-adherent, averaging <20 hours/week.
Table 1.
Comparison of intake PSS, ISI, and ESS total scores, subjective sleep indices, psychiatric history, objective sleep breathing diagnostic data, and objective titration data for complex sleep apnea cases
| Measures | Total sample (n=102) |
C-RU (n=59) |
SC-RU (n=19) |
NC-MU (n=24) |
P or C valuea | C-RU vs NC-MU
|
SC-RU vs NC-MU
|
|---|---|---|---|---|---|---|---|
| Effect sizeb | Effect sizeb | ||||||
| Intake questionnaire scores | |||||||
| PSS, total scorec | 30.63 (8.03) | 29.71 (7.46) | 31.16 (7.38) | 32.46 (9.73) | P=0.35 | 0.33 | 0.15 |
| ISI, total scored | 20.53 (4.66) | 20.10 (4.95) | 20.21 (4.88) | 21.83 (3.55) | P=0.30 | 0.37 | 0.38 |
| ESS, total scoree | 11.34 (6.48) | 11.41 (6.50) | 11.74 (6.76) | 10.88 (6.44) | P=0.91 | 0.08 | 0.13 |
| Subjective sleep indices | |||||||
| SOL (minutes) | 95.82 (99.06) | 90.39 (95.61) | 93.47 (84.01) | 111.04 (119.15) | P=0.69 | 0.20 | 0.16 |
| TIB (hours) | 8.03 (1.86) | 8.10 (1.66) | 7.69 (1.81) | 8.15 (2.36) | P=0.68 | 0.03 | 0.21 |
| TST (hours) | 5.65 (1.73) | 5.93 (1.47) | 5.74 (1.81) | 4.88 (2.06) | P=0.04 | 0.63 | 0.43 |
| SE (%) | 71.86 (19.09) | 74.55 (16.71) | 76.93 (20.88) | 61.21 (19.93) | P=0.01 | 0.75 | 0.76 |
| WASO (minutes) | 135.67 (119.31) | 117.14 (108.30) | 104.89 (106.37) | 205.58 (131.58) | P=0.003 | 0.76 | 0.82 |
| Psychiatric history | |||||||
| Psychiatric history positive | 100 (98.04) | 57 (96.61) | 19 (100.00) | 24 (100.0) | C=0.12 | 3.39% | 0.00% |
| Anxiety clusterf positive | 78 (76.47) | 44 (74.58) | 14 (73.68) | 20 (83.33) | C=0.09 | 8.75% | 9.65% |
| Depression clusterg positive | 84 (82.35) | 46 (77.97) | 18 (94.74) | 20 (83.33) | C=0.16 | 5.36% | −11.41% |
| Objective diagnoses | |||||||
| OSA | 88 (86.27) | 51 (86.44) | 16 (84.21) | 21 (87.50) | C=0.13 | 1.06% | 3.29% |
| UARS | 12 (11.76) | 6 (10.17) | 3 (15.79) | 3 (12.50) | −0.35% | −3.29% | |
| Primary CSA | 2 (1.96) | 2 (3.39) | 0 (0.00) | 0 (0.00) | −2.39% | – | |
| Objective breathing indices | |||||||
| RDIh (events/hour) | 56.52 (31.98) | 55.45 (35.69) | 60.20 (29.14) | 55.81 (26.17) | P=0.86 | 0.01 | 0.16 |
| AHIi (events/hour) | 26.14 (28.01) | 28.66 (32.14) | 21.42 (24.48) | 23.76 (18.49) | P=0.56 | 0.17 | 0.11 |
| CAI (events/hour) | 2.75 (8.35) | 3.93 (10.50) | 0.89 (1.63) | 1.75 (5.86) | P=0.34 | 0.23 | 0.19 |
| RERAI (events/hour) | 31.35 (24.82) | 28.30 (23.37) | 38.78 (27.35) | 31.71 (25.45) | P=0.30 | 0.14 | 0.26 |
| CompSA diagnosis and indexj | |||||||
| Complex sleep apnea | 64 (62.75) | 33 (55.93) | 15 (78.95) | 16 (66.67) | C=0.18 | 10.74% | −12.28% |
| CAI (events/hour) | 15.59 (23.21) | 14.79 (23.38) | 15.76 (14.73) | 17.53 (28.44) | P=0.90 | 0.11 | 0.07 |
| AHI (events/hour) | 18.90 (27.02) | 18.19 (28.06) | 19.22 (16.90) | 20.51 (31.31) | P=0.94 | 0.08 | 0.05 |
| CAI/AHI ratio | 81.03 (16.48) | 81.61 (16.42) | 83.49 (14.45) | 77.71 (18.55) | P=0.67 | 0.23 | 0.34 |
Notes: Continuous variables are expressed as mean (SD), whereas dichotomous variables are expressed as total count (%) of columns.
P-values were determined using ANOVA for continuous variables. Contingency coefficient (C) was used for comparison of dichotomous variables.
