Abstract
Background
22q11.2 deletion syndrome (22q11DS) associates with schizophrenia spectrum disorders (SSDs), autism spectrum disorders (ASDs), and other psychiatric disorders, but co-occurrence of diagnoses are not well described.
Methods
We evaluated the co-occurrence of SSDs, ASDs and other axis I psychiatric diagnoses in 31 adolescents and adults with 22q11DS, assessing ASDs using either stringent Collaborative Program for Excellence in Autism (ASD-CPEA) criteria, or less stringent DSM-IV criteria alone (ASD-DSM-IV).
Results
Ten (32%) individuals met criteria for an SSD, five (16%) for ASD-CPEA, and five others (16%) for ASD-DSM-IV. Of those with ASD-CPEA, one (20%) met SSD criteria. Of those with ASD-DSM-IV, four (80%) met SSD criteria. Depressive disorders (8 individuals; 26%) and anxiety disorders (7; 23%) sometimes co-occurred with SSDs and ASDs. SSDs, ASDs, and anxiety occurred predominantly among males and depression predominantly among females.
Conclusions
Individuals with 22q11DS can manifest SSDs in the presence or absence of ASDs and other axis I diagnoses. The results suggest that standard clinical care should include childhood screening for ASDs, and later periodic screening for all axis I diagnoses.
Keywords: Schizophrenia and psychosis, Autism, Affective disorders, Anxiety disorders, Genetics
1. Introduction
22q11.2 deletion syndrome (22q11DS) is a chromosomal deletion disorder that occurs in approximately 1/4000 live births [9,27]. Somatic phenotypes associated with 22q11DS include facial dysmorphia, conotruncal heart anomalies, cleft palate, T-cell related immune deficiencies and parathyroid dysfunction. 22q11DS also associates with neurodevelopmental and behavioral disorders, including language impairments and specific learning disabilities [7,13,28], schizophrenia spectrum disorders (SSDs), autism spectrum disorders (ASDs), and a range of mood and anxiety symptoms and disorders [2,11,26,38,39].
Strong evidence supports an association between 22q11DS and risk for schizophrenia and related psychotic disorders. Following an initial report of early-onset psychosis in patients with velocardio-facial syndrome (VCFS; another name for 22q11DS) [36], Pulver et al. completed two landmark studies, examining psychiatric symptoms in adults with VCFS [30] and testing for 22q11DS in a cohort of patients ascertained for schizophrenia [14]. Pulver et al. [30] found that 29% of adults with VCFS, ranging in age from 17 to 41 years, had a schizophrenia or schizoaffective disorder. Karayiorgou et al. [14], using fluorescent in situ hybridization (FISH), identified two 22q11DS cases in 200 patients with schizophrenia. Subsequent FISH studies [6] or genome-wide scans of single-nucleotide polymorphisms [10,18] have verified that 0.3–1% of patients ascertained for schizophrenia have 22q11DS, a rate least 15 times higher than in the general population. Patients with idiopathic schizophrenia and those with 22q11DS and schizophrenia exhibit highly similar symptom profiles [5]. Moreover, adolescents and adults with 22q11DS show a profile of prodromal symptoms comparable to that of individuals with schizotypal personality disorder, suggesting that the risk state, and possibly illness progression, is equivalent across groups [5,16,35].
Studies of children with 22q11DS consistently identify ASDs, although rates vary across studies. Vorstman et al. [38] evaluated 60 Dutch children and adolescents with FISH-confirmed 22q11DS, concluding that 30% of them satisfied DSM-IV criteria for an ASD. Antshel et al. [1] found that 17 of 41 children (41.5%) recruited from a 22q11DS clinic met criteria for an ASD, with eight (19.5%) meeting criteria for autistic disorder. In contrast, Fine et al. identified an ASD in only 14% of 98 children with 22q11DS [11]. While differences in ascertainment strategies or diagnostic evaluation methods might contribute to varying rates of ASDs, the evidence clearly suggests that ASDs occur in a substantial minority of individuals with 22q11DS. Further evidence of an association between 22q11DS and ASDs comes from large-scale studies of copy number variation in which genome-wide analysis identified previously undiagnosed deletions at 22q11.2 in patients with ASDs at a rate of approximately 1% [19,34].
Other studies of psychopathology in 22q11DS have described an increased prevalence of mood disorders, including unipolar and bipolar depression [3,4,29], and a variety of anxiety disorders [4]. For example, Baker et al. [4] found the respective odds of a mood or anxiety disorder to be 2.6 or 2.0 times higher among individuals with 22q11DS compared to controls. While many studies have assessed the occurrence of specific psychiatric diagnoses in 22q11DS, there are surprisingly few studies that have specifically assessed psychiatric co-morbidities.
The primary goal of the current study was to evaluate occurrence and co-occurrence of SSDs, ASDs and other DSM-IV axis I diagnoses in adolescents and young adults with FISH-confirmed 22q11DS. Secondary goals were to assess whether ASD severity was associated with probability of an SSD diagnosis and to explore sex distributions among diagnostic categories.
