Abstract
Osteoporosis is a silent disease with increasing prevalence due to the global ageing population. Decreased bone strength and bone quality is the hallmark of osteoporosis which leads to an increased risk of fragility fractures in elderly. It has been estimated that approximately ~50% of women will suffer during their lifetime from an osteoporotic fracture. This must be considered as a major health concern, as it has previously been established that fragility fracture has been associated with decreased quality of life due to increased disability, more frequent hospital admission and most importantly, osteoporotic fractures have been related to an augmented mortality risk. Anti-osteoporotic drugs are available for improving bone quality. Although there is access to these therapeutic options, there remain multiple unmet needs in the field of osteoporosis and fracture care, for example, the primary prevention of osteoporosis in young individuals (to reach a high peak bone mass), the optimization of the use of imaging techniques [dual-energy X-ray absorptiometry (DXA), vertebral fracture assessment (VFA) and new techniques measuring bone quality], the use of nonmedical treatment options and surgical techniques of fracture healing. In this review, we will discuss topics that play a role in the occurrence and prevention of fractures, and we give an overview of and insight into the critical issues and challenges around osteoporosis and fracture prevention.
Keywords: bone mineral density, bone strength, fracture, osteoporosis, surgical treatment, treatment
Introduction
Osteoporosis is a silent and asymptomatic disease, characterized by loss of bone mass and bone strength, leading to an increased susceptibility of low-energy or fragility fractures.1 This is illustrated by the observation that fractures are common at the age of 50 years, as it has been estimated that the lifetime risk for women is 40–45%.2,3 Although the lifetime fracture risk, estimated between 15–27%, is lower for men, the risk for subsequent fractures after an initial fracture is the same in both men and women,2–4 which indicates that secondary fracture prevention after an initial fracture is important both in women and in men. Since age is the dominant risk factor for osteoporotic fractures, roughly 90% of the fragility fractures occur in patients 60 years and over, the fracture rate is world widely expected to increase, as the number of elderly is rising.5,6 From this point of view, it is relevant to note that fractures have a large impact: relatively uncomplicated fractures such as wrist fractures are usually associated with devastating pain and mild disability (and sometimes to limitations in daily work),7 while severe fractures, such as hip fractures, usually lead to hospital admissions and operative procedures, which may be complicated by infection, myocardial infarction, thromboembolism and delirium.8 In addition, patients with hip fractures may have persistent postoperative limitations in walking and daily living, and more importantly, mortality risk is increased.9,10
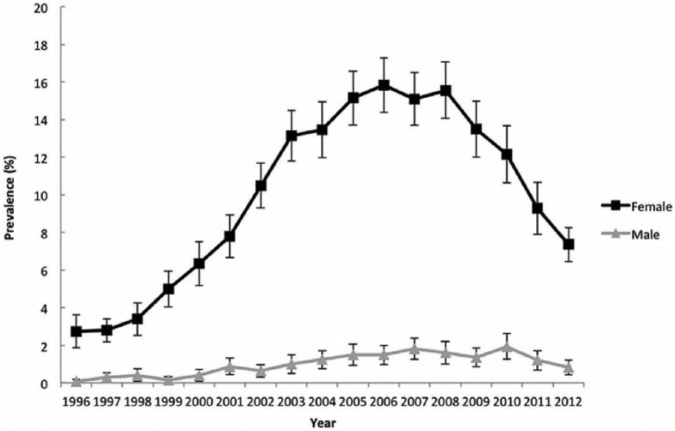
Therefore, the question is: which measures are needed to prevent fractures in the elderly? For two decades, we have had access to antiresorptive drugs (bisphosphonates and, later, denosumab) that substantially lower the risk of vertebral and nonvertebral fractures (including hip fractures),11–14 and even to anabolic drugs (e.g. teriparatide), that have the capability to build up new bone,15 while new, promising drugs like abaloparatide and monoclonal antibodies against sclerostin are in phase III trials.16–19 Recently, an alarming decrease of around 50% from 2008 to 2012 in bisphosphonate users was documented in the USA, the so-called ‘crisis in osteoporosis’, indicating that bisphosphonate prescription is suboptimal.20,21 This fall in anti-osteoporotic drug prescriptions was confirmed in a recently published study performed in the United Kingdom.22 In other words, the currently available drugs are very welcome, but are certainly not the full answer for fracture prevention in the ageing population (Figure 1).
Figure 1.
Crisis in osteoporosis treatment adapted from Journal of Bone Mineral Research (JBMR), 2015.20
Obviously, there is an unmet need in the field of osteoporosis (Table 1). This was recently discussed in manuscripts focusing on pathophysiology, screening and treatment of osteoporosis and on prevention of hip fractures.23,24 In our opinion, many other factors play a role: e.g. prevention of osteoporosis in young individuals (to reach a high peak bone mass), the optimization of the use of imaging techniques [dual-energy X-ray absorptiometry (DXA), vertebral fracture assessment (VFA) and new techniques measuring bone quality], the use of medical and nonmedical treatment options, and surgical techniques of fracture healing. Since we will discuss all the above-mentioned topics that play a role in the occurrence and prevention of fractures, this manuscript gives an overview of and insight into the critical issues and challenges around osteoporosis and fracture prevention.
Table 1.
Unmet needs in the field of osteoporosis.
| Optimizing peak bone mass in young adults |
| Structural implementation of a four-step diagnostic procedure in patients with clinical risk factors for osteoporotic fractures (DXA, VFA, fall risk and secondary osteoporosis) |
| More adequate measurement of bone strength |
| Reduction in the treatment gap |
| New drugs with a better efficacy/safety profile |
| Shared decision making with optimal nonmedical and medical treatment |
| New strategies: treat to target and definition of high-risk patients |
DXA, dual-energy X-ray absorptiometry; VFA, vertebral fracture assessment.
Primary prevention of osteoporosis
A good understanding of factors that influence peak bone mass is crucial to apply primary prevention of osteoporosis, which must be considered as optimization of peak bone mass to prevent future fractures. During youth and young adulthood, skeletal growth leads to accrual of peak bone mass, usually after the first 20–30 years of life.25 It is obvious that higher peak bone mass reduces and protects against fracture risk in later life.26
Unfortunately, it is unclear what the optimal strategies are to build up a strong skeleton in the first decades of life. From twin studies, we have learned that several genetic factors play a crucial role: it has been estimated that approximately 60–80% of peak bone mass is determined by genetic factors.27,28 Another important nonmodifiable factor is sex, as it has been shown that boys and males have higher peak bone mass, also illustrated by the observation that lumbar vertebral bodies in girls are, on the average, 11% smaller than in boys.29,30 So, in general, only 20–40% of the peak bone mass acquisition is influenced by modifiable factors with potential positive and negative effects on peak bone mass. The modifiable factors that influence peak bone mass include physical activity with mechanical stress on the bone, body composition and dietary intake of nutrients like calcium and vitamin D.31 In general, young patients with chronic diseases show more often the presence of modifiable factors with negative effects on peak bone mass.25,32 Although these modifiable risk factors have only a relatively small impact on bone mass and bone strength, they can be theoretically influenced by all individuals.
Factors that negatively influence peak bone mass
Without doubt, a low calcium intake is a risk factor for a low peak bone mass. Weaver and colleagues mentioned that in several randomized controlled trials, a skeletal gain of 3% versus 19% after calcium supplementation was observed in young individuals versus healthy individuals without calcium supplementation.25 Thus, calcium supplementation is useful in those young individuals with a low calcium intake. Unfortunately, it is not very clear what the optimal calcium intake is for boys and girls. Moreover, it is unknown what the optimal intake is for different races and different age groups. Another important modifiable factor is vitamin D, as vitamin D has a positive effect on intestinal calcium absorption, calcium metabolism and bone strength. However, there are still questions on how to screen for vitamin D deficiency and what are appropriate cutoff values for diagnosing vitamin D deficiency or vitamin D insufficiency.33 Theoretically, supplementation of vitamin D will increase bone density and this has indeed been illustrated by four out of eight randomized controlled trials.34–37 Moreover, the question remains: how much vitamin D should be supplemented and how we have to deal with racial differences in vitamin D metabolism in the skin.33 A very striking example on the relationship between nutrition and bone is that a strong association was demonstrated between lower bone mineral density (BMD) and eating disorders like anorexia nervosa and bulimia in adolescents with eating disorders.38 Finally, the increase of alcohol abuse and other intoxications like smoking are a major problem in adolescents. Alcohol and smoking have both direct negative effects on the bone, and may have also negative effects on heart and lungs and organ systems. Without doubt, no smoking and no or limited alcohol are crucial steps in the strategy to reach an optimal peak bone mass in young adulthood.39,40
How can we improve peak bone mass?
