Abstract
Objective
We aimed to determine the differences in the pattern and magnitude of thrombin generation between patients with preeclampsia (PE) and those with a small-for-gestational-age (SGA) fetus.
Methods
This cross-sectional study included women in the following groups: 1) normal pregnancy (NP) (n=49); 2) PE (n=56); and 3) SGA (n=28). Maternal plasma thrombin generation (TGA) was measured, calculating: a) lag time (LT); b) velocity index (VI); c) peak thrombin concentration (PTC); d) time-to-peak thrombin concentration (TPTC); and e) endogenous thrombin potential (ETP).
Results
1) The median TPTC, VI, and ETP differed among the groups (p=0.001, p=0.006, p<0.0001); 2) the median ETP was higher in the PE than in the NP (p<0.0001) and SGA (p=0.02) groups; 3) patients with SGA had a shorter median TPTC and a higher median VI than the NP (p=0.002, p=0.012) and PE (p<0.0001, p=0.006) groups.
Conclusions
1) Patients with PE have higher in vivo thrombin generation than women with NP and those with an SGA fetus; 2) the difference in TGA patterns between PE and SGA suggests that the latter group had faster TGA, while patients with PE had a longer reaction, generating more thrombin. This observation is important for the identification of a subset of patients who might benefit from low molecular-weight heparin.
Keywords: endogenous thrombin potential, fetal growth, hypertension, pregnancy, velocity index
Introduction
As opposed to the non-pregnant state, pregnancy is associated with a prothrombotic state and increased thrombin generation [1–5]. This is the result of 1) an increase in the maternal plasma concentration of fibrinogen as well as clotting factors VII-X and XII [1,2,6–9]; 2) a decrease in the concentration of anticoagulation proteins such as protein S [10–14] as well as a decrease in activated protein C sensitivity [14–16]; and 3) reduced fibrinolysis [6, 7–21] due to a decrease of activation of plasminogen activator inhibitors I and II [22–24].
Increased thrombin generation and thrombosis are considered to be one of the mechanisms of disease in preeclampsia [25–29], intrauterine growth restriction [29–33], stillbirth [34], recurrent pregnancy losses [35], and preterm delivery [36–38]. This concept is supported by the following observations: 1) an excessive rate of thrombotic lesions in the placental villi [39] and decidual vessels [36, 39] was reported in the placentas of patients who had any of these obstetrical syndromes; and 2) women who developed preeclampsia or delivered a small-for-gestational-age (SGA) neonate had higher maternal plasma concentrations of the thrombin-anti- thrombin (TAT) complex than women who had a normal pregnancy [40].
Given the technical difficulties of thrombin measurement in the plasma [41], its generation was previously assessed by surrogate markers such as prothrombin fragment 1+2 and TAT III complex. However, current thrombin generation assays enable us to measure the kinetics, quantity, and potential of thrombin generation [41]. The parameters measured by the thrombin generation assay include the following (Figure 1): 1) lag time (LT) – the time from the beginning of the reaction until thrombin formation starts; 2) peak thrombin concentration (PTC) – maximal concentration of thrombin; 3) PTC time (TPTC) – the time interval until the maximal concentration of thrombin is reached; 4) velocity index (VI) – the slope of the kinetics of thrombin generation, which is the ratio PTC/(TPTC-LT); and 5) endogenous thrombin potential (ETP) – the area under the curve that represents the amount of thrombin generated (Figure 1).
Figure 1.
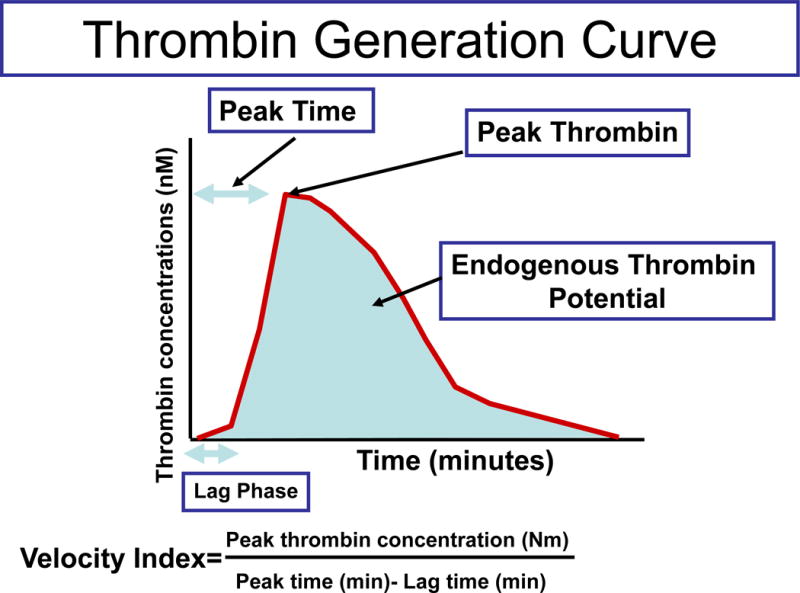
Component of the thrombin generation assay. The generation of thrombin during is measured through the fluorometric reaction. The parameters are calculated as follows: (1) lag time (LT) – the time interval from the beginning of the reaction to the detection of thrombin by the fluorometric assay; (2) estimated peak thrombin concentration (PTC) – the highest concentration of thrombin generated in a single measurement; (3) time-to-peak thrombin concentration (TPTC) – the interval from the beginning of the reaction and the generation of the highest thrombin concentration at a specific measurement; (4) velocity index (VI) – the rate of thrombin generation during the reaction; and (5) endogenous thrombin potential (ETP) – the calculation of how much thrombin has been generated during the time of the reaction (60 min). The figure was reproduced from www.Diapharma.com with permission.
During normal pregnancy, the ETP and PTC increased significantly with gestational age [5,42]; at term, the average peak of plasma thrombin generation was 22% higher compared to the non-pregnant state [43]. Both parameters correlated with maternal plasma prothrombin concentration [5]. In contrast, the LT and the TPTC did not change significantly with gestational age [5].
The purpose of this study was to determine whether there are differences in the pattern and magnitude of thrombin generation between patients with preeclampsia and women with an SGA fetus and without preeclampsia.
Material and methods
Study groups and inclusion criteria
A cross-sectional study included patients in the following groups: 1) normal pregnancy (n=49); 2) preeclampsia (n=56); and 3) SGA (n=28). Patients with multiple pregnancies or fetuses with congenital and/or chromosomal anomalies were excluded.
Samples and data were retrieved from our Bank of Biological Materials and clinical databases. Many of these samples have previously been employed to study the biology of inflammation, hemostasis, angiogenesis regulation, and growth factor concentrations in non-pregnant women, women with normal pregnancy, and those with pregnancy complications.