2. Subjects and methods
2.1. Subjects
Participants were ascertained in reverse-age order (oldest to youngest) from a 22q11DS case registry at Children’s Health Care of Atlanta, with two additional participants referred by local psychiatrists (total n = 32). Genetic diagnoses were confirmed with FISH using the standard TUPLE probe, which does not distinguish between the most common 3-Mb deletion and the second-most common 1.5-Mb deletion. All study participants or legal guardians provided written informed consent and/or assent following a protocol approved by the Emory University Institutional Review Board. The current analysis is based on 31 of 32 individuals (14 males, 17 females), as one participant was excluded due to an absence of spoken language that precluded completion of all assessments. Race/ethnicity included 81% (n = 25) Caucasian, 10% (n = 3) African American, 6% (n = 2) Hispanic, and 3% (n = 1) East Asian. The average age was 19.3 (SD = 4.1) with a range of 14 to 29 years. The average education level was 11.5 (SD = 2.2) years.
2.2. Estimated verbal IQ
All participants completed subtests from the Wechsler Adult Intelligence Scale-Second Edition (WAIS-II) or the Wechsler Intelligence Scale for Children-Third Edition (WISC-III). To derive an estimated verbal IQ, the vocabulary and similarities subtest t-scores were converted to standard scores and averaged (i.e., 85.5, SD = 16.3). Seventeen individuals had estimated verbal IQs of 85 or below, nine had estimates between 85 and 100, and five had estimates above 100.
2.3. Diagnostic assessments
Diagnostic assessments, behavioral questionnaire data, medical history, and neuropsychological data were collected during a one-day office visit as described in [32]. Diagnoses were determined in a consensus meeting involving a psychiatrist, a Ph.D.-level psychologist, and a masters-level research assistant, based on the following assessments: the Structured Clinical Interview for DSM-IV (SCID-I) [12], the Structured Interview for Prodromal Syndromes (SIPS) [22–24], the Autism Diagnostic Interview-Revised (ADI-R) [21], and the Autism Diagnostic Observation Schedule (ADOS) [20]. The currrent study employed module 4 of the ADOS [8] for all participants but one, who received a module 3 assessment.
2.4. Diagnostic categories
For the purposes of this study, participants were considered to have a schizophrenia spectrum disorder (SSD) if they met SCID-I criteria for schizophrenia, schizoaffective disorder or psychotic disorder – not otherwise specified (NOS), or if they met SIPS criteria for schizotypal personality disorder or any of the SIPS-defined clinical high-risk (CHR) syndromes (i.e., psychotic syndrome, brief intermittent psychotic symptom syndrome, attenuated positive symptom (APS) syndrome, or genetic risk and deterioration syndrome) [23]. Defining a broad group of SSDs allowed for the greatest overlap between SSDs and ASDs, thereby increasing confidence that non-overlapping cases represented genuinely distinct diagnostic groups.
We used two definitions for identifying ASDs. First, we applied a stringent definition for ASDs, following the criteria of the Collaborative Programs of Excellence in Autism (CPEA). A CPEA diagnosis of an ASD requires that:
the individual attain cut-off scores for autism or PDD-NOS from the ADI-R and the ADOS;
the clinician’s best estimate diagnosis is autism, Asperger’s, or PDD-NOS according to DSM-IV criteria.
In accordance to methods used in the Simons Simplex Collection study (Simons Foundation Autism Research Initiative; http://sfari.org/resources/sfari-base), we followed a hierarchical classification yielding diagnoses of autism, Asperger’s disorder, or PDD-NOS. This diagnostic category will be referred to as “ASD-CPEA.” We also identified individuals who met DSM-IV-defined autistic disorder, Asperger’s disorder, or pervasive developmental disorder – not otherwise specified, but who did not meet the ASD-CPEA criteria. These diagnoses were decided upon according to the study team’s best estimate, based on all available information. This diagnostic category will be referred to as “ASD-DSM-IV.”
Participants were considered to have a depressive or anxiety-related diagnosis if they met DSM-IV criteria for a mood or anxiety disorder based on the SCID-I. For one individual who completed only part of the SCID (due to emotional distress), diagnoses were formulated using all information available from the partially completed SCID, from self-report questionnaires such as the Beck Depression Inventory and Achenbach Adult Self-Report, and from the SIPS, ADI-R, and ADOS.
2.5. Data analysis
We used SPSS (Version 20) for all data analyses. We examined the proportions of individuals with SSDs, ASDs and other axis I diagnoses and assessed the degree of co-occurrence among these diagnoses. We used a t-test to compare mean verbal IQ estimates between those who did and did not meet criteria for axis I disorders. Since schizophrenia symptoms usually manifest in late adolescence and early adulthood, a one-way between subjects ANOVA was performed to compare the mean age between those with a schizophrenia diagnosis (n = 3), those who were clinically high-risk (n = 7) and those without an SSD (n = 21). The SSD subgroups were collapsed and a t-test was performed to compare the mean age of those with and without an SSD. T-tests were performed comparing the mean age of males and females overall and within the SSD and non-SSD subgroups.
