Abstract
Objectives
To assess the efficacy of mycophenolate mofetil (MMF) and cyclophosphamide (CYC) on the modified Rodnan skin score (mRSS) in participants enrolled in the Scleroderma Lung Study (SLS)-I and II.
Methods
SLS-I participants received daily oral CYC or matching placebo for one year, whereas SLS-II participants received daily MMF for 2 years or daily oral CYC for 1 year followed by placebo for second year. We assessed the impact of MMF and CYC on the mRSS in SLS-II over 24-month period. We also compared the change in mRSS in patients with diffuse cutaneous systemic sclerosis (dcSSc) assigned to CYC and MMF in SLS-II and SLS-I vs. placebo in SLS-I over a 24-month period using a linear mixed model.
Results
In SLS-II, the baseline (mean±SD) mRSS was 14.0±10.6 units for CYC and 15.3±10.4 units for MMF; 58.5% were classified as dcSSc. CYC and MMF were associated with statistically significant improvements in mRSS from baseline over the period of 24 months in dcSSc (p< 0.05 at each time point) but there were no differences between the two groups. In the dcSSc subgroup, the change in mRSS from baseline to all 6 month visits was similar in SLS-II groups— MMF, CYC, pooled cohort (MMF+ CYC) and SLS-I CYC groups and showed statistically significant improvements compared to SLS-I placebo at 12-, 18-, and 24-month period (p< 0.05).
Conclusions
In SLS-II, MMF and CYC resulted in improvements in mRSS in dcSSc over 24 months. In addition, MMF and CYC resulted in statistically significant improvements in mRSS in patients with dcSSc when compared with the SLS-I placebo group.
INTRODUCTION
Systemic sclerosis (SSc) is an autoimmune disease characterized by skin thickening and internal organ involvement. Skin thickening is a hallmark of SSc, which is present in approximately 90% of patients. The severity and distribution of skin thickening can be quantified using the modified Rodnan skin score (mRSS). mRSS meets the Outcome Measures of Rheumatology filters of truth, feasibility, and discrimination, and has been shown to differentiate potentially disease modifying drugs from placebo in randomized controlled trials(1–3).
Various immunosuppressive agents have been studied as potential disease-modifying therapies for skin thickening and interstitial lung disease in SSc. Methotrexate (MTX) was evaluated in two randomized, double-blinded, placebo-controlled studies in early diffuse cutaneous SSc (dcSSc), which demonstrated a trend towards statistically significant improvement in mRSS over a 12-month period with oral MTX and a significant improvement over a 24-week period with injectable MTX (4, 5). Two pivotal studies assessed cyclophosphamide (CYC) vs. placebo in SSc-associated ILD: Scleroderma Lung Study (SLS)-I and the Fibrosing Alveolitis in Scleroderma trial (FAST). Both studies demonstrated statistically significant or trends favoring efficacy in forced vital capacity percent predicted (FVC%) with either oral CYC for 1 year (6), or intravenous monthly infusions of CYC for 6 months followed by daily azathioprine for 6 additional months (7). The SLS-I trial also demonstrated a statistically significant difference in mRSS between the two groups (CYC vs. placebo) in participants with dcSSc over a 12-month period largely driven by the dcSSc group (6). In addition, the recently completed SLS-II study demonstrated that oral daily CYC over a one-year period, followed by a year on placebo, is equally effective in improving FVC% as daily mycophenolate mofetil (MMF) over a two-year period (8). In addition, improvement in mRSS was similar in the 2 groups. Furthermore, CYC and MMF have been assessed in several uncontrolled studies showing a beneficial effect on mRSS (9–13).
Although the SLS-I and II trials provided top-line results on the impact of CYC and MMF on mRSS, an in-depth analysis on the effect of CYC and MMF on mRSS has not been performed. With widespread use of immunosuppressives, especially MMF, for management of SSc skin involvement without evidence of its efficacy in a randomized controlled fashion, we evaluated the 2 patient-level data from the SLS-I and II to assess if CYC and MMF are superior to placebo for management of skin thickness. Therefore, our objectives for post-hoc analyses were to:
Assess the separate and comparative impact of each study drug in SLS-II (MMF and CYC) on the mRSS over 24 months, and
Compare the improvement in mRSS in the placebo arm of SLS-I arm and the CYC arm of SLS-I versus the CYC and MMF arms of SLS- II and MMF arm of SLS-II at 6, 12, 18, and 24 months.