All participants provided written informed consent prior to the collection of maternal blood. The Institutional Review Boards of Wayne State University and the National Institute of Child Health and Human Development (NICHD/NIH/DHHS) approved the collection and utilization of samples for research purposes.
Clinical definitions
Women included in the normal pregnancy group met the following criteria: 1) no medical, obstetrical, or surgical complications at the time of the study; 2) gestational age ranging from 20 to 41 weeks; and 3) delivery of a term infant, appropriate for gestational age, without complications. Preeclampsia was defined as the presence of hypertension (systolic blood pressure ≥140 mmHg or diastolic blood pressure ≥90 mmHg on at least two occasions, 4 hours to 1 week apart) and proteinuria (≥300 milligrams in a 24-hour urine collection or dipstick measurement ≥ 2+) [44]. An SGA neonate was defined by a birthweight <10th percentile [45]. Placental histologic findings were classified according to a diagnostic schema proposed by Redline et al [46].
Sample collection
All blood samples were collected in a vacutainer containing 0.109 M of trisodium citrate anticoagulant solution (BD Biosciences; San Jose, CA. The samples were centrifuged at 1300 g for 10 minutes at 4°C and stored at −70°C until assay.
Thrombin generation assay
The thrombin generation assay (Technothrombin® TGA, DiaPharma, Columbus, OH) was performed according to the manufacturer’s recommendations. In brief, 10 μL of a tissue factor phospholipid (TF/PL) solution (DiaPharma, Columbus, OH) were added to 50 μL of 1 mM of thrombin peptide substrate Z-Gly-Gly-Arg-AMC (DiaPharma, Columbus, OH) and calcium, 15 mM of CaCl2. The reaction was started by adding 40 μL plasma or control. The fluorophore effect of the reaction was monitored continuously and measured every minute for 60 minutes in a BIO-TEK FLx800 microplate fluorescence reader (DiaPharma, Columbus, OH). Thrombin concentrations (nM) were calculated by the conversion of the measurement of the thrombin generation as expressed in relative fluorescence units according to the reference curve prepared by purified thrombin. Characteristic parameters (LT, VI, PTC, TPTC, and ETP) were calculated by software adapted from TECHNOTHROMBIN® TGA (DiaPharma, Columbus, OH).
Statistical analysis
Characteristic parameters [lag time (LT), velocity index (VI), estimated peak thrombin concentration (PTC), time to peak thrombin concentration (TPTC), and the endogenous thrombin potential (ETP)] did not have a normal distribution curve. Thus, the Kruskal-Wallis test with post-hoc analysis was used for comparisons of continuous variables. Comparison of proportions was performed by Chi-square and Fisher’s exact tests. The Spearman’s rho test was used to detect a correlation between the characteristic parameters: a) lag time (LT); b) velocity index (VI); c) estimated peak thrombin concentration (PTC); d) time to peak thrombin concentration (TPTC); and e) the endogenous thrombin potential (ETP) to gestational age at sample collection. A p value < 0.05 was considered statistically significant. Analysis was performed with SPSS, version 12 (SPSS Inc., Chicago, IL, USA).
Results
Demographic and clinical characteristics
Patients in the preeclampsia and SGA groups had a lower median gestational age at delivery and birthweight compared to women with normal pregnancies (Table 1).
Table I.
Demographic and clinical characteristics of the study population
| Normal pregnancy (n=49) |
Preeclampsia (n=56) |
SGA (n=28) |
|
|---|---|---|---|
| Maternal age (years) | 24 (21–27) | 26.5(21–31) | 24 (20–26) |
| Gravidity€ | |||
| 1 | 8 (16.7) | 14(25.0) | 6 (22.2) |
| 2–5 | 32 (66.7) | 34 (60.7) | 20 (74.1) |
| ≥6 | 8 (16.7) | 8 (14.3) | 1 (3.7) |
| Parity§ | |||
| 1 | 26 (53.1) | 40 (71.4) | 18 (67.7) |
| 2–5 | 22 (44.9) | 14(25.0) | 8 (29.6) |
| ≥6 | 1 (2.0) | 2 (3.6) | 1 (3.7) |
| Ethnic origin¥ | |||
| African–American | 38 (82.6) | 42(76.4) | 21(80.8) |
| Caucasian | 5 (10.8) | 9 (16.4) | 5(19.2) |
| Hispanic | 1 (2.2) | 3(5.5) | 0 |
| Asian | 2 (4.4) | 1(1.8) | 0 |
| GA at blood collection (weeks) | 30.2 (23.6–32.4) |
30.2 (27.7–32.6) |
32.0 (29.3–33.4) |
| GA at delivery (weeks) | 39.3 (38.6–40.4) |
31.4* (28.6–33.0) |
33.2* (31.0–36.0) |
| Neonatal birthweight (grams) | 3245 (2972–3614) |
1260* (820–1700) |
1280* (1005–1968) |
Data are presented as median (minimum, maximum) or as numbers (%)
GA: gestational age; SGA: small for gestational age
= Normal pregnancy (n=48); SGA (n=27)
= SGA (n=27)
= Normal pregnancy (n=46); Preeclampsia (n=55); SGA (n=26)
p<0.05 in comparison to normal pregnancy
Changes in the parameters of thrombin generation in women with normal pregnancy
Among women with a normal pregnancy, gestational age at sample collection positively correlated with PTC in the maternal plasma (r=0.341, p=0.017). The lag phase positively correlated with the TPTC (r=0.313, p=0.03). Peak thrombin concentration positively correlated with the slope of thrombin generation (r=0.87, p<0.001) and the ETP (r=0.83, p<0.001) and negatively correlated with the TPTC (r= −0.612, p<0.001).
Changes in the parameters of thrombin generation in preeclampsia and SGA
1) The median maternal TPTC, VI, and ETP differed among the study groups (Kruskal-Wallis, p=0.001, p=0.006, and p˂0.0001, respectively); 2) the median maternal plasma ETP of patients with preeclampsia was higher than that of women who had a normal pregnancy (preeclampsia: median 8173.1 nM, range 418.6–10054.8 vs. normal pregnancy: median 7231.0 nM, range 1537.1–8599.8, p<0.0001) and patients with SGA (median 7597.8 nM, range 28.6–9233.0, p=0.02) (Figure 2); 3) patients who delivered an SGA neonate had a lower median TPTC than women with a normal pregnancy (SGA: median 12.5 min, range 3.8–28.8 vs. normal pregnancy: median 16.3 min, range 6.3–58.8, p=0.002), and patients with preeclampsia (median 15.9 min, range 8.8–58.8, p ˂ 0.0001) (Figure 3); and 4) patients with SGA had a higher median VI than women with normal pregnancy (SGA: median 146.4 nM/min, range 0.1–679.4 vs. normal pregnancy: 92.4 nM/min, range 1.0–587.3, p=0.012) and patients with preeclampsia (median 85.2 nM/min, range 0.6–315.9, p=0.006) (Figure 4). Among patients with preeclampsia, the presence of an SGA neonate was not associated with a significant difference in the parameters of thrombin generation.
