Abstract
Introduction
This study examined whether World Trade Center (WTC)-related exposures and posttraumatic stress disorder (PTSD) were associated with cognitive function and whether WTC responders' cognition differed from normative data.
Methods
A computer-assisted neuropsychological battery was administered to a prospective cohort study of 1193 WTC responders with no history of stroke or WTC-related head injuries. Data were linked to information collected prospectively since 2002. Sample averages were compared to published norms.
Results
Approximately 14.8% of sampled responders had cognitive dysfunction. WTC responders had worse cognitive function compared to normative data. PTSD symptom severity and working >5 weeks on-site was associated with lower cognition.
Discussion
Results from this sample highlight the potential for WTC responders to be experiencing an increased burden of cognitive dysfunction and linked lowered cognitive functioning to physical exposures and to PTSD. Future research is warranted to understand the extent to which cognitive dysfunction is evident in neural dysfunction.
Keywords: World Trade Center, Cognitive dysfunction, Trauma, Particulate exposure, Posttraumatic stress disorder
Alzheimer's disease (AD) is a neurodegenerative disease that affects as many as 40% of the US population [1], [2]. It is the fifth largest cause of death [3], and the only cause of death in the top 10 for which neither a prevention nor a cure is currently available. AD is a costly disease, with current estimates suggesting that health care, treatment, and long-term care associated with the disease cost approximately $236 billion annually [4]. It is increasingly clear that AD is preceded by long declines in cognitive functioning that occur for at least a decade before diagnosis, which broadly affect a range of cognitive domains [5], [6], [7] and also cause changes in other domains of functioning [8], [9]. Noting that these broad declines in functioning may result in interpretation and in reliably identifying risk factors for disease, a number of researchers have sought to identify risk factors for milder forms of cognitive dysfunction. These milder forms of cognitive dysfunction, sometimes characterized as preclinical dementia or mild cognitive impairment, can be meaningful and problematic for some patients and are the primary risk factor for incident AD.
Recent work has documented a potentially large burden of cognitive impairment among the men and women who participated in rescue and recovery work at the World Trade Center (WTC) attacks of 9/11/2001 [10]. To date, little is known about the reasons for cognitive dysfunction in this population, but contemporary theories suggest that exposure to dust received on-site and severe and chronic posttraumatic stress disorder (PTSD) likely play a role. During operations, responders who helped in rescue and recovery efforts were exposed to traumatic events and also inhaled and dug through the dust and debris from the collapsed buildings [11]. A number of other studies have found that PTSD is associated with reduced memory and slower processing speed [12], as well as with increased risk of cognitive impairment and dementia [10], [13], [14], [15]. At the same time, long-term exposure to air pollutants has been linked with increased risk of cognitive impairment [16], [17] and, increasingly, with indicators of neurodegenerative disease including decreased brain volume, increased β-amyloid, and white matter hyperintensities [18], [19], [20]. Since then, responders have shown elevated rates of chronic PTSD, asthma and other pulmonary disorders, and gastroesophageal reflux disease that may have implications for cognitive functioning [21].
Prior analyses of cognitive impairment in WTC responders were limited in using (1) screening tests that may be biased by problems with attention that are common among individuals with PTSD and (2) tests that are scored by individuals who may themselves be biased observers of patient personality or behaviors associated with PTSD. Finally, prior work on WTC responders has been unable to compare existing data with data from normative controls, thereby limiting our ability to understand the potential for any exposure, common across most WTC responders, to affect cognitive functioning. This study fills these gaps by fielding a computerized battery that measures six domains of cognition, including attention, to test two hypotheses in a sample of responders who were monitored for WTC-related conditions in 2015. We hypothesized that WTC responders would have worse cognitive performance when compared to age-matched cognitively healthy adults. Furthermore, we hypothesized that WTC exposures and PTSD would be associated with deficits in overall cognitive dysfunction and in a number of cognitive domains implicated in neurodegenerative diseases.
1. Methods
1.1. Overview of WTC responder monitoring study
The Centers for Disease Control and Prevention created a monitoring and treatment program for WTC responders with centers in areas where responders reside. More than 33,000 responders have enrolled in the WTC Health Program, forming what is known as the general responder's cohort (GRC) [11]. Stony Brook University (SBU) operates the second largest program and regularly monitors >8000 WTC responders residing on Long Island, NY. Monitoring appointments occur regularly every 12–18 months; a team of outreach and retention specialists is constantly engaged in ensuring that care is available and routine for all members of the cohort. Compared to the GRC, SBU responders had similar exposures and were similar in age on 9/11/2001 (GRC = 38.7, SBU = 38.4) [11], but the SBU population includes more law enforcement personnel, more men, and fewer responders without a high school degree.
For the present study, a prospective cohort of WTC responders completed a computer-assisted neuropsychological battery starting November 2015–June 2016. Trained research staff administered the cognitive tasks in a standard way in a quiet examination room at the clinic after monitoring visits were completed. Reasons for study refusal were recorded for all eligible responders. Responders who assented to the study were asked to first complete a short training in each task.
1.2. Ethics
The SBU Institutional Review Board approved this study; responders provided informed written consent.
1.3. Measures
1.3.1. Cognitive functioning
Cognition was measured using a computer-administered neuropsychological battery (www.cogstate.com). Cogstate was developed to precisely detect small changes in cognitive functioning across multiple assessments [22], [23], [24] and has been shown to be sensitive to dementia [25]. Using computer administration [26], it measures cognitive functioning using three game-like tasks that involve repeated trials using a virtual deck of playing cards displayed on a green background. Participants first received instructions and then interacted with the games using two keys on a keyboard (marked “Y” for yes and “N” for no).
Each task includes 30–88 independent trials, with overall measures being averaged across all trials within each task. The first task (“detection”: a simple reaction-time task) virtually flips over one card at a time and asks individuals to answer as quickly as possible “yes” when the card (always a Black Joker) is flipped over. The second task (“identification”: a choice reaction-time task) flips over either a Black or Red Joker card and then asks that individuals respond “yes” if the card is Red and “no” if it is Black. Finally, the third task (“one-card learning”: an n-back task) begins by sequentially flipping over a card that randomly displays one of the 52 cards found in a common deck of playing cards, and responders were then asked to answer whether they had seen that card before.