PATIENTS AND METHODS
One hundred and fifty-eight participants with SSc-ILD were enrolled in SLS-I and 142 in SLS-II. SLS-I and II received IRB approval at each medical center and all participants signed an informed consent form. Both trials were registered with clinicaltrials.gov (NCT00004563 for SLS-I and NCT00883129 for SLS-II). Inclusion criteria for enrollment in both studies were similar and included: age over 18 years, duration of disease within 7 years from onset of the first non-Raynaud’s symptom of SSc, FVC% 40–85 %, DLCO ≥40 % predicted (or 30–39 % predicted in the absence of clinical evidence of pulmonary hypertension), and the evidence of any ground glass opacities and/or positive bronchoalveolar lavage (≥3 % neutrophils and/or ≥2 % eosinophils). In SLS-I, participants received daily oral CYC (≤2 mg per kilogram of body weight per day as tolerated) or matching placebo for one year, and were followed for an additional year (6). mRSS was assessed at baseline and then every 3 months up to 24 months. The mean absolute difference in the primary outcome measure, the adjusted 12-month FVC% between the CYC and placebo groups was 2.53%, favoring CYC (P<0.03) but the effect on FVC% dissipated at 24 months(14). There were also treatment-related differences in physiological and symptom outcomes at 12 months. There was a greater frequency of adverse events in the CYC group, but the difference between the two groups in the number of serious adverse events was not significant.
In SLS-II, participants received daily MMF (≤3 g daily as tolerated) for two years or daily oral CYC (≤2 mg per kilogram of body weight as tolerated) for one year, followed by placebo twice daily for an additional year(15). mRSS was assessed at baseline and then every 3 months up to 24 months. The adjusted FVC% improved from baseline to 24 months by 2.19% in the MMF group and 2.88% in the CYC group; the course of the FVC% did not differ significantly between the two treatment groups based on the pre-specified primary analysis (p=0·24). MMF was better tolerated than CYC with fewer patients on MMF than on CYC prematurely withdrawing from study drug.
Statistical analysis
Demographic and clinical characteristics were compared using Student’s t-test for continuous variables and the chi-square test for categorical variables. Change in mRSS was calculated as the difference between mRSS at baseline and at 6-, 12-, 18-, and 24-months; a linear mixed effects model with a random subject effect and fixed effects for group (SLS-I CYC, SLS-I placebo, SLS-II CYC, and SLS II MYC), month, the interaction between group and month, and baseline mRSS was used to predict change in mRSS. Stratified analyses were conducted by study (SLS-I vs. SLS-II), SSc subtype, and by treatment group. Minimum clinically important difference (MCID), the smallest difference in a measure or instrument of interest that is considered to be “worthwhile or important” to the patient, was evaluated in the dcSSc subset and defined as change in the mRSS of ≥ 5.0 units (16).
Missing data was handled by the linear mixed model and the results were considered significant with a p ≤ 0.05, and all statistical analyses were performed using SAS version 9.4.
RESULTS
Patient Characteristics
A total of 158 participants were enrolled in SLS- I, and of those 145 participants (91.8 percent), including 73 in the CYC and 72 in the placebo subgroups, were evaluated for the primary outcome. In SLS- II, at baseline the total cohort included 142 participants (lcSSc: 33 CYC and 26 MMF; dcSSc: 40 CYC and 43 MMF). Total cohort was defined as combined SLS-I and II participants. Participants enrolled in the SLS-II trial were significantly older than SLS-I (Mean age± SD: 52.3 ±9.7 years vs. 48.5 ±12.3 years; p = 0.004; Supplementary Table S1). The percentage of participants classified as dcSSc and limited cutaneous SSc (lcSSc) were comparable in both trials (59% dcSSc vs. 41% lcSSc each in SLS-I and II). The baseline mean disease duration (defined as the first sign or symptom other than Raynaud’s phenomenon) was statistically shorter in SLS-II compared to SLS-I (Mean ± SD: 2.6 ±1.8 years vs. 3.2 ±2.1 years; p = 0.01), and a greater percentage of participants had a disease duration ≤ 24 months in SLS-II (N, %: 70, 50%) vs. the SLS-I (53, 33%) (p = 0.003). Baseline mRSS score was comparable in both trials: 14.8 ±10.9 in SLS-I and 14.7 ±10.5 in SLS-II (p = 0.89). In participants classified as dcSSc, the baseline mRSS in SLS-I vs. SLS-II trials were similar (Mean ± SD: 21.0 ±9.8 in SLS-I vs. 20.8 ±9.4 in SLS-II; p = 0.85; Supplementary Table S1).
