Abstract
Background
Hypertension and chronic kidney disease (CKD) are common comorbidities. Guidelines recommend treating hypertension in children with CKD because it is a modifiable risk factor for subsequent cardiovascular disease. Children with CKD are frequently excluded from antihypertensive drug trials. Consequently, safety and efficacy data for antihypertensive drugs are lacking in children with CKD.
Methods
We determined the incidence of adverse events in 10 pediatric antihypertensive trials to determine the effect of renal function on antihypertensive safety and efficacy in children. These trials were submitted to the U.S. Food and Drug Administration from 1998–2005. We determined the number and type of adverse events reported during the trials and compared these numbers between participants with normal renal function and those with decreased function (defined as an estimated glomerular filtration rate [eGFR] <90 mL/min/1.73m2 calculated using the original Schwartz equation).
Results
Among the 1703 children in the 10 studies, 315 had decreased renal function. We observed no difference between the two cohorts in the incidence of adverse events or adverse drug reactions related to study drug. Only 5 participants, all with decreased renal function, experienced a serious adverse event; none were recorded by investigators to be study drug-related. Among treated participants, children with decreased renal function who received a high dose of study drug had a significantly larger drop in diastolic blood pressure compared to children with normal renal function.
Conclusions
These data show that antihypertensive treatment in children with renal dysfunction can be safe and efficacious, and consideration should be given for their inclusion in select drug-development programs.
Keywords: chronic kidney disease, hypertension, pediatrics, antihypertensive drugs
INTRODUCTION
Hypertension and chronic kidney disease (CKD) are common comorbidities. While each disease individually is relatively uncommon in children, approximately 50% of children with CKD also suffer from hypertension [1,2]. The relationship between hypertension and CKD is cyclic. Hypertension can lead to more rapid progression of renal disease [1–3]; and CKD can also cause hypertension, primarily though fluid overload and increased systemic vascular resistance. Because of the early onset of CKD-related hypertension, children have a high lifetime risk for developing cardiovascular complications.
Current guidelines recommend treating hypertension in children with CKD because it is a modifiable risk factor for subsequent cardiovascular disease [4–6]. There is evidence that treatment can slow the progression of disease and, in some cases, reverse the cardiovascular changes [3,7,8]. Beneficial effects include decreased proteinuria and left ventricular hypertrophy [9–11]. Angiotensin-converting enzyme (ACE) inhibitors and angiotensin receptor blockers (ARBs) are first-line agents for adults with CKD, and there is a growing body of literature that supports their use in children with CKD [3,10,12–19]. Because of differences in pediatric physiology and drug metabolism and elimination, dedicated pediatric studies are essential to ensure safe and efficacious use in children [20].
Between 1998 and 2005, 10 oral antihypertensive drugs were studied under recent pediatric legislative initiatives [21,22]. Pediatric labeling changes were made for 7 of the10 drugs studied [23]. Efficacy was established for six drugs in children ≥6 years of age and one drug in children <6 years of age. Three drugs did not get a pediatric labeling change pursuant to the pediatric studies. While these 10 studies are excellent examples of the recent advancements in drug studies in children, as with most pediatric studies, these trials excluded children with severely decreased glomerular filtration rates (generally defined as <30 mL/min/1.73m2). Lack of data from patients with this degree of renal dysfunction is of great significance because of the frequency of kidney disease in children with hypertension and because altered renal function is known to affect the safety and efficacy of drugs by its impact on pharmacokinetics (PK) and dosing. Kidney disease can decrease clearance for renally eliminated drugs and increase the risk for drug-related adverse events (AEs). This has been demonstrated in adult studies where certain AEs were higher in patients with renal disease [24]. Given the close relationship between hypertension and kidney disease in the pediatric age group, we sought to determine the effect of renal function on antihypertensive safety and efficacy in children using data from these 10 antihypertensive trials.
METHODS
Study Cohort
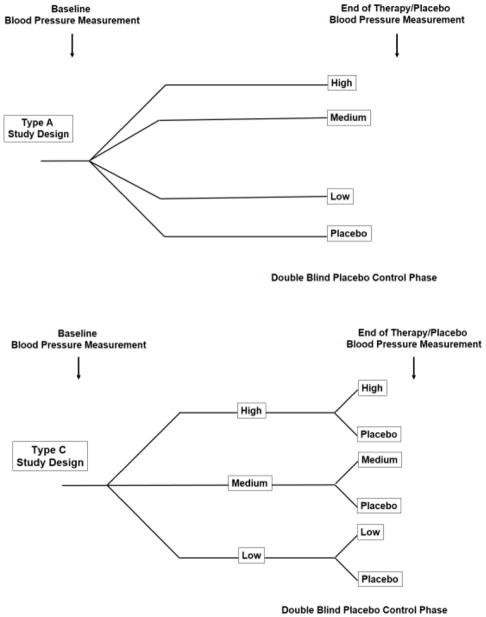
Between January 1998 and December 2005, pediatric data for 10 antihypertensive drugs were submitted to the U.S. Food and Drug Administration (FDA) pursuant to the Best Pharmaceuticals for Children Act for pediatric labeling (Table 1). Each submission included a multi-center, placebo-controlled safety and efficacy trial. The placebo-controlled phase of these 10 trials ranged from 2 to 4 weeks. Two of the trials were type A design (felodipine and quinapril), and the other eight trials were type C (Figure 1). All trials excluded children with severe hypertension and severe renal dysfunction, with the latter generally defined as an estimated glomerular filtration rate (eGFR) <30 mL/min/1.73m2.
