Abstract
Multiple sclerosis (MS) is a chronic demyelinating disease of the central nervous system characterized by recurrent and progressive demyelination/remyelination cycles, neuroinflammation, oligodendrocyte loss, and axonal pathology. Baicalein isolated from the roots of Scutellaria baicalensis has been shown to exert anti-inflammatory and antioxidant effects. The cuprizone model is an established mouse model of MS and causes demyelination and motor dysfunction and induces neuroinflammation, such as glial activation and pro-inflammatory cytokine production. To determine whether Baicalein attenuates cuprizone-induced demyelination, we administrated Baicalein to cuprizone-exposed mice. Baicalein attenuated weight loss (P<0.05) and motor dysfunction (P<0.05) in the cuprizone model mice. Baicalein treatment effectively suppressed the demyelination (P<0.01) and gene expressions of CNP (P<0.05) and MBP (P<0.05). Baicalein treatment also inhibited the cuprizone-induced increase in Iba1-positive microglia (P<0.001), GFAP-positive astrocytes (P<0.001), and the gene expressions of CD11b (P<0.01), GFAP (P<0.05), TNFα (P<0.05), IL-1β (P<0.05), and iNOS (p<0.01). We found that Baicalein treatment attenuated cuprizone-induced demyelination, glial activation, pro-inflammatory cytokine expression, and motor dysfunction. Our results suggest that Baicalein may be a useful therapeutic agent in demyelinating diseases to suppress neuroinflammation.
Keywords: Baicalein, Microglia, Multiple sclerosis, Myelin, Oligodendrocyte
1. Introduction
Recurrent and progressive demyelination/remyelination cycles, neuroinflammation, oligodendrocyte loss, and axonal pathology are characteristic of multiple sclerosis, which is a chronic inflammatory demyelinating disease of the central nervous system (Bramow et al., 2010; Ozawa et al., 1994; Stangel, 2012). The most commonly studied animal models of multiple sclerosis are the experimental autoimmune encephalomyelitis (EAE) model (Steinman and Zamvil, 2006; Weber et al., 2014) and the cuprizone-induced demyelination model (Matsushima and Morell, 2001; Torkildsen et al., 2008). A detailed immunopathological investigation of demyelination lesions in multiple sclerosis revealed four distinct patterns (Lucchinetti et al., 2000). In patterns I and II, demyelination occurs as a consequence of an autoimmune reaction against myelin, whereas demyelination is independent of immune activation and is caused by oligodendrocyte primary cell loss in patterns III and IV. Previous studies have revealed that the induction of EAE and the administration of cuprizone reproduces the pathology observed in patterns I/II and patterns III/IV, respectively (Lucchinetti et al., 2000). In this study, we used the cuprizone model, which induced demyelination involving the apoptotic death of mature oligodendrocytes, glial activation, enhanced cytokine production, axonal damage, and motor dysfunction.
Baicalein is a major flavonoid originally isolated from the roots of a Chinese medicinal herb and has anti-inflammatory properties such as antioxidant and free radical scavenging activities (Hamada et al., 1993). Baicalein has neuroprotective effects against ischemic brain injury (Liu et al., 2010), experimental parkinsonism(Chen et al., 2004; Mu et al., 2009), Aβ-induced toxicity (Lebeau et al., 2001), and experimental traumatic brain injury (Chen et al., 2008). These pharmacological properties suggest that Baicalein may be a useful agent to treat demyelination associated with neuroinflammation. The present study aimed to determine whether Baicalein attenuated demyelination and motor dysfunction in a cuprizone-exposed mouse model.
2. Materials and Methods
2.1. Animal procedures
The mice were housed in appropriate animal care facilities at Saitama Medical University (Iwasa et al., 2014). C57BL/6 male mice (Tokyo Laboratory Animals Science, Tokyo, Japan) were received at our facility at 10 weeks of age and fed ad libitum a powdered diet containing 0.2% cuprizone (bis-cyclohexanone oxaldihydrazone; Merck, KGaA, Darmstadt, Germany). Mice were maintained on a 12h/12h light-dark cycle and body weight was measured weekly. Mice were euthanized and tissue from the corpus callosum was collected for RNA extraction as previously reported (Yamamoto et al., 2014). Briefly, gross coronal cuts were sectioned at approximately Bregma −0.25 mm and −1.25 mm. Sagittal cuts were performed through the cingulum, medial to each lateral ventricle, followed by cuts above and below the corpus callosum to remove most of the cortex and fornix Corpus callosum tissue samples were stored at −80 °C until required for further processing. For histology, mice were intracardially perfused with 4% paraformaldehyde in phosphate buffered saline (PBS). Brains were removed and postfixed overnight in 4% paraformaldehyde in PBS, and subsequently cryoprotected in 30% sucrose solution in PBS, snap frozen, and stored at −80 °C until required.
2.2. Treatment with Baicalein
Baicalein (Sigma-Aldrich, St. Louis, MI) was dissolved in saline with 5% dimethyl sulfoxide, 25% ethylene glycol. Baicalein was administered at a dose of 100 mg/kg by intraperitoneal (i.p.) injection once-daily for the last 2 weeks (week 4 to 6) of cuprizone exposure.
