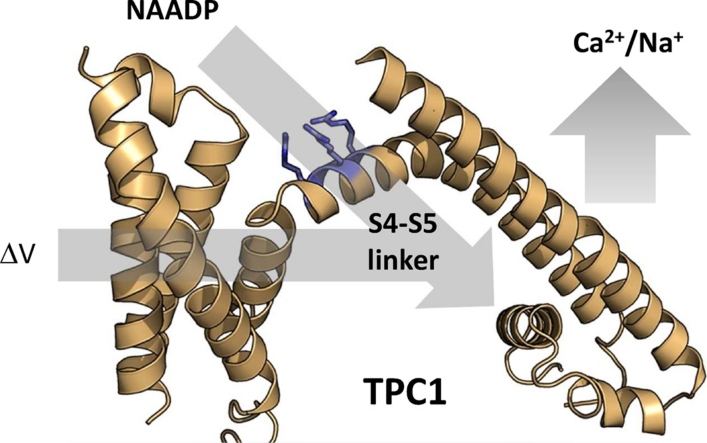
Graphical abstract
Highlights
-
•
The S4–S5 linker of TPCs harbours conserved basic residues.
-
•
Arginine residues in the S4–S5 linker are required for activation of TPC1 by NAADP.
-
•
The S4–S5 linker emerges as a potential common determinant for TPC activation.
Abstract
Two-pore channels (TPCs) are two-domain members of the voltage-gated ion channel superfamily that localize to acidic organelles. Their mechanism of activation (ligands such as NAADP/PI(3,5)P2 versus voltage) and ion selectivity (Ca2+ versus Na+) is debated. Here we report that a cluster of arginine residues in the first domain required for selective voltage-gating of TPC1 map not to the voltage-sensing fourth transmembrane region (S4) but to a cytosolic downstream region (S4-S5 linker). These residues are conserved between TPC isoforms suggesting a generic role in TPC activation. Accordingly, mutation of residues in TPC1 but not the analogous region in the second domain prevents Ca2+ release by NAADP in intact cells. Our data affirm the role of TPCs in NAADP-mediated Ca2+ signalling and unite differing models of channel activation through identification of common domain-specific residues.
1. Introduction
TPCs are ancient members of the voltage-gated ion channel superfamily [1] with widespread physiological roles ranging from germination and stomatal movement in plants [2] to receptor and virus trafficking in animals [3], [4], [5]. They are structurally characterized by two homologous domains each comprising six trans-membrane regions (S1–S6) organized as a voltage sensing module (S1–S4) connected to the pore (S5–S6) by a cytosolic linker [6], [7]. TPCs assemble as dimers [8] and are likely evolutionary intermediates between tetrameric one domain and monomeric four domain channels exemplified by voltage gated K+ and Ca2+ channels, respectively [9].
Although it is established that TPCs localize to acidic organelles such as and plant vacuoles and animal lysosomes [10], [11], their mechanism of activation and ion selectivity are unclear [12], [13]. Plant TPC1 encodes the SV channel which is a vacuolar non-selective Ca2+-permeable channel regulated by voltage and Ca2+ [2]. Recent X-ray crystal structures have illuminated molecular mechanisms underlying voltage sensing and Ca2+ regulation [14], [15], [16]. But whether plant TPCs contribute to physiological cellular Ca2+ signals is debated [17]. Similarly, animal TPCs (encoded by up to 3 genes) were originally functionally identified as Ca2+-permeable endo-lysosomal ion channels activated by NAADP [18], [19], [20], [21], [22]. NAADP is a potent Ca2+ mobilizing messenger produced in response to numerous physiological stimuli such as hormones and neurotransmitters and long known to release Ca2+ from acidic organelles [23], [24], [25], [26]. But other studies suggest that TPCs are NAADP-insensitive Na+ channels regulated by the endo-lysosomal phosphoinositide, PI(3,5)P2 [27] and/or voltage [28], [29]. Evidence for [30] and against [31] the original findings continues to accrue. Little at present is known concerning the molecular determinants of TPC activation in animals.