Impact of MMF and CYC on mRSS in SLS-II over 24 months
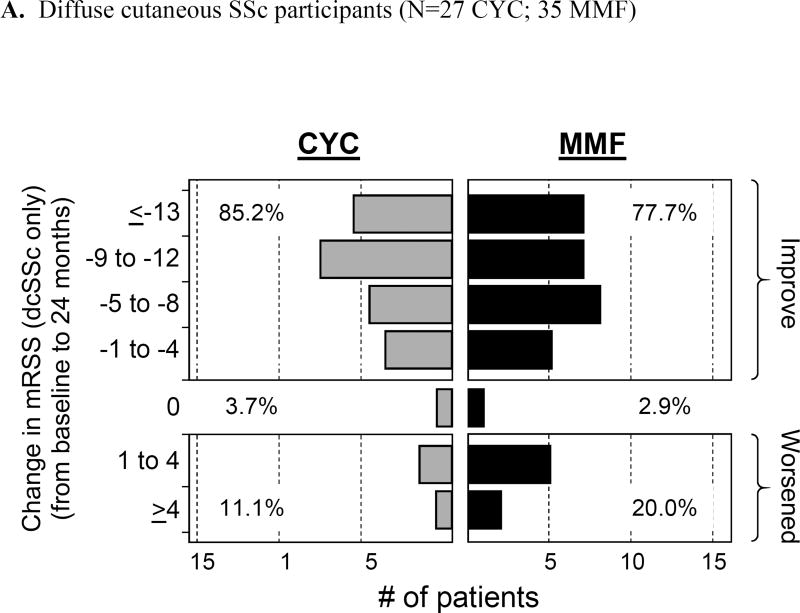
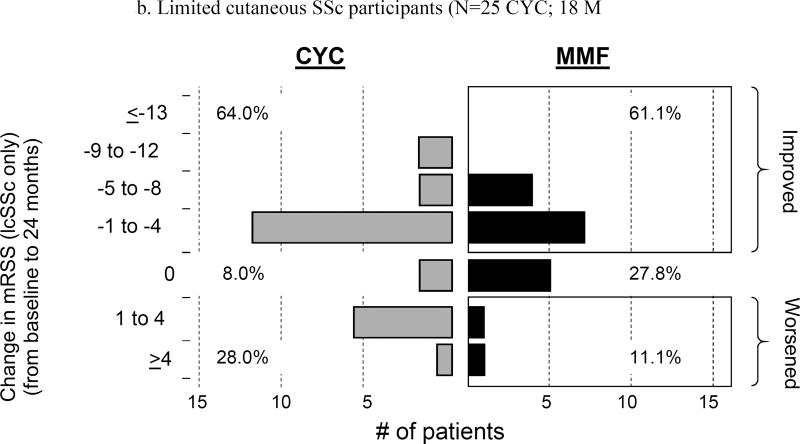
In the SLS-II participants, we compared mRSS scores at 6, 12, 18, and 24 months with those at baseline. Mean baseline mRSS scores were similar for CYC and MMF (14.0±10.6 units and 15.3±10.4 units, respectively). In lcSSc, the mean mRSS was 5.8 ±3.6 units and in the dcSSc it was 20.9 ±9.6 units at baseline. Using observed data, there was a statistically significant decline [improvement] in mRSS at all follow-up visits compared to baseline (p ≤ 0.05) both for the dcSSc and lcSSc subgroups combined and the dcSSc subgroup separately, but there was no difference between the 2 treatment arms at any follow-up evaluation (Figure 1). There was also a trend for improvement in the lcSSc subgroup over a 24-month period, but this did not achieve statistical significance (data not shown). The frequency distribution of observed skin changes at 24 months from baseline showed an improvement in the mRSS scores in each cutaneous subgroup: lcSSc (CYC: 64% and MMF: 61.1% improvement); and dcSSc (CYC: 85.2% and MMF: 77.7% improvement), (Figure 2 A and B).
