Abstract
Background:
The Multiple Sclerosis Severity Score (MSSS) is obtained by normalising the Expanded Disability Status Scale (EDSS) score for disease duration and has been a valuable tool in cross-sectional studies.
Objective:
To assess whether use of age rather than the inherently ambiguous disease duration was a feasible approach.
Method:
We pooled disability data from three population-based cohorts and developed an Age Related Multiple Sclerosis Severity (ARMSS) score by ranking EDSS scores based on the patient’s age at the time of assessment. We established the power to detect a difference between groups afforded by the ARMSS score and assessed its relative consistency over time.
Results:
The study population included 26058 patients from Sweden (n = 11846), Canada (n = 6179) and the United Kingdom (n = 8033). There was a moderate correlation between EDSS and disease duration (r = 0.46, 95% confidence interval (CI): 0.45–0.47) and between EDSS and age (r = 0.44, 95% CI: 0.43–0.45). The ARMSS scores showed comparable power to detect disability differences between groups to the updated and original MSSS.
Conclusion:
Since age is typically unbiased and readily obtained, and the ARMSS and MSSS were comparable, the ARMSS may provide a more versatile tool and could minimise study biases and loss of statistical power caused by inaccurate or missing onset dates.
Keywords: MSSS, EDSS, multiple sclerosis, disease severity, disability
Introduction
Kurtzke’s Expanded Disability Status Scale (EDSS) score1 is the most widely used measure of disability in multiple sclerosis (MS). The EDSS is a standard outcome in MS clinical trials and commonly used in natural history studies and often applied in clinical practice to monitor patient’s progression over time.
In 2005, Roxburgh et al.2 proposed the MS Severity Score (MSSS), a useful method of using and comparing cross-sectional patient-level disability (EDSS) data. An MSSS score was assigned according to how a patient’s EDSS ranked in comparison to all those patients with similar disease duration (±2 years). This offered advantages over other more crude approaches, such as the progression index (PI), which is estimated by dividing an individual’s EDSS by disease duration. The MSSS has been widely used in different settings,3–5 can be used in a variety of models and was shown to have improved statistical power, when compared to other available measures of disease progression, to detect differences in disability between groups of patients. One notable drawback of the approach is its reliance on the date of disease onset, which is generally assigned retrospectively and is necessarily imprecise. More importantly, an onset date is frequently missing or unobtainable in some data sets, resulting in loss of data and subsequently loss of statistical power.
Natural history studies have shown that many aspects of the clinical course of MS, including clinical symptoms,6 occurrence of relapses7 and disability progression, are associated with age.6,8–10 In light of these observations, we examined the suitability of using chronological age rather than years since date of onset in compensating for the effects of time on disability. To do this, we adapted the MSSS algorithm and developed an Age Related Multiple Sclerosis Severity (ARMSS) score by ranking EDSS scores based on the patient’s age at the time of assessment. Using age for the calculation of a severity score offers several advantages, not least of which are its availability, ease of measurement and absence of bias. In the work presented here, we have compared the proposed ARMSS score with its forerunner, the MSSS, and with other previously considered methods in terms of power to detect a difference in disability between groups. We also provided an updated version of the global MSSS and a new global ARMSS matrix by accessing three large population-based cohorts of MS patients.
Methods
Patients and data source
We used data from cohorts in Sweden, Canada (from the British Columbia MS database) and the United Kingdom (Cambridge MS cohort). These cohorts have been described before.6,7,11–13 Sex, date of birth, date of disease (first symptom) onset (recorded retrospectively through the neurologist–patient encounter), clinical course at onset (relapsing vs progressive), EDSS score and date of EDSS examination were obtained. From these cohorts, two data sets were constructed: cross-sectional and longitudinal. The cross-sectional data set was used for construction of the global ARMSS and the updated global MSSS matrices and included the most recent EDSS scores for all individuals in the three cohorts to ensure that individuals with varying rates of progression and different numbers of clinic visits contributed equally. The longitudinal data set comprised the Swedish and Canadian cohorts and contained serial EDSS scores recorded at different time points; this was used for testing the stability of the ARMSS.
Statistical analyses
As for derivation of the MSSS, the ARMSS was created using Weibull plotting positions, which is calculated as follows:
We also considered alternate versions of the ARMSS including the following:
Ranking within different age ranges. The original MSSS ranked EDSS scores within 2 years of disease duration. Since this can impact the power of the score within short time periods, we examined the performance of the ARMSS scores by ranking EDSS scores within the same year (±0), 2 years (±2) and 5 years (±5) of age.