Figure 2.
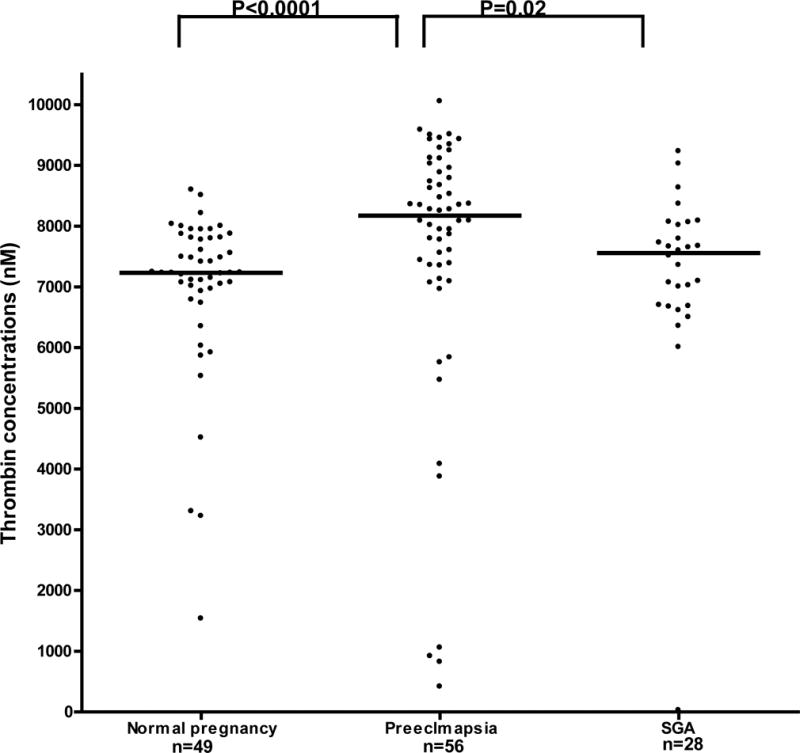
The differences in endogenous thrombin potential among the study groups. Normal pregnancy: median 7231.0 nM, range 1537.1–8599.8; preeclampsia: median 8173.1 nM, range 418.6–10054.8; SGA: median 7597.8 nM, range 28.6–9233.0, p = 0.0.
Figure 3.
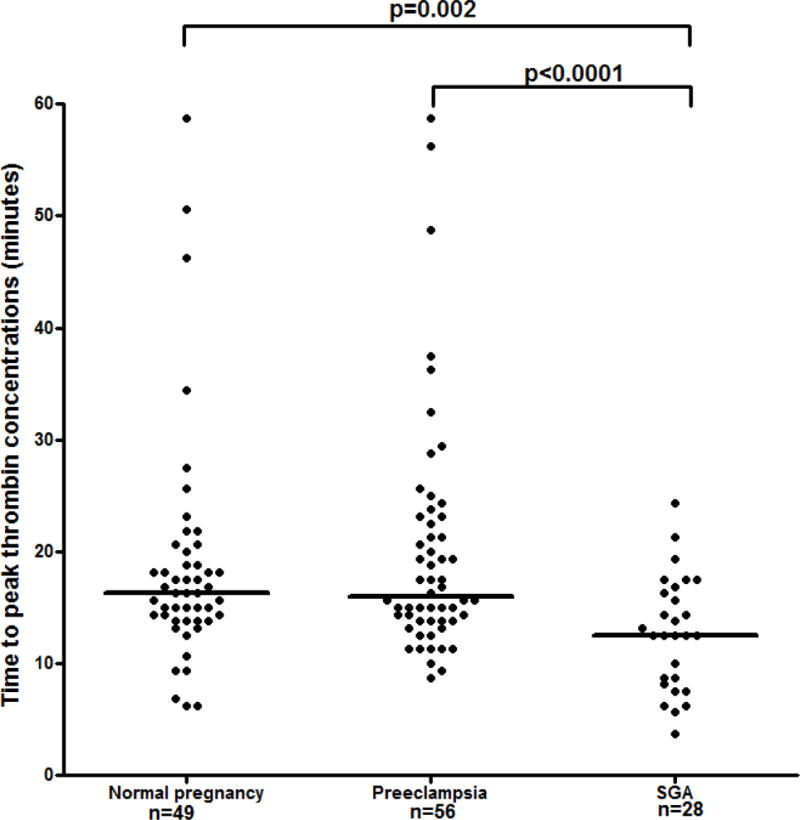
The differences in the time interval from the beginning of the reaction to the peak of thrombin generation among the study groups. Normal pregnancy: median 16.3 min, range 6.3–58.8; preeclampsia: median 15.9 min, range 8.8–58.8; SGA: median 12.5 min. range 3.8–28.8.
Figure 4.
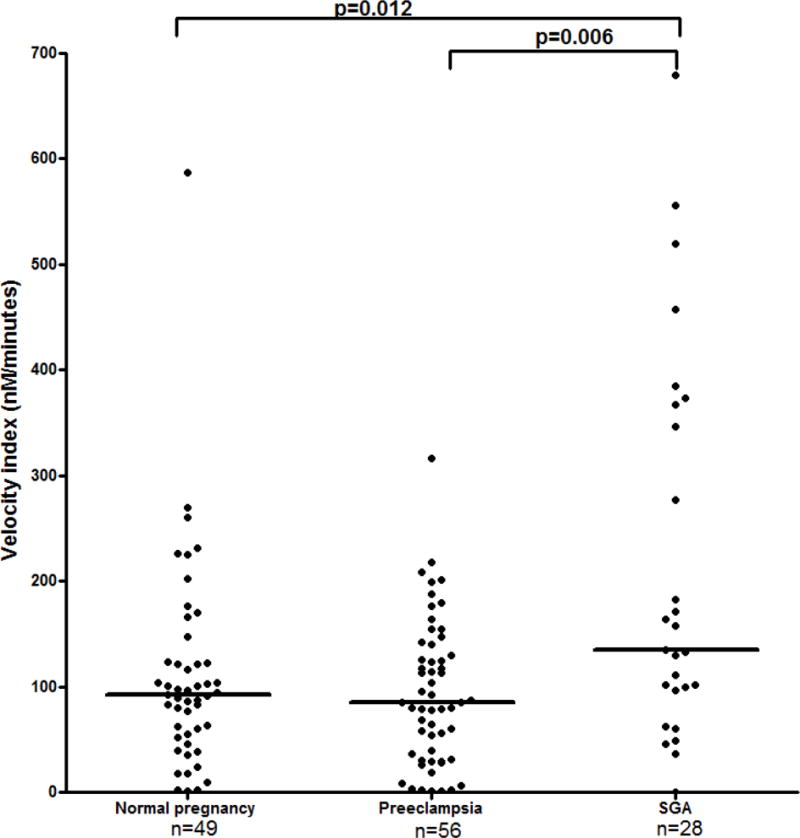
The differences in the velocity index among the study groups. Normal pregnancy: 92.4 nM/min, range 1.0–587.3; preeclampsia: median 85.2 nM/min, range 0.6–315.9; SGA: median 146.4 nM/min, range 0.1–679.4.
