Abstract
BACKGROUND
In neonates, the validation of urinary biomarkers to diagnose acute kidney injury is a rapidly evolving field. The neonatal population poses unique challenges when assessing the collection, storage, and processing of urinary samples for biomarker analysis. Given this, establishing optimal and consistent sample processing in this population for meaningful use in ongoing clinical trials is important.
METHODS
Urine from a cohort of 19 hospitalized neonatal intensive care unit patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (Clinical Trial NCT01378273) was collected for biomarker analysis by indirect techniques using Fisher-brand cotton balls placed in the diapers. Fourteen urinary biomarkers were measured using commercially available kits via electrochemiluminescence on multiarray plates and compared between paired samples processed with centrifugation prior to storage vs. prior to analysis.
RESULTS
None of the biomarker concentrations differed between samples undergoing centrifugation prior to storage vs. prior to analysis. The difference between samples was within 2 percent of the estimated concentration for the protein in 12 of 14 biomarkers (86%) and all paired biomarker concentrations were within 4 percent. The percentage error analysis did not show a difference between paired samples, with biomarker percentage errors smaller than the stated immunoassay Coefficient of Variance.
CONCLUSIONS
The urinary concentrations of biomarkers were comparable between paired samples, demonstrating that indirectly collected neonatal urine samples do not require centrifugation after collection and before storage. The ability to use routine urine collection and storage methods to obtain samples for subsequent quantitative immunoassay analysis should facilitate studies of newborns and young children.
Keywords: Acute kidney injury (AKI), urinary biomarkers, urine storage, neutrophil gelatinase-associated lipocalin (NGAL), kidney injury molecule 1 (KIM-1), interleukin 18 (IL-18), neonates
Introduction
The development and discovery of novel urinary biomarkers is an area of active research within nephrology. In neonates, validation of biomarkers in the setting of acute kidney injury (AKI) and chronic kidney disease (CKD) is a rapidly evolving field. Early detection, diagnosis, risk profiling and prognosis are areas of expanding investigation [1]. A number of large, ongoing clinical studies collect serial urine samples using a variety of methodologies for biomarker collection, storage and processing, allowing for batch analysis at a later time. Therefore, it is important to establish optimal and consistent processing methods for urine samples in neonates that do not affect biomarker concentration results.
Neonates pose additional challenges compared to other populations when assessing the collection, storage, and processing of urine samples for biomarker analysis. First, collection of adequate urine volume from infants is often difficult. Indwelling bladder catheters provide a reliable and accurate method of urine collection; however, concerns for catheter-associated urinary tract infections have limited placement to less than 3% of hospitalized infants [2]. Another option for urine sample collection is use of adhesive bags applied to the perineum. These are prone to leakage and/or sample loss, and can cause significant irritation to the skin in preterm infants. Routine clinical and research protocols frequently rely on cotton balls placed in the diapers or special diapers to obtain urine for clinical evaluation of neonates, infants and young children. Although indirectly collected urine samples produce reliable biochemical results, the processing of these samples is not standardized [3]. A 2010 National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) workshop concluded that there are variations in storage and processing in samples in the repository, and that protocols are often based on convenience and expert opinion [4]. Several recent studies have sought to evaluate processing and storage of urinary samples for biomarker analysis in children and adults and found no differences in biomarker concentration based on processing and storage methods; however, these studies evaluated urine samples collected directly from patients via catheterization of the bladder [5, 6].
In neonates, existing processing protocols require further study. Many study protocols employ processing (centrifugation) prior to storage as investigators worry that residual cotton fibers may affect biomarker results. Selective absorption of urine proteins (e.g. albumin, retinol binding protein) by cotton fibers has also been described [7]. The goal of this study was to determine whether biomarker concentrations would differ between neonatal urine samples collected indirectly via cotton balls centrifuged prior to storage in comparison to those centrifuged prior to analysis. Based on previous studies, we hypothesized that there would be a difference in biomarker concentrations between samples centrifuged prior to storage vs. those centrifuged prior to analysis.
Subjects and Methods
All hospitalized neonatal intensive care unit (NICU) patients enrolled in the Preterm Erythropoietin Neuroprotection Trial (PENUT, Clinical Trial Number NCT01378273) were eligible for enrollment in this study. PENUT is a multi-center phase III clinical study evaluating the effect of recombinant erythropoietin treatment on the combined outcome of death or severe neurodevelopmental impairment in prematurely born neonates [8]. Urine was collected at the time of study enrollment, per existing protocol using Fisher brand non-sterile large cotton balls placed in the diapers. A prospectively identified cohort of infants were included in this study based on availability of urine for paired biomarker analysis. Informed consent was obtained as part of the PENUT study, and all study procedures were performed in accordance with ethical standards of the local Institutional Review Board and Good Clinical Practice guidelines.