Double ranked scores. These scores were calculated by ranking each EDSS score first by age and then by disease duration (range: ±2).
Creation of the global ARMSS matrix
A global ARMSS matrix was constructed using the cross-sectional data set. This matrix included the ARMSS scores obtained for EDSS scores recorded between ages of 18 and 75 years.
Update of the global MSSS matrix
We also calculated an updated version of the global MSSS matrix using the same (original) approach as reported previously.2
Comparison of power to detect a 0.5 EDSS score difference between two groups
The power of a study refers to the probability of correctly rejecting the null hypothesis of no difference between groups where there is a true effect. Comparisons of the power of the global ARMSS, the local ARMSS (i.e. scores calculated using a specific data set), the global and the local MSSS, PI, and the EDSS score were made in a simulation study. Since all of the scenarios proposed by Roxburgh et al.2 yielded similar results, we replicated only their first simulation scenario on one subset of randomly selected patients (n = 500) from the cross-sectional data set. The test sample was excluded from the data set before the construction of the global matrices and was doubled in size to create two identical groups of 500 patients each, here called exposed and non-exposed. The scenario simulated the situation in which exposure to a hypothetical risk factor (e.g. a genetic risk allele) in the exposed group resulted in a 0.5 increase in the EDSS score (the ‘effect’). An increased EDSS score in the exposed group was simulated in 10 gradual steps; each step represented a 0.5 increase in the EDSS in 10% of the exposed patients. The Wilcoxon–Mann–Whitney test was then performed on 5000 bootstrap samples and the number of significant p values (defined as < 0.05) were counted. The quotient of ‘significant’ p values divided by the number of tests determined the power of the score. The gradual increase allowed us to determine which score gave a greater number of significant p values as the proportion of exposed patients increased from zero. The half point EDSS score increases were not applied to EDSS scores of 0.0 and 9.5. For this simulation, the local ARMSS score was ranked within different age ranges (±0, ±2 and ±5 years).
To test whether the power was influenced by the age distribution, we compared the power of the global scores in three patient samples where the mean ages were 30 (n = 1000), 50 (n = 1000) and 70 (n = 1000). Power analyses were also performed on a randomly selected sample of patients (n = 1000) from the Swedish cohort with less than 1 year of exposure to a disease-modifying treatments (DMTs) before the last EDSS record, and on simulated samples with missing onset dates in 0%, 20% and 40% of individuals to explore whether exposure to DMTs and missing onset date can influence power.
Stability over time of the ARMSS and MSSS measurements
We measured the correlation between two succeeding scores to assess the long-term stability of the measurement. Spearman’s rank correlation coefficient between assessments at ages of 20, 25, 30, 35, 40, 45 and 50 and assessment at 5, 10 and 15 years later (±1 year) were calculated using the pooled longitudinal data set. Comparisons were made between the correlation coefficients for the global ARMSS score, updated and original MSSS and the double ranked score.
Statistical analyses were performed using Stata (StataCorp, 2009, Stata Statistical Software: Release 11; College Station, TX: StataCorp LP).
Ethical approval
Ethical approval was obtained from the respective regional ethical committees.
Results
Patient population
The final population included 26058 patients with clinically definite MS from Sweden (n = 11846, covering EDSS scores recorded between 1979 and 2014 period), Canada (n = 6179, 1980–2009 period) and the United Kingdom (n = 8033, 1991–2014 period). Characteristics of the three cohorts are shown in Tables 1 and 2. At the last recorded EDSS score, the mean age was 46.9 years (±12.4), the median EDSS score was 3.0 (interquartile range (IQR): 1.5–6.0) and the mean disease duration was 14 years (±10.8). Overall, 72.1% of the patients were female.
Table 1.
Demographic and clinical characteristics of the 26,058 included MS patient at the last recorded EDSS.