Table 1.
Antihypertensive drugs studied in pediatric clinical trials from 1998–2005, with enrollment stratified by renal function
| Drug | GFR exclusion Criteria (mL/min/1.73m2) | Number of children enrolled | ||||
|---|---|---|---|---|---|---|
| Total | GFR ≥90 | GFR 60–89 | GFR 30–59 | GFR <30 | ||
| Amlodipine | <40 | 256 | 160 | 57 | 33 | 6 |
| Benazipril | <30 | 85 | 64 | 14 | 7 | 0 |
| Enalapril | <30 | 100 | 70 | 15 | 12 | 3 |
| Felodipine | <40 | 132 | 128 | 2 | 2 | 0 |
| Fosinopril | <25 | 235 | 209 | 24 | 2 | 0 |
| Irbesartan | <30 | 295 | 270 | 23 | 2 | 0 |
| Lisinopril | <30 | 104 | 76 | 18 | 9 | 1 |
| Losartan | <30 | 165 | 136 | 21 | 8 | 0 |
| Quinapril | NR | 112 | 91 | 17 | 4 | 0 |
| Ramipril | <40 | 219 | 184 | 24 | 11 | 0 |
| TOTAL | 1703 | 1388 | 215 | 90 | 10 | |
GFR was not an exclusion criterion for quinapril. In the quinapril study, children were excluded if urea or serum creatinine levels were >3 times the upper limit of normal for age.
GFR indicates glomerular filtration rate.
Figure 1.
Trial Designs
Data Management
We accessed the FDA’s Document Archiving, Reporting, and Regulatory Tracking System (DARRTS) and the FDA Electronic Document Room (EDR) to obtain study datasets. Safety and efficacy datasets were combined at the patient level to generate one record per AE. If there was no AE for a given child, that child was assigned one record.
From each trial we extracted the following variables: study drug, patient identification number, age, sex, race, height, weight, body mass index, baseline blood pressure, blood pressure after treatment, serum creatinine, AE preferred terms, body system Medical Dictionary for Regulatory Activities (MedDRA) terms, investigator opinion of the causal relationship between the study drug and AE, severity of AE, phase of study in which AE occurred, and therapy received during the placebo-controlled phase of the study (placebo or active drug).
All children were categorically grouped into normal renal function (eGFR ≥90 mL/min/1.73m2) or decreased renal function (eGFR <90 mL/min/1.73m2) [25]. eGFR was calculated for each child using the original Schwartz equation [26,27],
where k is a constant based on age and sex, Ht is height, and Crserum is serum creatinine. The highest Crserum measured during the placebo-controlled phase of the study was used to calculate eGFR for each child.
AEs were recorded and classified as serious or not by the trial investigator. Although safety data were recorded in other phases of some trials (e.g., dose-response) to be consistent across trials, we limited our analysis to the placebo-controlled phase of the trial.
Analysis
All analyses were stratified by renal function (normal vs. decreased). In order to compare efficacy between the two cohorts, we compared changes in systolic and diastolic blood pressures for all drugs at each dose level (high, medium, low, and placebo). Normal baseline blood pressure is age-dependent. To account for this, we also compared relative decrease in blood pressure calculated as follows,
where BPe refers to blood pressure measured at the end of therapy or placebo and BPi refers to blood pressure prior to therapy or placebo.
In order to assess safety, we examined the percentage of participants with AEs (AE prevalence), the mean number of AEs per patient (incidence of AEs), and identified participants with serious AEs. We also examined the prevalence of adverse drug reactions (ADRs), defined as AEs determined by the trial investigator to be possibly, probably, or definitely related to the study drug for each antihypertensive dose level (high, medium, low, and placebo).
We reported 2-sided P values calculated by t test for continuous variables or Fisher’s exact test for count outcomes. STATA v14.2 (College Station, TX) was used to perform the statistical analysis. Significance for all tests was established at P<0.05.
RESULTS
Demographics
The 10 studies included 1703 children between 1 and 17 years of age. Of the 1703 participants, 1388 (81.5%) had an eGFR ≥90 mL/min/1.73m2 and 315 (18.5%) had an eGFR <90 (Table 2). Children with decreased renal function were younger (11.1 vs. 12.4 years), shorter (141.4 vs. 158.7 cm), weighed less (47.2 vs. 72.1 kg), had a lower mean body mass index (22.3 vs. 27.8 kg/m2) and were more likely to be non-white (51.7% vs. 43.0%) (all P<0.01). Furthermore, when anthropometric measurements were normalized using z-scores, the children with decreased renal function had lower z-scores for weight (0.29 vs. 1.67), height (−0.77 vs. 0.55), and body mass index (0.82 vs. 1.54) (all P<0.001).