Discussion
Principal findings
1) Patients with preeclampsia had higher in vivo thrombin generation than patients with a normal pregnancy and those with an SGA fetus; 2) the difference in the pattern of thrombin generation between patients with preeclampsia and those with SGA suggests that the latter group had faster thrombin generation while patients with preeclampsia had a longer reaction, which generates more thrombin.
Measurement of thrombin generation
The activation of the extrinsic pathway of coagulation through the TF-FVIIa complex is essential for thrombin generation [47, 48]. This process has three steps: initiation, propagation, and termination [49–54]. In the initiation phase, TF and FVIIa form a complex that generates small amounts of FXa and FIXa [51, 54]. Factor Xa, assisted by phospholipids on the membrane surface of endothelial cells, generates a small amount of thrombin [51, 54]. The latter activates platelets as well as FV and FVIII that, together with FX, form the prothrombinase complex [51, 54]. At this stage of the cascade, there is a switch between the extrinsic and intrinsic pathways of coagulation, and the latter becomes the main source of thrombin generation. This switch is accomplished through the inhibition of the extrinsic pathway by tissue factor pathway inhibitor (TFPI) and the activation of FX, the catalytic activity of the FVIIIa-FIXa complex [50,54,55]. This process leads to the propagation phase in which more than 96% of thrombin is generated [51].
Given the technical difficulties of thrombin measurement in plasma [41], its generation was previously assessed by surrogate markers such as prothrombin fragments 1+2 and the TAT complex, the indicators for ongoing thrombin generation in the plasma. However, the recent development of the thrombin generation assay now enables us to measure the kinetics, quantity, and potential of thrombin generation.
Thrombin generation in normal pregnancy
The changes in the coagulation system during pregnancy are adaptive mechanisms that can prevent hemorrhage at the time of delivery [56–60]. Indeed, normal pregnancy is associated with a substantial increase in TF concentrations in the decidua and myometrium [61–64]. Similarly, high TF concentrations have been detected in the fetal membranes (mainly the amnion) and amniotic fluid [59, 65–67]. In addition to the changes in TF, normal pregnancy is associated with excessive thrombin generation [4,48,56], as determined by increased maternal concentrations of fibrinopeptide A, prothrombin fragment 1 + 2, and the TAT complex [59, 68–70]. The concentration of these complexes increases further during and after normal labor [71] and delivery [69, 71], and decreases later during puerperium [69,71].
Our finding of a positive correlation between the PTC and gestational age at sample collection is in agreement with previous studies [5, 48]. The authors reported that the PTC was higher during the third compared to the first trimester. However, they found no significant differences in the PTC between the first and second trimesters as well as between the second and third trimesters [5].
In our study, the ETP did not correlate with gestational age at sample collection. Similarly, Rosenkranz et al [5] reported a significantly higher ETP in the second and third trimesters when compared to the first, but no significant difference between the second and third trimesters.
Thrombin generation in complicated pregnancies
Patients with preeclampsia, an SGA neonate [40], or a fetal demise [37], as well those with preterm labor and intact membranes [4,72,73] or preterm PROM [4], had higher continuous thrombin generation than women with a normal pregnancy, reflected by the higher maternal plasma TAT complex concentration. However, the plasma profiles of coagulation factors and anti-coagulation proteins differ among these pregnancy complications. Evidence in support of this view includes the following: 1) patients with preeclampsia have higher maternal plasma TF [3,74], fibrinogen [75], thrombomodulin [76] and TFPI [74,77] concentrations as well as factor VIII activity [75], and lower protein Z [78] plasma concentrations than women with normal pregnancy; 2) patients with preterm PROM have higher TF concentrations and lower TFPI concentrations than the controls [79]; 3) patients with preterm labor with intact membranes have lower anticoagulation proteins, e.g., TFPI [80], as well as protein Z [81] concentrations, and higher TF activity [80] than women with normal pregnancy; 4) women who delivered an SGA fetus have a lower TF plasma concentration [74], yet no significant differences in median maternal plasma TFPI [74] and protein Z [78] concentrations compared to women with normal pregnancy; and 5) patients with a fetal demise have low TFPI concentrations but no significant changes in maternal plasma TF [37] and protein Z concentrations [78]. Thus, the increased ongoing thrombin generation observed in all these obstetrical syndromes resulted from an alteration in the balance between coagulation factors and their inhibitors. Interestingly, these profiles varied among the different syndromes.
The differences between thrombin generation pattern in patients with preeclampsia and those who delivered an SGA neonate
The findings that patients with preeclampsia have a significantly higher ETP than that of women with normal pregnancy and those who delivered an SGA neonate and that women with an SGA neonate had a higher VI and a shorter time interval to PTC than women with normal pregnancy and those with preeclampsia are novel.
The findings that patients with preeclampsia have a significantly higher ETP than that of women with normal pregnancy is in agreement with a recent report [82]. Possible explanations for these observations are 1) the activation of maternal coagulation due to the release of placental macrovesicles: indeed, Gardiner et al [83] extracted syncytiotrophoblast macrovesicles (STBM) from the placentas of patients with preeclampsia as well as from those with normal pregnancy, and demonstrated that STBM obtained from those with preeclampsia exhibit increased TF activity, resulting in higher thrombin generation kinetics compared to women with normal pregnancy; and 2) women who had preeclampsia have an a priori increased thrombin generation, even in the non-pregnant state. Indeed, among primiparous women at six months post-delivery, those who had preeclampsia had higher thrombin generation and parameters of thrombin generation kinetics than those with normal pregnancy [84]. Collectively, this suggests that women with preeclampsia may have a tendency toward higher thrombin generation that, at least in a subset of patients, persists even in the non-pregnant state. These women may benefit from prophylactic administration of low molecular-weight heparin in subsequent pregnancies for secondary prevention of preeclampsia [85].