Effect sizes for comparison of unequal sample sizes are expressed as Hedge’s g for continuous variables and as percentage differences for dichotomous variables; NC-MU was used as reference group for comparisons.
PSS scores ≥21 are consistent with moderate-to-severe PTSD symptom severity.
ISI scores ≥15 are indicative of clinically significant insomnia.
ESS scores >10 are indicative of clinically significant daytime sleepiness.
Anxiety cluster – subjective report of at least one of the following psychiatric conditions: anxiety disorder, panic attacks, or obsessive–compulsive disorder.
Depression cluster – subjective report of at least one of the following psychiatric conditions: depression or manic depression.
RDI – total apneas + total hypopneas + total RERAs/total hours of sleep.
AHI – total apneas + total hypopneas/total hours of sleep.
CAI >5 and AHI/CAI >50%.
Abbreviations: PSS, PTSD Symptom Scale; ISI, Insomnia Severity Index; ESS, Epworth Sleepiness Scale; C-RU, compliant regular users; SC-RU, sub-compliant regular users; NC-MU, noncompliant minimal users; SOL, sleep onset latency; TIB, time in bed; TST, total sleep time; SE, sleep efficiency; WASO, wake after sleep onset; OSA, obstructive sleep apnea; UARS, upper airway resistance syndrome; CSA, central sleep apnea; RDI, respiratory disturbance index; AHI, apnea–hypopnea index; CAI, central apnea index; RERAI, RERA index; CompSA, complex sleep apnea; PTSD, posttraumatic stress disorder; RERAs, respiratory effort-related arousals.
Data analysis
One-way ANOVA compared continuous variables, and Hedge’s g calculated effects between unequal-sized subsamples. Chi-square analyzed dichotomous variables, and effect sizes were based on proportional differences between groups. A P-value of 0.05 was considered statistically significant. Data were analyzed with IBM SPSS Statistics, version 11.0 for Windows, 2002 (IBM Corporation). All continuous variables are expressed as mean (SD).
Results
Sociodemographics and baseline sleep metrics
The final 102 patients were predominantly middle aged (average age = 49.0 years), overweight (average BMI = 32.5), Caucasian (55.9%) or Hispanic (32.4%), females (56.0%) who were married/living with partner (55.9%), and completed some college or less (62.8%). At intake, the mean PSS score (30.63 [8.03]) equated to moderately severe symptoms, mean ISI score (20.53 [4.66]) moderately severe insomnia, and mean Epworth Sleepiness Scale score (11.34 [6.48]) was in the mild range for daytime sleepiness (Table 1). The average time to follow-up was 12.72 (12.71) months.
Nearly 90% of patients were diagnosed with OSA (n=88), and the remainder suffered UARS (n=12) and primary central sleep apnea (n=2). By diagnostic breathing event indices, sleep breathing was severe based on RDI, moderate based on AHI, and very mild based on CAI. Ultimately, 64 patients (62.75%) were diagnosed with complex sleep apnea, necessitating the use of ASV mode of treatment (Table 1). Thirty-eight remaining patients used ABPAP.
CMS compliance metric
By standard CMS compliance metrics (Figure 1), there were 59 C-RU, 19 SC-RU, and 24 NC-MU patients. Thus, 58% of patients were compliant, and 42% noncompliant. On ODD among the 43 noncompliant patients, there were 19 SC-RU patients (partial users) within minutes from averaging 4 hours/night or a few percentage points from attaining adherence on 70% of nights. And, 17 of 19 SC-RU patients averaged >4 hours of PAP use on nights used yet were noncompliant due to insufficient nights used per week. PTSD symptom severity and insomnia severity did not correlate significantly with adherence status, albeit small nonsignificant effects were present demonstrating higher PSS and ISI scores in the non-adherent group compared to the adherent group (Table 1).
Figure 2 highlights SC-RU patients’ PAP use relative to C-RU and NC-MU patients. The boxed area demarcates a “transitional zone” comprising 3–6 hours of nightly use (horizontal sides of box) and 10–30 hours of weekly use (vertical sides of box). Note the two C-RU patients (black squares) within this transitional zone were deemed CMS compliant, whereas by comparison numerous SC-RU patients (gray diamonds) exceeded the nightly or the weekly hours of use of these two compliant patients, including 17 who documented ≥20 weekly hours. As seen in Figure 2, 15 of 19 SC-RU patients averaged >4 hours/night but were noncompliant due to not meeting the CMS 70% requirement. One example (circled gray diamond) is the patient who averaged 4.0 hours/night and 28.0 hours/week but was noncompliant because PAP use was 7 hours on 4 of 7 nights of the week, thus achieving compliance on only 56% of nights.
Figure 2.