2.3. Rotarod test
We used an accelerating rotarod treadmill for mice (Mouse rotarod, UgoBasile, Italy) to measured motor balance and coordination after 6 weeks of cuprizone exposure. All mice were given 2 days practice trials at 20 rpm for 5 min. Following the practice trials, mice were tested on the rod at 20 rpm (n = 8–9 per group). The time each mouse was able to stay on the rod (locomotion time) was recorded by a trip switch under the floor of each rotating drum with a maximum recording time of 300 s.
2.4. Histology
Coronal brain section (20 μm thick) were cut on a cryostat t (LEICACM1900, Wetzlar, Germany) and mounted on gelatin-coated glass slides. Sections were stained for myelin using Black Gold II (Histo-Chem, Jefferson, AR) as previously described (Iwasa et al., 2014; Yoshikawa et al., 2011). Briefly, sections were incubated in a 0.3% Black Gold II solution for 10 min, rinsed in distilled water, fixed in 1% sodium thiosulfate, rinsed in tap water, and air-dried. Sections were cover-slipped using Poly-Mount (Polysciences Inc. Boston, MA). Black Gold II stained sections were selected between Bregma −0.22 mm and −0.58 mm. Sections were photographed at 10 × magnification on a KEYENCE BZ-9000 microscope (Keyence Corporation, Osaka, Japan). Images were captured using a KEYENCE BZ-9000 BZ-II Analyzer, and imported into Image J 1.46r, which was used to measure the mean optical density (OD) within the middle of the corpus callosum (Iwasa et al., 2014; Yoshikawa et al., 2011). The OD of the tissue-free area used as a background blank was subtracted from the ODs for tissue. The resulting ODs for myelin in each mouse were normalized against values in unchallenged mice using the following formula: myelin score (%) = (density reading/ unchallenged density average) × 100.
For immunofluorescence, sections were incubated for 1 h in a blocking buffer (PBS, 5% Goat serum, 0.3% Triton X-100). Immunohistochemistry was performed using rabbit anti-Iba1 antibody (1:1000; Wako, Osaka, Japan) and rabbit anti-GFAP antibody (1:1000; Abcam, Cambridge, UK) as the primary antibody at 4 °C overnight, followed by incubation at room temperature for 1 h with horseradish peroxidase (HRP)-conjugated goat anti-rabbit IgG as the secondary antibody (1:1000; Enzo life Science, NY) with 0.1 mg/mL. Sections were then cover slipped using DPX (Merck KGaA, Darmstadt, Germany). Sections were photographed at 40 × magnification on a KEYENCE BZ-9000 microscope. Images were captured using a KEYENCE BZ-9000 BZ-II Analyzer. Iba1-positive microglia and GFAP-positive astrocyte in the corpus callosum were counted and cells per mm2 were calculated. The scoring was performed in a blinded fashion.
2.5. RNA extraction and quantitative real-time PCR (Q-PCR)
Fresh frozen mouse corpus callosum (n = 5 per group) was processed for RNA extraction using the Qiagen RNeasy Lipid Tissue Mini kit (Qiagen, Valencia, CA) following the manufacturer’s procedure. For Q-PCR, total RNA (5 μg) was reverse transcribed using a High Capacity cDNA Archive kit (Applied Biosystems, Foster City, CA). Q-PCR was performed using the ABI PRISM 7000 Sequence Detection System (Applied Biosystems). Q-PCR results were normalized to phosphoglycerate kinase 1 (PGK1; Mm_00435617_m1) expression levels. Gene expression was analyzed using the following assays on demand: Myelin Basic Protein (MBP; Mm01262035_m1); 2′,3′-Cyclic-nucleotide 3′-phosphodiesterase (CNP; Mm01306640-m1); Cluster of differentiation molecule 11b (CD11b; Mm00434455_m1); Glial Fibrillary Acidic Protein (GFAP; Mm01253033_m1); Tumor necrosis factor α (TNFα; Mm00443258_m1); Interleukin 1β (IL-1β; Mm99999061_m1); inducible nitric oxide synthase (iNOS; NM_010927); neuronal nitric oxide synthase (nNOS; NM_008712). Q-PCR conditions were 50°C for 2 min, 95°C for 10 min, followed by 40 cycles of 15 s at 95°C and 1 min at 60°C. The amount of target gene expression was calculated using the ΔΔCT method (Livak and Schmittgen, 2001). Data were analyzed using the relative quantification technique. Relative changes in gene expression were expressed as arbitrary units.
2.6. Statistical analyses
Body weight and locomotion time were analyzed using a Mann-Whitney U-test. All other data were analyzed with one-way ANOVA followed by Newman-Keuls post-hoc tests. Statistical analyses were performed using GraphPad Prism Ver. 5.01 (GraphPad Software, Inc., San Diego, CA) and expressed as means ± SEM. p values < 0.05 were considered statistically significant.
3. Results
3.1. Baicalein improved cuprizone-induced motor dysfunction
The cuprizone exposed mice developed a progressive disease that manifested as weight loss and motor dysfunction. The significant weight loss of the cuprizone-exposed mice was reduced during the last 2 weeks of Baicalein treatment (Fig. 1A). To investigate the effects of Baicalein on the motor dysfunction, we assessed the locomotor coordination and balance of the mice using a rotarod apparatus. Compared to controls, cuprizone-exposed mice exhibited a decline in locomotion time, which was rescued by Baicalein treatment (Fig. 1B).
Figure 1. Body weight and motor performance.