| Sweden, n (%) | UK, n (%) | Canada, n (%) | |
|---|---|---|---|
| Total sample size (n = 26,058) | 11846 (45.5%) | 8033 (30.8%) | 6179 (23.7%) |
| Females | 8470 (71.5%) | 5879 (73.1%) | 4463 (72.2%) |
| Clinical course | |||
| Relapse onset Progressive onset |
10850 (91.6%) 996 (8.4%) |
7293 (90.8%) 740 (9.2%) |
5604 (90.7%) 575 (9.3%) |
| Age, years | |||
| <20 20–29 30–39 40–49 50–59 60–69 ⩾70 |
90 (0.7%) 1132 (9.5%) 2489 (21.0%) 3094 (26.1%) 2807 (23.7%) 1752 (14.8%) 482 (4%) |
32 (0.4%) 555 (6.9%) 1909 (23.7%) 2587 (32.2%) 1827 (22.7%) 906 (11.2%) 217 (2.7%) |
11 (0.1%) 314 (5.0%) 1151 (18.6%) 1914 (30.9%) 1757 (28.4%) 774 (12.5%) 258 (4.1%) |
| Disease duration, years | |||
| <5 5 to <10 10 to <15 15 to <20 20 to <25 25 to <30 ⩾30 Missing |
2797 (23.6%) 2370 (20%) 1810 (15.2%) 1307 (11%) 1014 (8.5%) 705 (5.9%) 1172 (9.8%) 671 (5.6%) |
1568 (19.5%) 1876 (23.3%) 1578 (19.6%) 1103 (13.7%) 709 (8.8%) 513 (6.4%) 686 (8.5%) 0 (0%) |
706 (11.4%) 1265 (20%) 1131 (18.3%) 944 (15.2%) 696 (11.2%) 514 (8.3%) 713 (11.5%) 210 (3.4%) |
| EDSS category | |||
| 0–2.5 3.0–4.0 4.5–6.0 6.5–8.0 ⩾8.5 |
5963 (50.3%) 2104 (17.7%) 1623 (13.7%) 1807 (15.2%) 349 (2.95%) |
2768 (34.4%) 1682 (20.9%) 2102 (26.1%) 1405 (17.5%) 76 (0.95%) |
2339 (37.8%) 1169 (18.9%) 994 (16.1%) 1415 (22.9%) 262 (4.2%) |
| Original global MSSS (mean (95% CI)) | 4.03 (3.97–4.08) | 4.77 (4.71–4.83) | 4.73 (4.65–4.80) |
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis; CI: confidence Interval; MSSS: Multiple Sclerosis Severity Score.
Table 2.
Proportion of MS patients in each EDSS category by disease duration.
| Disease duration (years) | EDSS greater than or equal to | This study (%) |
Roxburgha et al. (%) | |||
|---|---|---|---|---|---|---|
| Sweden | UK | Canada | Overall | |||
| 5 | 3 | 37 | 56 | 48 | 46 | 48 |
| 6 | 12 | 26 | 22 | 19 | 14 | |
| 8 | 1 | 1 | 4 | 2 | 4 | |
| 10 | 3 | 51 | 67 | 60 | 59 | 67 |
| 6 | 23 | 33 | 33 | 29 | 30 | |
| 8 | 5 | 3 | 8 | 5 | 5 | |
| 15 | 3 | 64 | 76 | 71 | 70 | 75 |
| 6 | 33 | 42 | 43 | 39 | 38 | |
| 8 | 7 | 4 | 12 | 8 | 11 | |
| 20 | 3 | 74 | 81 | 75 | 76 | 85 |
| 6 | 46 | 48 | 50 | 48 | 58 | |
| 8 | 16 | 6 | 16 | 13 | 14 | |
EDSS: Expanded Disability Status Scale; MS: multiple sclerosis.
Percentages from Roxburgh et al.2
There was a moderate correlation between EDSS and disease duration (r = 0.46, 95% confidence interval (CI): 0.45–0.47) and EDSS and age (r = 0.44, 95% CI: 0.43–0.45).
The global ARMSS and updated global MSSS matrices
The global ARMSS (n = 25,558) and updated global MSSS (n = 24,277) matrices were constructed as described above (see Supplementary files 1 and 2).
Software packages were created for Stata and R to calculate the global/local ARMSS and MSSS. An interactive online tool has also been developed to obtain scores for individual patients and data sets (https://aliman.shinyapps.io/ARMSS/).14
Comparison of power to detect a 0.5 EDSS score difference between two groups
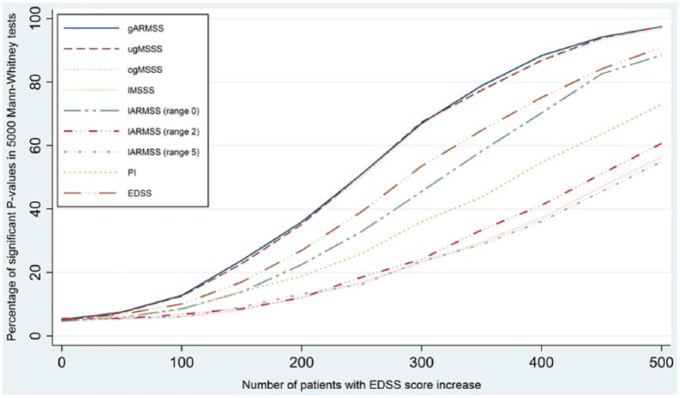
The results of the simulation study are shown in Figure 1. The global ARMSS, the updated global MSSS and the original global MSSS scores showed very similar power to capture EDSS changes which was considerably better than that of the other scales. For example, when half of the patients in the exposed group (n = 250) had increased by 0.5 on the EDSS, 50% of the tests were significant using the global scores compared to 40% using the actual EDSS score. This confirms that EDSS-based clinical changes are better reflected on global severity scores and these scores are more sensitive to capture changes in the course of disease. Similar results were found when power analyses were performed after including only individuals with less than 1 year exposure to DMTs (data not shown). Decrease in sample size by 20% or 40% resulted in approximately 15%–20% and 20%–50% decrease in analytical power, respectively. All three global scores showed similar power in samples with different age distributions.