Table 2.
Demographics
| eGFR ≥90 mL/min/1.73m2 (N=1388) | eGFR <90 mL/min/1.73m2 (N=315) | P | |
|---|---|---|---|
| Age, years | 12.4 (7.0, 16.0) | 11.1 (6.0, 16.0) | <0.001 |
| Weight, kg | 72.1 (29.5, 127.0) | 47.2 (19.2, 93.5) | <0.001 |
| Weight z-score | 1.67 (−0.69, 3.52) | 0.29 (−2.49, 2.76) | < 0.001 |
| Height, cm | 158.7 (126.0–183.0) | 141.4 (110.0, 170.8) | <0.001 |
| Height z-score | 0.55 (−1.50, 2.57) | −0.77 (−3.29, 1.57) | <0.001 |
| BMIa, kg/m2 | 27.8 (16.6, 42.8)a | 22.3 (14.8, 37.0) | <0.001 |
| BMI z-scorea | 1.54 (−0.65, 2.81)a | 0.82 (−1.03, 2.57) | <0.001 |
| Male | 892 (64.3) | 165 (52.4) | <0.001 |
| White | 791 (57.0) | 152 (48.3) | <0.001 |
| eGFR, mL/min/1.73m2 | 128 (94, 175) | 68 (36, 89) | <0.001 |
Values are presented as mean (5th, 95th percentiles) for continuous variables and n (%) for categorical variables.
1 participant was <2 years of age and did not have BMI or BMI z-score.
BMI indicates body mass index; eGFR, estimated glomerular filtration rate.
Blood Pressure Response
Among children who received high-dose study drug, children with decreased renal function had a significantly larger drop in diastolic blood pressure compared to children with normal renal function (Table 3). Children with decreased renal function had a statistically significant but not clinically relevant decrease in relative systolic blood pressure in the high-dose cohort.
Table 3.
Blood pressure response
| eGFR ≥90 mL/min/1.73m2 (n=1388) | eGFR <90 mL/min/1.73m2 (n=315) | P | eGFR ≥90 mL/min/1.73m2 (n=1388) | eGFR <90 mL/min/1.73m2 (n=315) | P | |
|---|---|---|---|---|---|---|
| Change in diastolic blood pressure, mmHg | Relative change in diastolic blood pressure, % | |||||
| Placebo | −3.4 (−17.0, 10.3) | −1.4 (−17.8, 19.7) | 0.03 | −3.7 (−20.2, 15.9) | −0.7 (−20.8, 29.5) | 0.01 |
| Low dose | −3.5 (−18.3, 10.0) | −5.9 (−27.3, 11.0) | 0.05 | −4.0 (−21.2, 14.7) | −6.4 (−28.6, 14.3) | 0.12 |
| Medium dose | −5.1 (−20.3, 7.0) | −5.6 (−26.3, 16.0) | 0.68 | −6.3 (−24.1, 10.7) | −5.9 (−30.2, 27.4) | 0.81 |
| High dose | −6.3 (−23.5, 10.0) | −16.1 (−30.0, −1.3) | <0.001 | −7.3 (−26.5, 12.5) | −18.4 (−34.6, −1.9) | <0.001 |
| Change in systolic blood pressure, mmHg | Relative change in systolic blood pressure, % | |||||
| Placebo | −5.8 (−23.7, 10.7) | −3.6 (−24.0, 21.0) | 0.05 | −4.2 (−16.9, 7.6) | −2.6 (−16.4, 17.0) | 0.05 |
| Low dose | −6.3 (−24.0, 11.3) | −8.8 (−32.0, 9.7) | 0.09 | −4.6 (−17.2, 8.9) | −6.6 (−25.3, 8.4) | 0.06 |
| Medium dose | −8.2 (−25.0, 6.7) | −8.4 (−31.0, 11.0) | 0.88 | −5.9 (−18.6, 5.4) | −6.4 (−24.0, 9.2) | 0.68 |
| High dose | −10.4 (−28.3, 6.7) | −13.8 (−35.5, 0.0) | 0.05 | −7.7 (−21.2, 5.7) | −10.7 (−24.1, 0.0) | 0.02 |
Values are presented as mean (5, 95 percentiles).
eGFR indicates estimated glomerular filtration rate.
Adverse Events
There was no significant difference in the incidence of AEs between children with decreased renal function compared to those with normal renal function (P=0.25, Table 4). Of the children with an eGFR ≥90 mL/min/1.73m2, 532 (38.3%) experienced at least one AE. Of the children with an eGFR <90 mL/min/1.73m2, 132 (41.9%) experienced an AE. Further, there was no significant difference in the number of children with an ADR whether they had normal or decreased renal function (P=0.84). When stratified by treatment vs. placebo, there remained no difference in the incidence of AEs or ADRs (Table 4).