In general, it can be stated that peak bone mass can be influenced not only negatively, but also positively during young adult age. Therefore, it is important to realize that due to welfare, there is a change in lifestyle not only due to changes in nutrition and diet, but adolescents also seem to have a more sedentary lifestyle. Nowadays, youth has changed activities during leisure to a more sedentary relaxation with game consoles and other video games. This may be a difficult but necessary challenge as several studies showed that sedentary behavior in young children is associated with a lower bone density and ultimately lower peak bone mass.41,42 Recently, it could be demonstrated that more hours of watching television per day was associated with a lower BMD.43
On the other hand, there is a chance to initiate intervention programmes to increase peak bone mass for young adults, as it was observed that physical activity was associated with increased BMD levels.44 In this study, it was shown that moderate activity, e.g. walking, cycling or exercises, for at least 4 h a week, participation in recreational sports for at least 4 h a week or participation in hard training or sports competitions several times each week may increase BMD up to 11% and 13%, respectively in girls and boys aged 15–19 years.44 Moreover, a retrospective cross-sectional study in prepubertal girls that observed global physical activity and not only activities related to sports was associated with a greater peak bone mass.45 Nevertheless, although physical exercise may have a positive effect on BMD and peak bone mass, there are remaining questions about the optimal intensity and duration of therapy. Promising results were shown in a recent study by Mitchell and colleagues, as this study observed an improvement of bone after physical activity in children genetically predisposed to lower bone density.46 Another point is how to change behavior from a sedentary type to a lifestyle with more physical exercise in large groups of young adults. Therefore, there is an urgent need for not only limiting the negative modifiable factors (low calcium, low vitamin D, smoking and alcohol), but also investing in positive modifiable factors (mainly exercise) to aid in achieving optimal peak bone mass values in many individuals.
Prevention of osteoporosis: who is responsible?
The most intriguing question is who must take responsibility for optimizing peak bone mass in young adults. While physicians (such as rheumatologists, geriatricians and endocrinologists), and fracture nurses working at the fracture liaison service play an important role in the diagnosis of underlying osteoporosis and subsequent treatment, this topic is probably more the responsibility of other individuals and organizations. In our opinion, the lifestyle measures are more under the responsibility of the young individuals themselves and their parents, general practitioners, physicians working in the field of social medicine, health insurance companies and governments. The first step is to create awareness of the burden of fracture risk in later life. The next step will be primary prevention of osteoporosis by education programmes for adolescents and young adults, preferably with support of their parents. Implementation of programmes around primary prevention are usually difficult, but certainly worthwhile, given the threat of an enormous increase in the number of fractures in the coming years.47
Osteoporosis and underdiagnosis
Osteoporosis is defined as a skeletal disorder characterized by compromised bone strength, predisposing a person to an increased risk of fracture.1 One aspect of bone strength is measured by bone density and can be assessed by both peak bone mass and amount of bone loss. The other aspect of bone strength is related to bone quality and reflects bone mineralization, the microarchitecture of the bone and bone turnover.48 The balance between these characteristics determines whether an individual has a high fracture risk. To assess BMD, dual-energy X-ray absorptiometry (DXA) was introduced three decades ago. Since its implementation, DXA has been considered the gold standard for measuring, noninvasively, BMD. In 1994, the World Health Organization (WHO) recommended easy-to-use thresholds for BMD by comparing an individual’s BMD levels with mean BMD levels of a young healthy reference population by incorporating the T-score (referring to the inventor Dr Thomas Kelly).49
It should be realized that the T-scores are arbitrarily chosen and were implemented for diagnosis of osteoporosis but not for treatment decisions. Moreover, for all DXA devices from several manufacturers, different reference groups were used and therefore similar BMD scores in different DXA systems do not reflect the same T-scores.50,51 For this reason, it was thought that a simple step forward in the diagnosis and treatment of osteoporosis would be the introduction of a normative database for all DXA devices. From this point of view, the International Society for Clinical densitometry was founded and commissioned in 2013 a Task Force on normative databases. This Task Force recommended manufacturers of DXA devices to use North American data [National Health and Nutrition Examination Survey (NHANES III)] as international reference for total hip and femoral neck. Moreover, as reference for the spine, it was recommended to continue the use of data of the manufacturers themselves. Although this recommendation may be practical, the question raises whether this method of implementation should be applied for all patients. An important issue in this case is that it should be realized there are marked differences (>10-fold variation) in hip fracture risk worldwide.52 For example, in Europe, the annual incidence of hip fracture was highest in Denmark and Sweden (>500/100,000) compared with (~250/100,000) in Spain and the Netherlands. Moreover, the highest mortality due to fractures was in the same countries with highest hip fracture rate; in Denmark and Sweden there were over 35 deaths compared with ~15 deaths/100,000 inhabitants aged 50 years and over in Spain and the Netherlands. These numbers illustrate the burden of the disease on the general health of all individuals. For this reason, the question raises whether geographical standardized normative databases may be more appropriate for assessing patients’ BMD levels.
It has been well documented that fracture risk is highest in those individuals with a T-score in the spine or in the hips ⩽2.5, and that for each decrease in SD, the fracture risk roughly increases by 1.5–2.5.53 One of the main disadvantages of DXA is the low sensitivity for fracture prediction, in other words, several patients who suffer from a fracture, do not have a T-score in the osteoporotic range. This is the so-called osteoporosis prevention paradox: fracture risk is highest in osteoporotic patients, but most fractures occur in osteopenic patients (because their number is much higher).54
So far, we are not able to clearly define which patients out of the large group of individuals with osteopenia have the highest risk for future fractures. Is it because we only measure BMD and we do not incorporate bone quality or vertebral fractures in the prediction models? Is it because we frequently neglect muscle power and fall risk? In general, there is agreement that prescription of anti-osteoporotic drugs is necessary in high-risk individuals, thus it can be advocated to treat osteopenic patients with vertebral fracture(s) or those with chronic use of glucocorticosteroids. Recently, the National Bone Health Alliance Working Group proposed offering treatment to all osteopenic patients with a recent severe fracture of vertebra, proximal humerus, pelvis and hip.55 For clinicians, this proposal is more relevant for individuals with a T-score of −2.3 than for individuals with a T-score of −1.1.
In 2010, it was estimated that approximately 28 million individuals will have osteoporosis in Europe. In all European Union countries, the prevalence of osteoporosis in women and men 50 years and over is 20–25% and ~6%, respectively.56 Therefore, a team of osteoporosis experts developed an independent project to create awareness of osteoporosis and fracture care in Europe.56 This project aimed to identify unmet needs in the prevention of first (primary prevention) and subsequent (secondary prevention) fractures. But more importantly, this project advocates that all individuals living in the European Union should have access to optimal diagnostic tools and the best treatment options for osteoporosis. For this reason, this project developed a scorecard with multiple indicators of osteoporosis care to identify the gaps and needs in the European countries. Therefore, good and early access, and reimbursement for DXA is essential. In the 24 of 27 countries of the European Union there is reimbursement for DXA investigation. This seems good osteoporosis care but in most countries, there are some barriers to clinical practice, for example, restriction to females or patients over 65-years old, reimbursement only for secondary osteoporosis or income-dependent reimbursement. In (only) 10 out of 27 countries of the European Union there is good access to DXA. However, this illustrates that more than half of the European Union countries do not have a good access to DXA, still one of the most essential tools to diagnose osteoporosis. To improve fracture care and decrease fracture risk, a better access to and a higher number of DXA devices is urgently needed, as DXA assesses low BMD, which is related to fracture risk.53,57
However, the clinical utility of DXA is strongly related to the scan acquisition, analysis, interpretation and reporting. Obviously, when one of these four steps is not properly (regularly) controlled or is suboptimal, the value of the BMD measurement is limited.58,59 Since the clinical consequences of a DXA measurement are very helpful for treatment decisions, measurements of BMD should be more accurate at improving the estimation of fracture risk.
Diagnosis of osteoporosis: more than dual-energy X-ray absorptiometry alone
It could be argued that performing a VFA (vertebral fracture assessment) in all patients for whom a DXA is indicated and performed would be beneficial.60 With this technique, (asymptomatic) vertebral deformities can be detected. For example, it was recently documented in a cross-sectional study that vertebral fractures were found in 13% of rheumatoid arthritis (RA) patients.61 Vertebral fractures are clinically relevant: although only one third of the vertebral deformities are associated with clinical signs and symptoms of an acute vertebral fracture, they are a good predictor of subsequent vertebral and hip fractures, and may have impact on quality of life.62 Moreover, assessment of vertebral fractures in addition to BMD enhances fracture risk prediction.63 Thus, the finding of one or more moderate or severe vertebral deformities in patients with osteopenia may make the difference between starting treatment with anti-osteoporotic medication or not.
The recently published European League Against Rheumatism (EULAR)/ European Federation of National Associations of Orthopaedics and Traumatology (EFORT) recommendations advocate that in all patients 50 years and over with a recent fracture in addition to DXA/VFA, fall risk evaluation and screening for secondary causes of osteoporosis needs to be performed.64 In patients with an elevated fall risk, it is clinically relevant to establish whether modifiable risk factors can be identified; the same is true for potentially treatable causes of secondary osteoporosis and other metabolic bone disorders. Obviously, both high fall rate and untreated secondary osteoporosis may limit the effect of both nonmedical and drug treatment. There are hardly any data on the implementation of these four crucial diagnostic steps in daily practice. However, we suppose that with the abovementioned suboptimal access to DXA, the implementation of the other three steps (VFA incorporation in DXA, fall risk assessment and active screening for secondary causes of osteoporosis) is even worse, which emphasizes that there is an urgent need for better diagnostic procedures in patients at risk for fractures.65
Another way to assess patient fracture risk is the use of fracture risk algorithms with clinical fracture risk factors. FRAX® is a computer-based algorithm that estimates an individual’s 10-year probability of a major fracture (e.g. vertebral, hip, humerus or wrist fracture), the so-called ‘absolute fracture risk’.66 The probability for a major fracture in FRAX® is based on several clinical risk factors, including previous fractures, parental history of hip fracture, body mass index, age, smoking, alcohol consumption, long-term use of glucocorticoids and RA or other secondary causes of osteoporosis. However, FRAX® underestimates fracture risk in patients with a recent fracture, does not consider glucocorticoid dosage and duration and has a relatively low positive predictive value and high negative predictive value.67,68 Nowadays, in 21 out of the 27 European Union countries, fracture risk assessment is based on FRAX®, but treatment is guided in <50% of the European Union nations on this validated risk score.56 It can be argued that the FRAX® has some limitations (not incorporating fall risk, vertebral fractures), but the prediction of the absolute fracture risk is very helpful for patients (and clinicians!) as a starting point for making decisions about treatment with anti-osteoporotic drugs or not (in contrast to the relative risk frequently reported in clinical trials). Therefore, there is an urgent need for structural implementation of fracture risk calculation algorithms like FRAX® in daily clinical practice.