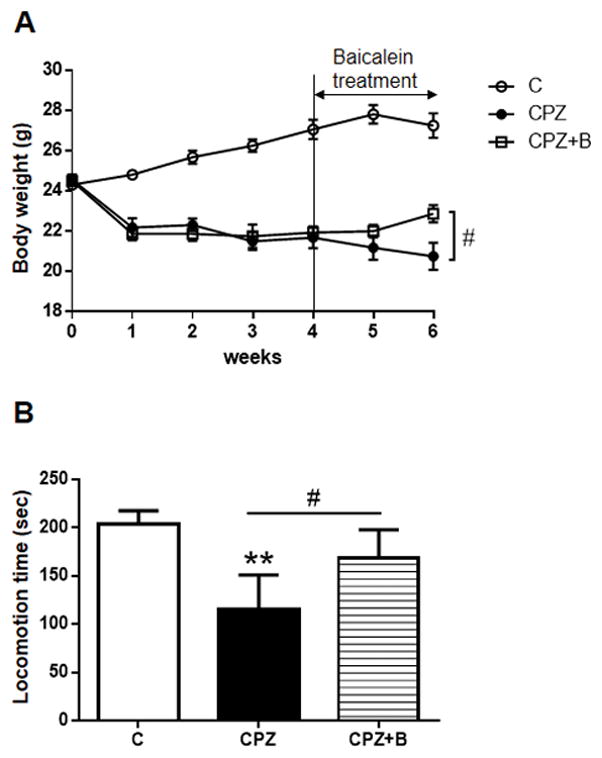
Body weight was measured weekly after 6 weeks of cuprizone (CPZ) exposure (A). Data are mean ± SEM, n = 8 animals. Statistical analysis was performed using one-way ANOVA followed by post-hoc Mann-Whitney U-test (#p < 0.05 vs. CPZ). Motor performance on the rotarod (B). Mice were assessed for locomotion time during a period of 300 s. Data are mean ± SEM, n = 8–9 animals. Statistical analysis was performed using Mann-Whitney U-test (**P < 0.01 vs. Control, #p < 0.05 vs. CPZ).
3.2. Baicalein suppressed cuprizone-induced demyelination
To investigate the effects of Baicalein on cuprizone-induced demyelination, the myelin content was quantified in the corpus callosum using Black Gold II staining (Fig. 2A–C). In the control mice, the corpus callosum appeared to retain sufficient myelin content (Fig. 2A). Six weeks of cuprizone exposure resulted in significant demyelination in the corpus callosum (Fig. 2B and D). Baicalein treatment effectively suppressed this cuprizone-induced demyelination (Fig. 2C and D). Cuprizone decreased both CNP and MBP mRNA levels, and Baicalein treatment attenuated this effect (Fig. 2 E and F).
Figure 2. Black Gold II staining of myelin and gene expression levels of CNP and MBP.
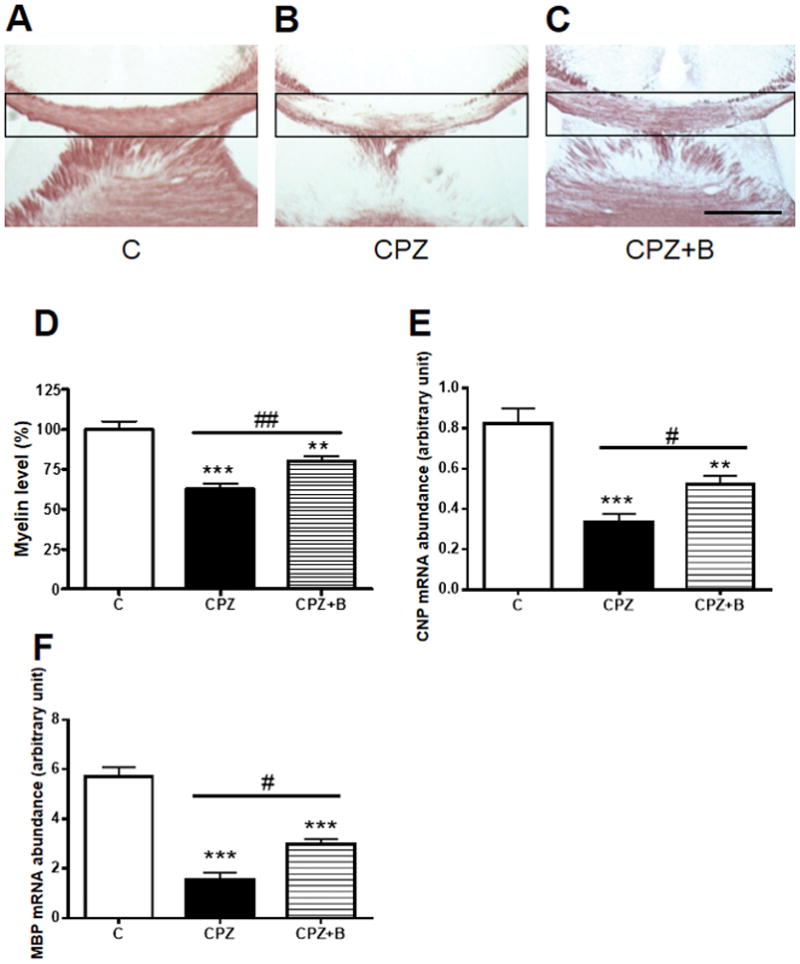
Representative photomicrographs of coronal brain sections at the level of the fimbria demonstrate progressive demyelination of the corpus callosum after 6 weeks of cuprizone (CPZ) exposure. Black-Gold II staining of control (A), CPZ (B), CPZ + Baicalein (C). Myelin densities in the corpus callosum (D) were compared with controls and expressed as a percentage of the control value using the ImageJ analysis program. Data are mean ± SEM, n = 7. Scale bar for = 500 μm. Gene expression of CNP (E) and MBP (F) relative to PGK1, as determined by real-time PCR in the corpus callosum after 6 weeks of cuprizone exposure. Data are mean ± SEM, n = 5 (***p < 0.001, **p < 0.01 vs. controls; ##p < 0.01, #p < 0.05 vs. CPZ).