From those three tasks, Cogstate recommends utilizing three specific measures to identify cognitive dysfunction. Reaction speed measures the average speed (answers/second) with which the detection tasks are completed. Processing speed (answers/second) measures the average number of correct answers during the identification task. Memory estimates the average arcsine probability of correct responses during the one-card learning task. However, we also derived the following cognitive measures from these tasks. First, because attention may be an indicator of PTSD and a mechanism linking PTSD to lower cognitive scores without cognitive dysfunction [27], attention was measured as the number of times that the respondent correctly identified the color of the card in the identification task. Response variability, a measure theorized to indicate the risk of a compromise in the central nervous system [28], [29], was measured using the within-person variability in processing speed required during the identification task. Cognitive efficiency was calculated by dividing accuracy on the one-card learning task by average time required to complete the one-card learning tasks.
The previously mentioned measures were used to indicate cognitive subdomains. To create an overall index of cognitive function, the measures were standardized [∼N (0,1)] and the average across measures was used. Response variability scores were reverse coded in the cognitive index. At the task level (Table S1), cognitive domains were relatively strongly interassociated with the exception of attention, which was weakly correlated with other domains of cognition used in these analyses and was therefore excluded from the overall index (Cronbach's α = 0.70). The index of overall cognitive functioning was excellent (area under the receiver operating curve = 0.94; Table S2) at identifying severe cognitive impairment in a subsample (n = 659) who were concurrently screened using the Montreal Cognitive Assessment [30] and was independently associated (r = 0.73) with the ratio of hippocampal volume to intracranial volume in a pilot imaging study of these responders (n = 7). For descriptive purposes, a cutoff of 1 standard deviation (SD) below the mean indicates cognitive dysfunction [26].
Two individuals consented to, but did not complete, cognitive testing. In both cases, completed test scores suggested that they had substantially worse performance compared with those who completed tasks. To limit exclusion bias, these data were used for both normative comparisons, and when creating an overall cognitive index, these scores were included.
1.4. Covariates
More than 98% of responders reported being severely exposed to WTC dust, and WTC exposures tended to be overlapping and were chronic for many who were working on-site full time for months. Chronic exposure therefore indicated responders who had spent more than 5 weeks (the median amount of time) working at the WTC.
Researchers have noted that head trauma, commonly received during many experiences that cause PTSD, may play a role in causing similar declines [31], [32]. We used clinical data to identify WTC-related head injuries and further used semi-structured head injury histories to identify WTC-related head injuries or head injuries received while in military service. Owing to small numbers, responders with WTC-related head injuries or head injuries due to military service were excluded from analyses.
PTSD symptoms (Diagnostic and Statistical Manual of Mental Disorders, fourth edition [DSM-IV]) were assessed at each monitoring visit using the PTSD Checklist (PCL), tailored to the WTC disaster (PCL-S) [33]. Items were summed in a standard way; scores ranged from 17 to 85. Since prior work has identified increases in PTSD symptoms over time among those who are experiencing cognitive impairment, we relied on PTSD at enrollment. For descriptive purposes, probable PTSD was defined using a common cutoff (≥44) [34], [35]. In multivariable analyses, utilization of such cutoffs to determine disease burden results in substantial loss of information [36]; to maintain power in multivariable analyses but to facilitate comparison of effect sizes with commonly accepted cutoffs, PCL scores were transformed by first dividing the overall score by 44 and then subtracting the minimum score. To supplement this measure, sensitivity analyses were conducted using a diagnostic measure of PTSD (the Structured Clinical Interview for DSM-IV [37]) in a subsample of responders (n = 757) and rescoring the PCL to better replicate DSM-IV diagnostic criteria.
1.4.1. Predisposing variables
Since >98% of responders had at least a high school degree, education was categorized into those with at least some college versus those with less education. Occupation on 9/11/2001 was dichotomized into law enforcement versus nontraditional responders (construction and utility workers). Sex, year of enrollment in the monitoring study, and present age were also included.
1.4.2. Post-WTC health behaviors
Four concurrent indicators of health and health behaviors were included: smoking status; obesity, operationalized as objectively measured body mass index >30; and a history of heart disease, stroke or transient ischemic attack, hypertension, or diabetes reported by the individual or recorded on clinical charts [38]. Owing to small sample sizes those who reported strokes or transient ischemic attacks were excluded from further analysis.
1.4.3. History of stressful experiences
PTSD may occlude a history of stress before and after the WTC events, which may also have implications for cognitive functioning. We included a count of adverse childhood experiences and a count of post-WTC stressful life events in these analyses.
1.4.4. WTC medical conditions
We also examined whether other WTC-related conditions served as possible causal factors, including diagnoses of major depressive disorder, upper respiratory disease, obstructive airway disease, all-cause cancer, and gastroesophageal reflux disease.
1.5. Normative comparison data
Aggregated normative data for outcomes were available from the Cogstate normative data [39], which were derived from participants of clinical trials. Age groups were created to match groups provided in normative data. These were separated into 10-year age groups among individuals aged 50 years and older and 15-year age groups among individuals aged 35–50 years due to data limitations. Sex was not associated with Cogstate-measured cognitive function in either normative or WTC data, so normative data were pooled by sex.
1.6. Statistical analysis
Descriptive statistics for all potential risk factors included means, SDs, and percentages. For descriptive purposes, we compared responders with high versus low overall cognitive function. Unadjusted relative risks and 95% confidence intervals (CIs) were reported to indicate effect sizes; t tests were used to compare continuous variables between groups, and χ2 tests were used to provide P values for categorical predictors.
To compare cognition with normative data, we utilized t tests to detect significant differences between published age-specific means and age-matched WTC averages. Since PTSD alone may increase risk among WTC responders, analyses were stratified into those with and without probable PTSD.
Ordinary least-squares regression was used to compare cognitive functioning across domains. Estimates included a robust variance to account for possible heteroskedasticity. R2 statistics were reported to examine model fit. Models were entered hierarchically in five blocks to provide estimates linking PTSD to cognitive dysfunction when adjusting for predisposing factors, WTC exposure severity, PTSD, post-WTC health behaviors concurrent with the cognitive assessment, stressful life events, and diagnoses of WTC-related diseases.
Since increases in psychiatric symptoms sometimes occur as part of some types of dementia [40], sensitivity analyses utilized longitudinal multilevel modeling with random intercepts and slopes on prospective monitoring data [41] to examine the potential for changes in PTSD symptoms since 2002.