The differences in the kinetics of thrombin generation between patients with preeclampsia and those who deliver an SGA neonate suggest that the former have a longer phase of thrombin generation, which can eventually generate more thrombin. However, women who deliver an SGA fetus have a faster reaction leading to a shorter VI and TPTC, yet they do not generate more thrombin than women with normal pregnancy. A possible explanation for the differences in the magnitude and pattern of thrombin generation in patients with preeclampsia and those who deliver an SGA neonate is that the activity/concentration of the anti-coagulation proteins in comparison to the coagulation factors may be lower in those with preeclampsia [74], resulting in increased thrombin generation.
Conclusion
1) Patients with preeclampsia have higher in vivo thrombin generation than patients with normal pregnancy and those who deliver an SGA fetus; 2) the difference in the pattern of thrombin generation between patients with preeclampsia and those with SGA suggests that the latter group has faster thrombin generation while patients with preeclampsia had a longer reaction, which generates more thrombin. This could be due to the lower activity of natural anti-coagulant factors that has been reported in preeclampsia. This observation is important because the identification of those with lower activity of the anticoagulation proteins may help us to distinguish patients who can benefit from the administration of low molecular-weight heparin.
Acknowledgments
This research was supported, in part, by the Perinatology Research Branch, Program for Perinatal Research and Obstetrics, Division of Intramural Research, Eunice Kennedy Shriver National Institute of Child Health and Human Development, National Institutes of Health, Department of Health and Human Services (NICHD/NIH/DHHS); and, in part, with Federal funds from NICHD/NIH/DHHS under Contract No. HHSN275201300006C.
Footnotes
Disclosure Statement: The authors report no conflicts of interest.
References
- 1.Beller FK, Ebert C. The coagulation and fibrinolytic enzyme system in pregnancy and in the puerperium. Eur J Obstet Gynecol Reprod Biol. 1982;13:177–197. doi: 10.1016/0028-2243(82)90028-4. [DOI] [PubMed] [Google Scholar]
- 2.Stirling Y, Woolf L, North WR, Seghatchian MJ, Meade TW. Haemostasis in normal pregnancy. Thromb Haemost. 1984;52:176–182. [PubMed] [Google Scholar]
- 3.Bellart J, Gilabert R, Angles A, et al. Tissue factor levels and high ratio of fibrinopeptide A:D-dimer as a measure of endothelial procoagulant disorder in pre-eclampsia. Br J Obstet Gynaecol. 1999;106:594–597. doi: 10.1111/j.1471-0528.1999.tb08330.x. [DOI] [PubMed] [Google Scholar]
- 4.Chaiworapongsa T, Espinoza J, Yoshimatsu J, et al. Activation of coagulation system in preterm labor and preterm premature rupture of membranes. J Matern Fetal Neonatal Med. 2002;11:368–373. doi: 10.1080/jmf.11.6.368.373. [DOI] [PubMed] [Google Scholar]
- 5.Rosenkranz A, Hiden M, Leschnik B, et al. Calibrated automated thrombin generation in normal uncomplicated pregnancy. Thromb Haemost. 2008;99:331–337. doi: 10.1160/TH07-05-0359. [DOI] [PubMed] [Google Scholar]
- 6.Hellgren M, Blomback M. Studies on blood coagulation and fibrinolysis in pregnancy, during delivery and in the puerperium. I. Normal condition. Gynecol Obstet Invest. 1981;12:141–154. doi: 10.1159/000299596. [DOI] [PubMed] [Google Scholar]
- 7.Donohoe S, Quenby S, Mackie I, et al. Fluctuations in levels of antiphospholipid antibodies and increased coagulation activation markers in normal and heparin-treated antiphospholipid syndrome pregnancies. Lupus. 2002;11:11–20. doi: 10.1191/0961203302lu132oa. [DOI] [PubMed] [Google Scholar]
- 8.Bremme KA. Haemostatic changes in pregnancy. Best Pract Res Clin Haematol. 2003;16:153–168. doi: 10.1016/s1521-6926(03)00021-5. [DOI] [PubMed] [Google Scholar]
- 9.Brenner B. Haemostatic changes in pregnancy. Thromb Res. 2004;114:409–414. doi: 10.1016/j.thromres.2004.08.004. [DOI] [PubMed] [Google Scholar]
- 10.Clark P, Brennand J, Conkie JA, McCall F, Greer IA, Walker ID. Activated protein C sensitivity, protein C, protein S and coagulation in normal pregnancy. Thromb Haemost. 1998;79:1166–1170. [PubMed] [Google Scholar]
- 11.Faught W, Garner P, Jones G, Ivey B. Changes in protein C and protein S levels in normal pregnancy. Am J Obstet Gynecol. 1995;172:147–150. doi: 10.1016/0002-9378(95)90104-3. [DOI] [PubMed] [Google Scholar]
- 12.Oruc S, Saruc M, Koyuncu FM, Ozdemir E. Changes in the plasma activities of protein C and protein S during pregnancy. Aust N Z J Obstet Gynaecol. 2000;40:448–450. doi: 10.1111/j.1479-828x.2000.tb01179.x. [DOI] [PubMed] [Google Scholar]
- 13.Lefkowitz JB, Clarke SH, Barbour LA. Comparison of protein S functional and antigenic assays in normal pregnancy. Am J Obstet Gynecol. 1996;175:657–660. doi: 10.1053/ob.1996.v175.a73866. [DOI] [PubMed] [Google Scholar]
- 14.Mahieu B, Jacobs N, Mahieu S, et al. Haemostatic changes and acquired activated protein C resistance in normal pregnancy. Blood Coagul Fibrinolysis: Int J Haemost Thromb. 2007;18:685–688. doi: 10.1097/MBC.0b013e3282f09835. [DOI] [PubMed] [Google Scholar]
- 15.Mimuro S, Lahoud R, Beutler L, Trudinger B. Changes of resistance to activated protein C in the course of pregnancy and prevalence of factor V mutation. Aust N Z J Obstet Gynaecol. 1998;38:200–204. doi: 10.1111/j.1479-828x.1998.tb03002.x. [DOI] [PubMed] [Google Scholar]