To evaluate whether variation in ASD symptom severity associated with the likelihood of an SSD diagnosis, we examined ADOS total algorithm scores from the 30 participants who completed module 4; data from the one individual completing module 3 were excluded because the scoring algorithm for module 3 differs from that of module 4. We compared mean module 4 total scores between those with and without SSDs and, further breaking down the SSD group, those with CHR SIPS diagnoses or schizotypal personality disorder (n = 7) compared to those without SSD (n = 21). The schizophrenia diagnosis subgroup (n = 2 after exclusion of the participant who completed ADOS module 3) was not included in the comparative analysis of ADOS scores due to the small sample size. We next performed two binary logistic regression analyses:
a simple analysis with an SSD diagnosis (yes/no) as the outcome and ADOS module 4 total as the predictor;
a repeat of this analysis while accounting for verbal IQ, gender and age.
These covariates were selected to allow for comparability with previous research [37].
Fisher’s exact tests evaluated male-female ratios for each diagnosis as compared to the male-female ratio for the rest of the sample (i.e., those without the diagnosis in question). For the ASD diagnoses, ASD-CPEA and ASD-DSM-IV were grouped together and compared to those with no ASD diagnosis.
3. Results
Of 31 participants, 32% (n = 10) met criteria for an SSD, which includes those who met criteria for schizoaffective disorder and CHR syndromes. Furthermore, 16% (n = 5) met criteria for ASD-CPEA and an additional 16% (n = 5) met criteria for ASD-DSM-IV. Considering other axis I disorders, 26% (n = 8) met criteria for a depressive disorder and 23% (n = 7) met criteria for an anxiety-related disorder. Twenty-six percent of participants (n = 8) did not meet criteria for a psychiatric disorder (Table 1).
Table 1.
Sex, age, and psychiatric diagnoses of adolescents and young adults with 22q11 deletion syndrome.
| ID | Male | Female | Age | Schizophrenia spectrum | Autism spectrum | Depressive disorder | Anxiety disorder |
|---|---|---|---|---|---|---|---|
| 1 | X | 21 | Schizotypal, prodromal | ||||
| 2 | X | 17 | Schizotypal | ||||
| 3 | X | 27 | Schizoaffective | ||||
| 4 | X | 17 | PDD-NOS-CPEA | ||||
| 5 | X | 18 | PDD-NOS-CPEA | ||||
| 6 | X | 19 | Autism-CPEA | ||||
| 7 | X | 20 | Autism-CPEA | ||||
| 8 | X | 16 | PDD-NOS-DSM-IV | ||||
| 9 | X | 20 | Depressive disorder NOS | ||||
| 10 | X | 22 | Depressive disorder NOS | ||||
| 11 | X | 29 | Depressive disorder NOS | ||||
| 12 | X | 17 | Major depressive disorder | ||||
| 13 | X | 22 | Bipolar disorder | ||||
| 14 | X | 15 | Specific phobia | ||||
| 15 | X | 21 | OCD | ||||
| 16 | X | 15 | Schizoaffective | PDD-NOS-CPEA | OCD, specific phobia | ||
| 17 | X | 20 | Prodromal | PDD-NOS-DSM-IV | |||
| 18 | X | 26 | Schizoaffective, schizotypal | PDD-NOS-DSM-IV | |||
| 19 | X | 17 | Schizotypal | PDD-NOS-DSM-IV | Depressive disorder NOS | ||
| 20 | X | 24 | Prodromal | PDD-NOS-DSM-IV | Major depressive disorder | Social phobia | |
| 21 | X | 15 | Prodromal | Social phobia | |||
| 22 | X | 15 | Prodromal | OCD | |||
| 23 | X | 21 | Major depressive disorder | Specific phobia | |||
| 24–31 | 2 | 6 | 18.1 (4.6) | Criteria not met for any axis I diagnosis or prodromal syndrome |
Identified schizophrenia spectrum disorders (SSDs) included schizotypal personality disorder, prodromal syndrome, and schizoaffective disorder. Identified autism spectrum disorders (ASDs) included autism and pervasive developmental disorder – not otherwise specified (PDD-NOS) and were evaluated using both DSM-IV diagnostic criteria and the Collaborative Programs of Excellence in Autism (CPEA) diagnostic criteria, which requires individuals to meet ASD criteria on the ADI-R, ADOS and clinician’s best judgment. Depressive disorders included major depressive disorder, bipolar disorder, and depressive disorder – not otherwise specified (NOS). Anxiety disorders included specific phobia, obsessive-compulsive disorder (OCD), and social phobia. There were eight individuals, ranging in age from 14–26 years (mean = 18.1, SD = 4.6), who did not meet criteria for any psychiatric diagnosis.
Verbal IQ scores were not significantly different between those who met criteria for a psychiatric disorder compared to those who did not (mean = 85.0, SD = 22.5; mean = 86.5 SD = 12.0, respectively; t = 0.21, df = 29, P = 0.84). Furthermore, there was no significant difference in the mean age between those with a schizoaffective disorder (n = 3; mean age = 22.7, SD = 6.7), those with CHR, (n = 7; mean age = 18.4, SD = 3.4), and those without any SSD (n = 21; mean age = 19.1, SD = 4.0; F(2, 28) = 1.17, P = 0.32). Considering SSDs as a whole, the age difference between those with and without an SSD remained non-significant (SSD mean age = 19.7 years; SD = 4.6; t = 0.345, df = 29, P = 0.73). Additionally, there was not a significant age difference between males and females when evaluated overall (male mean age = 19.21, SD = 4.15; female mean age = 19.41 SD = 4.25; t = 0.13, df = 29, P = 0.77), within the SSD subgroup (SSD male mean age = 20.13 SD = 4.91; female mean age = 18.00 SD = 4.24; t = −0.56, df = 8, P = 0.48), nor within the non-SSD subgroup (non-SSD male mean age = 18.00 SD = 2.83; female mean age = 19.60 SD = 4.37; t = 0.83, df = 19, P = 0.24).