3.3. Baicalein attenuated neuroinflammation associated glial activation
We investigated the effect of Baicalein on neuroinflammation-associated glial activation, using immunofluorescence staining of a microglial marker (Iba-1) and an astrocyte marker (GFAP) in the corpus callosum. In control mice, microglia and astrocytes were only sporadically observed in the corpus callosum (Fig. 3A and B). Mice exposed to cuprizone showed microglial and astrocytic hypertrophy, which were attenuated by Baicalein treatment (Fig. 3A and B). Cuprizone induced an increase in the numbers of microglia and astrocytes, which were inhibited by Baicalein administration in the corpus callosum (Fig. 3C and D). Furthermore, Baicalein treatment attenuated the cuprizone-increased mRNA expression of microglial (CD11b) and astrocytic (GFAP) markers (Fig. 3E and F). Taken together, Baicalein treatment effectively attenuated cuprizone-induced microglial and astrocytic activation.
Figure 3. Immunofluorescence of microglial and astrocyte markers, and gene expression levels of glial markers.
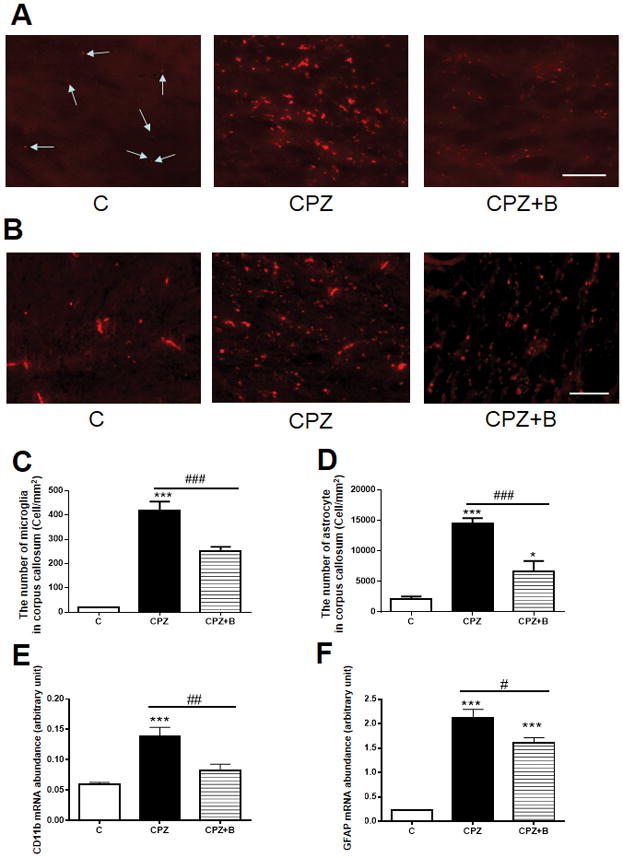
Immunofluorescence staining of microglial marker (Iba-1) (A) and astrocyte marker (GFAP) (B) in the corpus callosum. Arrows indicate Iba-1-positive rest microglia in (A). The number of microglia in the corpus callosum (C). Data are means ± SEM, n = 4 (***p < 0.001 vs. control; ###p < 0.001 vs. CPZ). The number of astrocyte in the corpus callosum (D) Data are means ± SEM, n = 5 (***p < 0.001, *p <0.05 vs. control; ###p < 0.001 vs. CPZ). Statistical analysis was performed using one-way ANOVA followed by post-hoc Newman-Keuls tests. Scale bars = 50 μm. Gene expression of microglial marker CD11b (E) and astrocytic marker GFAP (F) relative to PGK1, as determined by real-time PCR in the corpus callosum after 6 weeks of cuprizone exposure. Data are means ± SEM, n = 5 (***p < 0.001, **p < 0.01 vs. control; ###p < 0.001, #p < 0.05 vs. CPZ). Statistical analysis was performed using one-way ANOVA followed by post-hoc Newman-Keuls tests.
3.4. Baicalein attenuated pro-inflammatory cytokines and nitric oxide synthases expressions
Cuprizone exposure for 6 weeks increased the mRNA expression of the pro-inflammatory cytokines, TNFα and IL-1β (Fig. 4A and B), which was attenuated by Baicalein treatment. Cuprizone increased the mRNA levels of iNOS, but not nNOS. Baicalein treatment effectively attenuated cuprizone-induced iNOS mRNA expression (Fig. 4C and 4D).
Figure 4. Gene expression levels of pro-inflammatory cytokines and nitric oxide synthases.