- 16.Cumming AM, Tait RC, Fildes S, Yoong A, Keeney S, Hay CR. Development of resistance to activated protein C during pregnancy. Br J Haematol. 1995;90:725–727. doi: 10.1111/j.1365-2141.1995.tb05610.x. [DOI] [PubMed] [Google Scholar]
- 17.Biezenski JJ, Moore HC. Fibrinolysis in normal pregnancy. J Clin Pathol. 1958;11:306–310. doi: 10.1136/jcp.11.4.306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Naidoo SS, Hathorn M, Gillman T. Fibrinolytic and antifibrinolytic activity in pregnancy. J Clin Pathol. 1960;13:224–225. doi: 10.1136/jcp.13.3.224. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thorsen S. The inhibition of tissue plasminogen activator and urokinase-induced fibrinolysis by some natural proteinase inhibitors and by plasma and serum from normal and pregnant subjects. Scand J Clin Lab Invest. 1973;31:51–59. doi: 10.3109/00365517309082418. [DOI] [PubMed] [Google Scholar]
- 20.Kleiner GJ, Greston WM. Current concepts of defibrination in the pregnant woman. J Reprod Med. 1976;17:309–317. [PubMed] [Google Scholar]
- 21.Arias F, Andrinopoulos G, Zamora J. Whole-blood fibrinolytic activity in normal and hypertensive pregnancies and its relation to the placental concentration of urokinase inhibitor. Am J Obstet Gynecol. 1979;133:624–629. doi: 10.1016/0002-9378(79)90008-5. [DOI] [PubMed] [Google Scholar]
- 22.Kruithof EK, Tran-Thang C, Gudinchet A, et al. Fibrinolysis in pregnancy: a study of plasminogen activator inhibitors. Blood. 1987;69:460–466. [PubMed] [Google Scholar]
- 23.Ishii A, Yamada S, Yamada R, Hamada H. t-PA activity in peripheral blood obtained from pregnant women. J Perinat Med. 1994;22:113–117. doi: 10.1515/jpme.1994.22.2.113. [DOI] [PubMed] [Google Scholar]
- 24.Wright JG, Cooper P, Astedt B, et al. Fibrinolysis during normal human pregnancy: complex inter-relationships between plasma levels of tissue plasminogen activator and inhibitors and the euglobulin clot lysis time. Br J Haematol. 1988;69:253–258. doi: 10.1111/j.1365-2141.1988.tb07630.x. [DOI] [PubMed] [Google Scholar]
- 25.Brosens I, Renaer M. On the pathogenesis of placental infarcts in pre-eclampsia. J Obstet Gynaecol Br Commonw. 1972;79:794–799. doi: 10.1111/j.1471-0528.1972.tb12922.x. [DOI] [PubMed] [Google Scholar]
- 26.Robertson WB, Brosens I, Dixon G. Uteroplacental vascular pathology. Eur J Obstet Gynecol Reprod Biol. 1975;5:47–65. doi: 10.1016/0028-2243(75)90130-6. [DOI] [PubMed] [Google Scholar]
- 27.Pijnenborg R, Anthony J, Davey DA, et al. Placental bed spiral arteries in the hypertensive disorders of pregnancy. Br J Obstet Gynaecol. 1991;98:648–655. doi: 10.1111/j.1471-0528.1991.tb13450.x. [DOI] [PubMed] [Google Scholar]
- 28.Gersell DJ. Selected vascular lesions of the placenta. Clin Lab Med. 1995;15:611–629. [PubMed] [Google Scholar]
- 29.Sikkema JM, Franx A, Bruinse HW, van der Wijk NG, de Valk HW, Nikkels PG. Placental pathology in early onset pre-eclampsia and intra-uterine growth restriction in women with and without thrombophilia. Placenta. 2002;23:337–342. doi: 10.1053/plac.2001.0785. [DOI] [PubMed] [Google Scholar]
- 30.Rolschau J. Infarctions and intervillous thrombosis in placenta, and their association with intrauterine growth retardation. Acta Obstet Gynecol Scand Suppl. 1978;72:22–27. [PubMed] [Google Scholar]
- 31.Arias F, Romero R, Joist H, Kraus FT. Thrombophilia: a mechanism of disease in women with adverse pregnancy outcome and thrombotic lesions in the placenta. J Matern Fetal Med. 1998;7:277–286. doi: 10.1002/(SICI)1520-6661(199811/12)7:6<277::AID-MFM5>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- 32.Mitra SC, Seshan SV, Riachi LE. Placental vessel morphometry in growth retardation and increased resistance of the umbilical artery Doppler flow. J Matern Fetal Med. 2000;9:282–286. doi: 10.1002/1520-6661(200009/10)9:5<282::AID-MFM5>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 33.Sugimura M, Ohashi R, Kobayashi T, Kanayama N. Intraplacental coagulation in intrauterine growth restriction: cause or result? Semin Thromb Hemost. 2001;27:107–113. doi: 10.1055/s-2001-14068. [DOI] [PubMed] [Google Scholar]
- 34.Ornoy A, Crone K, Altshuler G. Pathological features of the placenta in fetal death. Arch Pathol Lab Med. 1976;100:367–371. [PubMed] [Google Scholar]
- 35.Preston FE, Rosendaal FR, Walker ID, et al. Increased fetal loss in women with heritable thrombophilia. Lancet. 1996;348:913–916. doi: 10.1016/s0140-6736(96)04125-6. [DOI] [PubMed] [Google Scholar]
- 36.Kim YM, Bujold E, Chaiworapongsa T, et al. Failure of physiologic transformation of the spiral arteries in patients with preterm labor and intact membranes. Am J Obstet Gynecol. 2003;189:1063–1069. doi: 10.1067/s0002-9378(03)00838-x. [DOI] [PubMed] [Google Scholar]
- 37.Elovitz MA, Baron J, Phillippe M. The role of thrombin in preterm parturition. Am J Obstet Gynecol. 2001;185:1059–1063. doi: 10.1067/mob.2001.117638. [DOI] [PubMed] [Google Scholar]
- 38.Arias F, Rodriquez L, Rayne SC, Kraus FT. Maternal placental vasculopathy and infection: two distinct subgroups among patients with preterm labor and preterm ruptured membranes. Am J Obstet Gynecol. 1993;168:585–591. doi: 10.1016/0002-9378(93)90499-9. [DOI] [PubMed] [Google Scholar]
- 39.Moldenhauer JS, Stanek J, Warshak C, Khoury J, Sibai B. The frequency and severity of placental findings in women with preeclampsia are gestational age dependent. Am J Obstet Gynecol. 2003;189:1173–1177. doi: 10.1067/s0002-9378(03)00576-3. [DOI] [PubMed] [Google Scholar]