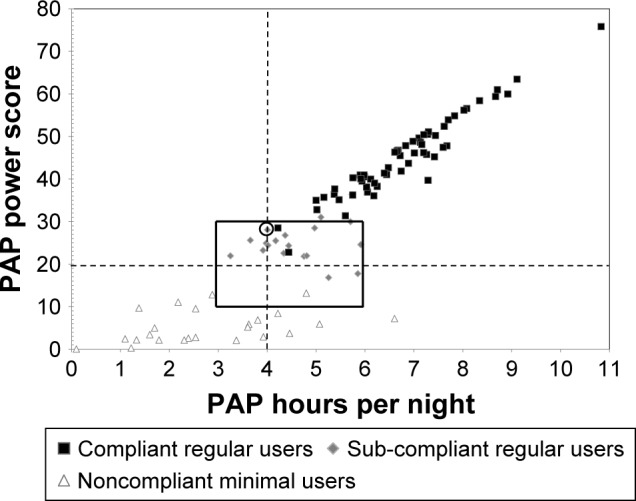
Scatter plot of weekly PAP hours used as a product of nightly PAP average (hours/night) and percentage of nights used (nights/week) for C-RU, SC-RU, and NC-MU.
Notes: Vertical dashed line signifies compliance standard of 4 hours per night, while horizontal dashed line signifies CMS minimum standard of 4 hours/night and 70% of nights used (19.6 hours per week). Transitional zone (box) contains patients with borderline PAP use values that are just above (C-RU: n=2) or just below (SC-RU: n=17) compliance guidelines. Circled diamond is reference SC-RU patient with average nightly use of 4 hours and weekly use of 28 hours (uses PAP 7 hours/night on 4 nights/week) but not meeting compliance standards due to PAP use >4 hours on only four (56%) instead of five (70%) nights/week.
Abbreviations: PAP, positive airway pressure; C-RU, compliant regular users; SC-RU, sub-compliant regular users; NC-MU, noncompliant minimal users; CMS, Center of Medicare and Medicaid Services.
Last, of potential clinical import, the proportions of ASV and ABPAP users were nearly identical for C-RU and NC-MU groups, but the SC-RU group demonstrated a significantly higher proportion of patients using ASV (n=15) compared to ABPAP (n=4) (contingency coefficient =0.50). Regarding residual breathing events on ODD, there was no significant difference (P=0.50) in AHI between C-RU (2.10 (3.11) events/hour) and SC-RU (2.68 [4.04]) patients.
Alternate metric (weekly use hours)
Rounding up to 20 hours/week, our sample would have yielded an additional 17 patients for an adherence total of 74.5% (76/102 patients) and a non-adherence total of 25.5% (26/102 patients) if CMS permitted total hours of use for adherence criteria.
Discussion
This research provides evidence for the use of advanced PAP therapies such as ABPAP or ASV to treat subjective and objective EPI in PTSD patients who failed CPAP therapy for the treatment of their comorbid OSA or UARS. The findings are consistent with a clinically meaningful comfort effect69 that counters distress experienced by patients with anxiety sensitivity or somatosensory amplification who struggle to exhale against fixed pressurized airflow. Due to the retrospective, uncontrolled design of the study, however, these findings can only be classified as an association.
This study also highlights the lack of a patient-centric perspective in the CMS compliance metric, which could have led to termination of therapy in 17 patients (Figure 2, gray diamonds) who were minutes or portions of nights removed from the 4 hours/70% nights standard despite >20 hours/week of use. Given the time course required for vulnerable psychiatric patients to adapt to PAP therapy,46 we have previously raised the question on whether the concept of hours of use is a more pragmatic and appropriate metric in the management of more complex sleep-disordered breathing patients,30 which also aligns with Stepnowsky’s “dose–response” model of PAP care.23,24 Moreover, by neglecting this clinical care principle, current insurance directives may discourage sub-threshold partial PAP users who need a longer interval of adaptation to meet coverage standards; likewise, a partial use framework would also promote a longer transitional window for problem-solving mask and pressure complaints as well as other adaptation issues, all essential ingredients to achieving adherence.12–14,19,21,22,61,64
Along the same lines, whereas adherence is largely an insurance-driven policy,44 partial use offers the potential for a patient-centric model of care because the latter provides opportunities to more accurately investigate dose–response relationships between the symptoms reported by sleep-disordered breathing patients and hours of use, a more precise continuous variable. In our recent study on adherence, outcomes improved in subthreshold compliant patients, and small correlation coefficients indicated that increasing hours of use were associated with decreasing symptoms of insomnia (r=0.20) and nocturia episodes (r=0.25). On the other hand, Orr et al’s study demonstrated a small effect for decreased PTSD symptoms associated with an increased number of nights used, whereas neither hours of use nor number of nights >4 hours showed significant effects for improvement in PTSD symptoms, albeit their sample size was small.7 Once again, these distinctions highlight how the two constructs of adherence and use may lead to paradoxical findings, which may interfere with our ability to more effectively treat OSA and UARS patients. Prospective research is needed to determine the clinical advantages and disadvantages in the application of adherence or use.