The fracture liaison service
Another very crucial tool to reduce future fracture risk is the implementation of fracture liaison services (FLS) in elderly patients who experienced a low trauma fracture. It has been shown in a systematic review and meta-analysis, that the FLS is the most effective organizational structure for risk evaluation and treatment initiation compared with other forms of interventions [e.g. patient education or alerting the primary care physician (PCP) by a discharge letter containing medical information on the fracture of the patient].69 In the FLS, a dedicated coordinator, often a well-educated nurse, who works under the supervision of a rheumatologist or endocrinologist, takes care of all aspects of the process (identification, investigation and intervention with therapy). Unfortunately, FLS has only been implemented widely in 8 of the 27 European Union countries.56 From studies with FLS services, we do know that only around 50% of elderly with a recent fracture who are invited for a visit to the FLS, are willing and able to attend the FLS and accept the invitation to have DXA scans and other diagnostic procedures.70 Obviously, here is a large unmet need: in the very high fracture-risk group of elderlies with a recent fracture, 50% will not undergo diagnostic procedures to detect underlying osteoporosis! Of course, it is crucial to know what are the main reasons for not accepting the invitation of the FLS. Severe immobility related to hip or pelvis fractures was found in 10%, while ‘not interested’ occurs in 30%. Other causes include no contact, death, already treated, living elsewhere.70 For optimal treatment of hip fracture patients, new initiatives have been developed in which hip fracture patients are treated in a joint care model between geriatrician and orthopaedic surgeon on a dedicated orthogeriatric ward. This model has been shown to have the shortest time to surgery, the shortest length of inpatient stay and the lowest inpatient mortality rate.71,72 Another topic is the large group of individuals who are ‘not interested’, which may be related to financial issues in some of them. In our opinion, education to the lay public and to health professionals is an enormous challenge, as it may lead to a greater awareness of the burden of fragility fractures, and may have a positive effect on the percentage of individuals that accept invitations from FLS services for diagnostic procedures. Obviously, these are new and promising initiatives, however, education alone is not effective, as it will take time and energy before it will be implemented broadly.
Another important issue is that several studies have shown a substantial number of the patients with high fracture risk for osteoporosis do not receive treatment with anti-osteoporotic drugs, also called the treatment gap of osteoporosis.73,74 Estimations of the treatment need showed that the treatment gap in Europe’s best performing country was 25%. But most of the countries showed a high treatment gap ranging from 40–95%. This means that even in relatively rich European Union countries, for approximately 50% or more patients with high fracture risk, drug treatment was not received. So, in general, there is an underuse of DXA, leading to an underdiagnosis of osteoporosis in many patients.
Measuring bone strength
Thus, many patients are not optimally assessed for osteoporosis with only DXA, even when they are at high risk for subsequent fractures, for example, after a fragility fracture in the elderly, and on top of that, there is a substantial treatment gap. But not only the treatment gap needs to be improved; early recognition of osteoporosis in individuals before the development of fracture is crucial to reduce the disease burden and improve the quality of health in these patients.
Although the DXA device is an easy-to-use tool for diagnosing osteoporosis, a limitation is that the DXA device measures only one aspect of bone strength, that is, bone density, which can be considered as the amount of hydroxyapatite (Ca10(PO4)6(OH)2) per bone area. Therefore, the BMD value measured by DXA is influenced by degenerative changes, atherosclerosis (aortic calcifications) and fractured lumbar vertebrae, as these conditions are characterized by calcifications potentially increasing BMD values.75,76
Another limitation of DXA is that it creates a two-dimensional image of bone structures and therefore details cannot be identified. A large proportion of fractures occur in individuals not identified by a low augmented BMD (aBMD), as abovementioned. For these reasons, new and more advanced techniques such as trabecular bone score (TBS), high-resolution peripheral quantitative computed tomography (HR-pQCT), ultrasound, finite element analysis (FEA) and magnetic resonance imaging (MRI) are under development.
TBS is a surrogate marker for bone microarchitecture and has been associated with prevalent and incident fractures.77 Although TBS changes during treatment, TBS is less sensitive to change than aBMD. Although TBS may have a role in predicting future fracture risk in specific disorders like hyperparathyroidism and diabetes, its precise role in osteoporotic care remains to be elucidated.
HR-pQCT is probably a more promising technique: one of the biggest advantages of HR-pQCT is that it constructs a three-dimensional image of the bone and it has the additional value of measuring the microarchitecture of bone, that is, both cortical and trabecular aspects of bone. Previous studies showed that several HR-pQCT-derived bone parameters, with or without FEA, are associated with previous fractures.78–80 More recently, it was shown that cortical area and cortical bone mass by HR-pQCT analysis was independently of aBMD associated with fracture risk, suggesting that HR-pQCT may have additional value on fracture risk calculation.81 Moreover, it was demonstrated that in individuals with identical BMD at distal radius area, differences in bone microarchitecture were observed by HR-pQCT due to differences in morphological and biomechanical differences, especially at the cortical level of bone.82,83
Although promising, an important point is that some clinical questions remain: up to now, we do not know what is the most clinically relevant and prognostically optimal region of interest to report in clinical practice. Furthermore, standardization of repetitive measurements of the same region of interest needs to be improved. Therefore, incorporation of this modern diagnostic tool is promising but remains challenging. In addition, HR-pQCT may have clinical relevance for certain rheumatic diseases like ankylosing spondylitis (AS) characterized by bone formation. In AS patients, suboptimal bone microarchitecture in both axial and peripheral skeleton (distal radius) was demonstrated,84 which is an important finding, as lumbar spine bone density measurement by DXA in AS patients may give an overestimation of BMD due to syndesmophytes or bamboo-spine development. Recently, HR-pQCT imaging made it possible to monitor the healing process of fractures by a non-invasive manner, as this technique identified differences in cortices and trabeculae during a follow up period of 2 years in the fractured and nonfractured site, whereas BMD was similar at both sites.85 Moreover, a recent collaboration between different bone specialists showed that HR-pQCT imaging is a promising tool to define erosions in RA patients instead of using plane X-rays.86 Another interesting observation is that HR-pQCT can measure changes in microarchitecture during treatment for a disease. This was illustrated in coeliac disease patients who underwent treatment with gluten-free diet, where it was observed that both BMD as microarchitectural parameters at the trabecular and cortical level improved during intervention.87 Very recently, data were presented of an observational study in 589 French postmenopausal women with 135 incident fractures, who were followed over 9.4 years. The authors compared the Structure Fragility Score (SFS) combining trabecular and cortical indices by HR-pQCT at the distal radius, with the BMD of the femoral neck and the FRAX® score: the predictive value seems to be comparable for all methods, with no additional value of the SFS on top of the BMD or the FRAX®.88
Although these studies do not demonstrate that HR-pQCT is superior to DXA for fracture risk assessment, it clearly illustrates that new modern techniques may have additional value and may be promising in the future to have a better fracture risk assessment, especially in certain high-risk patient groups.
The abovementioned concerns illustrate that the prevention of subsequent fractures after an initial fracture care needs to be improved. An important step forward may be intensification of collaboration between different medical specialists and general practitioners. The recently published recommendations by EULAR, in collaboration with the European Federation of National Associations of Orthopaedics and Traumatology (EFORT), for patients with fractures is a good example, in which 10 recommendations are advocated for optimal fracture care of patients older than 50 years with a fragility fracture, to prevent subsequent fractures.64
Nonpharmacological treatment: what is the evidence for the prevention of fractures?
Although for several anti-osteoporotic drugs it has been demonstrated that they reduce vertebral and nonvertebral fracture rate,89 there are no data on the effects of any nonpharmacological treatment on fracture incidence, with the exception of vitamin D. Nonpharmacological treatment (including adequate calcium intake and vitamin D levels, and exercise) has an important overlap with a healthy lifestyle, which is crucial for patients with a high fracture risk, since a nonhealthy lifestyle may have negative effects on BMD, bone quality and fall risk.90 So what are the arguments for advocating a healthy lifestyle? Firstly, there are a lot of preclinical data that point in the direction of a positive effect of healthy lifestyle elements (calcium, vitamin D, exercise) on bone. In addition, the balance between a possible, but not proven, effect of a healthy lifestyle on bone, in combination with the absence of side effects, is attractive. Another argument is that all phase III studies with anti-osteoporotic drugs have been performed in patients in which calcium and vitamin D were supplemented.