Here, we identify arginine residues within the first S4-S5 linker of TPC1 critical for NAADP-evoked Ca2+ signalling and propose that this region is a common determinant for channel activation.
2. Methods
2.1. Bioinformatics
Human TPC1 (accession: AAI50204.1) was modelled based on Arabidopsis thaliana TPC1 (PDB:5E1J) using Phyre2 [32] in intensive mode and presented using the PyMOL Molecular Graphics System, Version 1.8 (Schrödinger, LLC). Multiple sequence alignments were performed using T-Coffee [33], Clustal Omega [34] and MUSCLE [35].
2.2. Plasmids
Constructs encoding human TPC1 tagged at its C-terminus with GFP or mRFP were described in [20]. Site-directed mutagenesis of TPC1-mRFP was performed using QuikChange (Stratech).
2.3. Other methods
Culture of SKBR3 cells, transfection, confocal microscopy, Ca2+ imaging and microinjection were performed as described in [36].
3. Results and discussion
3.1. The first S4-S5 linker of TPCs harbours conserved basic residues
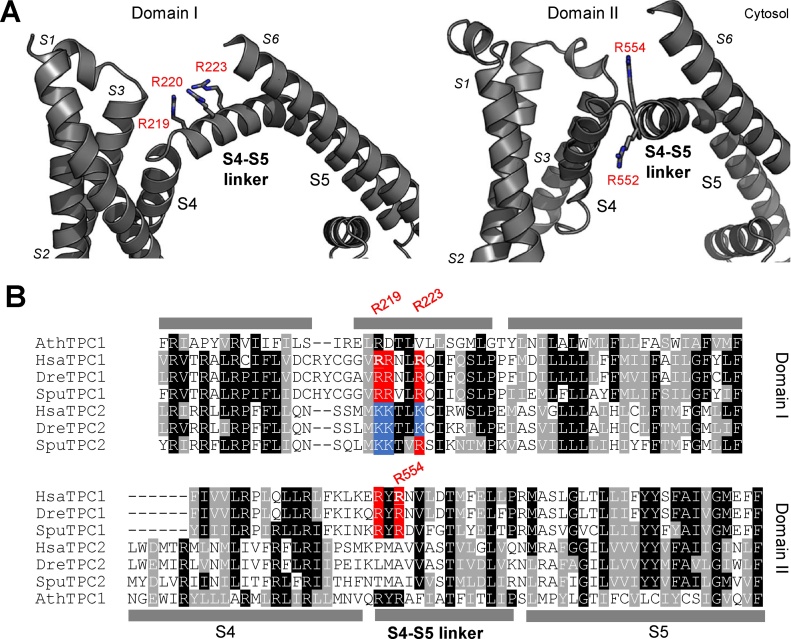
Previous work concluded that human TPC1 but not TPC2 is a voltage-gated Na+ channel that confers endo-lysosomal excitability through arginine residues within putative voltage sensors [28]. S4 helices are established in mediating voltage sensing in a number of voltage-gated ion channels including plant TPC1 [14]. Taking advantage of recent plant TPC1 crystal structures [14], we modelled human TPC1 (Fig. 1A). Inspection of domain I revealed that R219, R220 and R223, which upon combined mutation eliminated voltage sensitivity [28] do not map to S4 (Fig. 1A). Instead, the residues were located downstream in the cytosolic linker connecting S4 to S5 (Fig. 1A–B).
Fig. 1.
The first S4-S5 linker of TPCs harbours conserved basic residues. A. Structural model of human TPC1. Arginine residues in the S4-S5 linker of domain I (left) and domain II (right) are shown as sticks. B, T-Coffee multiple sequence alignment of TPC1 and TPC2 from Arabidopsis thaliana (Ath), Homo sapiens (Hsa), Danio rerio (Dre) and Stronglyocentrotus purpuratus (Spu). Conserved arginine (red) and lysine (blue) residues within the S4-S5 linkers of animal TPCs are highlighted. Arginine residues subjected to site-directed mutagenesis in human TPC1 are numbered. Secondary structure refers to that of AthTPC1. (For interpretation of the references to colour in this figure legend, the reader is referred to the web version of this article.)