The current study is limited by the retrospective, uncontrolled design. Future studies using randomized controlled trials may investigate the benefits of advanced vs traditional PAP devices in PTSD patients with comorbid sleep-disordered breathing. And prospective studies should also compare outcomes between PTSD and non-PTSD patients with OSA to discern how clinical care models may require different resources for each group. In particular, studies should compare the adherence rates of physician-prescribed APAP pressure ranges at setup (per Orr et al7) vs in-laboratory, manually titrated settings for dual-pressure auto-adjusting devices. In addition, selection bias is a concern in this cohort because we were unable to obtain data downloads in 28 (22%) of the user group and thus could not confirm if they were adherent, albeit it is worth reiterating that based on our clinical follow-up encounters, 130 (88%) of these 147 total PAP attempters appeared to be current users.
This distinction between use and adherence raises another clinical question on how to delineate the proper baseline in a sample of PAP patients assessed for adherence. We chose a construct similar to Orr et al (“willingness-to-use PAP”)7 by creating a baseline of patients who filled a PAP prescription. Arguments, pro and con could be made to use more or less stringent starting points in determining adherence rates in various cohorts of OSA/UARS patients.
In summary, though not well described in the sleep literature, certain sleep-disordered breathing patients describe CPAP attempts as “traumatizing experiences”. Related theories point to the potential for a CPAP user to experience a phobic response40 to the mask itself or pressurized air, and OSA patients may also be susceptible to activation of a “false suffocation alarm” due to rapid changes in carbon dioxide, leading to panic attacks.41 Taken together, at-risk OSA patients with anxiety disorders, as the best examples, are already vulnerable to claustrophobic tendencies prior to attempting CPAP because they are at risk for anxiety sensitivity and somatosensory amplification. When initiating fixed pressurized air treatment, these patients develop intolerance and irregular breathing patterns, including instances of iatrogenic central apneas.32,45,46 PTSD patients in particular and psychiatric patients in general who cannot adapt rapidly to PAP therapy may be served by monitoring their graded levels of adherence through which they would be encouraged to traverse steps from minimal use to subthreshold levels (partial users) and eventually full adherence during extended time periods.23,24,30,46 These time frames may prove longer than the standard 60- to 90-day CMS mandated window for clinical follow-up. A testable hypothesis is proposed to determine whether advanced PAP modes facilitate this process.
Acknowledgments
The authors thank the Los Alamos Medical Center and the Los Alamos Medical Center Sleep Laboratory for administrative and clinical assistance in the completion of this research project.
Abbreviations
- ABPAP
auto-bilevel PAP
- AHI
apnea–hypopnea index
- APAP
auto-CPAP
- ASV
adaptive servo-ventilation
- BPAP
bilevel positive airway pressure
- CAI
central apnea index
- CMS
Center of Medicare and Medicaid Services
- CPAP
continuous positive airway pressure
- C-RU
compliant regular PAP users
- EPI
expiratory pressure intolerance
- EPR
expiratory pressure relief
- ISI
insomnia severity index
- MSAS
Maimonides Sleep Arts & Sciences
- NC-MU
non-compliant minimal PAP users
- ODD
objective data download
- OSA
obstructive sleep apnea
- PAP
positive airway pressure
- PSG
polysomnography
- PSS
PTSD Symptom Scale
- PTSD
posttraumatic stress disorder
- RDI
respiratory disturbance index
- SC-RU
sub-compliant regular PAP users
- UARS
upper airway resistance syndrome
Footnotes
Author contributions
Ms Obando and Mr Ulibarri were responsible for acquisition of data. Ms Obando, Mr Ulibarri, Dr Krakow, and Ms McIver were responsible for data analysis and interpretation. Dr Krakow was responsible for the conception and design of the study. All authors contributed to the revision of manuscript, approved the final manuscript version for publication, and have agreed to be accountable for all aspects of this manuscript.
Disclosure
Dr Krakow is involved in six main activities related to his work on sleep medicine: He owns and operates six sites that provide education and offer products and services (www.nightmaretreatment.com, www.ptsdsleepclinic.com, www.sleeptreatment.com, www.sleepdynamictherapy.com, www.soundsleepsoundmind.com, and www.nocturiacures.com). He is the medical director of a national DME company Classic Sleep Care in which his sole functions are consultation and QA; he has neither patient encounters nor does he benefit from the sale of any DME equipment. He markets and sells three books for patients with sleep disorders (Insomnia Cures, Turning Nightmares into Dreams, and Sound Sleep, Sound Mind). He owns and operates one commercial sleep center (Maimonides Sleep Arts & Sciences). He conducts CME/CEU educational programs for medical and mental health providers to learn about sleep disorders. Sometimes, programs involve the attendee paying a fee directly to their center. Other times, the workshops at other locations may be paid for by vendors such as Respironics and RESMED or other institutions such as the AMEDDC&S, VAMC, and regional sleep center conferences. He is the president of a nonprofit sleep research center, the Sleep & Human Health Institute (www.shhi.org), that occasionally provides consultation services or receives grants for pilot studies, the most recent of which was ResMed (~$400,000, January 2015, funding for randomized control trial of PAP treatment in insomnia patients). Ms Obando, Mr Ulibarri, and Ms McIver report no conflicts of interest in this work.