Table 4.
Children with adverse events
| Adverse events per cohorta | Adverse drug reactions per cohortb | |||||
|---|---|---|---|---|---|---|
| Cohort | eGFR ≥90 mL/min/1.73m2 (n=1388) | eGFR <90 mL/min/1.73m2 (n=315) | P | eGFR ≥90 mL/min/1.73m2 (n=1388) | eGFR <90 mL/min/1.73m2 (n=315) | P |
| Treatment | 314/819 (38.3) | 87/201 (43.3) | 0.20 | 94/819 (11.5) | 22/201 (11.0) | 0.90 |
| Placebo | 218/569 (38.3) | 45/114 (39.5) | 0.83 | 54/569 (9.5) | 10/114 (8.8) | >0.99 |
| All participants | 532/1388 (38.3) | 132/315 (41.9) | 0.25 | 148/1388 (10.7) | 32/315 (10.2) | 0.84 |
Values are the number of children with adverse events/the total number of children in the respective cohort (% of children in respective cohort).
An adverse event is any untoward medical occurrence associated with the use of a drug, whether or not it is considered drug-related.
An adverse drug reaction is an undesirable effect reasonably associated with the use of a drug. See Methods for full definition. Because investigators were blinded, some adverse drug reactions were assigned to the placebo.
eGFR indicates estimated glomerular filtration rate.
We further evaluated AEs in children on antihypertensive medications by MedDRA System Organ Class categories. Among the 532 children with an AE and eGFR ≥90 mL/min/1.73m2, 859 AEs were recorded; and among the 132 children with an AE and eGFR <90 mL/min/1.73m2, 232 AEs were recorded. Children with an eGFR ≥90 mL/min/1.73m2 had significantly more nervous system AEs (P<0.01) than children with an eGFR <90. Otherwise, there were no significant differences in the incidence of AEs among the MedDRA categories.
When the analysis was limited to children on therapy and 10 categories of AEs that are commonly observed with antihypertensive drugs, there were significantly more headaches in children with an eGFR <90 mL/min/1.73m2 compared to those with an eGFR ≥90 (P=0.03, Table 5). Otherwise, there were no significant differences between groups, including no reports of hypotension in the lower eGFR group, despite the significantly greater decreases in diastolic blood pressure.
Table 5.
Adverse events associated with antihypertensive drugs that were reported in children on therapy
| Adverse events | eGFR ≥90 mL/min/1.73m2 | eGFR ≥90 mL/min/1.73m2 | P |
|---|---|---|---|
| N (%) | N (%) | ||
| Hypertension | - | 1 (0.5) | 0.20 |
| Hypotension | 1 (0.1) | - | >0.99 |
| Cardiaca | 9 (1.1) | 2 (1.0) | >0.99 |
| Headache | 7 (0.9) | 6 (3.0) | 0.03 |
| Neuro/psychb | 14 (1.7) | 6 (3.0) | 0.26 |
| Syncopec | 7 (0.9) | - | 0.36 |
| Gastrointestinald | 25 (3.1) | 10 (5.0) | 0.19 |
| Asthma/SOB | 7 (0.9) | 5 (2.5) | 0.07 |
| Elevated LFTs | 6 (0.7) | - | 0.60 |
| Muscle aches | 21 (2.6) | 4 (2.0) | 0.80 |
| Total | 155 (100) | 48 (100) | NA |
Includes tachycardia, palpitations, and chest pain.
Includes agitation, fatigue, seizures, tremors, and depression.
Includes blurry vision and dizziness.
Includes nausea, vomiting, and diarrhea.
eGFR indicates estimated glomerular filtration rate; LFT, liver function test; NA, not applicable; SOB, shortness of breath.
DISCUSSION
The aim of this study was to assess the safety and efficacy of 10 antihypertensive drugs submitted to the FDA for pediatric labeling in children with renal dysfunction compared to those with normal renal function. There were significant demographic differences between the children with an eGFR ≥90 mL/min/1.73m2 and those with an eGFR <90. Children in the lower eGFR group were noted to be younger, smaller, and less likely to be white. The age discrepancy is likely attributable to the different etiologies of hypertension in different age groups. Hypertension in younger children is more likely to be a result of CKD, whereas adolescents usually have primary hypertension that is less commonly associated with secondary renal disease [28]. In a recent North American Pediatric Renal Trials and Collaborative Studies report, the majority of CKD in young children was due to congenital causes, while glomerulonephritis was the leading cause of kidney disease in children older than 12 years of age [29].