Urine samples were divided into 2 equal aliquots. The study design is shown in Figure 1. One urine aliquot (before storage) was centrifuged at 4,000g at 4°C for 10 minutes, followed by immediate storage at −80°C. The second aliquot (before analysis) was stored at −80°C without centrifugation, and centrifuged as above at the time of thaw just prior to analysis. Urine samples were stored between 1 – 4 months prior to batch analysis.
Fig. 1.
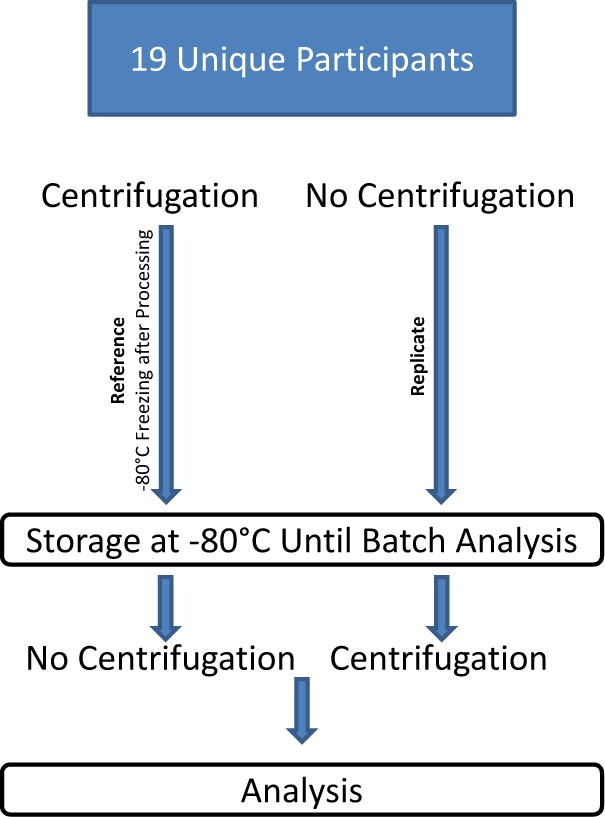
Schematic representation of the experimental protocol
Urinary biomarker analysis was performed by electrochemiluminescence on multiarray plates using Sector Image 2400 (Meso Scale Discovery [MSD], Gaithersburg, MD). α glutathione S-transferase (αGST), calbindin, clusterin, kidney injury molecule-1 (KIM-1), osteoactivin, trefoil factor 3 (TFF3), and vascular EGF (VEGF) were measured using MSD Human Kidney Injury Panel 3 Kit Assay. Albumin, β-2-microglobulin (β2M), cystatin C, epithelial growth factor (EGF), neutrophil gelatinase-associated lipocalin (NGAL), osteopontin (OPN), and uromodulin (UMOD) were measured in urine using MSD Human Kidney Injury Panel 5 Kit Assay. Samples for panel 3 were diluted 10-fold and samples for panel 5 were diluted 500-fold before plating. Samples were added to plates and prepared as stated in the manufacturer’s protocols and analyzed using standard methodology and manufacturer reported interassay coefficients of variation (CV) for low, medium and high concentration analytes [9, 10]. Urine creatinine levels were used to normalize biomarker concentration for urine dilution per standard protocol. However, as the paired aliquots were from the same urine sample, there was no need to normalize for creatinine level for comparisons. All samples were assessed in duplicate on the same plates under similar conditions.
Data analysis was performed using R Version 3.3.1 [11]. Missing data were excluded rather than imputed as it was not clear if samples were above or below the limits of the standardized curve. Paired t-test was utilized to more accurately account for inter-sample differences and was computed for each biomarker. A standard curve was generated using a four parameter logistic model, and used to estimate concentrations for urine samples, and p-values adjusted for 13 simultaneous comparisons using the Hochberg method [12]. Mean percentage error was calculated as:
and total percentage error was calculated as absolute mean percentage error + 2 SD, to calculate error for a continuous variable without bounds. Percentage error and total percentage error was used instead of regression analysis to evaluate for agreement between two methods for the same clinical measurement [13]. Bland-Altman plots were used to display the mean difference between paired samples with centrifugation prior to storage vs. prior to analysis [14, 15].