References
- 1.Jaoude P, Vermont LN, Porhomayon J, El-Solh AA. Sleep-disordered breathing in patients with post-traumatic stress disorder. Ann Am Thorac Soc. 2015;12(2):259–268. doi: 10.1513/AnnalsATS.201407-299FR. [DOI] [PubMed] [Google Scholar]
- 2.Krakow BJ, Ulibarri VA, Moore BA, McIver ND. Posttraumatic stress disorder and sleep-disordered breathing: a review of comorbidity research. Sleep Med Rev. 2015;24:37–45. doi: 10.1016/j.smrv.2014.11.001. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Ai L, Luo J, et al. Effect of adherence on daytime sleepiness, fatigue, depression and sleep quality in the obstructive sleep apnea/hypopnea syndrome patients undertaking nasal continuous positive airway pressure therapy. Patient Prefer Adherence. 2017;11:769–779. doi: 10.2147/PPA.S128217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Krakow B, Lowry C, Germain A, et al. A retrospective study on improvements in nightmares and post-traumatic stress disorder following treatment for co-morbid sleep-disordered breathing. J Psychosom Res. 2000;49(5):291–298. doi: 10.1016/s0022-3999(00)00147-1. [DOI] [PubMed] [Google Scholar]
- 5.Youakim JM, Doghramji K, Schutte SL. Posttraumatic stress disorder and obstructive sleep apnea syndrome. Psychosomatics. 1998;39(2):168–171. doi: 10.1016/S0033-3182(98)71365-9. [DOI] [PubMed] [Google Scholar]
- 6.El-Solh AA, Vermont L, Homish GG, Kufel T. The effect of continuous positive airway pressure on post-traumatic stress disorder symptoms in veterans with post-traumatic stress disorder and obstructive sleep apnea: a prospective study. Sleep Med. 2017;33:145–150. doi: 10.1016/j.sleep.2016.12.025. [DOI] [PubMed] [Google Scholar]
- 7.Orr JE, Smales C, Alexander TH, et al. Treatment of OSA with CPAP is associated with improvement in PTSD symptoms among Veterans. J Clin Sleep Med. 2017;13(1):57–63. doi: 10.5664/jcsm.6388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lettieri CJ, Walter RJ. Group education on CPAP – a response. J Clin Sleep Med. 2013;9(9):975–976. doi: 10.5664/jcsm.3012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lettieri CJ, Williams SG, Collen JF. OSA syndrome and posttraumatic stress disorder: clinical outcomes and impact of positive airway pressure therapy. Chest. 2016;149(2):483–490. doi: 10.1378/chest.15-0693. [DOI] [PubMed] [Google Scholar]
- 10.Collen JF, Lettieri CJ, Hoffman M. The impact of posttraumatic stress disorder on CPAP adherence in patients with obstructive sleep apnea. J Clin Sleep Med. 2012;8(6):667–672. doi: 10.5664/jcsm.2260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.El-Solh AA, Ayyar L, Akinnusi M, Relia S, Akinnusi O. Positive airway pressure adherence in veterans with posttraumatic stress disorder. Sleep. 2010;33(11):1495–1500. doi: 10.1093/sleep/33.11.1495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sawyer AM, Gooneratne NS, Marcus CL, Ofer D, Richards KC, Weaver TE. A systematic review of CPAP adherence across age groups: clinical and empiric insights for developing CPAP adherence interventions. Sleep Med Rev. 2011;15(6):343–356. doi: 10.1016/j.smrv.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chen X, Chen W, Hu W, Huang K, Huang J, Zhou Y. Nurse-led intensive interventions improve adherence to continuous positive airway pressure therapy and quality of life in obstructive sleep apnea patients. Patient Prefer Adherence. 2015;9:1707–1713. doi: 10.2147/PPA.S90846. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.La Piana GE, Scartabellati A, Chiesa L, et al. Long-term adherence to CPAP treatment in patients with obstructive sleep apnea: importance of educational program. Patient Prefer Adherence. 2011;5:555–562. doi: 10.2147/PPA.S24018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kovacevic I, Wallace DM, Vargas SS, Shafazand S. PAP compliance in hispanic veterans with psychiatric disorders. Sleep. 2011;34:A252. [Google Scholar]
- 16.Lajos LE, Molina PE, Im SS, Gonzales TA, Garza PC, Ingmundson PT. Continuous positive airway pressure adherence among veterans with and without posttraumatic stress disorder. Sleep. 2004;27:A228. [Google Scholar]
- 17.Means MK, Ulmer CS, Edinger JD. Ethnic differences in continuous positive airway pressure (CPAP) adherence in veterans with and without psychiatric disorders. Behav Sleep Med. 2010;8(4):260–273. doi: 10.1080/15402002.2010.509255. [DOI] [PubMed] [Google Scholar]