Twenty-six percent (n = 8) of the sample met criteria for two or more diagnoses. The majority of these individuals (n = 7 of 8) met criteria for an SSD. Sixty three percent (n = 5 of 8) of the co-morbid individuals met criteria for both an SSD and an ASD (CPEA or DSM-IV defined). Therefore, of the 10 individuals meeting criteria for an SSD, half also met criteria for an ASD (n = 1 CPEA-ASD, n = 4 DSM-IV ASD). Further breaking down the overlap between ASDs and SSDs, we found that only one of five individuals with ASD-CPEA had additional diagnoses (i.e. schizoaffective disorder and anxiety-related disorder) while the rest had no other diagnosis. However, four of the five individuals with ASD-DSM-IV defined, 80% (n = 4 of 5) exhibited a co-morbid SSD diagnosis (i.e. schizoaffective, schizotypal personality disorder, or CHR syndrome).
The association between ASD symptom severity, measured using the total algorithm score on module 4 of the ADOS, and the likelihood of an SSD diagnosis was also assessed. The average ADOS module 4 score was not significantly different between the SSD and non-SSD groups (SSD group = 7.0, SD = 3.42; non-SSD group = 5.0, SD = 3.56; P = 0.17). CHR individuals within the SSD group (n = 7) had an average ADOS module 4 score of 7.4 (3.8), which was also not significantly different from the non-SSD group (P = 0.14). Results from the binary logistic regression demonstrated that total ADOS score did not predict an SSD diagnosis by itself (P = 0.17), or after adjusting for verbal IQ, gender and age (P = 0.37).
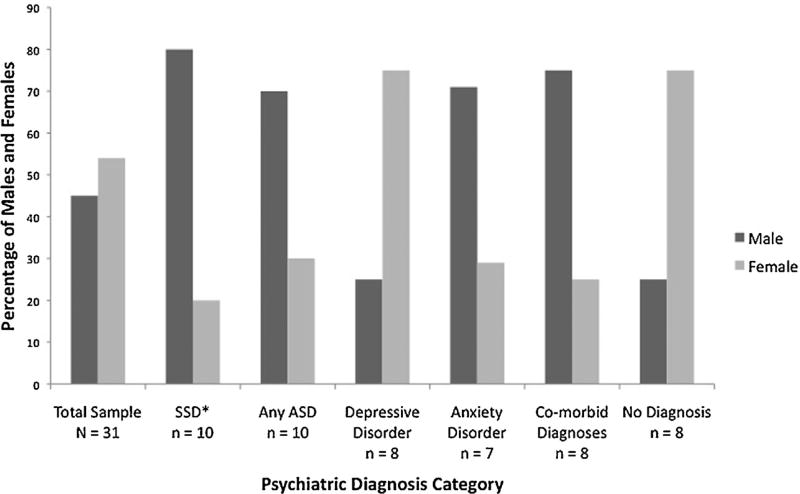
We examined the male to female ratio for each diagnostic category and, using Fisher’s exact test, also determined if the male to female ratio differed from that for the remaining individuals (i.e., those who did not meet criteria for the diagnosis in question). Although our overall sample consisted of 14 males and 17 females, the male to female sex ratios across the diagnostic subgroups varied substantially (Fig. 1). The SSD subgroup was comprised of more males than females (i.e., 8 males and 3 females; ratio = 2.7:1), and was significantly different than the ratio of those without an SSD (P = 0.018). Combining the CPEA and DSM-IV ASD groups and comparing the sex ratio for any ASD diagnosis (i.e., 7 males and 3 females; ratio = 2.3:1) against the ratio for those who did not meet criteria for an ASD diagnosis did not reach statistical significance (P = 0.12). Further examination showed that, within the ASD-CPEA diagnostic subgroup, there were three males and two females (ratio = 1.5:1); whereas, within the ASD-DSM-IV diagnostic subgroup there were four males and one female (ratio = 4:1). Fewer males than females had depressive disorders (i.e., 2 males and 6 females; ratio = 1:3), while more males than females had anxiety-related disorders (i.e., 5 males and 2 females; ratio = 2.5:1); however, these ratios were not significantly different from those who did not have these diagnoses (P = 0.24 for depression; P = 0.20 for anxiety). Six males and two females (ratio = 3:1) received co-morbid psychiatric diagnoses. This ratio did not differ significantly from the male to female ratio of those receiving one or no diagnosis (P = 0.24). Of those receiving no psychiatric diagnosis, the majority was female (i.e., 2 males and 6 females; ratio = 1:3; P = 0.10).
Fig. 1.