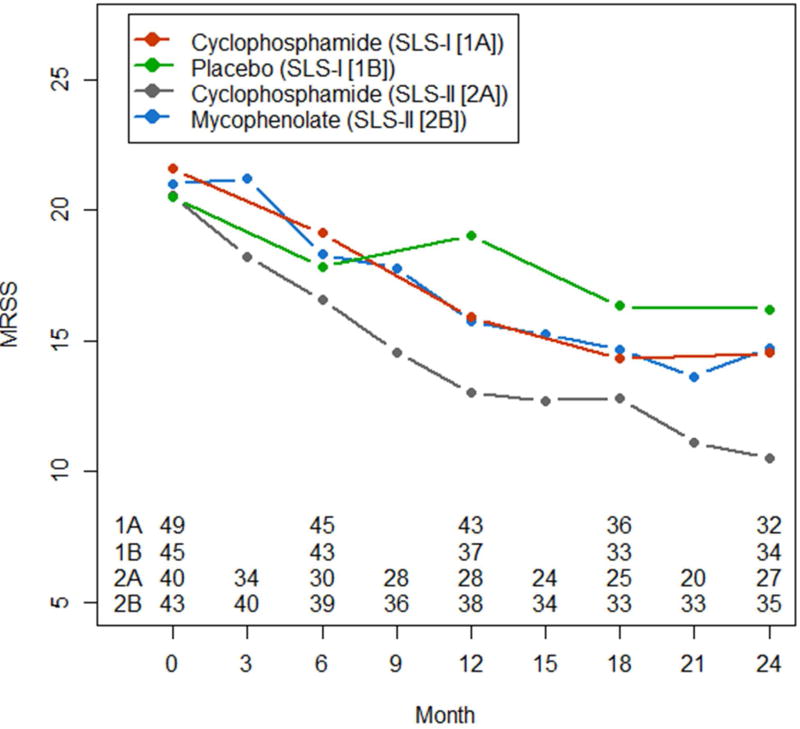
Figure 1.
Course of modified Rodnan Skin Score (MRSS; in absolute values) in diffuse cutaneous systemic sclerosis over 24-month period in participants assigned to Placebo, SLS-I(CYC), SLS- II(CYC) and SLS-II (MMF) using the observed data. The mRSS was assessed every 3 months in the SLS-II and every 6 months in the SLS-1. P <0.05 at each 12, 18, and 24 months between placebo groups vs. others whereas the p was ≥0.05 for other treatments at each time points.
Figure 2.
a. Frequency distribution of observed absolute changes at 24 months from baseline in modified Rodnan Skin Score (mRSS) in both limited and diffuse cutaneous SSc participants (N= 52 CYC; 53 MMF)
Comparing the improvement in mRSS in the SLS-I vs. SLS-II cohorts at 6, 12, 18 and 24 months
No significant differences in baseline mRSS were found in comparisons of dcSSc participants in the CYC arm of SLS-I (21.6), the pooled MMF and CYC arms of SLS-II (20.8), the pooled CYC arms from SLS-I and II (21.1), the MMF arm of SLS-II (21.0) and the placebo arm of SLS I (20.4; Table 1). Using the linear mixed model, the changes from baseline in mRSS at 6-, 12-, 18- and 24-month periods were statistically significant within each these groups singly and pooled (p≤0.05 for each comparison). In addition, no significant differences in the changes in mRSS from baseline at 6-, 12-, 18-, and 24- month periods were noted between the pooled CYC and MMF arms of SLS-II and the CYC arm of SLS-I (p≥0.05) but the mRSS was statistically different and improved in the treatments groups vs. placebo at 12, 18, and 24 months (p< 0.05) (Table 1 and Figure 1).
Table1.