Figure 1.
Comparison of power to detect 0.5 EDSS score difference between two patients groups in 500 randomly selected patients from the pooled data set. The power curve for the three Global scores overlap . Local scores are calculated within the same sample.
Local scores calculated using the same sample.
gARMSS: global ARMSS; ugMSSS: updated global MSSS; ogMSSS: original MSSS; lMSSS: local MSSS; lARMSS: local ARMSS; PI: progression index.
Stability over time of the ARMSS and MSSS measurement
The stability analysis included 68,240 observations from the Swedish data and 38,977 observations from the Canadian data. All scores showed relative stability after 5, 10 and 15 years and, for any age at first assessment, the 95% CIs of the correlation estimates for the different severity scores all overlapped (Supplementary Table 1). It should be noted that results of the stability analyses are based on average scores of patient groups. Results from the stability analyses demonstrate similar rate of progression by age; however, individual fluctuations are still significantly present. The individual fluctuation specifically at younger ages limits the use of ARMSS as a predictive tool. Ranking EDSS scores based on both disease duration and age (double ranked) did not provide any advantage.
Application as a patient monitoring/comparison tool
The MSSS has been used in several registers as a patient monitoring/comparison tool15,16 giving clinicians the ability to determine where a patient is relative to other patients of similar disease duration. Such an application is also possible for the ARMSS to define relative severity and rate of progression over time in an individual patient. Similar to MSSS, a patient having an ARMSS score of x progresses faster than 10x% and slower than (100 − 10x)% of MS patients in the population. For example, a patient with global ARMSS score of 3.51 (EDSS 3.0 at age 53 years) is progressing faster than 35.1% and slower than 64.9% (100 − 35.1) of 25,558 patients included in the global ARMSS matrix.
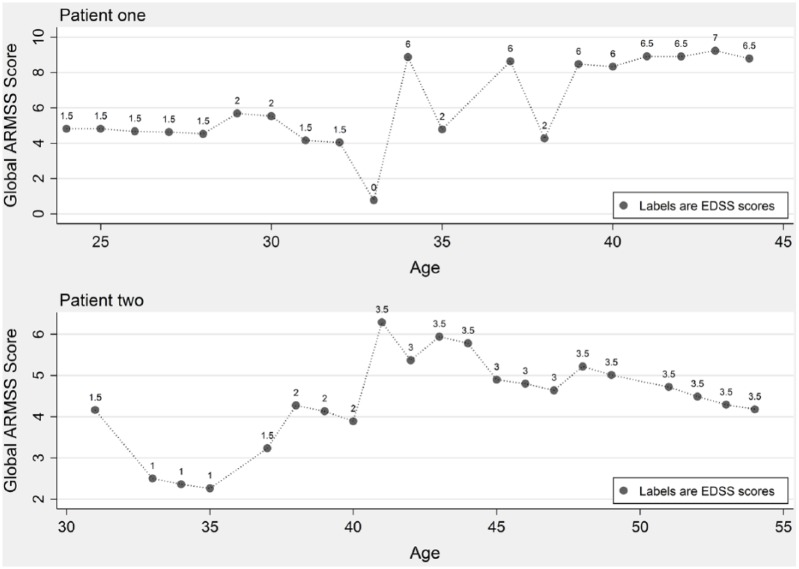
Figure 2 demonstrates the longitudinal assessments of the global ARMSS scores in two patients with consecutive EDSS measurements over time. While the disease course can be longitudinally assessed using EDSS scores, the global ARMSS score allows cross-sectional and longitudinal comparisons of disease severity in each individual compared to the patients of similar age.