Percent of males and females within each diagnostic category. The percent of males and females is shown for the following: the total sample, those meeting criteria for a schizophrenia spectrum disorder (SSD), an autism spectrum disorder (diagnosed with CPEA-defined or DSM-IV-defined criteria), a depressive disorder, and an anxiety disorder, as well as those meeting criteria for more than one psychiatric disorder (co-morbid diagnoses) or no psychiatric disorder (no diagnosis). Using Fisher’s exact test, the male to female ratio for the SSD group was significantly different than the male to female ratio for all research participants without an SSD diagnosis (P < 0.05). Other comparisons did not reach significance at the P < 0.05 level.
4. Discussion
This study confirms that 22q11DS associates with a broad variety of DSM-IV diagnoses, which can occur separately or as co-morbid conditions. The majority of the individuals in the study, 74% (n = 23 of 31), met criteria for at least one psychiatric diagnosis, with 26% (n = 8 of 31) of all individuals receiving co-morbid diagnoses. An SSD diagnosis was found in 32% (n = 10 of 31) of the individuals, a majority of whom also met criteria for additional psychiatric diagnoses (70%; n = 7 of 10). For those with an SSD, 50% also met criteria for an ASD, 20% met criteria for a depressive disorder, and 40% met criteria for an anxiety-related disorder. For the autism-related diagnoses, 16% of the entire sample (n = 5 of 31) met a strict research definition for an ASD (i.e., ASD-CPEA). An additional 16% (n = 5 of 31), who did not meet the research based CPEA-ASD criteria, met criteria for a best estimate ASD using DSM-IV criteria, which is the diagnostic method used in current clinical practice. As has been reported by others [4,7], we identified a substantial proportion of 22q11DS patients with depressive (26%; n = 8 of 31) or anxiety (23%; n = 7 of 31) disorders, including major depression, depressive disorder – not otherwise specified, specific phobia, and obsessive-compulsive disorder.
While there was substantial overlap between SSDs and ASDs when considering all ASD diagnoses (i.e., both CPEA and DSM-IV defined ASD), the overlap was less substantial when considering only those who met stringent ASD-CPEA criteria. Assessing the ASD-SSD overlap by ASD criteria grouping, we found that 20% (n = 1 of 5) of the individuals receiving an ASD-CPEA diagnosis also met criteria for an SSD (specifically, schizoaffective disorder); whereas, 80% (n = 4 of 5) of the individuals with an ASD-DSM-IV diagnosis had a co-morbid SSD. Thus, a novel finding of the study is that when stringent research diagnostic criteria are used for determining diagnostic classification, the diagnoses of ASDs and SSDs are more clearly separated though co-morbidity still occurs. These findings, if replicated in larger samples, would favor the hypothesis that 22q11DS associates with risk for an ASD diagnosis distinct from an SSD rather than the hypothesis that the association between 22q11DS and ASDs merely reflects manifestations of SSD symptomatology, as suggested, for example, by Karayiorgou et al. [15]. The finding that ASD symptom severity was not a significant predictor of likelihood of an SSD diagnosis is in accordance with previous research [37] and further supports the hypothesis that 22q11DS associates with ASDs independently of its association with SSDs. However, the limited sample size should be taken into account. Large prospective studies explicitly examining the overlap between SSD and ASD symptoms longitudinally in 22q11DS are needed.
Co-morbidity was observed between SSDs and depression and anxiety. Furthermore, depression and anxiety disorders also occurred independently. These findings suggest clinical psychiatric heterogeneity in 22q11DS. Alternatively, the constellation of symptoms across multiple diagnostic categories (i.e., depression and anxiety) could in some cases represent precursors to an SSD.
Although the size of our sample precludes definitive conclusions, the sex ratio distribution we observed within diagnostic subgroups may prove helpful in defining at-risk subgroups within 22q11DS. In particular, more males than females met SSD diagnostic criteria, and a comparison of the male to female ratio for those with an SSD (i.e., 2.7:1) to the ratio for those without a diagnosis of SSD (i.e., 1:2.3) revealed a statistically significant difference. We also found that more males than females had an ASD or anxiety-related diagnosis, and that more females than males had a depression diagnosis or no psychiatric diagnosis, although these findings did not reach statistical significance. These preliminary results, however, suggest a moderating role of sex on psychiatric outcomes in 22q11DS.
Due to the cross-sectional design of our study, we can only report the current presentation of psychiatric symptoms and do not know if new symptoms or diagnoses will emerge with the passage of time. Thus, the question remains as to whether the diagnostic classification of individuals will evolve or remain stable as they age and enter middle adulthood and beyond. As an indirect way of addressing this limitation, we examined the possibility that the presence of an SSD, which usually emerges in adolescence or early adulthood, associated with age; no significant age differences existed between those with and without an SSD nor between the CHR subgroup and those without an SSD.