Estimated mean changes in modified Rodnan Skin Score from baseline at 6-, 12-, 18-, and 24- months in SLS-I and II in dcSSc.
| SLS-I Placebo group |
SLS-I CYC group |
SLS-I and SLS-II CYC |
SLS-II MMF |
SLS-II Pooled data (CYC+ MMF) |
|||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| N | Mean (SE) | % Change |
N | Mean (SE)) | % Change |
N | Mean (SE) | % Change |
N | Mean (SE)) | % Change |
N | Mean (SE) | % Change |
|
| Baseline | 46 | 20.4 (9.4) | NA | 49 | 21.6 (10.3) | NA | 89 | 21.1 (9.7) | NA | 43 | 21.0 (8.5) | NA | 83 | 20.8 (9.4) | NA |
| 6 month | 43 | −2.6 (1.0)† | 12.74 | 45 | −2.6 (1.0)† | 12.5 | 75 | −2.6 (0.8)† | 12.32 | 39 | −2.5 (1.1)†* | 12.86 | 69 | −2.5 (0.8)† | 11.53 |
| 12 month | 37 | −1.5 (1.1) | 8.33 | 43 | −5.1 (1.0)*† | 24.53 | 72 | −5.4 (0.8)†* | 25.59 | 38 | −5.1 (1.1)†* | 24.29 | 66 | −5.4 (0.8)†* | 26.44 |
| 18 month | 33 | −3.2 (1.1)† | 16.66 | 36 | −5.7 (1.1) *† | 31.94 | 60 | −6.0 (0.8)†* | 31.27 | 33 | −6.1 (1.1)†* | 30.95 | 58 | −6.2 (0.9) †* | 30.76 |
| 24 month | 34 | −3.7 (1.1)† | 19.11 | 32 | −6.3 (1.1) †* | 33.33 | 59 | −7.2 (0.8)†* | 33.64 | 35 | −6.4 (1.1) †* | 30.00 | 62 | −7.3 (0.8) †* | 33.65 |
P< 0.05 for mRSS at follow up vs. baseline within each group.
P< 0.05 for active treatment groups compared to SLS-1 Placebo group.
Relative change (percentage) of observed (not-modeled) mRSS from baseline after 6, 12, 18 and 24 months of treatment. NA=Not applicable
Comparison of mRSS in SLS-I placebo vs. SLS-I CYC and SLS-II CYC and MMF in dcSSc at 12 months
mRSS improvements exceeding the MCID (≥ 5.0 units) were observed in 40% of the participants in the CYC arm of SLS-I, 37% of the participants in the pooled CYC and MMF arms of SLS-II and 38% of the participants in the MMF arm of SLS-II compared to 25% of the participants in the placebo arm of SLS-I. Conversely, worse scores that exceeded the MCID for mRSS were found in only 7% of participants in the CYC arm of SLS-I, 4% of the participants in the pooled CYC and MMF arms of SLS-II and 4% of the participants in the MMF arm of SLS-II, in contrast to 16% of participants in the placebo arm of SLS-I (p=0.009), Table 2.
Table 2.
Comparison of modified Rodnan Skin Score (mRSS) in Scleroderma Lung Study (SLS)- I (placebo) vs. SLS-I (CYC), SLS-II (CYC+MMF pooled) and SLS-II (MMF) in diffuse cutaneous systemic sclerosis at 12 months utilizing minimum clinically important difference (MCID) which is defined as ≥ 5units improvement and ≥ 5units worsening in mRSS.
| N | mRSS improvement (≥5.0 units)(%) |
mRSS worsening (≥5.0 units)(%) |
mRSS change between −5.0 and +5.0, % |
|
|---|---|---|---|---|
| SLS-I (Placebo group) | 63 | 25% | 16% | 59% |
| SLS-I (CYC group) | 68 | 40% | 7% | 53% |
| SLS-II (pooled group) | 113 | 37% | 4% | 59% |
| SLS-II (MMF group) | 58 | 38% | 4% | 59% |
DISCUSSION
Skin thickness is a surrogate for disease severity in patients with dcSSc, and is associated with increased risk of internal organ involvement and mortality (17). The mRSS is a feasible, reliable and valid measure of skin thickness that has been used as the primary outcome measure in clinical trials of SSc (3, 18). Herein, we utilized data from 2 RCTs to study the efficacy of CYC (in SLS-I and SLS-II) and MMF (in SLS-II) on mRSS in comparison with placebo (in SLS-I). In addition, we compared responses to these two active agents between patients with dcSSc and those with lcSSc subsets. We showed that both CYC and MMF led to clinically meaningful improvements in mRSS in dcSSc, and the improvements were significantly larger than those observed in the placebo arm. Our data support the role of oral CYC and MMF not only for SSc-ILD, but also for skin improvement in participants with dcSSc.