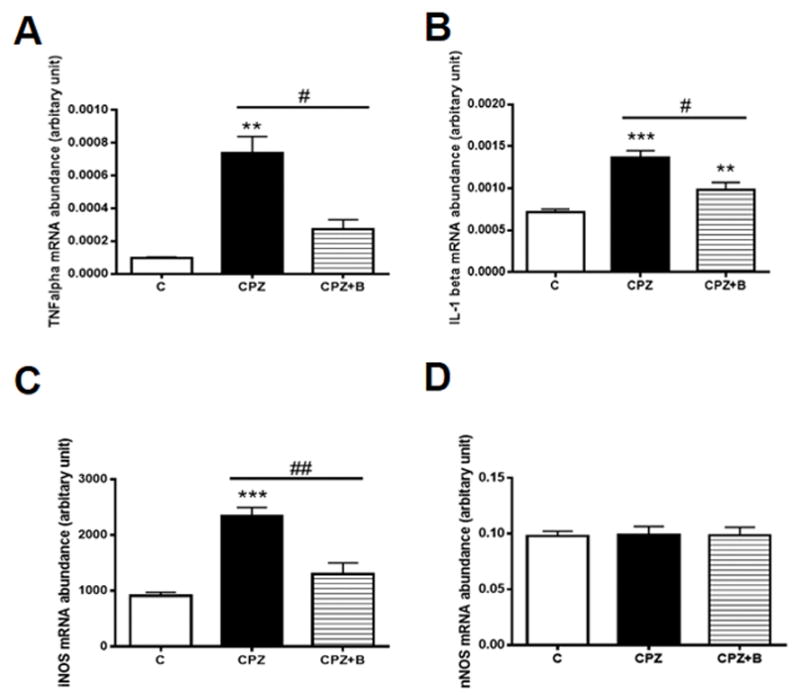
Gene expression of TNFα (A), IL-1β (B), iNOS (C) and nNOS (D) relative to PGK1, as determined by real-time PCR in the corpus callosum after 6 weeks of cuprizone exposure. Data are means ± SEM, n = 5 (***p < 0.001, **p < 0.01 vs. control; ##p < 0.01, #p < 0.05 vs. CPZ). Statistical analysis was performed using one-way ANOVA followed by post-hoc Newman-Keuls tests.
4. Discussion
Neuroinflammation, including microglial activation, enhanced pro-inflammatory cytokine production, demyelination, and axonal damage (Alfonso-Loeches et al., 2014; Pittock and Lucchinetti, 2007; van Noort, 1998), is a key component of the pathological progression of MS. The neuroinflammatory demyelination of the cuprizone experimental model reflects aspects of MS pathology. In the present study, we investigated the potential beneficial effects of Baicalein on cuprizone-induced demyelination. We demonstrated that Baicalein treatment attenuated cuprizone-induced demyelination, neuroinflammation, microglial activation, pro-inflammatory cytokine production, and motor dysfunction, suggesting that Baicalein is a useful therapeutic compound for MS.
After 5–6 weeks of cuprizone exposure, demyelination peaks in the corpus callosum. During the last stage of peak demyelination, spontaneous remyelination occurs, even during persistent cuprizone exposure (Hillis et al., 2016; Matsushima and Morell, 2001). Our results showed that the last 2 weeks of Baicalein treatment prevented cuprizone-induced demyelination at week 6. We speculate that Baicalein protects against demyelination rather than promoting remyelination. Although microglia and astrocytes play different roles in neuroinflammation, demyelination coincides with microglial and astrocytic activation in the cuprizone model (Groebe et al., 2009; Yamamoto et al., 2017). Activated microglia and astrocytes contribute to the death of oligodendrocytes through secretion of pro-inflammatory cytokines (Ambrosini et al., 2005; Taylor et al., 2010), leading to axonal damage (Howell et al., 2010; Wang et al., 2005). Microglia are known to develop diverse functional phenotypes of pro-inflammatory (M1) and alternative (M2) activations. M1 microglia are characterized to release pro-inflammatory cytokines, such as TNFα and IL-1β. In our results, Baicalein treatment attenuated the gene expressions of TNFα and IL-1β, suggesting that Baicalein suppressed microglial M1 pro-inflammatory activation. We demonstrated that treatment with Baicalein attenuated cuprizone-induced microglial activation. Similar studies have reported that the microglial activation inhibitor, minocycline reduced cuprizone-induced demyelination and improved motor activity (Skripuletz et al., 2010). Our results indicated that Baicalein may be beneficial for protecting oligodendrocytes and neuronal axons from an unfavorable microenvironment caused by excessive microglial activation under neuroinflammatory conditions.
The pro-inflammatory cytokines, TNFα and IL-1β, are potent enhancers of neuroinflammatory reactions and are associated with axonal damage (Dujmovic et al., 2009; Sharief and Hentges, 1991). Here, we showed that Baicalein attenuated the expression of these pro-inflammatory cytokines, consistent with previous reports that Baicalein attenuated TNFα and IL-1β up-regulation in neurodegenerative disease (Chen et al., 2004; Cheng et al., 2007; Yang et al., 2009). TNFα and IL-1β are known to induce iNOS and can produce high concentrations of NO, which is associated with neurodegenerative disease (Schuh et al., 2014; Steinert et al., 2010). A previous study reported that Baicalein suppress iNOS-derived NO production via NF-κB inhibition in inflammatory conditions (Suk et al., 2003). Our results also showed that Baicalein treatment attenuated cuprizone-induced iNOS gene expression. These results suggest that the Baicalein-mediated attenuation of axonal damage and motor dysfunction occurs via the suppression of TNFα, IL-1β, and iNOS.