Results
The median age of patients was 28 days, with a range of 3 to 72 days, and a majority were female (74%). Median gestational age was 26 weeks and 6 days (range from 24 weeks and 1 day to 28 weeks and 0 days) and median birth weight was 773 grams, with a range from 494 grams to 1,010 grams.
Urine biomarkers in our paired samples with centrifugation prior to storage vs. prior to analysis were closely correlated, with paired t-statistics for parameter estimates presented in Table 1. Positive estimates indicate that biomarker concentration was on average higher in the samples spun before storage, and negative estimates indicate that the concentration was on average higher in the samples spun before analysis. There was excellent concordance between the groups, with no differences in concentration for any of these biomarkers when comparing the samples processed before storage to samples processed before analysis (Figure 2).
Table 1.
Analysis of differences between samples processed with centrifugation prior to storage vs. prior to analysis
| Estimate | Std. Error | T value | Unadjusted p-value | Adjusted p-value | |
|---|---|---|---|---|---|
| αGST | 0.64 | 3.75 | 0.17 | 0.87 | 0.96 |
| Calbindin | 82.39 | 273.44 | 0.30 | 0.77 | 0.96 |
| Clusterin | 3840.07 | 3532.07 | 1.09 | 0.29 | 0.96 |
| KIM-1 | −0.94 | 17.89 | −0.05 | 0.96 | 0.96 |
| Osteoactivin | 68.90 | 90.76 | 0.76 | 0.46 | 0.96 |
| TFF3 | 183.08 | 195.21 | 0.94 | 0.36 | 0.96 |
| VEGF | 42.41 | 33.68 | 1.26 | 0.23 | 0.96 |
| Albumin | −671752.82 | 342794.67 | −1.96 | 0.06 | 0.81 |
| β2M | −354764.87 | 671433.16 | −0.53 | 0.60 | 0.96 |
| EGF | −52.73 | 67.20 | −0.78 | 0.44 | 0.96 |
| Cystatin-C | −7640.56 | 5506.46 | −1.39 | 0.17 | 0.96 |
| NGAL | 7535.28 | 8726.92 | 0.86 | 0.39 | 0.96 |
| OPN | 2715.86 | 14209.31 | 0.19 | 0.85 | 0.96 |
| UMOD | −47733.06 | 34211.38 | −1.40 | 0.17 | 0.96 |
Abbreviations: α glutathione S-transferase (αGST), Calbindin, Clusterin, Kidney injury molecule-1 (KIM-1), Osteoactivin, Trefoil factor 3 (TFF3), Vascular EGF (VEGF), Albumin, β-2-microglobulin (β2M), Epithelial growth factor (EGF), Cystatin-C, Neutrophil gelatinase-associated lipocalin (NGAL), Osteopontin (OPN), Uromodulin (UMOD)
Fig. 2.
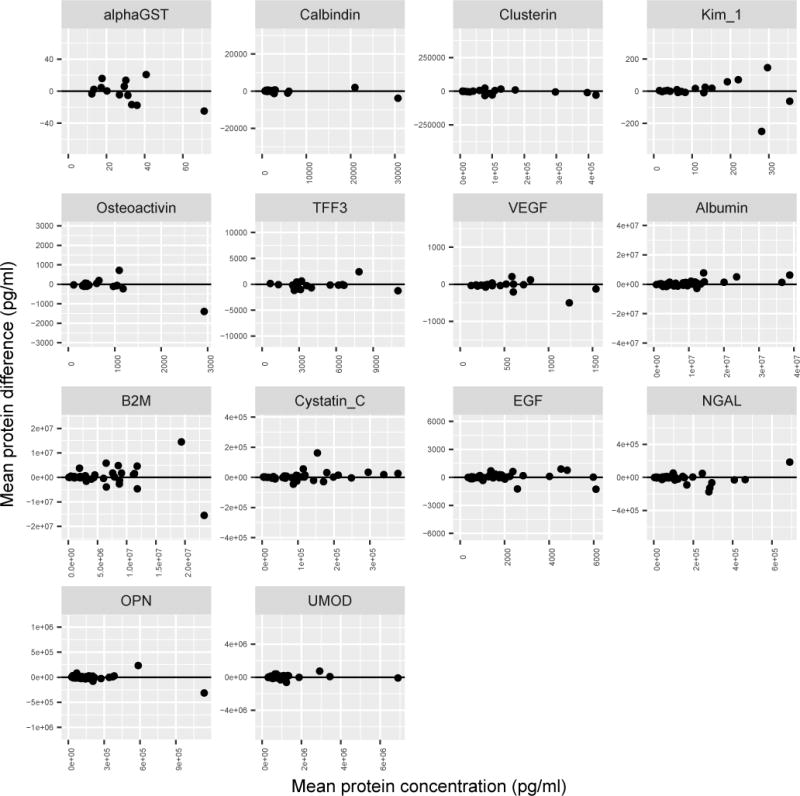
Bland-Altman Plot, showing the mean peptide concentration and mean difference between samples centrifuged prior to storage (before storage) vs. prior to analysis (before analysis) samples. The horizontal line at zero indicates no difference between paired samples centrifuged prior to storage (before storage) vs. prior to analysis (before analysis)
In addition, the concentration difference between paired samples processed prior to storage vs. prior to analysis was within 2 percent in 12 out of 14 biomarkers (86%) and within 4 percent in all biomarkers (Table 2). The largest mean percent error was in β-2-microglobulin (−3.7%) and the smallest percentage difference with epithelial growth factor (−0.61%). The most common clinically utilized biomarkers demonstrated excellence concordance as well, with mean percentage errors between paired samples 1.5% for neutrophil gelatinase-associated lipocalin (NGAL), −1.0% for kidney injury molecule-1 (KIM-1), and −0.95% for osteopontin (OPN).