- 18.Stepnowsky CJ, Jr, Bardwell WA, Moore PJ, Ancoli-Israel S, Dimsdale JE. Psychologic correlates of compliance with continuous positive airway pressure. Sleep. 2002;25(7):758–762. doi: 10.1093/sleep/25.7.758. [DOI] [PubMed] [Google Scholar]
- 19.Aloia MS, Arnedt JT, Stepnowsky C, Hecht J, Borrelli B. Predicting treatment adherence in obstructive sleep apnea using principles of behavior change. J Clin Sleep Med. 2005;1(4):346–353. [PubMed] [Google Scholar]
- 20.Budhiraja R, Parthasarathy S, Drake CL, et al. Early CPAP use identifies subsequent adherence to CPAP therapy. Sleep. 2007;30(3):320–324. [PubMed] [Google Scholar]
- 21.Smith SS, Lang CP, Sullivan KA, Warren J. A preliminary investigation of the effectiveness of a sleep apnea education program. J Psychosom Res. 2004;56(2):245–249. doi: 10.1016/S0022-3999(03)00545-2. [DOI] [PubMed] [Google Scholar]
- 22.Wickwire EM, Lettieri CJ, Cairns AA, Collop NA. Maximizing positive airway pressure adherence in adults: a common-sense approach. Chest. 2013;144(2):680–693. doi: 10.1378/chest.12-2681. [DOI] [PubMed] [Google Scholar]
- 23.Stepnowsky CJ, Dimsdale JE. Dose-response relationship between CPAP compliance and measures of sleep apnea severity. Sleep Med. 2002;3(4):329–334. doi: 10.1016/s1389-9457(02)00010-2. [DOI] [PubMed] [Google Scholar]
- 24.Stepnowsky CJ, Jr, Moore PJ. Nasal CPAP treatment for obstructive sleep apnea: developing a new perspective on dosing strategies and compliance. J Psychosom Res. 2003;54(6):599–605. doi: 10.1016/s0022-3999(03)00038-2. [DOI] [PubMed] [Google Scholar]
- 25.Antic NA, Catcheside P, Buchan C, et al. The effect of CPAP in normalizing daytime sleepiness, quality of life, and neurocognitive function in patients with moderate to severe OSA. Sleep. 2011;34(1):111–119. doi: 10.1093/sleep/34.1.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Faccenda JF, Mackay TW, Boon NA, Douglas NJ. Randomized placebo-controlled trial of continuous positive airway pressure on blood pressure in the sleep apnea-hypopnea syndrome. Am J Respir Crit Care Med. 2001;163(2):344–348. doi: 10.1164/ajrccm.163.2.2005037. [DOI] [PubMed] [Google Scholar]
- 27.Kingshott RN, Vennelle M, Hoy CJ, Engleman HM, Deary IJ, Douglas NJ. Predictors of improvements in daytime function outcomes with CPAP therapy. Am J Respir Crit Care Med. 2000;161(3 Pt 1):866–871. doi: 10.1164/ajrccm.161.3.9905053. [DOI] [PubMed] [Google Scholar]
- 28.Stradling JR, Davies RJ. Is more NCPAP better? Sleep. 2000;23(Suppl 4):S150–S153. [PubMed] [Google Scholar]
- 29.Weaver TE, Maislin G, Dinges DF, et al. Relationship between hours of CPAP use and achieving normal levels of sleepiness and daily functioning. Sleep. 2007;30(6):711–719. doi: 10.1093/sleep/30.6.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Krakow B, Ulibarri VA, Foley-Shea MR, Tidler A, McIver ND. Adherence and subthreshold adherence in sleep apnea subjects receiving positive airway pressure therapy: a retrospective study evaluating differences in adherence versus use. Respir Care. 2016;61(8):1023–1032. doi: 10.4187/respcare.04538. [DOI] [PubMed] [Google Scholar]
- 31.Anderson L. In Search of Compliance. Birmingham: Cahaba Media Group, Home Care; 2012. [Google Scholar]
- 32.Krakow B, Ulibarri V, Melendrez D, Kikta S, Togami L, Haynes P. A daytime, abbreviated cardio-respiratory sleep study (CPT 95807-52) to acclimate insomnia patients with sleep disordered breathing to positive airway pressure (PAP-NAP) J Clin Sleep Med. 2008;4(3):212–222. [PMC free article] [PubMed] [Google Scholar]
- 33.Collimore KC, McCabe RE, Carleton RN, Asmundson GJ. Media exposure and dimensions of anxiety sensitivity: differential associations with PTSD symptom clusters. J Anxiety Disord. 2008;22(6):1021–1028. doi: 10.1016/j.janxdis.2007.11.002. [DOI] [PubMed] [Google Scholar]
- 34.Kiliç EZ, Kiliç C, Yilmaz S. Is anxiety sensitivity a predictor of PTSD in children and adolescents? J Psychosom Res. 2008;65(1):81–86. doi: 10.1016/j.jpsychores.2008.02.013. [DOI] [PubMed] [Google Scholar]
- 35.Koteles F, Doering BK. The many faces of somatosensory amplification: the relative contribution of body awareness, symptom labeling, and anxiety. J Health Psychol. 2016;21(12):2903–2911. doi: 10.1177/1359105315588216. [DOI] [PubMed] [Google Scholar]