5. Conclusion
Our current results corroborate prior evidence that a number of distinct psychiatric outcomes associate with 22q11DS, but the investigation of psychiatric and neurodevelopmental outcomes in 22q11DS remains incomplete. 22q11DS appears to predispose individuals to a variety of syndromic presentations that sometimes share clinical features, such as the social impairments observed in SSDs and ASDs [17,25,31]; however, we do not have sufficiently large studies investigating the developmental trajectories and emergence of psychiatric illness in 22q11DS to fully characterize the overlap or distinction between SSDs and ASDs. Moreover, we do not know which risk factors are the best predictors of the most debilitating psychiatric outcomes. The current study highlights the importance of considering that a range of neurodevelopmental and psychiatric outcomes associate with the 22q11 deletion, that multiple psychiatric disorders can co-occur in the same individual, and that models of psychiatric risk in 22q11DS will likely need to consider how sex and other biological factors contribute to risk outcomes [33]. Finally, our results emphasize the need for clinicians to screen broadly across all major domains of psychopathology when monitoring patients with 22q11DS across the lifespan [7].
Acknowledgments
O.Y. Ousley Clinical trials: Collaborator in BioMarin Pharmaceutical study.
J.F. Cubells Clinical trials: Co-investigator for Seaside Therapeutics, Roche, Schering-Plough, Lilly, and Biomarin. Advisor Abott Laboratories.
Grant support: Robert W. Woodruff Fund (J.F. Cubells, O.Y. Ousley), NARSAD (O.Y. Ousley), the Autism Foundation of Georgia (J.F. Cubells), NIH Medical Scientist Training grant T32 GM008169 (E. Smearman), and the Burroughs Wellcome Fund (Grant number 008188; E. Smearman). A. Nichole Evans and Danielle Tidwell provided research assistance.
Footnotes
Disclosure of interest
E. Smearman, E.F. Walker, K. Coleman, K.A. Rockers, S. Fernandez-Carriba declare that they have no conflicts of interest concerning this article.
Portions of these data were presented at the 8th Biennial 22q11.2 Deletion Syndrome Meeting, Orlando, Florida, July 8, 2012.
References
- 1.Antshel KM, Aneja A, Strunge L, Peebles J, Fremont WP, Stallone K, et al. Autistic spectrum disorders in velo-cardio facial syndrome (22q11.2 deletion) J Autism Dev Disord. 2007;37(9):1776–86. doi: 10.1007/s10803-006-0308-6. [Epub 2006/12/21] [DOI] [PubMed] [Google Scholar]
- 2.Antshel KM, Fremont W, Roizen NJ, Shprintzen R, Higgins AM, Dhamoon A, et al. ADHD, major depressive disorder, and simple phobias are prevalent psychiatric conditions in youth with velo-cardio-facial syndrome. J Am Acad Child Adolesc Psychiatry. 2006;45(5):596–603. doi: 10.1097/01.chi.0000205703.25453.5a. [Epub 2006/05/04] [DOI] [PubMed] [Google Scholar]
- 3.Arnold PD, Siegel-Bartelt J, Cytrynbaum C, Teshima I, Schachar R. Velo-cardiofacial syndrome: Implications of microdeletion 22q11 for schizophrenia and mood disorders. Am J Med Genet. 2001;105(4):354–62. doi: 10.1002/ajmg.1359. [Epub 2001/05/30] [DOI] [PubMed] [Google Scholar]
- 4.Baker KD, Skuse DH. Adolescents and young adults with 22q11 deletion syndrome: psychopathology in an at-risk group. Br J Psychiatry. 2005;186:115–20. doi: 10.1192/bjp.186.2.115. [Epub 2005/02/03] [DOI] [PubMed] [Google Scholar]
- 5.Bassett AS, Chow EW, AbdelMalik P, Gheorghiu M, Husted J, Weksberg R. The schizophrenia phenotype in 22q11 deletion syndrome. Am J Psychiatry. 2003;160(9):1580–6. doi: 10.1176/appi.ajp.160.9.1580. [Epub 2003/08/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Bassett AS, Costain G, Alan Fung WL, Russell KJ, Pierce L, Kapadia R, et al. Clinically detectable copy number variations in a Canadian catchment population of schizophrenia. J Psychiatr Res. 2010;44(15):1005–9. doi: 10.1016/j.jpsychires.2010.06.013. [Epub 2010/07/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Bassett AS, McDonald-McGinn DM, Devriendt K, Digilio MC, Goldenberg P, Habel A, et al. Practical guidelines for managing patients with 22q11.2 deletion syndrome. J Pediatr. 2011;159(2):e1332–9. doi: 10.1016/j.jpeds.2011.02.039. [Epub 2011/05/17] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Bastiaansen JA, Meffert H, Hein S, Huizinga P, Ketelaars C, Pijnenborg M, et al. Diagnosing autism spectrum disorders in adults: the use of Autism Diagnostic Observation Schedule (ADOS) module 4. J Autism Dev Disord. 2011;41(9):1256–66. doi: 10.1007/s10803-010-1157-x. [Epub 2010/12/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Botto LD, May K, Fernhoff PM, Correa A, Coleman K, Rasmussen SA, et al. A population-based study of the 22q11.2 deletion: phenotype, incidence, and contribution to major birth defects in the population. Pediatrics. 2003;112(1 Pt 1):101–7. doi: 10.1542/peds.112.1.101. [Epub 2003/07/03] [DOI] [PubMed] [Google Scholar]