Although it is reported that Baicalein elicits apoptotic effects on cancer cells (Takahashi et al., 2011; Tong et al., 2002), our data suggest that Baicalein exhibited protective effects against demyelinating conditions. One possible mechanism that would potentially explain both the apoptotic and protective effects is the inhibitory action of NF-κB and its-regulated gene expression by Baicalein. Baicalein induced apoptosis in cancer cells by suppression of anti-apoptotic Bcl-2 family proteins via the inhibition of NF-κB (Li et al., 2016). Meanwhile, Baicalein exhibited protective effects mediated by the suppression of iNOS via NF-κB inhibition (Suk et al., 2003). The protective effect of Baicalein observed in our study could probably be attributed to the inhibitory action of NF-κB.
Xu et al. reported that Baicalein ameliorated experimental autoimmune encephalomyelitis (EAE) pathology through the attenuation of neuroinflammation (Xu et al., 2013). Our study also showed that Baicalein attenuated cuprizone-induced demyelination and motor dysfunction via the suppression of neuroinflammation. Taken together, these results showed the beneficial effects of Baicalein on the two most common models of MS: autoimmune-dependent EAE and immune system-independent cuprizone injury.
In conclusion, the results of our study suggest that Baicalein may be a valuable anti-inflammatory and neuroprotective agent to treat demyelination and therapeutically manage MS.
Acknowledgments
We thank the Division of Laboratory Animal Medicine, Biomedical Research Center, Saitama Medical University, for maintaining the mice. We thank K. Maeda. (Saitama Medical University) for research support. This work was supported by Intramural Research Program of NIH (National Institute on Aging), MEXT KAKENHI Grant Number 25870677, Ochiai Memorial Award Research Grant, Special Foundation Takeshi Nagao Research Grants Fund, and Saitama Medical University Internal Grant 16-B-1-05.
Abbreviations
- CD11b
Cluster of differentiation molecule 11b
- CNP
2′, 3′-Cyclic-nucleotide 3′-phosphodiesterase
- CPZ
Cuprizone
- EAE
Experimental autoimmune encephalomyelitis
- GFAP
Glial fibrillary acidic protein
- IL-1β
Interleukin-1 beta
- iNOS
Inducible nitric oxide synthase
- MBP
Myelin Basic Protein
- MS
Multiple sclerosis
- NF-κB
nuclear factor-kappa B
- NOS
Nitric oxide synthase
- NO
Nitric oxide
- PGK1
phosphoglycerate kinase 1
- TNFα
Tumor necrosis factor alpha
Footnotes
Author’s contributions
KeY and FB conceived and designed the experiments. MH, KeY, SY, KI and KoY performed the experiments. KeY and MH wrote the first draft of the manuscript. KeY, MI, KM and FB criteria for authorship read and met. MH, SY, KI, KoY, MI, KM, FB and KeY agree with manuscript results and conclusions.
References
- Alfonso-Loeches S, Urena-Peralta JR, Morillo-Bargues MJ, Oliver-De La Cruz J, Guerri C. Role of mitochondria ROS generation in ethanol-induced NLRP3 inflammasome activation and cell death in astroglial cells. Frontiers in cellular neuroscience. 2014;8:216. doi: 10.3389/fncel.2014.00216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ambrosini E, Remoli ME, Giacomini E, Rosicarelli B, Serafini B, Lande R, Aloisi F, Coccia EM. Astrocytes produce dendritic cell-attracting chemokines in vitro and in multiple sclerosis lesions. Journal of neuropathology and experimental neurology. 2005;64:706–715. doi: 10.1097/01.jnen.0000173893.01929.fc. [DOI] [PubMed] [Google Scholar]
- Bramow S, Frischer JM, Lassmann H, Koch-Henriksen N, Lucchinetti CF, Sorensen PS, Laursen H. Demyelination versus remyelination in progressive multiple sclerosis. Brain : a journal of neurology. 2010;133:2983–2998. doi: 10.1093/brain/awq250. [DOI] [PubMed] [Google Scholar]
- Chen CC, Chow MP, Huang WC, Lin YC, Chang YJ. Flavonoids inhibit tumor necrosis factor-alpha-induced up-regulation of intercellular adhesion molecule-1 (ICAM-1) in respiratory epithelial cells through activator protein-1 and nuclear factor-kappaB: structure-activity relationships. Molecular pharmacology. 2004;66:683–693. doi: 10.1124/mol.66.3.. [DOI] [PubMed] [Google Scholar]
- Chen SF, Hsu CW, Huang WH, Wang JY. Post-injury baicalein improves histological and functional outcomes and reduces inflammatory cytokines after experimental traumatic brain injury. British journal of pharmacology. 2008;155:1279–1296. doi: 10.1038/bjp.2008.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheng PY, Lee YM, Wu YS, Chang TW, Jin JS, Yen MH. Protective effect of baicalein against endotoxic shock in rats in vivo and in vitro. Biochemical pharmacology. 2007;73:793–804. doi: 10.1016/j.bcp.2006.11.025. [DOI] [PubMed] [Google Scholar]