Decreased height, weight, and body mass index in the lower eGFR group are also not surprising because the correlation between kidney disease and growth disturbance is well established [30,31]. The racial discrepancy is one that has been well documented in adult CKD and end-stage renal disease (ESRD) patients and is now being shown in pediatrics as well, particularly in adolescents with ESRD, where there is a large African American predominance [32]. In addition, whites have been shown to have a lower rate of progression from CKD to ESRD [33,34].
We did observe differences in efficacy at high doses, with a greater decrease in diastolic blood pressure in children with decreased renal function. Although there was a statistically significant decrease in diastolic blood pressure among the two cohorts in the placebo arm, the difference was not clinically important. This decrease in diastolic blood pressure in children with decreased renal function is likely due to 1) a larger effect due to higher baseline blood pressure or 2) a decreased clearance of the drug due to altered renal function, resulting in prolonged exposure. The former has been demonstrated in multiple studies of ACE inhibitors and ARBs in children with CKD [1,13,17,35]. Differences in decreased systolic blood pressure, on the other hand, were not observed between children with normal renal function and those with decreased renal function. The only excpetion was a statistically significant but not clinically important difference in the relative change in systolic blood pressure on high-dose study drug. As noted by Benjamin et al in their analysis of the endpoints and dose range of pediatric antihypertensive trials, the successful trials were those using diastolic blood pressure as their endpoint [36]. Those that used systolic blood pressure reduction as an endpoint failed. They hypothesize that because diastolic blood pressure has less physiologic variability in children than systolic blood pressure, significant reductions may be more readily apparent.
Surprisingly, we observed no significant difference in the incidence of AEs in children with decreased renal function compared to those with normal renal function. Similarly, when looking at ADRs at least possibly related to the study drug, there was no difference in the incidence of ADRs in children with decreased renal function versus those with normal renal function. These data show that antihypertensives can be safe and efficacious in treating children with renal dysfunction, and consideration should be given for their inclusion in select drug-development programs. This study is limited because children with severely decreased renal function were excluded, there were relatively few patients with decreased eGFR, and there were few young children included. Also, because we were unable to determine what method was used to measure serum creatinine (Jeffe vs. enzymatic), we used the original Schwartz equation to calculate eGFR. This may have resulted in overestimation of eGFR [37]. Nevertheless, our study combining patient-level data across 10 trials shows that dedicated studies in this population should be conducted based on the results from studies in children with decreased renal function.
Dedicated pediatric drug trials are increasingly common as a result of legislative incentives and requirements enacted under the FDA Modernization Act of 1997 [21] and made permanent in 2012 with the FDA Safety and Innovation Act [38]. These pediatric legislative initiatives led to almost 500 pediatric label changes between 1998 and 2012 [23]. However, approximately 50% of drug-product labeling still has insufficient data on the safety, efficacy, or dosing appropriate for use in children [39]. The lack of safety and efficacy data is especially pronounced in pediatric special populations, including children with renal dysfunction. Renal dysfunction can alter drug safety and efficacy in several ways, including: 1) decreased renal excretion and metabolism, resulting in higher exposure and potential toxicity; 2) altered plasma protein binding; and 3) changes in absorption and transport. In addition, renal disease may also affect hepatic metabolism, although the mechanism for this remains unclear [40]. FDA guidance recommends PK studies in patients with renal impairment when the drug is likely to be used in that population or when renal impairment might mechanistically alter the PK [41].
Antihypertensive drugs are a prime example of the utility of these guidelines because hypertension and renal dysfunction are frequent comorbidities, and many of these drugs are excreted by the kidneys. Of the 10 drugs studied in this analysis, all of them undergo at least partial renal elimination. All have been studied in adults with renal dysfunction and carry special renal dosing guidance, but these data are limited in children [17, 42]. The current study demonstrates the feasibility of studies in children with renal dysfunction and highlights the fact that pediatric clinical trials should be conducted initially for these drugs rather than evaluating them ad-hoc with dosing extrapolated from adults.
Acknowledgments
FUNDING: This study was funded by a grant from the Duke University O’Brien Center for Kidney Research, which is supported by a grant from the National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK), and by the Duke University School of Medicine and Department of Medicine.
Footnotes
Ethics
All trials were approved by the institutional review boards of the participating sites. Because the datasets obtained from DARRTS and the EDR contained no patient identifiers, we received a waiver of review from the Duke University Medical Center Institutional Review Board and a letter of exempt status from the FDA Research Involving Human Subjects Committee.
CONFLICT OF INTEREST
The work of KMW was supported under an Intergovernmental Personnel Agreement (IPA) between the U.S. Food and Drug Administration and Duke University.
K.M.W. receives support from the National Institute of Child Health and Human Development (NICHD) (1K23HD075891, 5K12HD047349) and the Thrasher Research Fund for his work in pediatric clinical pharmacology. D.K.B. Jr. receives support from the National Institutes of Health (NIH) (award 2K24HD058735-06, National Center for Advancing Translational Sciences (NCATS)award UL1TR001117, NICHD contract HSN275201000003I, and National Institute of Allergy and Infectious Diseases [NIAID] contract HHSN272201500006I); he also receives research support from Cempra Pharmaceuticals (subaward to HHSO100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp).