Previous uncontrolled studies have evaluated both CYC and MMF in dcSSc. Improvement in mRSS have been demonstrated by a combination of either intravenous or oral CYC (≤2 mg/ kg daily for 12 months and then maintained on ≤1 mg/kg daily), and prednisone over a 12-month period, compared to participants who received azathioprine (2.5 mg/kg daily for 12 months and then maintained on 2 mg/kg daily) in open label studies (9–11). The effectiveness of MMF in dcSSc was retrospectively investigated in a large UK cohort and was shown to be associated with an improved 5-year survival compared to other immunosuppressive therapies, whereas no significant differences in mRSS outcome were noted between those patients receiving MMF and those treated with other standard immunosuppressive therapies: anti-thymocyte globulin (32.1%), azathioprine (18.3%), IV CYC and MTX (14.7% each) (12). The effectiveness of MMF on dcSSc was further studied in a US Scleroderma center (13), and the change in mRSS from baseline was calculated at 3-month intervals up to 12 months. The results were compared to those observed in a historical control group derived, from a pooled analysis of three large multicenter-randomized clinical trials (1). A significant improvement in mRSS compared with baseline was detected at 6 and 9 months, and this effect was maintained throughout the 12-month follow-up period. There was no statistical significance achieved at 6 months in mRSS between MMF and the historical controls (MMF −3.05±7.4 vs recombinant relaxin −4.83±6.99, p=0.059), but was significantly lower at 12 months (MMF −7.59±10.1 vs D-penicillamine −2.47±8.6, p<0.001 and vs oral collagen −3.4±7.12, p=0.002) (13).
Our current post-hoc analysis supports the results of case series and uncontrolled trials showing that both CYC and MMF are efficacious in early dcSSc, and that MMF appears to be better tolerated than oral CYC (8), findings that further support the increasing use of MMF for the management of SSc (12, 19). However, the choice of the therapy depends on physician preferences and resources available in each health care system. In addition, significant improvement in mRSS compared to baseline was observed mainly beyond 6 months of treatment, an important point to consider when designing a clinical trial in SSc as shorter trial duration can result in a negative result using the traditional immunosuppressives. This may not be applicable for novel targeted therapeutics.
Our study has several strengths. It utilized 2 large SSc RCTs in which mRSS measurements were captured at regular intervals and performed by experienced researchers in SSc.
The study is not without limitations. First, our study is the pooled, post-hoc analysis. Both studies were designed primarily to evaluate the impact of treatment on ILD in patients with SSc-ILD, and only secondarily to assess the effect of therapy on mRSS. Second, there were missing data, and a few of the participants did not have mRSS measurements at each follow-up. However, we used linear mixed model to account for this.
In conclusion, our data further support the role of MMF and CYC in the improvement in skin thickness in patients with SSc. In the SLS-II trial, 2 years of daily MMF and 1-year of CYC were each associated with clinically meaningful and statistically significant improvements in mRSS vs. placebo arm in patients with dcSSc over a 24-month period.
Supplementary Material
Significance & Innovation.
Treatment of scleroderma-related interstitial lung disease with mycophenolate mofetil for 2 years or cyclophosphamide for 1 year in the diffuse cutaneous subset of the participants in two randomized controlled trials resulted in statistically significant improvements in skin thickness.
Acknowledgments
Acknowledgements and Funding
Drs Khanna, Wilhalme, and Tseng were funded by K24 NIH/NIAMS AR063120 (to Dr. Khanna).
SLS-I was supported by grants from the National Institutes of Health (U01 HL60587, U01 HL60606, and R01 HL089758) and SLS-II was supported by grants from the NHLBI/NIH: R01 HL089758 and R01 HL089901. Study drug (cyclophosphamide) was supplied by Bristol-Myers Squibb for use in SLS I and the study drug (mycophenolate) and matching placebo were supplied at no charge through Drug Supply Grant # CEL539 from Hoffmann-La Roche/Genentech in SLS II.