An adequate calcium balance is an important factor in bone strength. Obviously, an extremely low dietary calcium intake, particularly in patients with malabsorption, for example, after bariatric surgery, may induce a strong tendency to serum hypocalciemia and a subsequent elevated bone resorption. This can be counteracted by oral calcium supplementation, but earlier data have suggested that calcium supplementation and increased cardiovascular risk are associated.91 However, several studies have not confirmed a possible relationship between high dietary calcium intake and cardiovascular events,92,93 leading to a continuing debate about whether calcium supplementation may lead to an elevated myocardial infarction risk. This is even more crucial, since it was shown in a recent study that for patients with RA, the risk of a cardiovascular event was elevated after a fragility fracture: hazard ratio 1.8 (95% confidence interval: 0.85–1.63).94 Another point is that when we prescribe calcium in osteoporotic patients with a low dietary calcium intake, it is not easy to estimate the dietary calcium intake with a simple questionnaire,95 and we also do not know which percentage of the calcium is absorbed in the intestine, and which part of that is finally absorbed in the bone.
Lower serum 25-hydroxy(OH) vitamin D levels have a negative effect on bone mineralization and thus, on bone strength, and may also lead to muscular weakness and an increased fall risk.96,97 Indeed, low levels of serum 25(OH) vitamin D are often observed in persons with a hip fracture.96 Recent data also showed an association between low vitamin D levels and an increased risk of all-cause mortality, which could reflect a causal effect, but could also result from less exposure to sunshine in elderly individuals with severe underlying diseases and comorbidities.98 So the controversy remains, as calcium D supplementation lowers fracture risk on one hand, but may increase cardiovascular risk on the other hand. Recently, Abrahamsen summarized the evidence on this topic as follows:
Hence, despite the combination of CaD having shown the strongest anti-fracture and survival benefits but also the strongest suspicion of renal and cardiovascular potential for harm, there is currently a complete lack of clinical trial activity to resolve this central controversy and inform clinical guidance to patients and public health strategies.99
Another important modifiable risk factor is Vitamin D. In a large meta-analysis, it was shown that vitamin D supplementation (800 IU/day), in patients who also received calcium supplementation, is associated with a 20% reduction in nonvertebral fractures, and also with a 20% reduction in falls.100,101 In an RCT observing different dosages of vitamin D, in >95% of patients, a serum level of 50 nmol/l was found after 6 months of treatment.102 However, it is not clear to which patients vitamin D supplementation should be prescribed: to all osteoporotic patients, or only to those with a vitamin D level below 20, 30, 50 nmol/l, or 70 nmol/l? In other words, vitamin D deficiency, is there really a pandemic?33 It is also not clear whether we should perform measurements of serum 25(OH) vitamin D, to check whether the levels are in the optimal range, or not. Strikingly, very high-peak dosages of vitamin D (annually 500,000 IU/year) seem to be associated with increased fall risk and fracture risk,103 while a dosage of 2000 IU per day was associated with a higher fall risk than with a dosage of 800 IU per day.104
Smoking is another important nonpharmacological factor that has a negative effect on bone strength, mediated by direct negative effects on osteoblasts, upregulation of Receptor activator of nuclear factor-kB ligand (RANKL), alterations in calciotriopic hormones and decreased intestinal calcium absorption.39,105 In addition ‘heavy smokers’ are often physically inactive and have a low body weight, which are also important risk factors for fractures. Thus, there is much evidence that stopping smoking and starting with a healthier lifestyle is crucial in those individuals regarded as heavy smokers; unfortunately stopping smoking is not simple for those who are addicted to nicotine.
With regard to alcohol, more than four alcoholic beverages per day show deleterious effects on bone tissue, particularly a negative effect on bone formation.95 However, even more than two units of alcohol per day increases the risk of osteoporotic and hip fractures, not only because of the negative effect on bone, but also because of a negative effect on neuromuscular coordination and fall risk.40,106
Other dietary-modifiable factors that influence bone mass and future fracture risk include other nutritional factors like protein intake and fruit. Previous studies have shown an incremental increase in bone mass with protein intake in young adults and recently, different diets have been identified to decrease fracture risk by improving bone strength.25,107 Moreover, better milk intake improves bone mineral acquisition in adolescent girls.108 These observations may implicate clinical relevance, although the main question is how much intake of proteins, fruit or dairy is necessary in general; and the next question is whether these amounts can be applied to the individual patient in your outpatient clinic.
Ageing is not only associated with bone loss, but also affects the muscles. More than 90% of nonvertebral fractures occur after fall events, and we are able to diagnose low BMD reflecting low bone strength in these individuals, but unfortunately, we are not able to adequately test the mean muscle force, or even better, muscle strength.109,110 Finding a T-score of <–2.5 often leads to a simple diagnosis of osteoporosis by clinicians, but no operational and widely accepted definition of low muscle strength or sarcopenia exists. The development of an operational definition for muscle weakness or sarcopenia and the development of drug therapies that substantially improve muscle strength are among the biggest challenges (or unmet needs) in the field of osteoporosis and fracture prevention. For this reason, exercise programs and fall prevention programs are a hallmark of nonpharmacological treatment for the prevention of fractures, as well. One year or more of aerobic and strengthening exercises, of which at least a part is weight bearing, is related to positive effects on BMD.90,111,112 A meta-analysis of 17 trials involving 4305 participants studied exercise programmes designed to prevent falls in older adults; all injurious falls, falls resulting in medical care and falls resulting in fractures were reduced.113,114 Individual trials in patients with vertebral fractures for 1 year resulted in an improved quality of life, functional mobility and balance.115 Moreover, it was shown that a 2-year balance training programme, combining weekly and individual sessions, was effective in reducing falls, but not in serious injuries, and improves physical function in elderly women.114 In general, positive effects on both BMD and muscle strength are described in patients who exercise rigorously, as well as fall reduction, but the evidence for fracture reduction is limited.116 Recently, a meta-analysis of randomized controlled trials observed exercise prevents fractures related to fall in elderly.117
Another point is that not all exercise programmes are the same: there are burning questions on the optimal intensity, frequency and duration of the programme. Compared with the number of large trials with anti-osteoporotic drugs, there is a paucity of data in the field of exercise therapy, which is even more remarkably if one realizes that side effects of exercise therapy are limited compared to anti-osteoporotic drugs and since exercise therapy may also have some benefit on cardiovascular function. Clearly, there is a challenge or unmet need to unravel the positive effects of different types of exercise therapy on bone, muscle, falls and fractures.
Surgical therapy
From the process of making a collaborative set of recommendations together with orthopaedic surgeons, we learned that after a fragility fracture, optimal care in the preoperative, operative and postoperative phases all have an important effect on clinical outcome.64 Firstly, fragility fractures should be managed in the context of a multidisciplinary clinical system, guaranteeing adequate preoperative assessment and preparation of patients, including adequate pain relief, appropriate fluid management and surgery within 48 h of injury.118,119 Secondly, to improve functional outcome, and to reduce length of hospital stay and mortality, orthogeriatric comanagement should be provided, especially in elderly hip fracture patients.71,72 And thirdly, appropriate treatment of the fractures in these often elderly and multimorbid patients with frail bones requires a balanced approach with regard to operative versus nonoperative treatment and careful selection of fixation devices and techniques.64 As a consequence, it is very likely that limited mobility and a poor quality of life in the postoperative phase may be associated with an elevated risk of future fractures.
Anti-osteoporotic drugs: still unmet needs?
Recently, a decrease of around 50% from 2008 to 2012 in postmenopausal women using bisphosphonates was documented in the USA, the so-called ‘crisis in osteoporosis’.20,21 The reason for the crisis is probably multifactorial: a common public awareness about devastating side effects as atypical femur fractures and osteonecrosis of the jaw is probably the most important. Suboptimal communication by physicians that are not capable of achieving a large fracture reduction by bisphosphonates (30–70% vertebral fracture reduction) versus the very small risk of severe side effects, around 1 in 100,000 bisphosphonate users, may exacerbate this issue.120 It is important to realize that effective drugs reduce fracture rates, but do not fully prevent the occurrence of fractures. Other explanations are lack of education to and engagement with osteoporosis by physicians, who may regard osteoporosis as a low medical priority, poor coordination of health care systems, inadequate access to diagnostic tools such as DXA and VFA, low adherence and compliance to anti-osteoporotic drugs, and the treatment gap (Table 2).
Table 2.
Reasons for suboptimal fracture prevention.
| Why is fracture prevention suboptimal? |
|---|
| Fractures do occur, mainly in the elderly |
| Fear of severe side effects |
| Lack of education in professionals and in the lay public |
| Lack of engagement: osteoporosis is a low medical priority |
| Lack of coordination between health care systems |
| Inadequate access to diagnostics such as DXA and VFA |
| Suboptimal predictive value of diagnostic techniques |
| The treatment gap |
| Low adherence and compliance to anti-osteoporotic drugs |
| Generic drugs, nocebo-effect* |
| Lack of focus on muscle strength and fall prevention |
A negative expectation of a phenomenon causes it to have a more negative effect than it otherwise would.
DXA, dual-energy X-ray absorptiometry; VFA, vertebral fracture assessment.