- 10.Consortium IS. Rare chromosomal deletions and duplications increase risk of schizophrenia. Nature. 2008;455(7210):237–41. doi: 10.1038/nature07239. [Epub 2008/08/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Fine SE, Weissman A, Gerdes M, Pinto-Martin J, Zackai EH, McDonald-McGinn DM, et al. Autism spectrum disorders and symptoms in children with molecularly confirmed 22q11.2 deletion syndrome. J Autism Dev Disord. 2005;35(4):461–70. doi: 10.1007/s10803-005-5036-9. [Epub 2005/09/01] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gibbon M, Williams JBW. First MBSR. Structured clinical interview for DSM-IV-TR axis I disorders, research version, patient edition (SCID-I/P) New York: Biometrics Research, New York State Psychiatric Institute; 2002. [Google Scholar]
- 13.Gothelf D, Frisch A, Michaelovsky E, Weizman A, Shprintzen RJ. Velo-cardiofacial syndrome. J Ment Health Res Intellect Disabil. 2009;2(2):149–67. doi: 10.1080/19315860902756136. [Epub 2010/01/30] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karayiorgou M, Morris MA, Morrow B, Shprintzen RJ, Goldberg R, Borrow J, et al. Schizophrenia susceptibility associated with interstitial deletions of chromosome 22q11. Proc Natl Acad Sci U S A. 1995;92(17):7612–6. doi: 10.1073/pnas.92.17.7612. [Epub 1995/08/15] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karayiorgou M, Simon TJ, Gogos JA. 22q11.2 microdeletions: linking DNA structural variation to brain dysfunction and schizophrenia. Nat Rev Neurosci. 2010;11(6):402–16. doi: 10.1038/nrn2841. [Epub 2010/05/21] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kates WR, Antshel KM, Faraone SV, Fremont WP, Higgins AM, Shprintzen RJ, et al. Neuroanatomic predictors to prodromal psychosis in velo-cardio-facial syndrome (22q11.2 deletion syndrome): a longitudinal study. Biol Psychiatry. 2011;69(10):945–52. doi: 10.1016/j.biopsych.2010.10.027. [Epub 2011/01/05] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.King BH, Lord C. Is schizophrenia on the autism spectrum? Brain Res. 2011;1380:34–41. doi: 10.1016/j.brainres.2010.11.031. [Epub 2010/11/17] [DOI] [PubMed] [Google Scholar]
- 18.Levinson DF, Duan J, Oh S, Wang K, Sanders AR, Shi J, et al. Copy number variants in schizophrenia: confirmation of five previous findings and new evidence for 3q29 microdeletions and VIPR2 duplications. Am J Psychiatry. 2011;168(3):302–16. doi: 10.1176/appi.ajp.2010.10060876. [Epub 2011/02/03] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Levy D, Ronemus M, Yamrom B, Lee YH, Leotta A, Kendall J, et al. Rare de novo and transmitted copy number variation in autistic spectrum disorders. Neuron. 2011;70(5):886–97. doi: 10.1016/j.neuron.2011.05.015. [Epub 2011/06/11] [DOI] [PubMed] [Google Scholar]
- 20.Lord C, Risi S, Lambrecht L, Cook EH, Jr, Leventhal BL, DiLavore PC, et al. The autism diagnostic observation schedule-generic: a standard measure of social and communication deficits associated with the spectrum of autism. J Autism Dev Disord. 2000;30(3):205–23. [PubMed] [Google Scholar]
- 21.Lord C, Rutter M, Le Couteur A. Autism diagnostic interview-revised: a revised version of a diagnostic interview for caregivers of individuals with possible pervasive developmental disorders. J Autism Dev Disord. 1994;24(5):659–85. doi: 10.1007/BF02172145. [Epub 1994/10/01] [DOI] [PubMed] [Google Scholar]
- 22.McGlashan TH, Miller TJ, Woods SW. Pre-onset detection and intervention research in schizophrenia psychoses: current estimates of benefit and risk. Schizophr Bull. 2001;27(4):563–70. doi: 10.1093/oxfordjournals.schbul.a006896. [Epub 2002/02/05] [DOI] [PubMed] [Google Scholar]
- 23.Miller TJ, McGlashan TH, Rosen JL, Cadenhead K, Ventura J, McFarlane W, et al. Prodromal assessment with the structured interview for prodromal syndromes and the scale of prodromal symptoms: predictive validity, interrater reliability, and training to reliability. Schizophr Bull. 2003;29(4):703–15. doi: 10.1093/oxfordjournals.schbul.a007040. [DOI] [PubMed] [Google Scholar]
- 24.Miller TJ, McGlashan TH, Rosen JL, Somjee L, Markovich PJ, Stein K, et al. Prospective diagnosis of the initial prodrome for schizophrenia based on the structured interview for prodromal syndromes: preliminary evidence of interrater reliability and predictive validity. Am J Psychiatry. 2002;159(5):863–5. doi: 10.1176/appi.ajp.159.5.863. [DOI] [PubMed] [Google Scholar]