The absolute value of Cohen's d was reported to indicate effect sizes of overall estimates and, to maintain comparability between PTSD subdomains, compared differences between maximal PTSD symptom severity and minimal severity. The previously mentioned results relied on the index of cognitive functioning, and thus, P-values were reported; α = 0.05 was used to define statistical significance. Where multiple testing was conducted using subdomain analyses, significance values were reported adjusting for the false discovery rate [42].
2. Results
2.1. Sampling
Of the 1624 approached for cognitive assessment, 78.3% completed the cognitive battery (Fig. 1). The most common reason cited for nonresponse was concerns about the length of time that the additional measures would take after a long monitoring visit. On average, the SBU responders sampled were 39.1 years of age on 9/11/2001 and 53.7 years (SD = 8.6) when the Cogstate was administered. Those who refused testing did not differ with respect to age at 9/11/2001 (P = .310), chronic exposure (P = .103), and education (P = .145) but had slightly higher PCL values at baseline (difference = 0.06, P = .014) compared with those who were tested.
Fig. 1.
Sample inclusion and exclusion criteria, World Trade Center (WTC) Cognitive Aging Study 2015–2016.
This sample was mostly middle aged, male, with at least some college education, and worked in law enforcement in 2001 (Table 1). Overall, 14.8% of responders in this sample were characterized as having cognitive dysfunction. Compared to cognitively normal responders, those characterized as having a cognitive dysfunction had lower education, poorer health behaviors, and were more likely to be diagnosed with a WTC-related disease. In bivariable analyses, a PCL score of 44 (compared to 17) was associated with increased risk of cognitive dysfunction (RR = 2.25 [1.73–2.77]). Similarly, WTC responders who spent more than 5 weeks on-site were at greater risk of cognitive dysfunction (RR = 1.36 [1.03–1.80]).
Table 1.
Sample characteristics
| Characteristics | Total | Cognitively normal (n = 1018) | Low cognitive function (n = 175) |
|---|---|---|---|
| Predisposing Characteristics | |||
| Education, N (%) | |||
| High school or less | 299 (25.06) | 232 (22.79) | 67 (38.29)∗∗∗ |
| Some college | 567 (47.53) | 487 (47.84) | 80 (45.71) |
| University degree | 249 (20.87) | 232 (22.79) | 17 (9.71) |
| Graduate schooling | 78 (6.54) | 67 (6.58) | 11 (6.29) |
| Female, N (%) | 90 (7.54) | 71 (6.97) | 19 (10.86)∗∗ |
| Nontraditional responders, N (%) | 331 (27.75) | 117 (11.49) | 29 (16.57)∗∗∗ |
| World Trade Center exposures | |||
| Slept on-site at the WTC, N (%) | 210 (17.6) | 180 (17.68) | 30 (17.14) |
| Saw jumpers, N (%) | 180 (15.09) | 153 (15.03) | 27 (15.43) |
| More than 5 weeks on-site, N (%) | 548 (45.93) | 455 (44.7) | 93 (53.14) |
| PTSD | |||
| PCL score at enrollment, mean (SD) | 0.32 (0.38) | 0.3 (0.35) | 0.47 (0.48)∗∗∗ |
| Mental health | |||
| Diagnosed depression, N (%) | 121 (10.14) | 42 (4.46) | 16 (10.67)∗∗∗ |
| World Trade Center diseases | |||
| Gastroesophageal reflux disease, N (%) | 521 (43.67) | 565 (61.88) | 89 (58.17)* |
| Obstructive airway disease, N (%) | 417 (34.95) | 339 (33.3) | 78 (44.57)∗∗ |
| Cancer, N (%) | 146 (12.24) | 117 (11.49) | 29 (16.57) |
| Current health and behavioral risk factors | |||
| Heart disease, N (%) | 89 (7.46) | 72 (7.07) | 17 (9.71)∗∗ |
| Hypertension, N (%) | 364 (30.51) | 292 (28.68) | 72 (41.14)∗∗ |
| Diabetes, N (%) | 132 (11.06) | 96 (9.43) | 36 (20.57)∗∗∗ |
Abbreviations: PCL, PTSD Checklist; PTSD, posttraumatic stress disorder; SD, standard deviation.
NOTE. Two-tailed P values compare individuals with low cognitive functioning to those with normal cognitive function and were derived from χ2 tests for categorical variables and t tests for continuous variables. ∗P < .05, ∗∗P < .001, ∗∗∗P < .001.