Multiple sequence alignment showed that these residues were conserved mostly as lysine in human, zebrafish and sea urchin TPC2 (voltage-insensitive; Fig. 1B). Similar results were obtained using two additional alignment algorithms and a number of other species (data not shown). Our alignment agrees with those in [14], [15].
Together, this reappraisal suggests that the basic cluster is unlikely to directly mediate voltage-sensing by TPC1 and point to a more generic role in TPC function across isoforms.
3.2. Arginine residues in the first S4–S5 linker are required for activation of TPC1 by NAADP
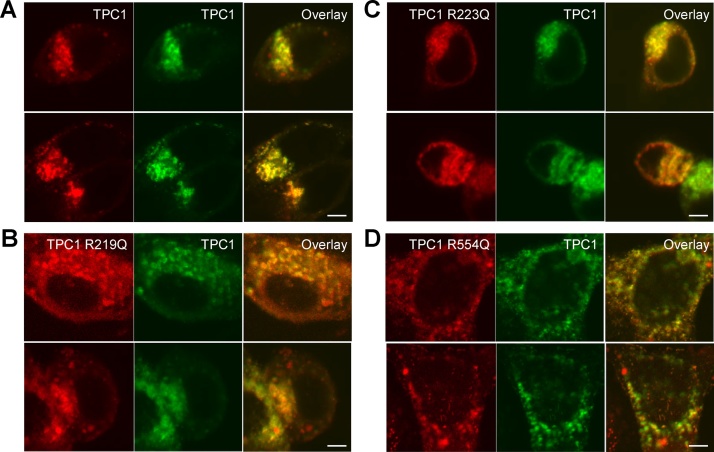
To examine the functional role of the S4–S5 linker, we examined the consequences of mutating conserved arginine residues on Ca2+ release by NAADP in intact cells. TPC1 constructs in which either R219 or R223 had been mutated individually to glutamine expressed and colocalized with wild type TPC1 (Fig. 2A–C). Microinjection of NAADP evoked robust Ca2+ responses in cells expressing wild type TPC1 (Fig. 3A). The responses however were inhibited in cells expressing either mutant (Fig. 3A).
Fig. 2.
TPC1 mutated in the S4-S5 linkers express. Confocal images of SKBR3 cells expressing GFP-tagged TPC1 (middle panels) together with mRFP-tagged TPC1 (A), TPC1 R219Q (B), TPC1 R221Q (C) and TPC1 R554Q (D, left panels). Image overlays are shown to the right. Scale bars 2 μm.
Fig. 3.
Arginine residues in the first S4-S5 linker are required for activation of TPC1 by NAADP. A, Cytosolic Ca2+ responses of cells expressing the indicated mRFP-tagged TPC1 construct and microinjected with NAADP (10 μM, pipette concentration). Responses of individual cells (left) and the average response of all cells are shown (right). B, Pooled data summarizing the change in [Ca2+]. Data are presented as mean ± S.E.M (n = 6–8).
We also mutated R554 in the S4–S5 linker of domain II. This residue together with R552 is conserved in TPC1 but not TPC2 across species, and like the conserved residues in domain I, is placed within a helix (Fig. 1). Again, the mutant channel expressed and co-localised with wildtype TPC1 (Fig. 2D). However, in contrast to the domain I mutants, this construct supported NAADP-evoked signals (Fig. 3A).
Collectively, these data summarised in Fig. 3B, identify novel molecular determinants underlying NAADP action and further link TPCs to Ca2+ fluxes in intact cells.
3.3. Conclusions
In sum, prompted by new structural data, we identify a domain-specific requirement for the first S4-S5 linker in activation of TPCs by NAADP. Together with previous work implicating this same region in voltage-sensing [28] identifies it as a key mediator of TPC functionality. This region is unlikely to directly sense voltage (due to its placing out of the membrane) or bind NAADP (which interacts with accessory proteins [37]). Given the conservation of positive charge in TPC1 and TPC2, we speculate that mutations within the linker may instead affect channel activity indirectly through perturbing electrostatic interactions. This could be an intramolecular (allosteric) defect or an intermolecular one that disrupts association with putative NAADP binding proteins or anionic lipids such as PI(3,5)P2, which regulates both TPC isoforms [27], [28], [36]. Further analyses of this region across isoforms may rationalize multimodal activation of TPCs.