Table 2.
The mean percent error and total percent error between samples centrifuged prior to storage vs. prior to analysis.
| Mean Percent Error | Total Percent Error (2SDs) | |
|---|---|---|
| αGST | −1.13 | 21.07 |
| Calbindin | −1.44 | 12.44 |
| Clusterin | 1.13 | 8.36 |
| KIM-1 | −1.02 | 14.66 |
| Osteoactivin | 1.40 | 12.72 |
| TFF3 | 1.51 | 9.86 |
| VEGF | 2.00 | 9.10 |
| Albumin | −0.35 | 23.96 |
| β2M | −3.70 | 23.96 |
| EGF | −0.61 | 9.09 |
| Cystatin-C | −1.71 | 13.66 |
| NGAL | 1.53 | 12.47 |
| OPN | −0.95 | 15.79 |
| UMOD | −1.37 | 10.32 |
Abbreviations: α glutathione S-transferase (αGST), Calbindin, Clusterin, Kidney injury molecule-1 (KIM-1), Osteoactivin, Trefoil factor 3 (TFF3), Vascular EGF (VEGF), Albumin, β-2-microglobulin (β2M), Epithelial growth factor (EGF), Cystatin-C, Neutrophil gelatinase-associated lipocalin (NGAL), Osteopontin (OPN), Uromodulin (UMOD)
The MSD documentation for these immunoassays indicates that the expected intra-plate coefficient of variation (CV) is between 2.5–12%, depending on the concentration of the analyte. Therefore, we would expect the standard deviation (SD) to be 5–6% of the mean, and two standard deviations (2SD) to be approximately 10–12% of the mean. The mean percentage error and total percent error analysis for all biomarkers are presented in Table 2; mean percentage error did not show a difference between paired samples processed by centrifugation prior to storage vs. prior to analysis, as these values were all smaller than the CV values (Table 2). Bland-Altman plots demonstrated symmetric clustering around the horizontal line at zero, suggesting minimal difference between paired samples with centrifugation prior to storage vs. prior to analysis (Figure 2).
Discussion
In this small neonatal cohort, the timing of centrifugation in relation to storage did not result in a difference in urinary biomarker values for each of the 14 biomarkers assessed. All biomarker concentration values were within 4%, which is well within the accepted error range of the laboratory measurement itself [9, 10]. This study suggests that performing centrifugation prior to storage of urine samples from neonates is not necessary when neonatal urine samples are collected with Fisher brand non-sterile large cotton balls placed in diapers, simplifying the process of specimen collection, processing and storage for urinary biomarker analysis. These findings could streamline the collection process of samples in both clinical care and research studies, likely saving time and money and enhancing feasibility, as processing could occur at the time of analysis.