- Dujmovic I, Mangano K, Pekmezovic T, Quattrocchi C, Mesaros S, Stojsavljevic N, Nicoletti F, Drulovic J. The analysis of IL-1 beta and its naturally occurring inhibitors in multiple sclerosis: The elevation of IL-1 receptor antagonist and IL-1 receptor type II after steroid therapy. Journal of neuroimmunology. 2009;207:101–106. doi: 10.1016/j.jneuroim.2008.11.004. [DOI] [PubMed] [Google Scholar]
- Groebe A, Clarner T, Baumgartner W, Dang J, Beyer C, Kipp M. Cuprizone treatment induces distinct demyelination, astrocytosis, and microglia cell invasion or proliferation in the mouse cerebellum. Cerebellum (London, England) 2009;8:163–174. doi: 10.1007/s12311-009-0099-3. [DOI] [PubMed] [Google Scholar]
- Hamada H, Hiramatsu M, Edamatsu R, Mori A. Free radical scavenging action of baicalein. Archives of biochemistry and biophysics. 1993;306:261–266. doi: 10.1006/abbi.1993.1509. [DOI] [PubMed] [Google Scholar]
- Hillis JM, Davies J, Mundim MV, Al-Dalahmah O, Szele FG. Cuprizone demyelination induces a unique inflammatory response in the subventricular zone. Journal of neuroinflammation. 2016;13:190. doi: 10.1186/s12974-016-0651-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howell OW, Rundle JL, Garg A, Komada M, Brophy PJ, Reynolds R. Activated microglia mediate axoglial disruption that contributes to axonal injury in multiple sclerosis. Journal of neuropathology and experimental neurology. 2010;69:1017–1033. doi: 10.1097/NEN.0b013e3181f3a5b1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iwasa K, Yamamoto S, Takahashi M, Suzuki S, Yagishita S, Awaji T, Maruyama K, Yoshikawa K. Prostaglandin F2alpha FP receptor inhibitor reduces demyelination and motor dysfunction in a cuprizone-induced multiple sclerosis mouse model. Prostaglandins, leukotrienes, and essential fatty acids. 2014;91:175–182. doi: 10.1016/j.plefa.2014.08.004. [DOI] [PubMed] [Google Scholar]
- Lebeau A, Esclaire F, Rostene W, Pelaprat D. Baicalein protects cortical neurons from beta-amyloid (25–35) induced toxicity. Neuroreport. 2001;12:2199–2202. doi: 10.1097/00001756-200107200-00031. [DOI] [PubMed] [Google Scholar]
- Li J, Ma J, Wang KS, Mi C, Wang Z, Piao LX, Xu GH, Li X, Lee JJ, Jin X. Baicalein inhibits TNF-alpha-induced NF-kappaB activation and expression of NF-kappaB-regulated target gene products. Oncology reports. 2016;36:2771–2776. doi: 10.3892/or.2016.5108. [DOI] [PubMed] [Google Scholar]
- Liu C, Wu J, Xu K, Cai F, Gu J, Ma L, Chen J. Neuroprotection by baicalein in ischemic brain injury involves PTEN/AKT pathway. Journal of neurochemistry. 2010;112:1500–1512. doi: 10.1111/j.1471-4159.2009.06561.x. [DOI] [PubMed] [Google Scholar]
- Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(−Delta Delta C(T)) Method. Methods (San Diego, Calif) 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- Lucchinetti C, Bruck W, Parisi J, Scheithauer B, Rodriguez M, Lassmann H. Heterogeneity of multiple sclerosis lesions: implications for the pathogenesis of demyelination. Annals of neurology. 2000;47:707–717. doi: 10.1002/1531-8249(200006)47:6<707::aid-ana3>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Matsushima GK, Morell P. The neurotoxicant, cuprizone, as a model to study demyelination and remyelination in the central nervous system. Brain pathology (Zurich, Switzerland) 2001;11:107–116. doi: 10.1111/j.1750-3639.2001.tb00385.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu X, He G, Cheng Y, Li X, Xu B, Du G. Baicalein exerts neuroprotective effects in 6-hydroxydopamine-induced experimental parkinsonism in vivo and in vitro. Pharmacology, biochemistry, and behavior. 2009;92:642–648. doi: 10.1016/j.pbb.2009.03.008. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Suchanek G, Breitschopf H, Bruck W, Budka H, Jellinger K, Lassmann H. Patterns of oligodendroglia pathology in multiple sclerosis. Brain : a journal of neurology. 1994;117(Pt 6):1311–1322. doi: 10.1093/brain/117.6.1311. [DOI] [PubMed] [Google Scholar]
- Pittock SJ, Lucchinetti CF. The pathology of MS: new insights and potential clinical applications. The neurologist. 2007;13:45–56. doi: 10.1097/01.nrl.0000253065.31662.37. [DOI] [PubMed] [Google Scholar]
- Schuh C, Wimmer I, Hametner S, Haider L, Van Dam AM, Liblau RS, Smith KJ, Probert L, Binder CJ, Bauer J, Bradl M, Mahad D, Lassmann H. Oxidative tissue injury in multiple sclerosis is only partly reflected in experimental disease models. Acta neuropathologica. 2014;128:247–266. doi: 10.1007/s00401-014-1263-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharief MK, Hentges R. Association between tumor necrosis factor-alpha and disease progression in patients with multiple sclerosis. The New England journal of medicine. 1991;325:467–472. doi: 10.1056/NEJM199108153250704. [DOI] [PubMed] [Google Scholar]