Figure 2.
Longitudinal assessments of the global ARMSS scores in two patients with consecutive EDSS measurements.
Discussion
Here, we demonstrate that the ARMSS score obtained by ranking of EDSS scores based on patients’ age offers a powerful method for measuring relative severity of disability in MS. The ARMSS score presents an outcome measure that offers reasonable stability over time and is able to capture an effect contributing to a change in disability scores.
The ARMSS score offers a major advantage over the MSSS by its use of a patient’s age, which is typically readily and accurately available as opposed to the date of disease onset, and has been consistently shown to be associated with disease progression.17 Date of symptom onset in MS is generally based on a patient’s ability to recall, date and articulate past events and might be influenced by factors such as type and nature of the first symptom(s), sex (or gender) and the initial MS clinical phenotype. The accuracy of the symptom onset date can also depend on the history taking skills of the evaluating physician and the consultation time available, and the record can be biased by factors such as an assessor’s (the patient or clinician) knowledge of the average age at MS onset.18 Using age not only increases accuracy when comparing between patients and across cohorts, but also increases the sample size by preventing case exclusions due to an unknown or undetermined date of onset. As shown here, as the proportion of the cohort with a missing onset date increased, the power of the MSSS significantly decreased as a result of the reduced sample size. Unlike MSSS, the ARMSS scores would not be influenced by the combined MS phenotypes in a single global cohort since patients with different MS phenotype (relapse- and progressive-onset) reach disability milestones at almost similar ages (but significantly different disease durations).
There are several applications of the ARMSS score. In a clinical setting, the ARMSS score enables practicing clinicians to compare a patient’s disease severity to that of a large global (as well as local) patient population to get an overall picture of the patient’s performance. When recorded at several time points, the ARMSS scores can offer a rather comprehensive overview of a patient’s relative disease severity with the impact of age already taken into account. This might be helpful when patient is being monitored for clinical purposes19 as a change in EDSS score is better reflected in the ARMSS score. In the context of research, the ARMSS scores offer an outcome measure that can make the best use of sparse clinical data or cross-sectional EDSS scores and detect small differences between groups when even a limited number of patients have experienced a change in the EDSS score. Examples of such research applications would be the genome-wide association studies (GWAS) of disease severity,20,21 studies of association between environmental factors and disease severity22,23 or studies investigating MS clinical course.3,4,24–26
A major strength of our study is that the data used for construction of the global ARMSS and the updated global MSSS were obtained from three large cohorts: two in Europe and one in North America. Although there are some population-based differences, the pooled disability data enabled us to include MS patients with a wide spectrum of age, disease duration and EDSS scores. As a result, compared with the original global MSSS, the updated global MSSS assigns a higher score to the same combination of EDSS and disease duration in the majority of the cases. Nevertheless, if patients with very severe MS had died before any EDSS assessment could be made, or very benign patents were never assessed, these extreme groups would be underrepresented in the data sets such that the global scores might either under or overestimate the actual MS severity. The Swedish, Canadian and UK MS cohorts are predominantly comprised of patients of Northern European descent. It would be of value to assess the ARMSS in other ethnic groups and country-specific data sets.
Our sample included patients with varying lengths of exposure to different DMTs. We did not include treatment data in the calculation of global scores as this was impractical and we did not have comprehensive access to this data across all sites. We believe that the data from our cohorts are representative of the real-world setting which includes patients with varying lengths of exposure to DMTs. Hence, the global scores obtained from these cohorts are generalisable to many contemporary clinical settings. Furthermore, the three global scores showed significantly better performance than the local ARMSS and MSSS, EDSS and PI in our power analysis of patients who have been exposed to a DMTs for less than a year (the group perceived as being benign (not needing/qualifying for treatment) or least influenced by DMTs that was available to us), implying the advantage of a big data set. It should be noted that no impact of DMTs on the MSSS has been reported24 and that any long-term influence of DMTs on EDSS progression remains hopeful but still uncertain.13,27
Useful applications of the global ARMSS might include baseline or cross-sectional comparisons of disease severity between two or more groups of MS patients, comparisons when disability data are sparse, or for matching groups of patients within or between studies. It should be noted that, regardless of its performance, the ARMSS score has limitations which are mainly due to its reliance on the EDSS score with its well-defined shortcomings such as its bimodal distribution and marked inter- and intra-rater variability.28 Nevertheless, the EDSS is the most widely used outcome measure in MS, it is of relatively low cost to obtain, neurologists are familiar with it worldwide, and it can reasonably capture disease worsening over the long term.