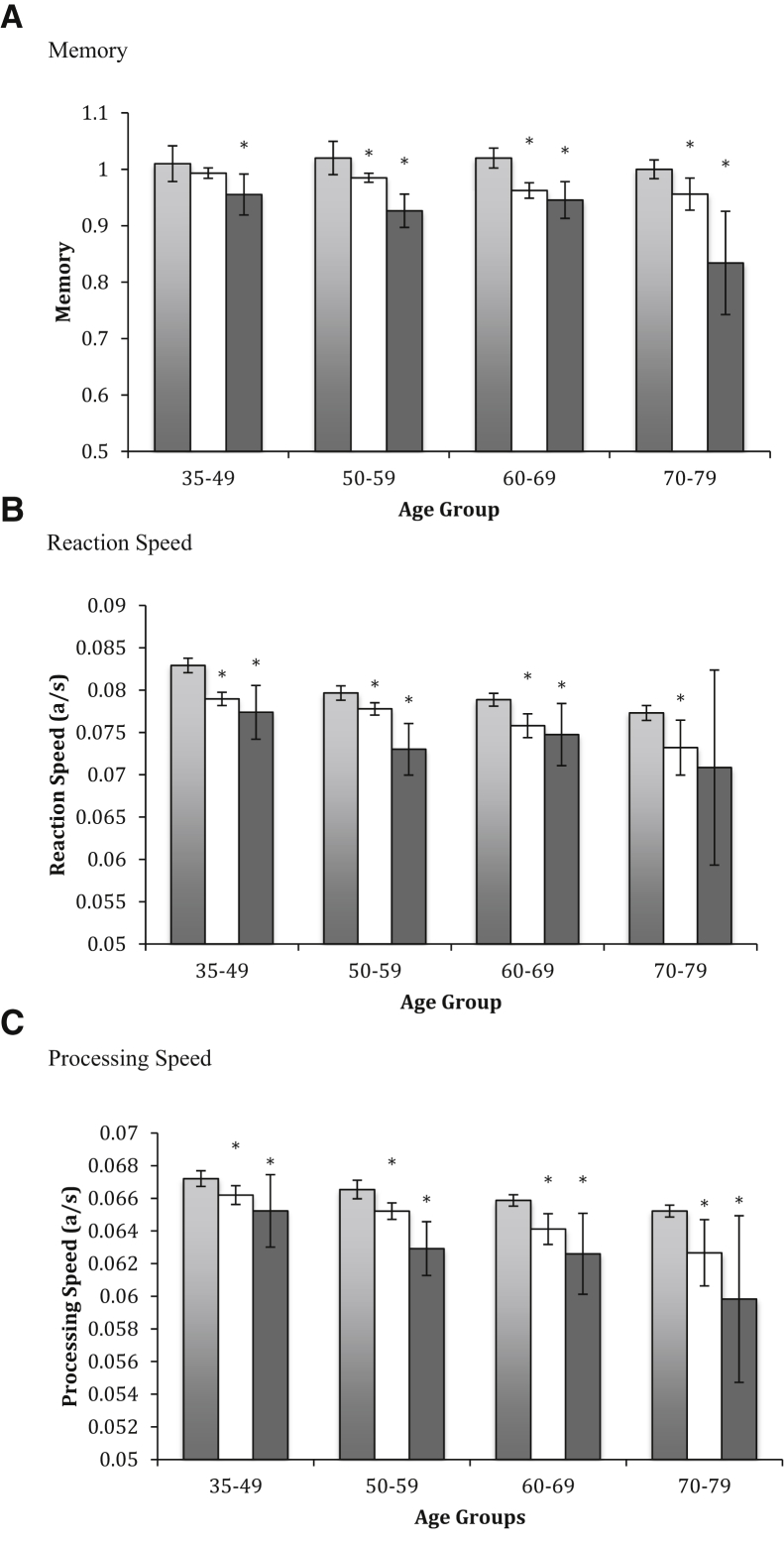
When compared to normative data, WTC responders without PTSD demonstrated slower reaction speed, processing speed, and worse memory (respectively, d = 0.38–0.44). Differences were evident within age groups (Fig. 2). Trend analyses further noted that WTC responders without PTSD also had larger age gradients than did normative controls for both memory and processing speed (d = 0.12-0.26, P < .001) but not for reaction speed.
Fig. 2.
Comparisons between World Trade Center responders and Cogstate normative data. Dark gray, WTC responders with possible PTSD; White, WTC responders without possible PTSD; Light gray, normative comparison population. Two-tailed t tests were used to compare groups and to compare estimated age effects between groups. (A) Shows differences between WTC responders with and without PTSD and normal controls in memory. (B) Shows differences between WTC responders with and without PTSD and normal controls in reaction speed. (C) Shows differences between WTC responders with and without PTSD and normal controls in processing speed. *Significantly different compared to normative data when adjusting for the false discovery rate. Abbreviations: a/s, answers per second; PTSD, posttraumatic stress disorder; WTC, World Trade Center.
Examining model fit (Table 2) revealed that the model adjusting only for predisposing characteristics (model 1) improved over the null model, and further that WTC exposures and PTSD also improved model fit (model 2). However, adding markers of any diagnosed WTC-related health conditions (model 3) or reported diagnoses of hypertension or diabetes (model 3) did not improve model fit (Table 2). Notably, while the addition of diagnosed major depression was marginally significant, the association between baseline PTSD symptom severity and cognitive functioning retained a strong overall association in model 3. Interpreting model 2 suggested that there was an association between longer times spent on-site at the WTC and lowered cognitive function (d = 0.14). Furthermore, associations were revealed linking increased PTSD symptom severity with lower cognitive functioning (d = 0.33).
Table 2.
β coefficients and SEs linking traumatic exposures and PTSD with overall cognitive functioning
| Characteristics | Model 1 |
Model 2 |
Model 3 |
Model 4 |
||||
|---|---|---|---|---|---|---|---|---|
| B | SE | B | SE | B | SE | B | SE | |
| Chronic exposure (>5 weeks) | −0.150 | 0.056∗∗ | −0.130 | 0.055∗ | −0.138 | 0.055∗ | −0.141 | 0.055∗ |
| PTSD symptom severity | −0.498 | 0.088∗∗∗ | −0.424 | 0.091∗∗∗ | −0.416 | 0.091∗∗∗ | ||
| Age | −0.027 | 0.004∗∗∗ | −0.027 | 0.004∗∗∗ | −0.026 | 0.004∗∗∗ | −0.017 | 0.007 |
| Female | −0.200 | 0.102∗ | −0.157 | 0.102 | −0.132 | 0.103 | −0.141 | 0.104 |
| Law enforcement | −0.159 | 0.074∗ | −0.075 | 0.071 | −0.064 | 0.071 | −0.062 | 0.072 |
| Some college | 0.166 | 0.071∗ | 0.163 | 0.069∗ | 0.166 | 0.069∗ | 0.173 | 0.070∗ |
| University degree | 0.395 | 0.082∗∗∗ | 0.360 | 0.081∗∗∗ | 0.364 | 0.081∗∗∗ | 0.366 | 0.081∗∗∗ |
| Graduate degree | 0.319 | 0.127∗ | 0.304 | 0.124∗ | 0.297 | 0.124∗ | 0.297 | 0.125∗ |
| Depression diagnosis | −0.248 | 0.111∗ | −0.244 | 0.111∗ | ||||
| Gastroesophageal reflux disease | 0.066 | 0.061 | 0.066 | 0.061 | ||||
| Obstructive airway disease | −0.113 | 0.066 | −0.110 | 0.066 | ||||
| Cancer | −0.051 | 0.085 | −0.050 | 0.085 | ||||
| Heart disease | −0.036 | 0.108 | ||||||
| Hypertension | −0.062 | 0.067 | ||||||
| Diabetes | −0.089 | 0.101 | ||||||
| Constant | 1.388 | 0.231 | 1.558 | 0.225 | 1.529 | 0.226 | 1.440 | 0.233 |
| R2 | 0.098 | ∗∗∗ | 0.132 | ∗∗∗ | 0.139 | ∗∗∗ | 0.141 | ∗∗∗ |
| ΔR2 | 0.033 | ∗∗∗ | 0.007 | 0.002 | ||||
Abbreviations: B, standardized β coefficient; PTSD, posttraumatic stress disorder; SE, standard error.