Author contributions
EB performed the Ca2+ imaging. DC performed the confocal microscopy. SP designed the study, performed the bioinformatics and molecular biology, and wrote the paper.
Competing financial interests statement
The authors declare that they have no competing interests.
Acknowledgements
This work was supported by BBSRC grants BB/G013721/1 and BB/N014524X/1. We thank Christopher J. Penny for useful discussion and Taufiq Rahman for advice.
References
- 1.Patel S. Function and dysfunction of two-pore channels. Sci. Signal. 2015;8:re7. doi: 10.1126/scisignal.aab3314. [DOI] [PubMed] [Google Scholar]
- 2.Peiter E. The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature. 2005;434(7031):404–408. doi: 10.1038/nature03381. [DOI] [PubMed] [Google Scholar]
- 3.Grimm C. High susceptibility to fatty liver disease in two-pore channel 2-deficient mice. Nat. Commun. 2014;5:4699. doi: 10.1038/ncomms5699. [DOI] [PubMed] [Google Scholar]
- 4.Sakurai Y. Two-pore channels control Ebola virus host cell entry and are drug targets for disease treatment. Science. 2015;347:6225. doi: 10.1126/science.1258758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kilpatrick B.S. An endosomal NAADP-Sensitive two-Pore Ca2+ channel regulates ER-endosome membrane contact sites to control growth factor signaling. Cell Rep. 2017;18(7):1636–1645. doi: 10.1016/j.celrep.2017.01.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hooper R. Membrane topology of NAADP-sensitive two-pore channels and their regulation by N-linked glycosylation. J. Biol. Chem. 2011;286:9141–9149. doi: 10.1074/jbc.M110.189985. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Churamani D. Domain assembly of NAADP-gated two-pore channels. Biochem. J. 2012;441(1):317–323. doi: 10.1042/BJ20111617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rietdorf K. Two-pore channels form homo- and heterodimers. J. Biol. Chem. 2011;286(43):37058–37062. doi: 10.1074/jbc.C111.289835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rahman T. Two-pore channels provide insight into the evolution of voltage-gated Ca2+ and Na+ channels. Sci. Signal. 2014;7(352):ra109. doi: 10.1126/scisignal.2005450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brailoiu E. An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 2010;285:38511–38516. doi: 10.1074/jbc.M110.162073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Larisch N. An N-Terminal dileucine motif directs two-Pore channels to the tonoplast of plant cells. Traffic. 2012;13:1012–1022. doi: 10.1111/j.1600-0854.2012.01366.x. [DOI] [PubMed] [Google Scholar]
- 12.Marchant J.S., Patel S. Questioning regulation of two-pore channels by NAADP. Messenger. 2013;2:113–119. doi: 10.1166/msr.2013.1027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Morgan A.J., Galione A. Two-pore channels (TPCs): current controversies. Bioessays. 2013;36:173–183. doi: 10.1002/bies.201300118. [DOI] [PubMed] [Google Scholar]
- 14.Guo J. Structure of the voltage-gated two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:196–201. doi: 10.1038/nature16446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kintzer A.F., Stroud R.M. Structure, inhibition and regulation of two-pore channel TPC1 from Arabidopsis thaliana. Nature. 2016;531:258–264. doi: 10.1038/nature17194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Patel S., Penny C.J., Rahman T. Two-pore channels enter the atomic era: Structure of plant TPC revealed. Trends Biochem. Sci. 2016;41:475–477. doi: 10.1016/j.tibs.2016.04.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hedrich R., Marten I. TPC1-SV channels gain shape. Mol. Plant. 2011;4(3):428–441. doi: 10.1093/mp/ssr017. [DOI] [PubMed] [Google Scholar]