In MS, age might be a better proxy of the cumulative effect of environmental and related exposures (including comorbidity) than disease duration. The burden of comorbidity in MS may increase with age and may also impact subsequent disease progression.29,30 Alternatively, the comparable performance of the ARMSS score to that of the MSSS in capturing effects on disability and its stability over time may indicate that irreversible disability in MS is just as much a function of chronologic age as it is of disease duration. These findings are in line with the reports on the effect of current age on reaching disability milestones.6,9 All said and as described before,31 significant heterogeneity in disability scores in individuals within the same age is still present, particularly in younger age groups. One might expect that the precision obtained using age lessens the variability in the EDSS scores. While EDSS scores at older ages were slightly more stable over time (Supplementary Table 1), the overall correlation between EDSS and age was only moderate and the power of global ARMSS score was comparable in samples with different age distributions. Hence, part of the correlation between age and disability levels may have resulted from the analyses of groups which can potentially obscure individual or subgroup heterogeneity. Nevertheless, judging by its effect, age is one of the most important factors in accumulation of disability in MS.
In conclusion, disability in MS as assessed by the EDSS correlates with age at a similar magnitude as with duration of disease. Since a patient’s age is nearly always available, and since the ARMSS and MSSS are comparable even when the onset date is known, the ARMSS offers a more versatile tool for comparing EDSS-based severity in MS between groups.
Acknowledgments
The authors would like to thank all of the neurologists, nurses and MS patients in Sweden, Canada and the United Kingdom for providing data for this study. They gratefully acknowledge the BC MS Clinic neurologists who contributed to the study through patient examination and data collection (current members listed here by primary clinic): UBC MS Clinic: A. Traboulsee, MD, FRCPC (UBC Hospital MS Clinic Director and Head of the UBC MS Programs); A.-L. Sayao, MD, FRCPC; V. Devonshire, MD, FRCPC; S. Hashimoto, MD, FRCPC (UBC and Victoria MS Clinics); J. Hooge, MD, FRCPC (UBC and Prince George MS Clinic); L. Kastrukoff, MD, FRCPC (UBC and Prince George MS Clinic); J. Oger, MD, FRCPC; Kelowna MS Clinic: D. Adams, MD, FRCPC; D. Craig, MD, FRCPC; S. Meckling, MD, FRCPC; Prince George MS Clinic: L. Daly, MD, FRCPC; Victoria MS Clinic: O. Hrebicek, MD, FRCPC; D. Parton, MD, FRCPC; K. Atwell-Pope, MD, FRCPC. The views expressed in this paper do not necessarily reflect the views of each individual acknowledged.
Footnotes
Author contribution: A.M. designed the study, analysed and interpreted data, wrote and revised the manuscript. H.W. analysed and interpreted data, wrote and revised the manuscript. E.K. analysed and interpreted data and revised the manuscript. F.Z. analysed and interpreted data and revised the manuscript. R.R. interpreted data and revised the manuscript. A.G. interpreted data and revised the manuscript. R.C. interpreted data and revised the manuscript. S.S. designed the study, interpreted data and revised the manuscript. M.B. interpreted data and revised the manuscript. H.T. facilitated data interpretation and revised the manuscript. J.H. designed the study, interpreted data and revised the manuscript.
Declaration of Conflicting Interests: The author(s) declared the following potential conflicts of interest with respect to the research, authorship and/or publication of this article: H.W., R.R., S.S., M.B., E.K. and F.Z. declare no conflict of interests. A.M. reports grants from Neuro Sweden (Neuroförbundet) during the conduct of the study. A.G. is receiving research support from Biogen. J.H. received honoraria for serving on advisory boards for Biogen, Sanofi-Genzyme and Novartis and speaker’s fees from Biogen, Merck-Serono, Bayer-Schering, Teva and Sanofi-Genzyme. He has served as P.I. for projects sponsored by, or received unrestricted research support from, Biogen, Merck-Serono, TEVA, Novartis, Sanofi-Genzyme and Bayer-Schering. H.T. is the Canada Research Chair for Neuroepidemiology and Multiple Sclerosis. She has received research support from the National Multiple Sclerosis Society, the Canadian Institutes of Health Research, the Multiple Sclerosis Society of Canada (Don Paty Career Development Award); the Michael Smith Foundation for Health Research (Scholar Award) and the UK MS Trust; speaker honoraria and/or travel expenses to attend conferences from the Consortium of MS Centres (2013), the National MS Society (2012, 2014), Bayer Pharmaceuticals (2010), Teva Pharmaceuticals (2011), ECTRIMS (2011, 2012, 2013, 2014, 2015), UK MS Trust (2011), the Chesapeake Health Education Program, US Veterans Affairs (2012), Novartis Canada (2012), Biogen (2014), American Academy of Neurology (2013, 2014, 2015). Unless otherwise stated, all speaker honoraria are either donated to an MS charity or to an unrestricted grant for use by her research group.