- 25.Moreno-De-Luca D, Cubells JF. Copy number variants: a new molecular frontier in clinical psychiatry. Curr Psychiatry Rep. 2011;13(2):129–37. doi: 10.1007/s11920-011-0183-5. [Epub 2011/01/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Niklasson L, Rasmussen P, Oskarsdottir S, Gillberg C. Neuropsychiatric disorders in the 22q11 deletion syndrome. Genet Med. 2001;3(1):79–84. doi: 10.1097/00125817-200101000-00017. [Epub 2001/05/08] [DOI] [PubMed] [Google Scholar]
- 27.Oskarsdottir S, Vujic M, Fasth A. Incidence and prevalence of the 22q11 deletion syndrome: a population-based study in Western Sweden. Arch Dis Child. 2004;89(2):148–51. doi: 10.1136/adc.2003.026880. [Epub 2004/01/23] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ousley O, Rockers K, Dell ML, Coleman K, Cubells JF. A review of neurocognitive and behavioral profiles associated with 22q11 deletion syndrome: implications for clinical evaluation and treatment. Curr Psychiatry Rep. 2007;9(2):148–58. doi: 10.1007/s11920-007-0085-8. [Epub 2007/03/29] [DOI] [PubMed] [Google Scholar]
- 29.Papolos DF, Faedda GL, Veit S, Goldberg R, Morrow B, Kucherlapati R, et al. Bipolar spectrum disorders in patients diagnosed with velo-cardio-facial syndrome: does a hemizygous deletion of chromosome 22q11 result in bipolar affective disorder? Am J Psychiatry. 1996;153(12):1541–7. doi: 10.1176/ajp.153.12.1541. [Epub 1996/12/01] [DOI] [PubMed] [Google Scholar]
- 30.Pulver AE, Nestadt G, Goldberg R, Shprintzen RJ, Lamacz M, Wolyniec PS, et al. Psychotic illness in patients diagnosed with velo-cardio-facial syndrome and their relatives. J Nerv Ment Dis. 1994;182(8):476–8. doi: 10.1097/00005053-199408000-00010. [Epub 1994/08/01] [DOI] [PubMed] [Google Scholar]
- 31.Rapoport J, Chavez A, Greenstein D, Addington A, Gogtay N. Autism spectrum disorders and childhood-onset schizophrenia: clinical and biological contributions to a relation revisited. J Am Acad Child Adolesc Psychiatry. 2009;48(1):10–8. doi: 10.1097/CHI.0b013e31818b1c63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Rockers K, Ousley O, Sutton T, Schoenberg E, Coleman K, Walker E, et al. Performance on the modified card sorting test and its relation to psychopathology in adolescents and young adults with 22q11.2 deletion syndrome. J Intellect Disabil Res. 2009;53(7):665–76. doi: 10.1111/j.1365-2788.2009.01178.x. [Epub 2009/05/23] [DOI] [PubMed] [Google Scholar]
- 33.Ross H, Guo Y, Coleman K, Ousley O, Miller A. Association of IL-12p70 and IL-6:IL-10 ratio with autism-related behaviors in 22q11.2 deletion syndrome: a preliminary report. Brain Behav Immun. 2013 doi: 10.1016/j.bbi.2012.12.021. [Epub 2013/01/29] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Sanders SJ, Ercan-Sencicek AG, Hus V, Luo R, Murtha MT, Moreno-De-Luca D, et al. Multiple recurrent de novo CNVs, including duplications of the 7q11.23 Williams syndrome region, are strongly associated with autism. Neuron. 2011;70(5):863–85. doi: 10.1016/j.neuron.2011.05.002. [Epub 2011/06/11] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shapiro DI, Cubells JF, Ousley OY, Rockers K, Walker EF. Prodromal symptoms in adolescents with 22q11.2 deletion syndrome and schizotypal personality disorder. Schizophr Res. 2011;129(1):20–8. doi: 10.1016/j.schres.2011.03.030. [Epub 2011/04/22] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Shprintzen RJ, Goldberg R, Golding-Kushner KJ, Marion RW. Late-onset psychosis in the velo-cardio-facial syndrome. Am J Med Genet. 1992;42(1):141–2. doi: 10.1002/ajmg.1320420131. [Epub 1992/01/01] [DOI] [PubMed] [Google Scholar]
- 37.Vorstman JA, Breetvelt EJ, Thode KI, Chow EW, Bassett AS. Expression of autism spectrum and schizophrenia in patients with a 22q11.2 deletion. Schizophr Res. 2013;143(1):55–9. doi: 10.1016/j.schres.2012.10.010. [Epub 2012/11/17] [DOI] [PubMed] [Google Scholar]
- 38.Vorstman JA, Morcus ME, Duijff SN, Klaassen PW, Heineman-de Boer JA, Beemer FA, et al. The 22q11.2 deletion in children: high rate of autistic disorders and early-onset of psychotic symptoms. J Am Acad Child Adolesc Psychiatry. 2006;45(9):1104–13. doi: 10.1097/01.chi.0000228131.56956.c1. [Epub 2006/08/24] [DOI] [PubMed] [Google Scholar]
- 39.Young AS, Shashi V, Schoch K, Kwapil T, Hooper SR. Discordance in diagnoses and treatment of psychiatric disorders in children and adolescents with 22q11.2 deletion syndrome. Asian J Psychiatry. 2011;4(2):119–24. doi: 10.1016/j.ajp.2011.03.002. [Epub 2011/07/12] [DOI] [PMC free article] [PubMed] [Google Scholar]