NOTE. Chronic exposure indicates individuals who worked the equivalent of more than 5 working weeks on-site. Model 1 examines associations between WTC exposures and adjusts for sociodemographics. Model 2 additionally incorporates PTSD symptom severity. Model 3 incorporates WTC-related diseases. Model 4 includes self-reported diagnoses. All models additionally adjust for age, sex, and year of responder's enrollment. ∗P < .05, ∗∗P < .001, ∗∗∗P < .001.
Subdomain-level analyses suggested that domains of PTSD were consistently associated with lower cognitive performance across domains of cognition (Table S3). Specifically, adjusted associations between PTSD subdomains measured at enrollment and cognition subdomains revealed relatively consistent associations (d = 0.06–0.30) with one caveat that associations were nonsignificant when linking re-experiencing symptoms, avoidance, and hyperarousal symptom subdomains with attention. On balance, associations suggested that linkages were strongest between PTSD and response variability, memory, and cognitive efficiency.
2.2. Sensitivity analyses
Consistent with the results presented previously, multivariable results using the diagnostic tool revealed that present PTSD status was associated with a –0.362 SD (P < .001) reduction in overall cognitive functioning. Utilizing patient diagnostic histories, rather than baseline symptom severity, revealed that diagnoses of PTSD were significantly related to cognitive function when adjusting for major depressive and generalized anxiety disorders (Table S3). Results further revealed that the self-reported PCL score was a better predictor of cognitive functioning. Multivariate analyses adjusting for, and stratifying by, year of enrollment in the parent monitoring study did not modify results. While PTSD symptom severity was associated with increased risk of post-WTC stressors, a summation of these stressful events was not associated with cognitive functioning (P = .725) after adjusting for PTSD.
3. Discussion
First, this study sought to examine whether responders were at greater risk of cognitive dysfunction and to re-examine whether WTC exposures and/or PTSD were associated with cognitive dysfunction using an objective computer-assisted battery of cognitive dysfunction. Second, this study sought to examine whether reductions could be explained by attentional difficulties common among individuals with mental health problems. In this cross-sectional cohort study of 1193 WTC responders monitored at WTC clinics in Long Island, NY, we assessed WTC responders using a computer-assisted cognitive battery. We found that responders were at greater risk of cognitive dysfunction compared to age-matched normative data (d = 0.38–0.44). We further found that PTSD was associated with moderately increased risk of cognitive dysfunction (RR = 2.64, 95% CI = [1.95–3.57]); and we identified an association between time working on-site and lower cognitive functioning (RR = 1.36, 95% CI = [1.03–1.80]). This study thus fills several gaps about the nature of physical and psychological exposures and highlights a concerning trend linking WTC experiences with cognitive troubles.
WTC responders demonstrated worse cognitive functioning compared to age-matched normative data. Period of time spent on-site at the WTC was also consistently associated with lower cognitive functioning. Most WTC responders (>98%) report having been severely exposed to WTC dust during their time on-site. Taken together, results may suggest that chronic exposures to WTC dust may have long-term negative effects on neural functioning affecting brain regions related to cognitive function, including the hippocampus. Several studies have posited that particulate matter, which contains neurotoxic contaminants such as polycyclic aromatic hydrocarbons [43], which have been linked with neural dysfunction earlier in life [44] and in which WTC responders dug for long periods, may have invaded the brain via the olfactory system [19], [20]. Prior work has identified biological mechanisms that may underpin associations between chronic stress and cognitive aging [45], with researchers suggesting proinflammatory [46] and neuroinflammatory [47] mechanisms. Notable among possible mechanisms, both PTSD and exposure to dust at the WTC have been associated with increased levels of systemic inflammation [48].
Results replicated prior work showing both that PTSD at baseline was associated with current cognitive function and increases in PTSD symptoms over time among those with cognitive dysfunction [10]. Results may signify important psychiatric changes occurring among those with cognitive dysfunction. Notably, these findings may be suggestive of mild behavioral impairments believed to precede some types of dementia [49].
Several cognitive domains were associated with PTSD, as was an index of cognitive function. Specifically, we found that PTSD was broadly associated with worse cognitive performances across measures linked to risk of neurodegenerative disease, including reaction speed, processing speed, and memory [12]. This study further showed that PTSD was associated with lower attention, increased response variability, and lowered cognitive efficiency. However, together these associations suggested relatively strong associations between all domains of PTSD and overall cognition and especially with memory, response speed, cognitive efficiency, and item-response variability.
3.1. Strengths and limitations
Implications of this work should be taken in light of its limitations. First, while we are concerned with aging and the risk of neurodegenerative disease, this study was unable to measure whether PTSD actually resulted in neurodegeneration. This study was not able to elucidate the etiology of the observed cognitive dysfunction and cannot be used to establish direction of association. Ongoing data collection is seeking to resolve directionality by collecting longitudinal data. Future work should also seek to use imaging to clarify neuropathological and functional correlates of this dysfunction. For example, cognitive dysfunction is commonly comorbid with, and can result from, underlying cardiovascular disease [50]. We tried to account for this by excluding those with a history of stroke and by adjusting for common cardiovascular diagnoses including hypertension and diabetes. Yet, possible unobserved microinfarctions might be problematic if they are common in this population. Future work using brain imaging may usefully clarify heretofore unobserved causes of WTC-related cognitive dysfunction.
Second, it was not possible here to determine the true extent of the link between physical exposures and cognitive dysfunction. We observed a small association between length of time on-site and cognitive dysfunction. However, since >98% of responders report having been severely exposed to WTC dust during their work on-site, and since only a fraction wore respirators throughout the work (<10%), this result may be conservative. Indeed, we found much larger differences between WTC responders and normative data, and stronger age-trends among WTC responders than normative data. Further research is warranted using unexposed occupationally matched controls to identify mechanisms through which WTC dust may impact cognitive functioning.
Third, this study did not measure cognitive function before the WTC events. Nevertheless, we tried to account for this issue in two ways. First, we relied upon measures, such as memory, processing speed, reaction speed, item-response variability, and cognitive efficiency that are consistently associated with the risk of neurodegenerative disease independent of intellectual capability at baseline [28], [29], [51]. Furthermore, we focused on measures, including cognitive efficiency and memory, that have been shown among Veterans to have no predictive power to determine the risk of PTSD [52], [53], and we note that in a study of civilians, pre-exposure differences in intellect [54] were not associated with the risk of PTSD.