Funding: The author(s) disclosed receipt of the following financial support for the research, authorship, and/or publication of this article: The lead site (Sweden) was received support for this study from Biogen and Neuro Sweden (Neuroförbundet). The participating sites (UK and Canada) received no direct funding for this study. The UK site was supported by the Cambridge NIHR Biomedical Research Centre. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript. Biogen provided a courtesy review and feedback on the paper to the authors. The authors had full editorial control of the paper and provided their final approval of all content.
Contributor Information
Ali Manouchehrinia, Department of Clinical Neuroscience (CNS), Karolinska Institutet, Stockholm, Sweden.
Helga Westerlind, Institute of Environmental Medicine (IMM), Karolinska Institutet, Stockholm, Sweden.
Elaine Kingwell, Division of Neurology, Faculty of Medicine, UBC Hospital, The University of British Columbia, Vancouver, BC, Canada.
Feng Zhu, Division of Neurology, Faculty of Medicine, UBC Hospital, The University of British Columbia, Vancouver, BC, Canada.
Robert Carruthers, Division of Neurology, Faculty of Medicine, UBC Hospital, The University of British Columbia, Vancouver, BC, Canada.
Ryan Ramanujam, Department of Clinical Neuroscience (CNS), Karolinska Institutet, Stockholm, Sweden.
Maria Ban, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK.
Anna Glaser, Department of Clinical Neuroscience (CNS), Karolinska Institutet, Stockholm, Sweden.
Stephen Sawcer, Department of Clinical Neurosciences, University of Cambridge, Cambridge, UK.
Helen Tremlett, Division of Neurology, Faculty of Medicine, UBC Hospital, The University of British Columbia, Vancouver, BC, Canada.
Jan Hillert, Department of Clinical Neuroscience (CNS), Karolinska Institutet, Stockholm, Sweden.
References
- 1. Kurtzke JF. Rating neurologic impairment in multiple sclerosis: An expanded disability status scale (EDSS). Neurology 1983; 33(11): 1444–1452. [DOI] [PubMed] [Google Scholar]
- 2. Roxburgh RH, Seaman SR, Masterman T, et al. Multiple Sclerosis Severity Score: Using disability and disease duration to rate disease severity. Neurology 2005; 64(7): 1144–1151. [DOI] [PubMed] [Google Scholar]
- 3. Petzold A, Eikelenboom MI, Keir G, et al. The new global Multiple Sclerosis Severity Score (MSSS) correlates with axonal but not glial biomarkers. Mult Scler 2006; 12(3): 325–328. [DOI] [PubMed] [Google Scholar]
- 4. Kister I, Chamot E, Bacon JH, et al. Trend for decreasing Multiple Sclerosis Severity Scores (MSSS) with increasing calendar year of enrollment into the New York State Multiple Sclerosis Consortium. Mult Scler 2011; 17(6): 725–733. [DOI] [PubMed] [Google Scholar]
- 5. Strange RC, Ramachandran S, Zeegers MP, et al. The Multiple Sclerosis Severity Score: Associations with MC1R single nucleotide polymorphisms and host response to ultraviolet radiation. Mult Scler 2010; 16(9): 1109–1116. [DOI] [PubMed] [Google Scholar]
- 6. Tremlett H, Paty D, Devonshire V. Disability progression in multiple sclerosis is slower than previously reported. Neurology 2006; 66(2): 172–177. [DOI] [PubMed] [Google Scholar]
- 7. Tremlett H, Zhao Y, Joseph J, et al. Relapses in multiple sclerosis are age- and time-dependent. J Neurol Neurosurg Psychiatry 2008; 79(12): 1368–1374. [DOI] [PubMed] [Google Scholar]
- 8. Confavreux C, Vukusic S. Age at disability milestones in multiple sclerosis. Brain 2006; 129(Pt 3): 595–605. [DOI] [PubMed] [Google Scholar]
- 9. Trojano M, Liguori M, Zimatore GB, et al. Age-related disability in multiple sclerosis. Ann Neurol 2002; 51(4): 475–480. [DOI] [PubMed] [Google Scholar]
- 10. Scalfari A, Neuhaus A, Daumer M, et al. Age and disability accumulation in multiple sclerosis. Neurology 2011; 77(13): 1246–1252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Andersen O. From the Gothenburg cohort to the Swedish multiple sclerosis registry. Acta Neurol Scand Suppl 2012; 195: 13–19. [DOI] [PubMed] [Google Scholar]
- 12. Beecham AH, Patsopoulos NA, Xifara DK, et al. Analysis of immune-related loci identifies 48 new susceptibility variants for multiple sclerosis. Nat Genet 2013; 45(11): 1353–1360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Shirani A, Zhao Y, Karim ME, et al. Association between use of interferon beta and progression of disability in patients with relapsing-remitting multiple sclerosis. JAMA 2012; 308(3): 247–256. [DOI] [PubMed] [Google Scholar]
- 14. Manouchehrinia A, Westerlind H. https://aliman.shinyapps.io/ARMSS (2017).