Percentage error and total percent error analyses assess for inherent biases of the processing as well as whether the expected differences between the two processing methods represent a clinically relevant difference. This measured error is a combination of the true difference between the analyte concentration in the paired samples as well as the variability of the immunoassay. Based on these standards, the observed difference between the paired samples is within the acceptable error for biomarker assays based on both previous studies and published manufacturer guidelines [3, 9, 10]. While total percent error values are large, it is entirely possible that the differences are solely the expected random variability of an immunoassay instead of true differences due to processing methodology. Additionally, these differences are no larger than would be expected for repeated biomarker sampling of the same urine sample [9, 10]. Therefore, the results for each of the sample pairs does not demonstrate a difference between centrifugation prior to storage vs. prior to analysis outside the variability in the accuracy of the immunoassay.
Previous studies have provided insights into the effects of various processing and storage conditions on biomarker stability, investigating factors including centrifugation, protease inhibitors and temperature [16]. Up to this point, these studies have been in adults or children with urine collected directly by catheterization. Parikh et al. investigated methods of processing samples for biomarker analysis in adult patients and found that with urine samples collected by catheterization, immediate storage without centrifugation did not influence biomarker results [5]. This study utilized similar methodology, and demonstrated that this is also true in neonates with urine collected indirectly via cotton balls. Previous investigations show comparable total protein values in spontaneously voided urine compared to urine collected indirectly via diapers, however these studies did note a 10% reduction in total protein values in samples [17]. Concerns about the impact of cotton fibers have long been noted in neonatal research protocols with urinary biomarker analysis, however the results of our study suggest that this processing step prior to storage is not necessary [4].
While we did not assess difference in biomarker stability based on short-term storage conditions, recent work by other groups have examined various storage techniques on the stability of urinary biomarkers. Schuh et al. assessed the impact of storage conditions on biomarker stability in pediatric cardiac surgery patients, finding that short-term storage for up to 24 hours at 4°C did not impact biomarker results and that biomarker concentrations were stable at −80°C for up to 5 years [6]. Based on the findings of this study, along with these other recent works, it appears that neonatal urine samples for biomarker analysis can be collected and stored without centrifugation, and processed at the time of biomarker analysis without any impact on biomarker concentrations for at least up to five years.
This study does have several limitations. First, we utilized a small convenience sample of infants with urine samples already collected as part of an ongoing research study. Additionally, while a paired analysis is the most powerful test for detecting differences between samples processed with different techniques, there may not have been enough samples to show small but consistent differences. Thirdly, with our study design, we cannot assess absorption of proteins by the cotton prior to centrifugation, which would result in absolute values systemically less than seen with direct catheterization. This would require a different methodology including direct urine collection (e.g. bladder catheterization) in order to test.
In summary, we suggest that centrifugation of urine samples in neonates as part of processing prior to sample storage is not required. Given the difficulties in obtaining adequate urine samples, researchers and clinicians frequently utilize disposable diapers with cotton balls placed in the diapers to obtain urine specimens for analysis. Based on the results of this study, urine samples can be processed and then centrifuged just prior to analysis to assess qualitative and quantitative urinary biomarker expression when using Fisher brand non-sterile large cotton balls placed in diapers. The ability to use this routine urine collection method to obtain samples for subsequent quantitative immunoassay analysis should greatly facilitate use of these techniques in studies of newborns and young children.
Acknowledgments
Support
We thank Elizabeth Howland, Amy Silvia, Stephanie Hauge, and Emily Pao for their assistance with this work. This study was supported by an internal grant from the Seattle Children’s Research Institute Center for Clinical and Translational Research (SH). It was also supported by the NIH R01 DK103608 (SH, DA, SG), U01NS077953 and NCT01378273 (SJ, DM), and T32DK007662 (MS). The authors declare that they have no other relevant financial interests.
Footnotes
Contributions: Research idea and study design: DA, SG, JM, PB, SJ, DM, SH; data acquisition: SJ, DM, SH; data analysis and interpretation: MS, DA, SG, JM, TB, ZA, PB, SH; statistical analysis: MS, JM, TB; supervision or mentorship: SH. Each author contributed important intellectual content during manuscript drafting or revision and accepts accountability for the overall work by ensuring that questions pertaining to the accuracy or integrity of any portion of the work are appropriately investigated and resolved. SH takes responsibility that this study is reported honestly, accurately, and transparently; that no important aspects of the study have been omitted; and that any discrepancies from the study as planned (and, if relevant, registered) have been explained.
Compliance with ethical standards
Informed consent was obtained as part of the PENUT study, and all study procedures were performed in accordance with ethical standards of the local Institutional Review Board and Good Clinical Practice guidelines.