- 36.Nakao M, Barskey AJ. Clinical application of somatosensory amplification in psychosomatic medicine. Biopsychosoc Med. 2007;1:1–7. doi: 10.1186/1751-0759-1-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yavuz BG, Aydinlar EI, Dikmen PY, Incesu C. Association between somatic amplification, anxiety, depression, stress and migraine. J Headache Pain. 2013;14:53. doi: 10.1186/1129-2377-14-53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sanders MH, Kern N. Obstructive sleep apnea treated by independently adjusted inspiratory and expiratory positive airway pressures via nasal mask. Physiologic and clinical implications. Chest. 1990;98(2):317–324. doi: 10.1378/chest.98.2.317. [DOI] [PubMed] [Google Scholar]
- 39.Kushida CA, Chediak A, Berry RB, et al. Clinical guidelines for the manual titration of positive airway pressure in patients with obstructive sleep apnea. J Clin Sleep Med. 2008;4(2):157–171. [PMC free article] [PubMed] [Google Scholar]
- 40.Casas I, de la Calzada MD, Guitart M, Roca A. Diagnastico y tratamiento de fobia a la terapia con presion nasal positiva continua de aire [Diagnosis and treatment of the phobia due to treatment with air using nasal continuous pressure] Rev Neurol. 2000;30(6):593–596. Spanish. [PubMed] [Google Scholar]
- 41.Chasens ER, Pack AI, Maislin G, Dinges DF, Weaver TE. Claustrophobia and adherence to CPAP treatment. West J Nurs Res. 2005;27(3):307–321. doi: 10.1177/0193945904273283. [DOI] [PubMed] [Google Scholar]
- 42.Edmonds JC, Yang H, King TS, Sawyer DA, Rizzo A, Sawyer AM. Claustrophobic tendencies and continuous positive airway pressure therapy non-adherence in adults with obstructive sleep apnea. Heart Lung. 2015;44(2):100–106. doi: 10.1016/j.hrtlng.2015.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Krakow B. Sound Sleep, Sound Mind: 7 Keys to Sleeping through the Night. New York: John Wiley & Sons; 2007. [Google Scholar]
- 44.Billings ME, Kapur VK. Medicare long-term CPAP coverage policy: a cost-utility analysis. J Clin Sleep Med. 2013;9(10):1023–1029. doi: 10.5664/jcsm.3074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Wang J, Wang Y, Feng J, Chen BY, Cao J. Complex sleep apnea syndrome. Patient Prefer Adherence. 2013;7:633–641. doi: 10.2147/PPA.S46626. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Krakow B, Ulibarri VA, McIver ND, et al. Reversal of PAP failure with the REPAP retitration protocol. Respir Care. 2017;62(4):396–408. doi: 10.4187/respcare.05032. [DOI] [PubMed] [Google Scholar]
- 47.Krakow B, Ulibarri VA, McIver ND, Nadorff MR. A novel therapy for chronic sleep-onset insomnia: a retrospective, nonrandomized controlled study of auto-adjusting, dual-level, positive airway pressure technology. Prim Care Companion CNS Disord. 2016;18(5) doi: 10.4088/PCC.16m01980. [DOI] [PubMed] [Google Scholar]
- 48.Krakow B, Ulibarri VA, Romero EA, Thomas RJ, McIver ND. Adaptive servo-ventilation therapy in a case series of patients with co-morbid insomnia and sleep apnea. J Sleep Disord Treat Care. 2013;2(1):1–10. [Google Scholar]
- 49.Su M, Zhang X, Huang M, Ding N. Adaptive pressure support servoventilation: a novel treatment for residual sleepiness associated with central sleep apnea events. Sleep Breath. 2011;15(4):695–699. doi: 10.1007/s11325-010-0424-6. [DOI] [PubMed] [Google Scholar]
- 50.Krakow B, Melendrez D, Warner TD, Dorin R, Harper R, Hollifield M. To breathe, perchance to sleep: sleep-disordered breathing and chronic insomnia among trauma survivors. Sleep Breath. 2002;6(4):189–202. doi: 10.1007/s11325-002-0189-7. [DOI] [PubMed] [Google Scholar]
- 51.Krakow B, Melendrez D, Haynes P. Integrating psychosocial and biomedical CPAP adherence models. A commentary on: “Improving CPAP use by patients with the sleep apnea/hypopnea syndrome (SAHS)” (HM Engleman & MR Wild) Sleep Med Rev. 2003;7(5):441–444. doi: 10.1053/smrv.2002.0288. [DOI] [PubMed] [Google Scholar]
- 52.Krakow B, Melendrez D, Lee SA, Warner TD, Clark JO, Sklar D. Refractory insomnia and sleep-disordered breathing: a pilot study. Sleep Breath. 2004;8(1):15–29. doi: 10.1007/s11325-004-0015-5. [DOI] [PubMed] [Google Scholar]