In addition, there is a low reimbursement for DXA investigations in the US. Finally, it is possible that pharmaceutical industries also play a role, as during the first years after introduction of osteoporotic drugs, an increase of bisphosphonate use was observed (in 2007 ~15% (!) of postmenopausal women used bisphosphonates).20 Currently, there is a growing market share of generics drugs and increased withdrawal of large pharmaceutical industries, which might be related to a decrease in bisphosphonate use. This emphasizes the certain unmet need for new drugs with an even better efficacy/safety profile.
Thus, the current situation is that we have the tools to diagnose osteoporosis, with DXA (and VFA), and we have effective anti-osteoporotic drugs that reduce the vertebral and nonvertebral fracture rate. Additionally, there is a separation between the growing number of elderly individuals in the population, and the lower risk of bisphosphonate users. In a large cohort study in >22,000 hip fracture patients, new prescription of bisphosphonates decreased from (only) 15% in 2004 to 4% in 2013.121 This is striking, since hip fracture patients have a high risk for subsequent fractures, and, even more strikingly, it has been shown that the use of zoledronic acid reduces not only vertebral and nonvertebral fractures after an initial hip fracture but also has an effect on mortality.122 For patients who start with oral anti-osteoporotic drugs, treatment adherence is one of the biggest challenges: after 1 year of treatment, 50% stopped their medication (!) and only 22% restarted with another anti-osteoporotic drug (!).123 Although it has been suggested that the use of bone markers might have a positive effect on adherence to therapy,124 this is not common practice. Mainly the costs of the measurement, the difficulty in collecting optimal samples and the variability of the measurements hamper the introduction of bone markers to daily practice. So, this raises the question how to improve the use of anti-osteoporotic drug treatment in those who urgently need it? One of the options is to improve the efficacy in terms of fracture reduction of the current medication: the available drugs reduce vertebral fractures by 30–70%, so it seems unlikely, nor very critical that that can be improved substantially. However, the reduction of nonvertebral fractures is (only) 20–25% for antiresorptive drugs, which is substantial, but probably not optimal. The ‘ideal’ drug for the prevention of osteoporotic fractures should reduce nonvertebral fractures versus placebo by 40–50% or more, which has been shown for the osteoanabolic drugs teriparatide and abaloparatide.15,19 In addition, the ‘ideal’ drug should not only reduce vertebral and nonvertebral fractures statistically significantly more than placebo, but should also be superior to an active comparator, for example, an oral bisphosphonate. This has never been investigated properly so far, but is currently studied in a randomized controlled trial with romosozumab versus alendronate and another controlled trial with teriparatide versus risedronate (the VERO trial), and the results of this trial are expected soon.
Another important issue is how to deal with osteoporotic medication use in a patient with a new fracture during the process of fracture healing.125 In general, there is consensus that bisphosphonates and denosumab will delay bone turnover with a delay in callus remodelling, although it has been observed that both bisphosphonates and denosumab increase callus volume with unchanged biomechanical properties. Intriguingly, the anabolic agent parathyroid hormone has shown a better functional outcome postoperatively,126 although a meta-analysis did not observe an improvement in the fracture healing process or pain outcome.127 Further clinical studies with these medications are needed to fully understand their effects on the healing process in order to simultaneously treat fragility fractures and underlying osteoporosis.
New treatment options
Another interesting option is to investigate whether a combination of drugs with a different working mechanism are superior to monotherapy;128 so far, no further fracture reduction has been demonstrated with combination therapy versus monotherapy. Finally, patients nowadays are usually treated with a bisphosphonate or denosumab, and with osteoanabolic drugs in those who fail with therapy, for instance, those who have fractures during adequate antiresorptive therapy. However, an attractive alternative is to start with an osteoanabolic drug in high-risk patients to improve BMD, preferably to normal ranges, and bone strength in 1–2 years, followed by maintenance therapy with an antiresorptive drugs. Age, a very low T-score (–3.5 or lower) and one or more previous fractures will probably be incorporated into the high-risk definition. The challenge, of course, is to find consensus on the definition of the high-risk group, which will be arbitrary. However, the threshold of T ⩽ 2.5 was also arbitrary but gave an enormous and positive impulse to the field of osteoporosis.
Another issue is that up till now, no targets have been defined in current fracture care. In other words: what is the goal of treatment in osteoporotic patients? Is this fracture prevention or an increase in BMD? Treat to target is useful in other situations, such as treatment of hypertension, gout and RA, but not yet proven in osteoporosis. One of the main reasons is that with bisphosphonates, the most commonly used drugs, normalization of BMD is usually not possible.129 However, with denosumab and with osteoanabolic drugs, substantially larger increases in BMD are a realistic goal of treatment. New and preliminary data suggest that treat to target will probably also be introduced the coming years in the field of osteoporosis. Black and colleagues showed at American Society for Bone and Mineral Research (ASBMR) that a larger increase in hip BMD was associated with a larger hip fracture reduction,130 and Ferrari and colleagues showed that 1-year nonvertebral fracture risk gradually decreases from a DXA hip T-score of −3 to a T-score of −1.5, but that no further improvement was observed, suggesting that a hip T-score of −1.5 is the optimal target for therapy.23 However, these data are from patients treated with denosumab in the Fracture Reduction Evaluation of Denosumab in Osteoporosis Every 6 Months (FREEDOM) study, and should be confirmed and validated in other studies and cohorts. However, it is very likely that side effects are a more important limitation than lack of efficacy. In fact, the side effects can be divided in relatively common (in 5–15%), but mild and reversible side effects, particularly dyspepsia, and severe, but infrequent side effects (1:100,000): atypical femur fractures and osteonecrosis of the jaw. Thus, the lower use of bisphosphonates is more likely to be related to fear for severe side effects than for the reoccurring (mild) side effects. Nowadays, shared decision making is a model that is characterized by communication between patients and physicians in which the pros and cons of any treatment will be discussed.131 For osteoporotic patients, showing the epidemiology with the very low incidence of the severe side effects versus the substantial reduction in fractures, combined with the individual fracture risk, probably estimated by FRAX®, may help the patient to make a clear decision to start with the anti-osteoporotic treatment, or not.
Acknowledgments
This review was initiated by a lecture around unmet needs, and was submitted following an unrestricted educational grant award by UCB. UCB had no influence on the final version and intellectual content of this manuscript. WFL and HGR both contributed substantially to the conception and design of the manuscript; both drafted the manuscript and revised it critically for important intellectual content and both approved the final version; and both agreed to be accountable for all aspects of the work by ensuring that questions relating to the accuracy or integrity of any part of the work were appropriately investigated and resolved.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare that there is no conflict of interest.
Contributor Information
Willem F. Lems, Amsterdam Rheumatology and Immunology Centre, VU University Medical Centre and Reade, P.O. Box 7057 1007 MB Amsterdam, The Netherlands.