- 40.Chaiworapongsa T, Yoshimatsu J, Espinoza J, et al. Evidence of in vivo generation of thrombin in patients with small-for-gestational-age fetuses and pre-eclampsia. J Matern Fetal Neonatal Med. 2002;11:362–367. doi: 10.1080/jmf.11.6.362.367. [DOI] [PubMed] [Google Scholar]
- 41.Hemker HC, Giesen P, AlDieri R, et al. The calibrated automated thrombogram (CAT): a universal routine test for hyper- and hypocoagulability. Pathophysiol Haemost Thromb. 2002;32:249–253. doi: 10.1159/000073575. [DOI] [PubMed] [Google Scholar]
- 42.Kovac MK, Lalic-Cosic SZ, Dmitrovic JM, Djordjevic VJ, Radojkovic DP. Thrombin generation, D-dimer and protein S in uncomplicated pregnancy. Clin Chem Lab Med. 2015;53:1975–1979. doi: 10.1515/cclm-2014-1030. [DOI] [PubMed] [Google Scholar]
- 43.Rice NT, Szlam F, Varner JD, Bernstein PS, Szlam AD, Tanaka KA. Differential contributions of intrinsic and extrinsic pathways to thrombin generation in adult, maternal and cord plasma samples. PLoS One. 2016;11:e0154127. doi: 10.1371/journal.pone.0154127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Bulletins–Obstetrics ACoP. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Obstet Gynecol. 2002;99:159–167. doi: 10.1016/s0029-7844(01)01747-1. [DOI] [PubMed] [Google Scholar]
- 45.Alexander GR, Himes JH, Kaufman RB, Mor J, Kogan M. A United States national reference for fetal growth. Obstet Gynecol. 1996;87:163–168. doi: 10.1016/0029-7844(95)00386-X. [DOI] [PubMed] [Google Scholar]
- 46.Redline RW, Heller D, Keating S, Kingdom J. Placental diagnostic criteria and clinical correlation–a workshop report. Placenta. 2005;26(Suppl A):S114–S117. doi: 10.1016/j.placenta.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 47.Levi M. Disseminated intravascular coagulation. Crit Care Med. 2007;35:2191–2195. doi: 10.1097/01.ccm.0000281468.94108.4b. [DOI] [PubMed] [Google Scholar]
- 48.McLean KC, Bernstein IM, Brummel-Ziedins KE. Tissue factor-dependent thrombin generation across pregnancy. Am J Obstet Gynecol. 2012;207:135.e131–e136. doi: 10.1016/j.ajog.2012.05.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lawson JH, Kalafatis M, Stram S, Mann KG. A model for the tissue factor pathway to thrombin. I. An empirical study. J Biol Chem. 1994;269:23357–23366. [PubMed] [Google Scholar]
- 50.Butenas S, van ’t Veer C, Mann KG. Evaluation of the initiation phase of blood coagulation using ultrasensitive assays for serine proteases. J Biol Chem. 1997;272:21527–21533. doi: 10.1074/jbc.272.34.21527. [DOI] [PubMed] [Google Scholar]
- 51.Brummel KE, Paradis SG, Butenas S, Mann KG. Thrombin functions during tissue factor-induced blood coagulation. Blood. 2002;100:148–152. doi: 10.1182/blood.v100.1.148. [DOI] [PubMed] [Google Scholar]
- 52.Butenas S, Mann KG. Blood coagulation. Biochemistry Mosc. 2002;67:3–12. doi: 10.1023/a:1013985911759. [DOI] [PubMed] [Google Scholar]
- 53.Butenas S, Bouchard BA, Brummel-Ziedins KE, Parhami-Seren B, Mann KG. Tissue factor activity in whole blood. Blood. 2005;105:2764–2770. doi: 10.1182/blood-2004-09-3567. [DOI] [PubMed] [Google Scholar]
- 54.Butenas S, Orfeo T, Brummel-Ziedins KE, Mann KG. Tissue factor in thrombosis and hemorrhage. Surgery. 2007;142:S2–S14. doi: 10.1016/j.surg.2007.06.032. [DOI] [PubMed] [Google Scholar]
- 55.Hockin MF, Jones KC, Everse SJ, Mann KG. A model for the stoichiometric regulation of blood coagulation. J Biol Chem. 2002;277:18322–18333. doi: 10.1074/jbc.M201173200. [DOI] [PubMed] [Google Scholar]
- 56.Bellart J, Gilabert R, Miralles RM, Monasterio J, Cabero L. Endothelial cell markers and fibrinopeptide A to D-dimer ratio as a measure of coagulation and fibrinolysis balance in normal pregnancy. Gynecol Obstet Invest. 1998;46:17–21. doi: 10.1159/000009989. [DOI] [PubMed] [Google Scholar]
- 57.Walker MC, Garner PR, Keely EJ, Rock GA, Reis MD. Changes in activated protein C resistance during normal pregnancy. Am J Obstet Gynecol. 1997;177:162–169. doi: 10.1016/s0002-9378(97)70456-3. [DOI] [PubMed] [Google Scholar]
- 58.Yuen PM, Yin JA, Lao TT. Fibrinopeptide A levels in maternal and newborn plasma. Eur J Obstet Gynecol Reprod Biol. 1989;30:239–244. doi: 10.1016/0028-2243(89)90007-5. [DOI] [PubMed] [Google Scholar]
- 59.de Boer K, ten Cate JW, Sturk A, Borm JJ, Treffers PE. Enhanced thrombin generation in normal and hypertensive pregnancy. Am J Obstet Gynecol. 1989;160:95–100. doi: 10.1016/0002-9378(89)90096-3. [DOI] [PubMed] [Google Scholar]
- 60.Sorensen JD, Secher NJ, Jespersen J. Perturbed (procoagulant) endothelium and deviations within the fibrinolytic system during the third trimester of normal pregnancy. A possible link to placental function. Acta Obstet Gynecol Scand. 1995;74:257–261. doi: 10.3109/00016349509024445. [DOI] [PubMed] [Google Scholar]
- 61.Kuczynski J, Uszynski W, Zekanowska E, Soszka T, Uszynski M. Tissue factor (TF) and tissue factor pathway inhibitor (TFPI) in the placenta and myometrium. Eur J Obstet Gynecol Reprod Biol. 2002;105:15–19. doi: 10.1016/s0301-2115(02)00113-6. [DOI] [PubMed] [Google Scholar]
- 62.Lockwood CJ, Krikun G, Schatz F. The decidua regulates hemostasis in human endometrium. Semin Reprod Endocrinol. 1999;17:45–51. doi: 10.1055/s-2007-1016211. [DOI] [PubMed] [Google Scholar]
- 63.Erlich J, Parry GC, Fearns C, et al. Tissue factor is required for uterine hemostasis and maintenance of the placental labyrinth during gestation. Proc Natl Acad Sci USA. 1999;96:8138–8143. doi: 10.1073/pnas.96.14.8138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Lockwood CJ, Krikun G, Schatz F. Decidual cell-expressed tissue factor maintains hemostasis in human endometrium. Ann N Y Acad Sci. 2001;943:77–88. doi: 10.1111/j.1749-6632.2001.tb03793.x. [DOI] [PubMed] [Google Scholar]