Conflict of interest
None to declare
References
- 1.Askenazi DJ, Koralkar R, Patil N, Halloran B, Ambalavanan N, Griffin R. Acute Kidney Injury Urine Biomarkers in Very Low-Birth-Weight Infants. Clin J Am Soc Nephrol. 2016;11:1527–1535. doi: 10.2215/CJN.13381215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Sohn AH, Garrett DO, Sinkowitz-Cochran RL, Grohskopf LA, Levine GL, Stover BH, Jarvis WR, Pediatric Prevention Network Prevalence of nosocomial infections in neonatal intensive care unit patients: Results from the first national point-prevalence survey. J Pediatr. 2001;139:821–827. doi: 10.1067/mpd.2001.119442. [DOI] [PubMed] [Google Scholar]
- 3.Muratore C, Dhanireddy R. Urine collection for disposable diapers in premature infants: Biochemical analysis. Clin Pediatr (Phila) 1993;32:314–315. doi: 10.1177/000992289303200516. [DOI] [PubMed] [Google Scholar]
- 4.Starr R, Kimmel P, Bonventre J. Best Practices for Sample Storage: A Report from the Workshop on Urine Biospecimen Handling [Internet] Available from: http://www3.niddk.nih.gov/fund/other/Best_Practices_for_sample_Storage.pdf.
- 5.Parikh CR, Butrymowicz I, Yu A, Chinchilli VM, Park M, Hsu CY, Reeves WB, Dervarjan P, Kimmel PL, Siew ED, Liu KD, ASSESS-AKI Study Investigators Urine Stability Studies for Novel Biomarkers of Acute Kidney Injury. Am J Kidney Dis. 2014;63:567–572. doi: 10.1053/j.ajkd.2013.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Schuh MP, Nehus E, Ma Q, Haffner C, Bennett M, Krawczeski CD, Devarajan P. Long-term stability of Urinary Biomarkers of Acute Kidney Injury in Children. Am J Kidney Dis. 2016;67:56–61. doi: 10.1053/j.ajkd.2015.04.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mock DM. Rayon balls and disposable-diaper material selectively adsorb creatinine. Am J Clin Nutr. 1992;55:326–330. doi: 10.1093/ajcn/55.2.326. [DOI] [PubMed] [Google Scholar]
- 8.Juul SE, Mayock DE, Comstock BA, Heagerty PJ. Neuroprotective potential of erythropoietin in neonates; design of a randomized trial. Matern Health Neonatol Perinatol. 2015;1:27. doi: 10.1186/s40748-015-0028-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kidney Injury Panel 3 (human) Kit: MDS Toxicology Assays [Internet] 2017 Jan; Available from: https://www.mesoscale.com/~/media/files/product%20inserts/kidney%20injury%20panel%203%20human.pdf.
- 10.Kidney Injury Panel 5 (human) Kit: MDS Multi-Spot Assay System [Internet] 2017 Jan; Available from: https://www.mesoscale.com/~/media/files/product%20inserts/kidney%20injury%20panel%205%20human.pdf.
- 11.R Core Team. R: A language and environment for statistical computing. R Foundations for Statistical Computing; Vienna, Austria: 2013. Available from: https://www.R-project.org. [Google Scholar]
- 12.Hochberg Y. A sharper bonferroni procedure for multiple tests of significance. Biometrika. 1988;75:800. [Google Scholar]
- 13.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 14.Hofman CS, Melis RJ, Donders AR. Adapted Bland-Altman method was used to compare measurement methods with unequal observations per case. J Clin Epidemiol. 2015;68:939–943. doi: 10.1016/j.jclinepi.2015.02.015. [DOI] [PubMed] [Google Scholar]
- 15.Bland JM, Altman DG. Comparing methods of measurement: Why plotting difference against standard method is misleading. Lancet. 1995;346:1085–1087. doi: 10.1016/s0140-6736(95)91748-9. [DOI] [PubMed] [Google Scholar]
- 16.Giesen C, Lieske JC. The Influence of Processing and Storage Conditions on Renal Protein Biomarkers. Clin J Am Soc Nephrol. 2016;11:1726–1728. doi: 10.2215/CJN.08800816. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Beeram MR, Dhanireddy R. Urinalysis: Direct versus diaper collection. Clin Pediatr (Phila) 1991;30:278–280. doi: 10.1177/000992289103000502. [DOI] [PubMed] [Google Scholar]