P.B.S. receives salary support for research from the NIH and the NCATS of the NIH (UL1TR001117), the NICHD (HHSN275201000003I and 1R01-HD081044-01), and the Food and Drug Administration (FDA) (1R18-FD005292-01); he also receives research support from Cempra Pharmaceuticals (subaward to HHS0100201300009C) and industry for neonatal and pediatric drug development (www.dcri.duke.edu/research/coi.jsp). The remaining authors have no disclosures. The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
References
- 1.Flynn JT, Mitsnefes M, Pierce C, Cole SR, Parekh RS, Furth SL, Warady BA Chronic Kidney Disease in Children Study Group. Blood pressure in children with chronic kidney disease: a report from the Chronic Kidney Disease in Children study. Hypertension. 2008;52(4):631–637. doi: 10.1161/HYPERTENSIONAHA.108.110635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mitsnefes M, Ho PL, McEnery PT. Hypertension and progression of chronic renal insufficiency in children: a report of the North American Pediatric Renal Transplant Cooperative Study (NAPRTCS) J Am Soc Nephro. 2003;14(10):2618–2622. doi: 10.1097/01.asn.0000089565.04535.4b. [DOI] [PubMed] [Google Scholar]
- 3.Wuhl E, Trivelli A, Picca S, Litwin M, Peco-Antic A, Zurowska A, Testa S, Jankauskiene A, Emre S, Caldas-Afonso A, Anarat A, Niaudet P, Mir S, Bakkaloglu A, Enke B, Montini G, Wingen AM, Sallay P, Jeck N, Berg U, Caliskan S, Wygoda S, Hohbach-Hohenfellner K, Dusek J, Urasinski T, Arbeiter K, Neuhaus T, Gellermann J, Drozdz D, Fischbach M, Möller K, Wigger M, Peruzzi L, Mehls O, Schaefer F ESCAPE Trial Group. Strict blood-pressure control and progression of renal failure in children. N Engl J Med. 2009;361(17):1639–1650. doi: 10.1056/NEJMoa0902066. [DOI] [PubMed] [Google Scholar]
- 4.Kidney Disease Outcomes Quality Initiative (K/DOQI) Clinical practice guidelines on hypertension and antihypertensive agents in chronic kidney disease. Am J Kidney Dis. 2004;43(Suppl):S1–S290. [PubMed] [Google Scholar]
- 5.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114(2 Suppl):555–576. 4th Report. [PubMed] [Google Scholar]
- 6.Kavey RE, Allada V, Daniels SR, Hayman LL, McCrindle BW, Newburger JW, Parekh RS, Steinberger J American Heart Association Expert Panel on Population and Prevention Science; American Heart Association Council on Cardiovascular Disease in the Young; American Heart Association Council on Epidemiology and Prevention; American Heart Association Council on Nutrition, Physical Activity and Metabolism; American Heart Association Council on High Blood Pressure Research; American Heart Association Council on Cardiovascular Nursing; American Heart Association Council on the Kidney in Heart Disease; Interdisciplinary Working Group on Quality of Care and Outcomes Research. Cardiovascular risk reduction in high-risk pediatric patients: a scientific statement from the American Heart Association Expert Panel on Population and Prevention Science; the Councils on Cardiovascular Disease in the Young, Epidemiology and Prevention, Nutrition, Physical Activity and Metabolism, High Blood Pressure Research, Cardiovascular Nursing, and the Kidney in Heart Disease; and the Interdisciplinary Working Group on Quality of Care and Outcomes Research: endorsed by the American Academy of Pediatrics. Circulation. 2006;114(24):2710–2738. doi: 10.1161/CIRCULATIONAHA.106.179568. [DOI] [PubMed] [Google Scholar]
- 7.Kupferman JC, Aronson Friedman L, Cox C, Flynn J, Furth S, Warady B, Mitsnefes M CKiD Study Group. BP control and left ventricular hypertrophy regression in children with CKD. J Am Soc Nephrol. 2014;25(1):167–174. doi: 10.1681/ASN.2012121197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Matteucci MC, Chinali M, Rinelli G, Wühl E, Zurowska A, Charbit M, Pongiglione G, Schaefer F ESCAPE Trial Group. Change in cardiac geometry and function in CKD children during strict BP control: a randomized study. Clin J Am Soc Nephrol. 2013;8(2):203–210. doi: 10.2215/CJN.08420811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Loirat C, Azancot A, Pillion G, Macher MA, Mouchet B, Gainet B, Mathieu H. Sequential echocardiographic study prior and during antihypertensive therapy in children with severe hypertension. Clin Exp Hypertens A. 1986;8(4–5):805–810. doi: 10.3109/10641968609046598. [DOI] [PubMed] [Google Scholar]