Fourth, while being a relatively large study, results may not be generalizable to other populations. Notably, more than 98% were working at the time of the disaster, many as law enforcement officers, who passed comprehensive personality and neuropsychological tests before being hired. This study is, therefore, a study of very healthy, but severely traumatized, individuals. Nevertheless, about 6%–7% of the general population will experience PTSD in their lifetime, due to a broad range of experiences, including child abuse, neglect, bullying, rape, combat, or violent victimization [55] that, for some, reflects a lifetime of traumatic experiences. In this aspect, this study is therefore of relevance to the wider population. However, the degree of dust exposure in WTC responders is unlikely to be experienced outside the environment of extreme rescue work.
4. Conclusion
In spite of these limitations, results of this study extend earlier work showing that PTSD is associated with cognitive dysfunction [10], by finding that these results were consistent across domains and were evident in WTC responders despite high educational backgrounds. This study further extended prior work by showing that WTC responders had worse cognitive functioning as a whole compared to general population norms and that spending 5 weeks or more at the WTC site was associated with cognitive dysfunction independently of PTSD. Clinicians caring for traumatized individuals in general, and for WTC and other first responders in particular, may want to be aware of potential cognitive dysfunction when treating traumatized patients.
Research in Context.
-
1
Systematic review: Authors sought to identify articles using traditional databases (e.g., PubMed). Although posttraumatic stress disorder (PTSD) has been associated with cognitive impairment, prior work has focused on the role of head injury. Those that have examined PTSD have largely relied on diagnoses and have not examined whether attention explains associations with overall cognitive functioning, and therefore results may be further biased by interviewer bias. Studies of World Trade Center (WTC) responders have further not compared expected cognitive dysfunction to normative data and have not examined whether physical exposures explain cognitive dysfunction.
-
2
Interpretation: WTC responders without a history of stroke or of WTC/military head injuries had worse functioning compared to published norms. PTSD was a risk factor for cognitive dysfunction, as was being on-site for a longer period of time. Results may suggest that WTC responders are aging more rapidly than expected.
-
3
Future directions: Future work should conduct longitudinal studies of cognitive decline should be fielded to examine the rate at which cognition is declining, and neural imaging should be utilized to quantify the extent to which observed cognitive dysfunction is associated with pathological changes indicative of early neurodegenerative diseases.
Acknowledgments
Funding was provided by the National Institutes of Health (R01 AG049953) and by the Centers for Disease Control and Prevention's National Institute of Occupational Safety and Health to administer the monitoring survey (CDC-200-2011-39361). A.S.III was supported by a Senior Research Career Scientist award from the US Department of Veterans Affairs, Clinical Science R&D Service. The funders played no role in data collection, analysis, interpretation, reporting, or the decision to submit for publication.
Footnotes
The authors have declared that no conflict of interest exists.
Supplementary data related to this article can be found at https://doi.org/10.1016/j.trci.2017.09.001.
Supplementary data
The following is/are the supplementary data related to this article:
References
- 1.McKhann G.M., Knopman D.S., Chertkow H., Hyman B.T., Jack C.R., Jr., Kawas C.H. The diagnosis of dementia due to Alzheimer's disease: recommendations from the National Institute on Aging-Alzheimer's Association workgroups on diagnostic guidelines for Alzheimer's disease. Alzheimers Dement. 2011;7:263–269. doi: 10.1016/j.jalz.2011.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Alzheimer's Association Alzheimer's disease: facts and figures. Alzheimer's Dement. 2014;8:131–168. doi: 10.1016/j.jalz.2012.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Heron M. Deaths: Leading Causes for 2013. National vital statistics reports: from the Centers for Disease Control and Prevention, National Center for Health Statistics. Natl Vital Stat Syst. 2016;65:1–14. [PubMed] [Google Scholar]
- 4.Alzheimer's Association 2016 Alzheimer's disease facts and figures. Alzheimers Dement. 2016;12:459–509. doi: 10.1016/j.jalz.2016.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Richards M., Deary I.J. A life course approach to cognitive capability. In: Kuh D., Cooper R., Hardy R., Richards M., Ben-Shlomo Y., editors. A Life Course Approach to Healthy Ageing. Oxford University Press; Oxford: 2014. pp. 32–45. [Google Scholar]
- 6.Petersen R.C. Mild cognitive impairment. N Engl J Med. 2011;364:2227–2234. doi: 10.1056/NEJMcp0910237. [DOI] [PubMed] [Google Scholar]
- 7.Richards M., Deary I.J. A life course approach to cognitive reserve: a model for cognitive aging and development? Ann Neurol. 2005;58:617–622. doi: 10.1002/ana.20637. [DOI] [PubMed] [Google Scholar]