Hennie G. Raterman, Amsterdam Rheumatology and Immunology Centre, VU University Medical Centre and Reade, North West Clinics, Alkmaar, The Netherlands
References
- 1. Kilbanski A, Adams-Campbell L, Bassford T, et al. Osteoporosis prevention, diagnosis, and therapy. JAMA 2001; 285: 785–795. [DOI] [PubMed] [Google Scholar]
- 2. Melton LJ, III, Chrischilles EA, Cooper C, et al. Perspective. How many women have osteoporosis? J Bone Miner Res 1992; 7: 1005–1010. [DOI] [PubMed] [Google Scholar]
- 3. Nguyen ND, Ahlborg HG, Center JR, et al. Residual lifetime risk of fractures in women and men. J Bone Miner Res 2007; 22: 781–788. [DOI] [PubMed] [Google Scholar]
- 4. Center JR, Bliuc D, Nguyen TV, et al. Risk of subsequent fracture after low-trauma fracture in men and women. JAMA 2007; 297: 387–394. [DOI] [PubMed] [Google Scholar]
- 5. Sambrook P, Cooper C. Osteoporosis. Lancet 2006; 367: 2010–2018. [DOI] [PubMed] [Google Scholar]
- 6. Kim SH, Choi HS, Rhee Y, et al. Prevalent vertebral fractures predict subsequent radiographic vertebral fractures in postmenopausal Korean women receiving antiresorptive agent. Osteoporos Int 2011; 22: 781–787. [DOI] [PubMed] [Google Scholar]
- 7. MacIntyre NJ, Dewan N. Epidemiology of distal radius fractures and factors predicting risk and prognosis. J Hand Ther 2016; 29: 136–145. [DOI] [PubMed] [Google Scholar]
- 8. Zuckerman JD. Hip fracture. N Engl J Med 1996; 334: 1519–1525. [DOI] [PubMed] [Google Scholar]
- 9. Haentjens P, Magaziner J, Colon-Emeric CS, et al. Meta-analysis: excess mortality after hip fracture among older women and men. Ann Intern Med 2010; 152: 380–390. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Kannegaard PN, van der Mark S, Eiken P, et al. Excess mortality in men compared with women following a hip fracture. National analysis of comedications, comorbidity and survival. Age Ageing 2010; 39: 203–209. [DOI] [PubMed] [Google Scholar]
- 11. Black DM, Cummings SR, Karpf DB, et al. Randomised trial of effect of alendronate on risk of fracture in women with existing vertebral fractures. Fracture Intervention Trial Research Group. Lancet 1996; 348: 1535–1541. [DOI] [PubMed] [Google Scholar]
- 12. Black DM, Delmas PD, Eastell R, et al. Once-yearly zoledronic acid for treatment of postmenopausal osteoporosis. N Engl J Med 2007; 356: 1809–1822. [DOI] [PubMed] [Google Scholar]
- 13. Cummings SR, San MJ, McClung MR, et al. Denosumab for prevention of fractures in postmenopausal women with osteoporosis. N Engl J Med 2009; 361: 756–765. [DOI] [PubMed] [Google Scholar]
- 14. McClung MR, Geusens P, Miller PD, et al. Effect of risedronate on the risk of hip fracture in elderly women. Hip Intervention Program Study Group. N Engl J Med 2001; 344: 333–340. [DOI] [PubMed] [Google Scholar]
- 15. Neer RM, Arnaud CD, Zanchetta JR, et al. Effect of parathyroid hormone (1-34) on fractures and bone mineral density in postmenopausal women with osteoporosis. N Engl J Med 2001; 344: 1434–1441. [DOI] [PubMed] [Google Scholar]
- 16. Cosman F, Crittenden DB, Adachi JD, et al. Romosozumab treatment in postmenopausal women with osteoporosis. N Engl J Med 2016; 375: 1532–1543. [DOI] [PubMed] [Google Scholar]
- 17. Rosen CJ, Ingelfinger JR. Building better bones with biologics—a new approach to osteoporosis? N Engl J Med 2016; 375: 1583–1584. [DOI] [PubMed] [Google Scholar]
- 18. Cosman F, Hattersley G, Hu MY, et al. Effects of abaloparatide-SC on fractures and bone mineral density in subgroups of postmenopausal women with osteoporosis and varying baseline risk factors. J Bone Miner Res 2017; 32: 17–23. [DOI] [PubMed] [Google Scholar]
- 19. Miller PD, Hattersley G, Riis BJ, et al. Effect of abaloparatide vs placebo on new vertebral fractures in postmenopausal women with osteoporosis: a randomized clinical trial. JAMA 2016; 316: 722–733. [DOI] [PubMed] [Google Scholar]
- 20. Jha S, Wang Z, Laucis N, et al. Trends in media reports, oral bisphosphonate prescriptions, and hip fractures 1996–2012: an ecological analysis. J Bone Miner Res 2015; 30: 2179–2187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Khosla S, Shane E. A crisis in the treatment of osteoporosis. J Bone Miner Res 2016; 31: 1485–1487. [DOI] [PubMed] [Google Scholar]
- 22. Van der Velde RY, Wyers CE, Teesselink E, et al. Trends in oral anti-osteoporosis drug prescription in the United Kingdom between 1990 and 2012: variation by age, sex, geographic location and ethnicity. Bone 2017; 94: 50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Ferrari S, Reginster JY, Brandi ML, et al. Unmet needs and current and future approaches for osteoporotic patients at high risk of hip fracture. Arch Osteoporos 2016; 11: 37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Jarvinen TL, Michaelsson K, Aspenberg P, et al. Osteoporosis: the emperor has no clothes. J Intern Med 2015; 277: 662–673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Weaver CM, Gordon CM, Janz KF, et al. The National Osteoporosis Foundation’s position statement on peak bone mass development and lifestyle factors: a systematic review and implementation recommendations. Osteoporos Int 2016; 27: 1281–1386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Berger C, Goltzman D, Langsetmo L, et al. Peak bone mass from longitudinal data: implications for the prevalence, pathophysiology, and diagnosis of osteoporosis. J Bone Miner Res 2010; 25: 1948–1957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Eisman JA. Genetics of osteoporosis. Endocr Rev 1999; 20: 788–804. [DOI] [PubMed] [Google Scholar]
- 28. Estrada K, Styrkarsdottir U, Evangelou E, et al. Genome-wide meta-analysis identifies 56 bone mineral density loci and reveals 14 loci associated with risk of fracture. Nat Genet 2012; 44: 491–501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Gilsanz V, Kovanlikaya A, Costin G, et al. Differential effect of gender on the sizes of the bones in the axial and appendicular skeletons. J Clin Endocrinol Metab 1997; 82: 1603–1607. [DOI] [PubMed] [Google Scholar]
- 30. McCormick DP, Ponder SW, Fawcett HD, et al. Spinal bone mineral density in 335 normal and obese children and adolescents: evidence for ethnic and sex differences. J Bone Miner Res 1991; 6: 507–513. [DOI] [PubMed] [Google Scholar]
- 31. Cameron MA, Paton LM, Nowson CA, et al. The effect of calcium supplementation on bone density in premenarcheal females: a co-twin approach. J Clin Endocrinol Metab 2004; 89: 4916–4922. [DOI] [PubMed] [Google Scholar]
- 32. Pitukcheewanont P, Austin J, Chen P, et al. Bone health in children and adolescents: risk factors for low bone density. Pediatr Endocrinol Rev 2013; 10: 318–335. [PubMed] [Google Scholar]
- 33. Manson JE, Brannon PM, Rosen CJ, et al. Vitamin D deficiency–is there really a pandemic? N Engl J Med 2016; 375: 1817–1820. [DOI] [PubMed] [Google Scholar]
- 34. El-Hajj FG, Nabulsi M, Tamim H, et al. Effect of vitamin D replacement on musculoskeletal parameters in school children: a randomized controlled trial. J Clin Endocrinol Metab 2006; 91: 405–412. [DOI] [PubMed] [Google Scholar]
- 35. Al-Shaar L, Nabulsi M, Maalouf J, et al. Effect of vitamin D replacement on hip structural geometry in adolescents: a randomized controlled trial. Bone 2013; 56: 296–303. [DOI] [PubMed] [Google Scholar]
- 36. Viljakainen HT, Natri AM, Karkkainen M, et al. A positive dose-response effect of vitamin D supplementation on site-specific bone mineral augmentation in adolescent girls: a double-blinded randomized placebo-controlled 1-year intervention. J Bone Miner Res 2006; 21: 836–844. [DOI] [PubMed] [Google Scholar]
- 37. Khadilkar AV, Sayyad MG, Sanwalka NJ, et al. Vitamin D supplementation and bone mass accrual in underprivileged adolescent Indian girls. Asia Pac J Clin Nutr 2010; 19: 465–472. [PubMed] [Google Scholar]
- 38. Robinson L, Aldridge V, Clark EM, et al. A systematic review and meta-analysis of the association between eating disorders and bone density. Osteoporos Int 2016; 27: 1953–1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kanis JA, Johnell O, Oden A, et al. Smoking and fracture risk: a meta-analysis. Osteoporos Int 2005; 16: 155–162. [DOI] [PubMed] [Google Scholar]
- 40. Kanis JA, Johansson H, Johnell O, et al. Alcohol intake as a risk factor for fracture. Osteoporos Int 2005; 16: 737–742. [DOI] [PubMed] [Google Scholar]
- 41. Sioen I, Michels N, Polfliet C, et al. The influence of dairy consumption, sedentary behaviour and physical activity on bone mass in Flemish children: a cross-sectional study. BMC Public Health 2015; 15: 717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Herrmann D, Buck C, Sioen I, et al. Impact of physical activity, sedentary behaviour and muscle strength on bone stiffness in 2–10-year-old children—cross-sectional results from the IDEFICS study. Int J Behav Nutr Phys Act 2015; 12: 112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. McVeigh JA, Zhu K, Mountain J, et al. Longitudinal trajectories of television watching across childhood and adolescence predict bone mass at age 20 years in the Raine Study. J Bone Miner Res 2016; 31: 2032–2040. [DOI] [PubMed] [Google Scholar]
- 44. Winther A, Dennison E, Ahmed LA, et al. The Tromso Study: Fit Futures: a study of Norwegian adolescents’ lifestyle and bone health. Arch Osteoporos 2014; 9: 185. [DOI] [PubMed] [Google Scholar]
- 45. Pasqualini L, Leli C, Ministrini S, et al. Relationships between global physical activity and bone mineral density in a group of male and female students. J Sports Med Phys Fitness 2017; 57: 238–243. [DOI] [PubMed] [Google Scholar]