- 10.Trachtman H, Gauthier B. Effect of angiotensin-converting enzyme inhibitor therapy on proteinuria in children with renal disease. J Pediatr. 1988;112(2):295–298. doi: 10.1016/s0022-3476(88)80073-8. [DOI] [PubMed] [Google Scholar]
- 11.Seeman T, Gilik J, Vondrák K, Simková E, Flögelová H, Hladíková M, Janda J. Regression of left-ventricular hypertrophy in children and adolescents with hypertension during ramipril monotherapy. Am J Hypertens. 2007;20(9):990–996. doi: 10.1016/j.amjhyper.2007.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Mirkin BL, Newman TJ. Efficacy and safety of captopril in the treatment of severe childhood hypertension: report of the International Collaborative Study Group. Pediatrics. 1985;75(6):1091–1100. [PubMed] [Google Scholar]
- 13.Franscini LM, Von Vigier RO, Pfister R, Casaulta-Aebischer C, Fossali E, Bianchetti MG. Effectiveness and safety of the angiotensin II antagonist irbesartan in children with chronic kidney diseases. Am J Hypertens. 2002;15(12):1057–1063. doi: 10.1016/s0895-7061(02)03083-2. [DOI] [PubMed] [Google Scholar]
- 14.Trachtman H, Frymoyer A, Lewandowski A, Greenbaum LA, Feig DI, Gipson DS, Warady BA, Goebel JW, Schwartz GJ, Lewis K, Anand R, Patel UD Best Pharmaceuticals for Children Act-Pediatric Trials Network Administrative Core Committee. Pharmacokinetics, Pharmacodynamics, and Safety of Lisinopril in Pediatric Kidney Transplant Patients: Implications for Starting Dose Selection. Clin Pharmacol Ther. 2015;98(1):25–33. doi: 10.1002/cpt.127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Seeman T, Dusek J, Vondrak K, Janda J. Ramipril in the treatment of proteinuria in children after renal transplantation. Pediatr Transplant. 2010;14(2):283–287. doi: 10.1111/j.1399-3046.2009.01216.x. [DOI] [PubMed] [Google Scholar]
- 16.Seeman T, Pohl M, Misselwitz J, John U. Angiotensin receptor blocker reduces proteinuria independently of blood pressure in children already treated with Angiotensin-converting enzyme inhibitors. Kidney Blood Press Res. 2009;32(6):440–444. doi: 10.1159/000266478. [DOI] [PubMed] [Google Scholar]
- 17.Wuhl E, Mehls O, Schaefer F, Group ET. Antihypertensive and antiproteinuric efficacy of ramipril in children with chronic renal failure. Kidney Int. 2004;66(2):768–776. doi: 10.1111/j.1523-1755.2004.00802.x. [DOI] [PubMed] [Google Scholar]
- 18.White CT, Macpherson CF, Hurley RM, Matsell DG. Antiproteinuric effects of enalapril and losartan: a pilot study. Pediatr Nephrol. 2003;18(10):1038–1043. doi: 10.1007/s00467-003-1190-5. [DOI] [PubMed] [Google Scholar]
- 19.Soergel M, Verho M, Wuhl E, Gellermann J, Teichert L, Scharer K. Effect of ramipril on ambulatory blood pressure and albuminuria in renal hypertension. Pediatr Nephrol. 2000;15(1–2):113–118. doi: 10.1007/s004670000422. [DOI] [PubMed] [Google Scholar]
- 20.Kearns GL, Abdel-Rahman SM, Alander SW, Blowey DL, Leeder JS, Kauffman RE. Developmental pharmacology--drug disposition, action, and therapy in infants and children. N Engl J Med. 2003;349(12):1157–1167. doi: 10.1056/NEJMra035092. [DOI] [PubMed] [Google Scholar]
- 21.US Food and Drug Administration Modernization Act of 1997, Pub L No. 105–115, 111 Stat 2296. 1997.
- 22.Best Pharmaceuticals for Children Act. Pub L no. 107–109, 115 Stat 1408 (2002). Available at: http://www.fda.gov/Drugs/DevelopmentApprovalProcess/DevelopmentResources/ucm049876.htm.2002.
- 23.US Food and Drug Administration. New Pediatric Labeling Information Database. Available at: www.accessdata.fda.gov/scripts/sda/sdNavigation.cfm?sd=labelingdatabase.