- Skripuletz T, Miller E, Moharregh-Khiabani D, Blank A, Pul R, Gudi V, Trebst C, Stangel M. Beneficial effects of minocycline on cuprizone induced cortical demyelination. Neurochemical research. 2010;35:1422–1433. doi: 10.1007/s11064-010-0202-7. [DOI] [PubMed] [Google Scholar]
- Stangel M. Neurodegeneration and neuroprotection in multiple sclerosis. Current pharmaceutical design. 2012;18:4471–4474. doi: 10.2174/138161212802502189. [DOI] [PubMed] [Google Scholar]
- Steinert JR, Chernova T, Forsythe ID. Nitric oxide signaling in brain function, dysfunction, and dementia. The Neuroscientist : a review journal bringing neurobiology, neurology and psychiatry. 2010;16:435–452. doi: 10.1177/1073858410366481. [DOI] [PubMed] [Google Scholar]
- Steinman L, Zamvil SS. How to successfully apply animal studies in experimental allergic encephalomyelitis to research on multiple sclerosis. Annals of neurology. 2006;60:12–21. doi: 10.1002/ana.20913. [DOI] [PubMed] [Google Scholar]
- Suk K, Lee H, Kang SS, Cho GJ, Choi WS. Flavonoid baicalein attenuates activation-induced cell death of brain microglia. The Journal of pharmacology and experimental therapeutics. 2003;305:638–645. doi: 10.1124/jpet.102.047373. [DOI] [PubMed] [Google Scholar]
- Takahashi H, Chen MC, Pham H, Angst E, King JC, Park J, Brovman EY, Ishiguro H, Harris DM, Reber HA, Hines OJ, Gukovskaya AS, Go VL, Eibl G. Baicalein, a component of Scutellaria baicalensis, induces apoptosis by Mcl-1 down-regulation in human pancreatic cancer cells. Biochimica et biophysica acta. 2011;1813:1465–1474. doi: 10.1016/j.bbamcr.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taylor DL, Pirianov G, Holland S, McGinnity CJ, Norman AL, Reali C, Diemel LT, Gveric D, Yeung D, Mehmet H. Attenuation of proliferation in oligodendrocyte precursor cells by activated microglia. Journal of neuroscience research. 2010;88:1632–1644. doi: 10.1002/jnr.22335. [DOI] [PubMed] [Google Scholar]
- Tong WG, Ding XZ, Witt RC, Adrian TE. Lipoxygenase inhibitors attenuate growth of human pancreatic cancer xenografts and induce apoptosis through the mitochondrial pathway. Molecular cancer therapeutics. 2002;1:929–935. [PubMed] [Google Scholar]
- Torkildsen O, Brunborg LA, Myhr KM, Bo L. The cuprizone model for demyelination. Acta neurologica Scandinavica Supplementum. 2008;188:72–76. doi: 10.1111/j.1600-0404.2008.01036.x. [DOI] [PubMed] [Google Scholar]
- van Noort JM. Antigen-specific therapies in multiple sclerosis. Biotherapy (Dordrecht, Netherlands) 1998;10:237–250. doi: 10.1007/BF02678302. [DOI] [PubMed] [Google Scholar]
- Wang D, Ayers MM, Catmull DV, Hazelwood LJ, Bernard CC, Orian JM. Astrocyte-associated axonal damage in pre-onset stages of experimental autoimmune encephalomyelitis. Glia. 2005;51:235–240. doi: 10.1002/glia.20199. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Steinman L, Zamvil SS. Neither T-helper type 2 nor Foxp3+ regulatory T cells are necessary for therapeutic benefit of atorvastatin in treatment of central nervous system autoimmunity. Journal of neuroinflammation. 2014;11:29. doi: 10.1186/1742-2094-11-29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J, Zhang Y, Xiao Y, Ma S, Liu Q, Dang S, Jin M, Shi Y, Wan B, Zhang Y. Inhibition of 12/15-lipoxygenase by baicalein induces microglia PPARbeta/delta: a potential therapeutic role for CNS autoimmune disease. Cell death & disease. 2013;4:e569. doi: 10.1038/cddis.2013.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto S, Gotoh M, Kawamura Y, Yamashina K, Yagishita S, Awaji T, Tanaka M, Maruyama K, Murakami-Murofushi K, Yoshikawa K. Cyclic phosphatidic acid treatment suppress cuprizone-induced demyelination and motor dysfunction in mice. European journal of pharmacology. 2014;741:17–24. doi: 10.1016/j.ejphar.2014.07.040. [DOI] [PubMed] [Google Scholar]
- Yamamoto S, Yamashina K, Ishikawa M, Gotoh M, Yagishita S, Iwasa K, Maruyama K, Murakami-Murofushi K, Yoshikawa K. Protective and therapeutic role of 2-carba-cyclic phosphatidic acid in demyelinating disease. Journal of neuroinflammation. 2017;14:142. doi: 10.1186/s12974-017-0923-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang LP, Sun HL, Wu LM, Guo XJ, Dou HL, Tso MO, Zhao L, Li SM. Baicalein reduces inflammatory process in a rodent model of diabetic retinopathy. Investigative ophthalmology & visual science. 2009;50:2319–2327. doi: 10.1167/iovs.08-2642. [DOI] [PubMed] [Google Scholar]
- Yoshikawa K, Palumbo S, Toscano CD, Bosetti F. Inhibition of 5-lipoxygenase activity in mice during cuprizone-induced demyelination attenuates neuroinflammation, motor dysfunction and axonal damage. Prostaglandins, leukotrienes, and essential fatty acids. 2011;85:43–52. doi: 10.1016/j.plefa.2011.04.022. [DOI] [PMC free article] [PubMed] [Google Scholar]