- 46. Mitchell JA, Chesi A, Elci O, et al. Physical activity benefits the skeleton of children genetically predisposed to lower bone density in adulthood. J Bone Miner Res 2016; 31: 1504–1512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Gullberg B, Johnell O, Kanis JA. World-wide projections for hip fracture. Osteoporos Int 1997; 7: 407–413. [DOI] [PubMed] [Google Scholar]
- 48. Felsenberg D, Boonen S. The bone quality framework: determinants of bone strength and their interrelationships, and implications for osteoporosis management. Clin Ther 2005; 27: 1–11. [DOI] [PubMed] [Google Scholar]
- 49. World Health Organisation. Assessment of fracture risk and its application to screening for postmenopausal osteoporosis. Technical report series, Geneva: WHO, 1994. [PubMed] [Google Scholar]
- 50. Pocock NA, Sambrook PN, Nguyen T, et al. Assessment of spinal and femoral bone density by dual X-ray absorptiometry: comparison of lunar and hologic instruments. J Bone Miner Res 1992; 7: 1081–1084. [DOI] [PubMed] [Google Scholar]
- 51. Faulkner KG, Roberts LA, McClung MR. Discrepancies in normative data between Lunar and Hologic DXA systems. Osteoporos Int 1996; 6: 432–436. [DOI] [PubMed] [Google Scholar]
- 52. Kanis JA, Oden A, McCloskey EV, et al. A systematic review of hip fracture incidence and probability of fracture worldwide. Osteoporos Int 2012; 23: 2239–2256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Marshall D, Johnell O, Wedel H. Meta-analysis of how well measures of bone mineral density predict occurrence of osteoporotic fractures. BMJ 1996; 312: 1254–1259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Siris ES, Chen YT, Abbott TA, et al. Bone mineral density thresholds for pharmacological intervention to prevent fractures. Arch Intern Med 2004; 164: 1108–1112. [DOI] [PubMed] [Google Scholar]
- 55. Siris ES, Adler R, Bilezikian J, et al. The clinical diagnosis of osteoporosis: a position statement from the National Bone Health Alliance Working Group. Osteoporos Int 2014; 25: 1439–1443. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Kanis JA, Borgstrom F, Compston J, et al. SCOPE: a scorecard for osteoporosis in Europe. Arch Osteoporos 2013; 8: 144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57. Cummings SR, Cawthon PM, Ensrud KE, et al. BMD and risk of hip and nonvertebral fractures in older men: a prospective study and comparison with older women. J Bone Miner Res 2006; 21: 1550–1556. [DOI] [PubMed] [Google Scholar]
- 58. Lewiecki EM, Binkley N, Morgan SL, et al. Best practices for dual-energy X-ray absorptiometry measurement and reporting: International Society for Clinical Densitometry Guidance. J Clin Densitom 2016; 19: 127–140. [DOI] [PubMed] [Google Scholar]
- 59. Morgan SL, Prater GL. Quality in dual-energy X-ray absorptiometry scans. Bone. Epub ahead of print 31 January 2017. DOI: 10.1016/j.bone.2017.01.033. [DOI] [PubMed] [Google Scholar]
- 60. Bultink IE, Lems WF. Performance of vertebral fracture assessment in addition to dual energy X-ray absorptiometry in patients with rheumatoid arthritis. Rheumatology (Oxford) 2014; 53: 775–776. [DOI] [PubMed] [Google Scholar]
- 61. Mohammad A, Lohan D, Bergin D, et al. The prevalence of vertebral fracture on vertebral fracture assessment imaging in a large cohort of patients with rheumatoid arthritis. Rheumatology (Oxford) 2014; 53: 821–827. [DOI] [PubMed] [Google Scholar]
- 62. Lems WF. Clinical relevance of vertebral fractures. Ann Rheum Dis 2007; 66: 2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63. Siris ES, Genant HK, Laster AJ, et al. Enhanced prediction of fracture risk combining vertebral fracture status and BMD. Osteoporos Int 2007; 18: 761–770. [DOI] [PubMed] [Google Scholar]
- 64. Lems WF, Dreinhofer KE, Bischoff-Ferrari H, et al. EULAR/EFORT recommendations for management of patients older than 50 years with a fragility fracture and prevention of subsequent fractures. Ann Rheum Dis 2017; 76: 802–810. [DOI] [PubMed] [Google Scholar]
- 65. Van der Velde RY, Bours SPG, Wyers CE, et al. Effect of implementation of guidelines on assessment and diagnosis of vertebral fractures in patients older than 50 years with a recent non-vertebral fracture. Osteoporos Int. Epub ahead of print 26 July 2017. DOI: 10.1007/s00198-017-4147-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66. Kanis JA, Hans D, Cooper C, et al. Interpretation and use of FRAX in clinical practice. Osteoporos Int 2011; 22: 2395–2411. [DOI] [PubMed] [Google Scholar]
- 67. Dagan N, Cohen-Stavi C, Leventer-Roberts M, et al. External validation and comparison of three prediction tools for risk of osteoporotic fractures using data from population based electronic health records: retrospective cohort study. BMJ 2017; 356: i6755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68. Roux S, Cabana F, Carrier N, et al. The World Health Organization fracture risk assessment tool (FRAX) underestimates incident and recurrent fractures in consecutive patients with fragility fractures. J Clin Endocrinol Metab 2014; 99: 2400–2408. [DOI] [PubMed] [Google Scholar]
- 69. Ganda K, Puech M, Chen JS, et al. Models of care for the secondary prevention of osteoporotic fractures: a systematic review and meta-analysis. Osteoporos Int 2013; 24: 393–406. [DOI] [PubMed] [Google Scholar]
- 70. Eekman DA, van Helden SH, Huisman AM, et al. Optimizing fracture prevention: the fracture liaison service, an observational study. Osteoporos Int 2014; 25: 701–709. [DOI] [PubMed] [Google Scholar]
- 71. Prestmo A, Hagen G, Sletvold O, et al. Comprehensive geriatric care for patients with hip fractures: a prospective, randomised, controlled trial. Lancet 2015; 385: 1623–1633. [DOI] [PubMed] [Google Scholar]
- 72. Grigoryan KV, Javedan H, Rudolph JL. Orthogeriatric care models and outcomes in hip fracture patients: a systematic review and meta-analysis. J Orthop Trauma 2014; 28: e49–e55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73. Diez-Perez A, Hooven FH, Adachi JD, et al. Regional differences in treatment for osteoporosis. The Global Longitudinal Study of Osteoporosis in Women (GLOW). Bone 2011; 49: 493–498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74. Guggina P, Flahive J, Hooven FH, et al. Characteristics associated with anti-osteoporosis medication use: data from the Global Longitudinal Study of Osteoporosis in Women (GLOW) USA cohort. Bone 2012; 51: 975–980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75. Rand T, Seidl G, Kainberger F, et al. Impact of spinal degenerative changes on the evaluation of bone mineral density with dual energy X-ray absorptiometry (DXA). Calcif Tissue Int 1997; 60: 430–433. [DOI] [PubMed] [Google Scholar]
- 76. Frohn J, Wilken T, Falk S, et al. Effect of aortic sclerosis on bone mineral measurements by dual-photon absorptiometry. J Nucl Med 1991; 32: 259–262. [PubMed] [Google Scholar]
- 77. Harvey NC, Gluer CC, Binkley N, et al. Trabecular bone score (TBS) as a new complementary approach for osteoporosis evaluation in clinical practice. Bone 2015; 78: 216–224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78. Keyak JH, Sigurdsson S, Karlsdottir G, et al. Male-female differences in the association between incident hip fracture and proximal femoral strength: a finite element analysis study. Bone 2011; 48: 1239–1245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79. Kopperdahl DL, Aspelund T, Hoffmann PF, et al. Assessment of incident spine and hip fractures in women and men using finite element analysis of CT scans. J Bone Miner Res 2014; 29: 570–580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80. Wang X, Sanyal A, Cawthon PM, et al. Prediction of new clinical vertebral fractures in elderly men using finite element analysis of CT scans. J Bone Miner Res 2012; 27: 808–816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81. Ohlsson C, Sundh D, Wallerek A, et al. Cortical bone area predicts incident fractures independently of areal bone mineral density in older men. J Clin Endocrinol Metab 2017; 102: 516–524. [DOI] [PubMed] [Google Scholar]
- 82. Kazakia GJ, Burghardt AJ, Link TM, et al. Variations in morphological and biomechanical indices at the distal radius in subjects with identical BMD. J Biomech 2011; 44: 257–266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83. Nicks KM, Amin S, Atkinson EJ, et al. Relationship of age to bone microstructure independent of areal bone mineral density. J Bone Miner Res 2012; 27: 637–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84. Klingberg E, Lorentzon M, Gothlin J, et al. Bone microarchitecture in ankylosing spondylitis and the association with bone mineral density, fractures, and syndesmophytes. Arthritis Res Ther 2013; 15: R179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85. De Jong JJ, Heyer FL, Arts JJ, et al. Fracture repair in the distal radius in postmenopausal women: a follow-up 2 years postfracture using HRpQCT. J Bone Miner Res 2016; 31: 1114–1122. [DOI] [PubMed] [Google Scholar]
- 86. Barnabe C, Toepfer D, Marotte H, et al. Definition for rheumatoid arthritis erosions imaged with high resolution peripheral quantitative computed tomography and interreader reliability for detection and measurement. J Rheumatol 2016; 43: 1935–1940. [DOI] [PubMed] [Google Scholar]
- 87. Zanchetta MB, Longobardi V, Costa F, et al. Impaired bone microarchitecture improves after one year on gluten-free diet: a prospective longitudinal HRpQCT study in women with celiac disease. J Bone Miner Res 2017; 32: 135–142. [DOI] [PubMed] [Google Scholar]