- 8.Ismail Z., Agüera-Ortiz L., Brodaty H., Cieslak A., Cummings J., Fischer C.E. The Mild Behavioral Impairment Checklist (MBI-C): a rating scale for neuropsychiatric symptoms in pre-dementia populations. J Alzheimers Dis. 2017;56:929–938. doi: 10.3233/JAD-160979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Singh-Manoux A., Dugravot A., Fournier A., Abell J., Ebmeier K., Kivimäki M. Trajectories of depressive symptoms before diagnosis of dementia: a 28-year follow-up study. JAMA psychiatry. 2017;74:712–718. doi: 10.1001/jamapsychiatry.2017.0660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Clouston S., Kotov R., Pietrzak R.H., Luft B.J., Gonzalez A., Richards M. Cognitive impairment among World Trade Center responders: long-term implications of re-experiencing the 9/11 terrorist attacks. Alzheimers Dement. 2016;4:67–75. doi: 10.1016/j.dadm.2016.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Dasaro C.R., Holden W.L., Berman K.D., Crane M.A., Kaplan J.R., Lucchini R.G. Cohort Profile: World Trade Center Health Program General Responder Cohort. Int J Epidemiol. 2017;46:e9. doi: 10.1093/ije/dyv099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sumner J.A., Hagan K., Grodstein F., Roberts A.L., Harel B., Koenen K.C. Posttraumatic stress disorder symptoms and cognitive function in a large cohort of middle-aged women. Depress Anxiety. 2017;34:356–366. doi: 10.1002/da.22600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Schuitevoerder S., Rosen J.W., Twamley E.W., Ayers C.R., Sones H., Lohr J.B. A meta-analysis of cognitive functioning in older adults with PTSD. J Anxiety Disord. 2013;27:550–558. doi: 10.1016/j.janxdis.2013.01.001. [DOI] [PubMed] [Google Scholar]
- 14.Veitch D.P., Friedl K.E., Weiner M.W. Military risk factors for cognitive decline, dementia and Alzheimer's disease. Curr Alzheimer Res. 2013;10:907–930. doi: 10.2174/15672050113109990142. [DOI] [PubMed] [Google Scholar]
- 15.Flatt J.D., Gilsanz P., Quesenberry C.P., Albers K.B., Whitmer R.A. Post-traumatic stress disorder and risk of dementia among members of a health care delivery system. Alzheimers Dement. 2017 doi: 10.1016/j.jalz.2017.04.014. http://dx.doi.org/10.1016/j.jalz.2017.04.014 [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Calderón-Garcidueñas L., Azzarelli B., Acuna H., Garcia R., Gambling T.M., Osnaya N. Air pollution and brain damage. Toxicol Pathol. 2002;30:373–389. doi: 10.1080/01926230252929954. [DOI] [PubMed] [Google Scholar]
- 17.Calderón-Garcidueñas L., Villarreal-Ríos R. Living close to heavy traffic roads, air pollution, and dementia. Lancet. 2017;389:675–677. doi: 10.1016/S0140-6736(16)32596-X. [DOI] [PubMed] [Google Scholar]
- 18.Chen R., Ling D., Zhao L., Wang S., Liu Y., Bai R. Parallel Comparative Studies on Mouse Toxicity of Oxide Nanoparticle-and Gadolinium-Based T1 MRI Contrast Agents. ACS Nano. 2015;9:12425–12435. doi: 10.1021/acsnano.5b05783. [DOI] [PubMed] [Google Scholar]
- 19.Wilker E.H., Preis S.R., Beiser A.S., Wolf P.A., Au R., Kloog I. Long-term exposure to fine particulate matter, residential proximity to major roads and measures of brain structure. Stroke. 2015;46:1161–1166. doi: 10.1161/STROKEAHA.114.008348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim S.H., Knight E.M., Saunders E.L., Cuevas A.K., Popovech M., Chen L.C. Rapid doubling of Alzheimer's amyloid-β40 and 42 levels in brains of mice exposed to a nickel nanoparticle model of air pollution. F1000Res. 2012;1:70. doi: 10.12688/f1000research.1-70.v1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Aldrich T.K., Gustave J., Hall C.B., Cohen H.W., Webber M.P., Zeig-Owens R. Lung Function in Rescue Workers at the World Trade Center after 7 Years. N Engl J Med. 2010;362:1263–1272. doi: 10.1056/NEJMoa0910087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lim Y.Y., Ellis K.A., Harrington K., Ames D., Martins R.N., Masters C.L. Use of the CogState Brief Battery in the assessment of Alzheimer's disease related cognitive impairment in the Australian Imaging, Biomarkers and Lifestyle (AIBL) study. J Clin Exp Neuropsychol. 2012;34:345–358. doi: 10.1080/13803395.2011.643227. [DOI] [PubMed] [Google Scholar]
- 23.Fredrickson J., Maruff P., Woodward M., Moore L., Fredrickson A., Sach J. Evaluation of the usability of a brief computerized cognitive screening test in older people for epidemiological studies. Neuroepidemiology. 2010;34:65–75. doi: 10.1159/000264823. [DOI] [PubMed] [Google Scholar]
- 24.Hammers D., Spurgeon E., Ryan K., Persad C., Heidebrink J., Barbas N. Reliability of repeated cognitive assessment of dementia using a brief computerized battery. Am J Alzheimers Dis Other Demen. 2011;26:326–333. doi: 10.1177/1533317511411907. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Lim Y.Y., Jaeger J., Harrington K., Ashwood T., Ellis K.A., Stöffler A. Three-month stability of the CogState brief battery in healthy older adults, mild cognitive impairment, and Alzheimer's disease: results from the Australian Imaging, Biomarkers, and Lifestyle-rate of change substudy (AIBL-ROCS) Arch Clin Neuropsychol. 2013;28:320–330. doi: 10.1093/arclin/act021. [DOI] [PubMed] [Google Scholar]
- 26.Maruff P., Thomas E., Cysique L., Brew B., Collie A., Snyder P. Validity of the CogState brief battery: relationship to standardized tests and sensitivity to cognitive impairment in mild traumatic brain injury, schizophrenia, and AIDS dementia complex. Arch Clin Neuropsychol. 2009;24:165–178. doi: 10.1093/arclin/acp010. [DOI] [PubMed] [Google Scholar]
- 27.Meewisse M.L., Reitsma J.B., Olff M., Kleber R., Gersons B.P.R. Longitudinal effects of posttraumatic stress disorder and depressive symptoms on attentional functioning in disaster survivors. In: Meewisse M.L., editor. Sequelae of Traumatic Stress: Psychopathology, Cortisol, and Attentional Function in the Aftermath of a Disaster. Academic Medical Centre Amsterdam; Amsterdam, Netherlands: 2011. [Google Scholar]
- 28.Strauss E., Bielak A.A., Bunce D., Hunter M.A., Hultsch D.F. Within-person variability in response speed as an indicator of cognitive impairment in older adults. Neuropsychol Dev Cogn B Aging Neuropsychol Cogn. 2007;14:608–630. doi: 10.1080/13825580600932419. [DOI] [PubMed] [Google Scholar]
- 29.MacDonald S.W., Li S.C., Bäckman L. Neural underpinnings of within-person variability in cognitive functioning. Psychol Aging. 2009;24:792. doi: 10.1037/a0017798. [DOI] [PubMed] [Google Scholar]
- 30.Nasreddine Z.S., Phillips N.A., Bédirian V., Charbonneau S., Whitehead V., Collin I. The Montreal Cognitive Assessment, MoCA: a brief screening tool for mild cognitive impairment. J Am Geriatr Soc. 2005;53:695–699. doi: 10.1111/j.1532-5415.2005.53221.x. [DOI] [PubMed] [Google Scholar]