- 18.Calcraft P.J. NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature. 2009;459(7246):596–600. doi: 10.1038/nature08030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zong X. The two-pore channel TPCN2 mediates NAADP-dependent Ca2+-release from lysosomal stores. Pflugers Arch. 2009;458(5):891–899. doi: 10.1007/s00424-009-0690-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Brailoiu E. Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 2009;186:201–209. doi: 10.1083/jcb.200904073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Brailoiu E. An ancestral deuterostome family of two-pore channels mediate nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 2010;285:2897–2901. doi: 10.1074/jbc.C109.081943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Cai X., Patel S. Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 2010;27(10):2352–2359. doi: 10.1093/molbev/msq122. [DOI] [PubMed] [Google Scholar]
- 23.Churchill G.C. NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell. 2002;111:703–708. doi: 10.1016/s0092-8674(02)01082-6. [DOI] [PubMed] [Google Scholar]
- 24.Yamasaki M. Role of NAADP and cADPR in the induction and maintenance of agonist-evoked Ca2+ Spiking in mouse pancreatic acinar cells. Curr. Biol. 2005;15:874–878. doi: 10.1016/j.cub.2005.04.033. [DOI] [PubMed] [Google Scholar]
- 25.Pandey V. Recruitment of NAADP-sensitive acidic Ca2+ stores by glutamate. Biochem. J. 2009;422:503–512. doi: 10.1042/BJ20090194. [DOI] [PubMed] [Google Scholar]
- 26.Galione A. A primer of NAADP-mediated Ca signalling: from sea urchin eggs to mammalian cells. Cell Calcium. 2015;58:27–47. doi: 10.1016/j.ceca.2014.09.010. [DOI] [PubMed] [Google Scholar]
- 27.Wang X. TPC proteins are phosphoinositide- activated sodium-selective ion channels in endosomes and lysosomes. Cell. 2012;151(2):372–383. doi: 10.1016/j.cell.2012.08.036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cang C., Bekele B., Ren D. The voltage-gated sodium channel TPC1 confers endolysosomal excitability. Nat. Chem. Biol. 2014;10(6):463–469. doi: 10.1038/nchembio.1522. [DOI] [PubMed] [Google Scholar]
- 29.Cang C., Aranda K., Ren D. A non-inactivating high-voltage-activated two-pore Na(+) channel that supports ultra-long action potentials and membrane bistability. Nat. Commun. 2014;5:5015. doi: 10.1038/ncomms6015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ruas M. Expression of Ca2+-permeable two-pore channels rescues NAADP signalling in TPC-deficient cells. EMBO J. 2015;34:1743–1758. doi: 10.15252/embj.201490009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Guo J., Zeng W., Jiang Y. Tuning the ion selectivity of two-pore channels. Proc. Natl. Acad. Sci. U. S. A. 2017;114(5):1009–1014. doi: 10.1073/pnas.1616191114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kelley L.A. The Phyre2 web portal for protein modeling: prediction and analysis. Nat. Protoc. 2015;10(6):845–858. doi: 10.1038/nprot.2015.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Notredame C., Higgins D.G., Heringa J. T-Coffee. A novel method for fast and accurate multiple sequence alignment. J. Mol. Biol. 2000;302(1):205–217. doi: 10.1006/jmbi.2000.4042. [DOI] [PubMed] [Google Scholar]
- 34.Sievers F. Fast, scalable generation of high-quality protein multiple sequence alignments using Clustal Omega. Mol. Syst. Biol. 2011;7:539. doi: 10.1038/msb.2011.75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Edgar R.C. MUSCLE: multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 2004;32(5):1792–1797. doi: 10.1093/nar/gkh340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jha A. Convergent regulation of the lysosomal two-pore channel-2 by Mg2+, NAADP, PI(3,5)P2 and multiple protein kinases. EMBO J. 2014;33:501–511. doi: 10.1002/embj.201387035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lin-Moshier Y. Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 2012;287:2296–2307. doi: 10.1074/jbc.M111.305813. [DOI] [PMC free article] [PubMed] [Google Scholar]