- 53.Krakow BJ, Melendrez DC, Johnston LG, et al. Sleep Dynamic Therapy for Cerro Grande Fire evacuees with posttraumatic stress symptoms: a preliminary report. J Clin Psychiatry. 2002;63(8):673–684. doi: 10.4088/jcp.v63n0804. [DOI] [PubMed] [Google Scholar]
- 54.Krakow B, Melendrez D, Warner TD, et al. Signs and symptoms of sleep-disordered breathing in trauma survivors: a matched comparison with classic sleep apnea patients. J Nerv Ment Dis. 2006;194(6):433–439. doi: 10.1097/01.nmd.0000221286.65021.e0. [DOI] [PubMed] [Google Scholar]
- 55.Krakow B, Krakow J, Ulibarri VA, McIver ND. Frequency and accuracy of “RERA” and “RDI” terms in the Journal of Clinical Sleep Medicine from 2006 through 2012. J Clin Sleep Med. 2014;10(2):121–124. doi: 10.5664/jcsm.3432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Krakow B, Ulibarri VA, Sanchez JN, Kikta S, McIver N, Melendrez D. Driving on “auto”: hands-on is more effective than hands-free. J Clin Sleep Med. 2012;8(3):343–344. doi: 10.5664/jcsm.1934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Means MK, Edinger JD. Graded exposure therapy for addressing claustrophobic reactions to continuous positive airway pressure: a case series report. Behav Sleep Med. 2007;5(2):105–116. doi: 10.1080/15402000701190572. [DOI] [PubMed] [Google Scholar]
- 58.American Academy of Sleep Medicine . International Classification of Sleep Disorders: Diagnostic & Coding Manual. 2nd ed. Westchester: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 59.Foa EB, Riggs DS, Dancu CV, Rothbaum BO. Reliability and validity of a brief instrument for assessing posttraumatic stress disorder. J Trauma Stress. 1993;6(4):459–473. [Google Scholar]
- 60.Kuzniar TJ, Golbin JM, Morgenthaler TI. Moving beyond empiric continuous positive airway pressure (CPAP) trials for central sleep apnea: a multi-modality titration study. Sleep Breath. 2007;11(4):259–266. doi: 10.1007/s11325-007-0118-x. [DOI] [PubMed] [Google Scholar]
- 61.Wang Y, Geater AF, Chai Y, et al. Pre- and in-therapy predictive score models of adult OSAS patients with poor adherence pattern on nCPAP therapy. Patient Prefer Adherence. 2015;9:715–723. doi: 10.2147/PPA.S83105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.McArdle N, Devereux G, Heidarnejad H, Engleman HM, Mackay TW, Douglas NJ. Long-term use of CPAP therapy for sleep apnea/hypopnea syndrome. Am J Respir Crit Care Med. 1999;159(4 Pt 1):1108–1114. doi: 10.1164/ajrccm.159.4.9807111. [DOI] [PubMed] [Google Scholar]
- 63.Reeves-Hoche MK, Meck R, Zwillich CW. Nasal CPAP: an objective evaluation of patient compliance. Am J Respir Crit Care Med. 1994;149(1):149–154. doi: 10.1164/ajrccm.149.1.8111574. [DOI] [PubMed] [Google Scholar]
- 64.Weaver TE, Kribbs NB, Pack AI, et al. Night-to-night variability in CPAP use over the first three months of treatment. Sleep. 1997;20(4):278–283. doi: 10.1093/sleep/20.4.278. [DOI] [PubMed] [Google Scholar]
- 65.Condos R, Norman RG, Krishnasamy I, Peduzzi N, Goldring RM, Rapoport DM. Flow limitation as a noninvasive assessment of residual upper-airway resistance during continuous positive airway pressure therapy of obstructive sleep apnea. Am J Respir Crit Care Med. 1994;150(2):475–480. doi: 10.1164/ajrccm.150.2.8049832. [DOI] [PubMed] [Google Scholar]
- 66.Stanchina M, Robinson K, Corrao W, Donat W, Sands S, Malhotra A. Clinical use of loop gain measures to determine continuous positive airway pressure efficacy in patients with complex sleep apnea. A pilot study. Ann Am Thorac Soc. 2015;12(9):1351–1357. doi: 10.1513/AnnalsATS.201410-469BC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Krakow B, McIver ND, Ulibarri VA, Nadorff MR. Retrospective, non-randomized controlled study on autoadjusting, dual-pressure positive airway pressure therapy for a consecutive series of complex insomnia disorder patients. Nat Sci Sleep. 2017;9:81–95. doi: 10.2147/NSS.S120048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Brooks R, Trimble M. The future of sleep technology: report from an American Association of Sleep Technologists summit meeting. J Clin Sleep Med. 2014;10(5):589–593. doi: 10.5664/jcsm.3720. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Wang Y, Gao W, Sun M, Chen B. Adherence to CPAP in patients with obstructive sleep apnea in a Chinese population. Respir Care. 2012;57(2):238–243. doi: 10.4187/respcare.01136. [DOI] [PubMed] [Google Scholar]