- 31.Davenport N.D., Lim K.O., Sponheim S.R. White matter abnormalities associated with military PTSD in the context of blast TBI. Hum Brain Mapp. 2015;36:1053–1064. doi: 10.1002/hbm.22685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Weiner M.W., Friedl K.E., Pacifico A., Chapman J.C., Jaffee M.S., Little D.M. Military risk factors for Alzheimer's disease. Alzheimers Dement. 2013;9:445–451. doi: 10.1016/j.jalz.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Blanchard E.B., Jones-Alexander J., Buckley T.C., Forneris C.A. Psychometric properties of the PTSD Checklist (PCL) Behav Res Ther. 1996;34:669–673. doi: 10.1016/0005-7967(96)00033-2. [DOI] [PubMed] [Google Scholar]
- 34.Kroenke K., Spitzer R.L. The PHQ-9: a new depression diagnostic and severity measure. Psychiatr Ann. 2002;32:509–515. [Google Scholar]
- 35.Terhakopian A., Sinaii N., Engel C.C., Schnurr P.P., Hoge C.W. Estimating population prevalence of posttraumatic stress disorder: an example using the PTSD checklist. J Trauma Stress. 2008;21:290–300. doi: 10.1002/jts.20341. [DOI] [PubMed] [Google Scholar]
- 36.MacCallum R.C., Zhang S., Preacher K.J., Rucker D.D. On the practice of dichotomization of quantitative variables. Psychol Methods. 2002;7:19–40. doi: 10.1037/1082-989x.7.1.19. [DOI] [PubMed] [Google Scholar]
- 37.First M., Spitzer R., Gibbon M., Williams J. New York Psychiatric Institute, Biometrics Research Department; New York, NY: 1996. Structured Clinical Interview for DSM-IV Axis I Disorders–Clinician Version (SCID-I/P, Version 2.0) [Google Scholar]
- 38.Barnes D.E., Yaffe K. The projected effect of risk factor reduction on Alzheimer's disease prevalence. Lancet Neurol. 2011;10:819–828. doi: 10.1016/S1474-4422(11)70072-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.CogState I. Final Normative Data. In: CogState I., editor. New Haven, CT; 2014. [Google Scholar]
- 40.Mortby M.E., Cherbuin N., Anstey K.J. Mild behavioral impairment: Association between neuropsychiatric symptoms and activities of daily living. Alzheimers Dement. 2015;11:P294. [Google Scholar]
- 41.Rabe-Hesketh S., Skrondal A. STATA press; College Station, TX: 2008. Multilevel and Longitudinal Modeling Using Stata. [Google Scholar]
- 42.Glickman M.E., Rao S.R., Schultz M.R. False discovery rate control is a recommended alternative to Bonferroni-type adjustments in health studies. J Clin Epidemiol. 2014;67:850–857. doi: 10.1016/j.jclinepi.2014.03.012. [DOI] [PubMed] [Google Scholar]
- 43.Lioy P.J., Weisel C.P., Millette J.R., Eisenreich S., Vallero D., Offenberg J. Characterization of the dust/smoke aerosol that settled east of the World Trade Center (WTC) in lower Manhattan after the collapse of the WTC 11 September 2001. Environ Health Perspect. 2002;110:703. doi: 10.1289/ehp.02110703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peterson B.S., Rauh V.A., Bansal R., Hao X., Toth Z., Nati G. Effects of prenatal exposure to air pollutants (polycyclic aromatic hydrocarbons) on the development of brain white matter, cognition, and behavior in later childhood. JAMA psychiatry. 2015;72:531–540. doi: 10.1001/jamapsychiatry.2015.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Greenberg M.S., Tanev K., Marin M.-F., Pitman R.K. Stress, PTSD, and dementia. Alzheimers Dement. 2014;10:S155–S165. doi: 10.1016/j.jalz.2014.04.008. [DOI] [PubMed] [Google Scholar]
- 46.Lindqvist D., Wolkowitz O.M., Mellon S., Yehuda R., Flory J.D., Henn-Haase C. Proinflammatory milieu in combat-related PTSD is independent of depression and early life stress. Brain Behav Immun. 2014;42:81–88. doi: 10.1016/j.bbi.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 47.Pasqualetti G., Brooks D.J., Edison P. The role of neuroinflammation in dementias. Curr Neurol Neurosci Rep. 2015;15:1–11. doi: 10.1007/s11910-015-0531-7. [DOI] [PubMed] [Google Scholar]
- 48.Kazeros A., Zhang E., Cheng X., Shao Y., Liu M., Qian M. Systemic inflammation associated with World Trade Center dust exposures and airway abnormalities in the local community. J Occup Environ Med. 2015;57:610–616. doi: 10.1097/JOM.0000000000000458. [DOI] [PubMed] [Google Scholar]
- 49.Hallam B.J., Silverberg N.D., LaMarre A.K., Mackenzie I.R., Feldman H.H. Clinical presentation of prodromal frontotemporal dementia. Am J Alzheimers Dis Other Demen. 2008;22:456–467. doi: 10.1177/1533317507308781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Sahathevan R., Brodtmann A., Donnan G.A. Dementia, stroke, and vascular risk factors; a review. Int J Stroke. 2012;7:61–73. doi: 10.1111/j.1747-4949.2011.00731.x. [DOI] [PubMed] [Google Scholar]
- 51.Jaffee C.M.S., Meyer K.S. A brief overview of traumatic brain injury (TBI) and post-traumatic stress disorder (PTSD) within the Department of Defense. Clin Neuropsychol. 2009;23:1291–1298. doi: 10.1080/13854040903307250. [DOI] [PubMed] [Google Scholar]
- 52.Vasterling J.J., Proctor S.P., Amoroso P., Kane R., Heeren T., White R.F. Neuropsychological outcomes of army personnel following deployment to the Iraq war. JAMA. 2006;296:519–529. doi: 10.1001/jama.296.5.519. [DOI] [PubMed] [Google Scholar]
- 53.Marx B.P., Brailey K., Proctor S.P., MacDonald H.Z., Graefe A.C., Amoroso P. Association of time since deployment, combat intensity, and posttraumatic stress symptoms with neuropsychological outcomes following Iraq war deployment. Arch Gen Psychiatry. 2009;66:996–1004. doi: 10.1001/archgenpsychiatry.2009.109. [DOI] [PubMed] [Google Scholar]
- 54.Vasterling J.J., Duke L.M., Brailey K., Constans J.I., Allain A.N., Sutker P.B. Attention, learning, and memory performances and intellectual resources in Vietnam veterans: PTSD and no disorder comparisons. Neuropsychology. 2002;16:5. doi: 10.1037//0894-4105.16.1.5. [DOI] [PubMed] [Google Scholar]
- 55.Kessler R.C., Berglund P., Demler O., Jin R., Merikangas K.R., Walters E.E. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.