- 65.Omsjo IH, Oian P, Maltau JM, Osterud B. Thromboplastin activity in amniotic fluid. Gynecol Obstet Invest. 1985;19:1–5. doi: 10.1159/000299000. [DOI] [PubMed] [Google Scholar]
- 66.Creter D. Amnioplastin: new reagent for coagulation tests. Lancet. 1977;2:251. doi: 10.1016/s0140-6736(77)92871-9. [DOI] [PubMed] [Google Scholar]
- 67.Lockwood CJ, Bach R, Guha A, Zhou XD, Miller WA, Nemerson Y. Amniotic fluid contains tissue factor, a potent initiator of coagulation. Am J Obstet Gynecol. 1991;165:1335–1341. doi: 10.1016/0002-9378(91)90363-v. [DOI] [PubMed] [Google Scholar]
- 68.Reinthaller A, Mursch-Edlmayr G, Tatra G. Thrombin-antithrombin III complex levels in normal pregnancy with hypertensive disorders and after delivery. Br J Obstet Gynaecol. 1990;97:506–510. doi: 10.1111/j.1471-0528.1990.tb02520.x. [DOI] [PubMed] [Google Scholar]
- 69.Uszynski M. Generation of thrombin in blood plasma of non-pregnant and pregnant women studied through concentration of thrombin-antithrombin III complexes. Eur J Obstet Gynecol Reprod Biol. 1997;75:127–131. doi: 10.1016/s0301-2115(97)00101-2. [DOI] [PubMed] [Google Scholar]
- 70.Reber G, Amiral J, de Moerloose P. Modified antithrombin III levels during normal pregnancy and relationship with prothrombin fragment F1 + 2 and thrombin-antithrombin complexes. Thromb Res. 1998;91:45–47. doi: 10.1016/s0049-3848(98)00043-7. [DOI] [PubMed] [Google Scholar]
- 71.Andersson T, Lorentzen B, Hogdahl H, Clausen T, Mowinckel MC, Abildgaard U. Thrombin-inhibitor complexes in the blood during and after delivery. Thromb Res. 1996;82:109–117. doi: 10.1016/0049-3848(96)00057-6. [DOI] [PubMed] [Google Scholar]
- 72.Erez O, Gotsch F, Mazaki-Tovi S, et al. Evidence of maternal platelet activation, excessive thrombin generation, and high amniotic fluid tissue factor immunoreactivity and functional activity in patients with fetal death. J Maternal–Fetal Neonatal Med. 2009;22:672–687. doi: 10.1080/14767050902853117. [DOI] [PubMed] [Google Scholar]
- 73.Elovitz MA, Saunders T, Ascher-Landsberg J, Phillippe M. Effects of thrombin on myometrial contractions in vitro and in vivo. Am J Obstet Gynecol. 2000;183:799–804. doi: 10.1067/mob.2000.108897. [DOI] [PubMed] [Google Scholar]
- 74.Erez O, Romero R, Hoppensteadt D, et al. Tissue factor and its natural inhibitor in pre-eclampsia and SGA. J Maternal–Fetal Neonatal Med. 2008;21:855–869. doi: 10.1080/14767050802361872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Williams VK, Griffiths AB, Carbone S, Hague WM. Fibrinogen concentration and factor VIII activity in women with preeclampsia. Hypertens Pregnancy. 2007;26:415–421. doi: 10.1080/10641950701548240. [DOI] [PubMed] [Google Scholar]
- 76.Hayashi M, Inoue T, Hoshimoto K, Negishi H, Ohkura T, Inaba N. Characterization of five marker levels of the hemostatic system and endothelial status in normotensive pregnancy and pre-eclampsia. Eur J Haematol. 2002;69:297–302. doi: 10.1034/j.1600-0609.2002.02691.x. [DOI] [PubMed] [Google Scholar]
- 77.Abdel Gader AM, Al-Mishari AA, Awadalla SA, Buyuomi NM, Khashoggi T, Al-Hakeem M. Total and free tissue factor pathway inhibitor in pregnancy hypertension. Int J Gynaecol Obstet. 2006;95:248–253. doi: 10.1016/j.ijgo.2006.07.014. [DOI] [PubMed] [Google Scholar]
- 78.Erez O, Hoppensteadt D, Romero R, et al. Preeclampsia is associated with low concentrations of protein Z. J Maternal–Fetal Neonatal Med. 2007;20:661–667. doi: 10.1080/14767050701495011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Erez O, Espinoza J, Chaiworapongsa T, et al. A link between a hemostatic disorder and preterm PROM: a role for tissue factor and tissue factor pathway inhibitor. J Maternal–Fetal Neonatal Med. 2008;21:732–744. doi: 10.1080/14767050802361807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Erez O, Romero R, Vaisbuch E, et al. High tissue factor activity and low tissue factor pathway inhibitor concentrations in patients with preterm labor. J Maternal–Fetal Neonatal Med. 2010;23:23–33. doi: 10.3109/14767050902994770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Kusanovic JP, Espinoza J, Romero R, et al. Plasma protein Z concentrations in pregnant women with idiopathic intrauterine bleeding and in women with spontaneous preterm labor. J Matern Fetal Neonatal Med. 2007;20:453–463. doi: 10.1080/14767050701398272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Macey MG, Bevan S, Alam S, et al. Platelet activation and endogenous thrombin potential in pre-eclampsia. Thromb Res. 2010;125:e76–e81. doi: 10.1016/j.thromres.2009.09.013. [DOI] [PubMed] [Google Scholar]
- 83.Gardiner C, Tannetta DS, Simms CA, Harrison P, Redman CW, Sargent IL. Syncytiotrophoblast microvesicles released from pre-eclampsia placentae exhibit increased tissue factor activity. PLoS One. 2011;6:e26313. doi: 10.1371/journal.pone.0026313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Rafik Hamad R, Curvers J, Berntorp E, Eriksson M, Bremme K. Increased thrombin generation in women with a history of preeclampsia. Thromb Res. 2009;123:580–586. doi: 10.1016/j.thromres.2008.03.022. [DOI] [PubMed] [Google Scholar]
- 85.Mastrolia SA, Novack L, Thachil J, et al. LMWH in the prevention of preeclampsia and fetal growth restriction in women without thrombophilia. A systematic review and meta-analysis. Thromb Haemost. 2016;116:868–878. doi: 10.1160/TH16-02-0169. [DOI] [PubMed] [Google Scholar]