- 24.Bakris GL, Sarafidis PA, Weir MR, Dahlöf B, Pitt B, Jamerson K, Velazquez EJ, Staikos-Byrne L, Kelly RY, Shi V, Chiang YT, Weber MA ACCOMPLISH Trial investigators. Renal outcomes with different fixed-dose combination therapies in patients with hypertension at high risk for cardiovascular events (ACCOMPLISH): a prespecified secondary analysis of a randomised controlled trial. Lancet. 2010;375(9721):1173–1181. doi: 10.1016/S0140-6736(09)62100-0. [DOI] [PubMed] [Google Scholar]
- 25.KDIGO CKD Work Group. KDIGO 2012 Clinical Practice Guideline for the Evaluation and Management of Chronic Kidney Disease. Kid Int Supp. 2013;3:1–150. doi: 10.1038/ki.2013.243. [DOI] [PubMed] [Google Scholar]
- 26.Schwartz GJ, Feld LG, Langford DJ. A simple estimate of glomerular filtration rate in full-term infants during the first year of life. J Pediat. 1984;104(6):849–854. doi: 10.1016/s0022-3476(84)80479-5. [DOI] [PubMed] [Google Scholar]
- 27.Schwartz GJ, Gauthier B. A simple estimate of glomerular filtration rate in adolescent boys. J Pediatr. 1985;106(3):522–526. doi: 10.1016/s0022-3476(85)80697-1. [DOI] [PubMed] [Google Scholar]
- 28.Wyszyńska T, Cichocka E, Wieteska-Klimczak A, Jobs K, Januszewicz P. A single pediatric center experience with 1025 children with hypertension. Acta Paediatr. 1992;81(3):244–246. doi: 10.1111/j.1651-2227.1992.tb12213.x. [DOI] [PubMed] [Google Scholar]
- 29.Harambat J, van Stralen KJ, Kim JJ, Tizard EJ. Epidemiology of chronic kidney disease in children. Pediatr Nephrol. 2012;27(3):363–373. doi: 10.1007/s00467-011-1939-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rees L, Mak RH. Nutrition and growth in children with chronic kidney disease. Nat Rev Nephrol. 2011;7(11):615–623. doi: 10.1038/nrneph.2011.137. [DOI] [PubMed] [Google Scholar]
- 31.Betts PR, Magrath G. Growth pattern and dietary intake of children with chronic renal insufficiency. Br Med J. 1974;2(5912):189–193. doi: 10.1136/bmj.2.5912.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Minnick ML, Boynton S, Ndirangu J, Furth S. Sex, race, and socioeconomic disparities in kidney disease in children. Semin Nephrol. 2010;30(1):26–32. doi: 10.1016/j.semnephrol.2009.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Hsu CY, Lin F, Vittinghoff E, Shlipak MG. Racial differences in the progression from chronic renal insufficiency to end-stage renal disease in the United States. J Am Soc Nephrol. 2003;14(11):2902–2907. doi: 10.1097/01.asn.0000091586.46532.b4. [DOI] [PubMed] [Google Scholar]
- 34.Sorof JM, Hawkins EP, Brewer ED, Boydstun II, Kale AS, Powell DR. Age and ethnicity affect the risk and outcome of focal segmental glomerulosclerosis. Pediatr Nephro. 1998;12(9):764–768. doi: 10.1007/s004670050542. [DOI] [PubMed] [Google Scholar]
- 35.von Vigier RO, Zberg PM, Teuffel O, Bianchetti MG. Preliminary experience with the angiotensin II receptor antagonist irbesartan in chronic kidney disease. Eur J Pediatr. 2000;159(8):590–593. doi: 10.1007/s004310000495. [DOI] [PubMed] [Google Scholar]
- 36.Benjamin DK, Jr, Smith PB, Jadhav P, Gobburu JV, Murphy MD, Hasselblad V, Baker-Smith C, Califf RM, Li JS. Pediatric antihypertensive trial failures: analysis of end points and dose range. Hypertension. 2008;51(4):834–840. doi: 10.1161/HYPERTENSIONAHA.107.108886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Schwartz GJ, Munoz A, Schneider MF, Mak RH, Kaskel F, Warady BA, Furth SL. New equations to estimate GFR in children with CKD. J Am Soc Nephrol. 2009;20(3):629–637. doi: 10.1681/ASN.2008030287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Food and Drug Administration Safety and Innovation Act, S. 3187–3 (2012).
- 39.Sachs AN, Avant D, Lee CS, Rodriguez W, Murphy MD. Pediatric information in drug product labeling. JAMA. 2012;307(18):1914–1915. doi: 10.1001/jama.2012.3435. [DOI] [PubMed] [Google Scholar]
- 40.Lam YW, Banerji S, Hatfield C, Talbert RL. Principles of drug administration in renal insufficiency. Clin Pharmacokinet. 1997;32(1):30–57. doi: 10.2165/00003088-199732010-00002. [DOI] [PubMed] [Google Scholar]
- 41.Research CfDEa, editor. General Clinical Pharmacology Considerations for Pediatric Studies for drugs and Biological Products: Guidance for Industry (Draft) Food and Drug Administration: U.S. Department of Health and Human Services; 2014. [Google Scholar]
- 42.von Vigier ROFL, Bianda ND, Pfister R, Casaulta Aebischer C, Bianchetti MG. Antihypertensive efficacy of amlodipine in children with chronic kidney diseases. J Hum Hypertens. 2001;15(6):387–391. doi: 10.1038/sj.jhh.1001203. [DOI] [PubMed] [Google Scholar]