- 88. Boutroy S. Measurement of cortical and trabecular deterioration identifies postmenopausal women at imminent risk for fracture: the OFELY study. ASBMR 2016; 2016: Abstract 1076. [Google Scholar]
- 89. Black DM, Rosen CJ. Postmenopausal osteoporosis. N Engl J Med 2016; 374: 2096–2097. [DOI] [PubMed] [Google Scholar]
- 90. Body JJ, Bergmann P, Boonen S, et al. Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int 2011; 22: 2769–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91. Bolland MJ, Avenell A, Baron JA, et al. Effect of calcium supplements on risk of myocardial infarction and cardiovascular events: meta-analysis. BMJ 2010; 341 DOI: 10.1136/bmj.c3691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92. Lewis JR, Calver J, Zhu K, et al. Calcium supplementation and the risks of atherosclerotic vascular disease in older women: results of a 5-year RCT and a 4.5-year follow-up. J Bone Miner Res 2011; 26: 35–41. [DOI] [PubMed] [Google Scholar]
- 93. Bauer DC. The calcium supplement controversy: now what? J Bone Miner Res 2014; 29: 531–533. [DOI] [PubMed] [Google Scholar]
- 94. Ni MO, Crowson CS, Gabriel SE, et al. Fragility fractures are associated with an increased risk for cardiovascular events in women and men with rheumatoid arthritis: a population-based study. J Rheumatol 2017; 44: 558–564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95. Rasch LA, de van der Schueren MA, van Tuyl LH, et al. Content validity of a short calcium intake list to estimate daily dietary calcium intake of patients with osteoporosis. Calcif Tissue Int 2017; 100: 271–277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96. Rizzoli R, Boonen S, Brandi ML, et al. Vitamin D supplementation in elderly or postmenopausal women: a 2013 update of the 2008 recommendations from the European Society for Clinical and Economic Aspects of Osteoporosis and Osteoarthritis (ESCEO). Curr Med Res Opin 2013; 29: 305–313. [DOI] [PubMed] [Google Scholar]
- 97. Bruyere O, Cavalier E, Souberbielle JC, et al. Effects of vitamin D in the elderly population: current status and perspectives. Arch Public Health 2014; 72: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98. Gaksch M, Jorde R, Grimnes G, et al. Vitamin D and mortality: individual participant data meta-analysis of standardized 25-hydroxyvitamin D in 26916 individuals from a European consortium. PLoS One 2017; 12: e0170791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Abrahamsen B. The calcium and vitamin D controversy. Ther Adv Musculoskelet Dis 2017; 9: 107–114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100. Bischoff-Ferrari HA, Dawson-Hughes B, Staehelin HB, et al. Fall prevention with supplemental and active forms of vitamin D: a meta-analysis of randomised controlled trials. BMJ 2009; 339 DOI: 10.1136/bmj.b3692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 101. Bischoff-Ferrari HA, Willett WC, Orav EJ, et al. A pooled analysis of vitamin D dose requirements for fracture prevention. N Engl J Med 2012; 367: 40–49. [DOI] [PubMed] [Google Scholar]
- 102. Gallagher JC, Sai A, Templin T, et al. Dose response to vitamin D supplementation in postmenopausal women: a randomized trial. Ann Intern Med 2012; 156: 425–437. [DOI] [PubMed] [Google Scholar]
- 103. Sanders KM, Stuart AL, Williamson EJ, et al. Annual high-dose oral vitamin D and falls and fractures in older women: a randomized controlled trial. JAMA 2010; 303: 1815–1822. [DOI] [PubMed] [Google Scholar]
- 104. Bischoff-Ferrari HA, Dawson-Hughes B, Orav EJ, et al. Monthly high-dose vitamin D treatment for the prevention of functional decline: a randomized clinical trial. JAMA Intern Med 2016; 176: 175–183. [DOI] [PubMed] [Google Scholar]
- 105. Yoon V, Maalouf NM, Sakhaee K. The effects of smoking on bone metabolism. Osteoporos Int 2012; 23: 2081–2092. [DOI] [PubMed] [Google Scholar]
- 106. Berg KM, Kunins HV, Jackson JL, et al. Association between alcohol consumption and both osteoporotic fracture and bone density. Am J Med 2008; 121: 406–418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107. De Jonge EA, Kiefte-de Jong JC, Hofman A, et al. Dietary patterns explaining differences in bone mineral density and hip structure in the elderly: the Rotterdam Study. Am J Clin Nutr 2017; 105: 203–211. [DOI] [PubMed] [Google Scholar]
- 108. Cadogan J, Eastell R, Jones N, et al. Milk intake and bone mineral acquisition in adolescent girls: randomised, controlled intervention trial. BMJ 1997; 315: 1255–1260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109. Reginster JY, Beaudart C, Buckinx F, et al. Osteoporosis and sarcopenia: two diseases or one? Curr Opin Clin Nutr Metab Care 2016; 19: 31–36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110. Beaudart C, McCloskey E, Bruyere O, et al. Sarcopenia in daily practice: assessment and management. BMC Geriatr 2016; 16: 170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111. Zehnacker CH, Bemis-Dougherty A. Effect of weighted exercises on bone mineral density in post-menopausal women. A systematic review. J Geriatr Phys Ther 2007; 30: 79–88. [DOI] [PubMed] [Google Scholar]
- 112. De Kam D, Smulders E, Weerdesteyn V, et al. Exercise interventions to reduce fall-related fractures and their risk factors in individuals with low bone density: a systematic review of randomized controlled trials. Osteoporos Int 2009; 20: 2111–2125. [DOI] [PubMed] [Google Scholar]
- 113. El-Khoury F, Cassou B, Charles MA, et al. The effect of fall prevention exercise programmes on fall induced injuries in community dwelling older adults. Br J Sports Med 2015; 49: 1348. [DOI] [PubMed] [Google Scholar]
- 114. El-Khoury F, Cassou B, Latouche A, et al. Effectiveness of two year balance training programme on prevention of fall induced injuries in at risk women aged 75-85 living in community: Ossebo randomised controlled trial. BMJ 2015; 351: h3830. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115. Evstigneeva L, Lesnyak O, Bultink IE, et al. Effect of twelve-month physical exercise program on patients with osteoporotic vertebral fractures: a randomized, controlled trial. Osteoporos Int 2016; 27: 2515–2524. [DOI] [PubMed] [Google Scholar]
- 116. Body JJ, Bergmann P, Boonen S, et al. Non-pharmacological management of osteoporosis: a consensus of the Belgian Bone Club. Osteoporos Int 2011; 22: 2769–2788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117. Zhao R, Feng F, Wang X. Exercise interventions and prevention of fall-related fractures in older people: a meta-analysis of randomized controlled trials. Int J Epidemiol 2017; 46: 149–161. [DOI] [PubMed] [Google Scholar]
- 118. Roberts KC, Brox WT. AAOS clinical practice guideline: management of hip fractures in the elderly. J Am Acad Orthop Surg 2015; 23: 138–140. [DOI] [PubMed] [Google Scholar]
- 119. Simunovic N, Devereaux PJ, Sprague S, et al. Effect of early surgery after hip fracture on mortality and complications: systematic review and meta-analysis. CMAJ 2010; 182: 1609–1616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120. Lewiecki EM. Risk communication and shared decision making in the care of patients with osteoporosis. J Clin Densitom 2010; 13: 335–345. [DOI] [PubMed] [Google Scholar]
- 121. Kim SC, Kim DH, Mogun H, et al. Impact of the U.S. food and drug administration’s safety-related announcements on the use of bisphosphonates after hip fracture. J Bone Miner Res 2016; 31: 1536–1540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122. Lyles KW, Colon-Emeric CS, Magaziner JS, et al. Zoledronic acid and clinical fractures and mortality after hip fracture. N Engl J Med 2007; 357: 1799–1809. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123. Netelenbos JC, Geusens PP, Ypma G, et al. Adherence and profile of non-persistence in patients treated for osteoporosis–a large-scale, long-term retrospective study in The Netherlands. Osteoporos Int 2011; 22: 1537–1546. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124. Diez-Perez A, Naylor KE, Abrahamsen B, et al. International osteoporosis foundation and european calcified tissue society working group. Recommendations for the screening of adherence to oral bisphosphonates. Osteoporos Int 2017; 28: 767–774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125. Hegde V, Jo JE, Andreopoulou P, et al. Effect of osteoporosis medications on fracture healing. Osteoporos Int 2016; 27: 861–871. [DOI] [PubMed] [Google Scholar]
- 126. Malouf-Sierra J, Tarantino U, Garcia-Hernandez PA, et al. Effect of teriparatide or risedronate in elderly patients with a recent pertrochanteric hip fracture: final results of a 78-week randomized clinical trial. J Bone Miner Res 2017; 32: 1040–1051. [DOI] [PubMed] [Google Scholar]
- 127. Shi Z, Zhou H, Pan B, et al. Effectiveness of teriparatide on fracture healing: a systematic review and meta-analysis. PLoS One 2016; 11: e0168691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128. Shen Y, Gray DL, Martinez DS. Combined pharmacologic therapy in postmenopausal osteoporosis. Endocrinol Metab Clin North Am 2017; 46: 193–206. [DOI] [PubMed] [Google Scholar]
- 129. Cummings SR, Cosman F, Lewiecki EM, et al. Goal-directed treatment for osteoporosis: a progress report from the ASBMR-NOF working group on goal-directed treatment for osteoporosis. J Bone Miner Res 2017; 32: 3–10. [DOI] [PubMed] [Google Scholar]
- 130. Black DM. Hip BMD by DXA can reliably estimate reduction in hip risk in osteoporosis trials: a meta regression. ASBMR 2016 2015. [Google Scholar]
- 131. Fried TR. Shared decision making–finding the sweet spot. N Engl J Med 2016; 374: 104–106. [DOI] [PubMed] [Google Scholar]