- 15. Swedish Multiple Sclerosis Register, http://www.neuroreg.se/en.html/multiple-sclerosis (2017).
- 16. Hughes SE, Spelman T, Bianchi E, et al. Investigators HB on behalf of the MSB. MS severity rank calculator, 2012, https://www.msbase.org/msbase/mscurves;jsessionid=15sndgq7w8504ppt4nsi32p7b
- 17. Tremlett H, Zhao Y, Rieckmann P, et al. New perspectives in the natural history of multiple sclerosis. Neurology 2010; 74(24): 2004–2015. [DOI] [PubMed] [Google Scholar]
- 18. Hróbjartsson A, Thomsen ASS, Emanuelsson F, et al. Observer bias in randomised clinical trials with binary outcomes: Systematic review of trials with both blinded and non-blinded outcome assessors. BMJ 2012; 344: e1119. [DOI] [PubMed] [Google Scholar]
- 19. Gava G, Bartolomei I, Costantino A, et al. Long-term influence of combined oral contraceptive use on the clinical course of relapsing-remitting multiple sclerosis. Fertil Steril 2014; 102(1): 116–122. [DOI] [PubMed] [Google Scholar]
- 20. International Multiple Sclerosis Genetics Consortium. Genome-wide association study of severity in multiple sclerosis. Genes Immun 2011; 12(8): 615–625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Baranzini SE, Wang J, Gibson RA, et al. Genome-wide association analysis of susceptibility and clinical phenotype in multiple sclerosis. Hum Mol Genet 2009; 18(4): 767–778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Gelfand JM, Cree BAC, McElroy J, et al. Vitamin D in African Americans with multiple sclerosis. Neurology 2011; 76(21): 1824–1830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Manouchehrinia A, Tench C, Maxted J. Tobacco smoking and disability progression in multiple sclerosis: United Kingdom cohort study. Brain 2013; 136(Pt 7): 2298–2304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Pachner AR, Steiner I. The Multiple Sclerosis Severity Score (MSSS) predicts disease severity over time. J Neurol Sci 2009; 278(1–2): 66–70. [DOI] [PubMed] [Google Scholar]
- 25. Kister I, Chamot E, Bacon JH, et al. Rapid disease course in African Americans with multiple sclerosis. Neurology 2010; 75(3): 217–223. [DOI] [PubMed] [Google Scholar]
- 26. Kister I, Bacon T, Chamot E, et al. Multiple Sclerosis Severity Scores decrease with calendar year of first EDSS assignment in MSBase. Mult Scler 2010; 16(S10): S208. [Google Scholar]
- 27. Munari L, Lovati R, Boiko A. Therapy with glatiramer acetate for multiple sclerosis. Cochrane Database Syst Rev 2004; 1: CD004678. [DOI] [PubMed] [Google Scholar]
- 28. Hobart J, Freeman J, Thompson A. Kurtzke scales revisited: The application of psychometric methods to clinical intuition. Brain 2000; 123(Pt 5): 1027–1040. [DOI] [PubMed] [Google Scholar]
- 29. Marrie RA, Horwitz RI. Emerging effects of comorbidities on multiple sclerosis. Lancet Neurol 2010; 9(8): 820–828. [DOI] [PubMed] [Google Scholar]
- 30. Brodin P, Jojic V, Gao T, et al. Variation in the human immune system is largely driven by non-heritable influences. Cell 2015; 160(1–2): 37–47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Weinshenker BG, Rice GPA, Noseworthy JH, et al. The natural history of multiple sclerosis: A geographically based study. Brain 1991; 114(2): 133–146. [DOI] [PubMed] [Google Scholar]