The following people and institutions participated in the Scleroderma Lung Study I: University of Michigan, Ann Arbor: D. Khanna; University of California at Los Angeles (UCLA), Los Angeles: P. J. Clements, D. P. Tashkin, R. Elashoff, J. Goldin, M. Roth, D. Furst, K. Bulpitt, W.-L. J. Chung, S. Viasco, M. Sterz, L. Woolcock, X. Yan, J. Ho, S. Vasunilashorn, and I. da Costa; University of Medicine and Dentistry of New Jersey, New Brunswick: J. R. Seibold, D. J. Riley, J. K. Amorosa, V. M. Hsu, D. A. McCloskey, and J. E. Wilson; University of Illinois at Chicago, Chicago: J. Varga, D. Schraufnagel, A. Wilbur, D. Lapota, S. Arami, and P. Cole-Saffold; Boston University, Boston, MA: R. Simms, A. Theodore, P. Clarke, J. Korn, K. Tobin, and M. Nuite; Medical University of South Carolina, Charleston: R. Silver, M. Bolster, C. Strange, S. Schabel, E. Smith, J. Arnold, K. Caldwell, and M. Bonner; The Johns Hopkins University School of Medicine, Baltimore, MD: R. Wise, F. Wigley, B. White, L. Hummers, M. Bohlman, A. Polito, G. Leatherman, E. Forbes, and M. Daniel; Georgetown University, Washington, DC: V. Steen, C. Read, C. Cooper, S. Wheaton, A. Carey, and A. Ortiz; University of Texas at Houston, Houston: M. Mayes, E. Parsley, S. Oldham, T. Filemon, S. Jordan, and M. Perry; University of California at San Francisco, San Francisco: K. Connolly, J. Golden, P. Wolters, R. Webb, J. Davis, C. Antolos, and C. Maynetto; University of Alabama at Birmingham, Birmingham: B. Fessler, M. Olman, C. Sanders, L. Heck, and T. Parkhill; University of Connecticut Health Center, Farmington: N. Rothfield, M. Metersky, R. Cobb, M. Aberles, F. Ingenito, and E. Breen; Wayne State University, Detroit, MI: M. Mayes, K. Mubarak, J. L. Granda, J. Silva, Z. Injic, and R. Alexander; Virginia Mason Research Center, Seattle, WA: D. Furst, S. Springmeyer, S. Kirkland, J. Molitor, R. Hinke and A. Mondt; Data Safety and Monitoring Board: Harvard Medical School, Boston, MA: T. Thompson; Veterans Affairs Medical Center, Brown University, Providence, RI: S. Rounds; Cedars Sinai Medical Center–UCLA Health System, Los Angeles, CA: M. Weinstein; Clinical Trials & Surveys, Baltimore, MD: B. Thompson; Mortality and Morbidity Review Committee: University of California, Los Angeles: H. Paulus and S. Levy; The Johns Hopkins University, Baltimore, MD: D. Martin.
The Scleroderma Lung Study II Research Group includes the authors listed in the mast head and the following support staff: E. Kissin, F.Y. Cheong (Boston University); G, Marlis, J. Mason-Berry, P. Saffold, M. Rodriguez, L. Guzman, J. Brook, G. Ibrahim, K. Largaespada (UCLA); C. Fridley, M. Zulmastashvili, A. Manu, S. Moore (Georgetown University); L. Hummers, G. Leatherman (Johns Hopkins University); F.N. Hant, K. Gibson (Medical University of South Carolina); M. Morrison (National Jewish Health); H. Donnelly, C. Marlin, J. Gangar (Northwestern University); D.A. McCloskey (Rutgers University); A. Eller, D. Leong, M. Lalosh, J. Obata (UCSF); S. Arami, D. Franklin (University of Illinois); E. Schiopu, M. Benedict-Blue, V. Leone, J. Shaw (University of Michigan); F. Tan, M. Perry, J. Anderson, A Saulino (University of Texas, Houston); P. Carey, M. Esplin (University of Utah); P. Carlson (University of Minnesota).
References
- 1.Amjadi S, Maranian P, Furst DE, Clements PJ, Wong WK, Postlethwaite AE, et al. Course of the modified Rodnan skin thickness score in systemic sclerosis clinical trials: analysis of three large multicenter, double-blind, randomized controlled trials. Arthritis and rheumatism. 2009;60(8):2490–8. doi: 10.1002/art.24681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Clements P, Lachenbruch P, Siebold J, White B, Weiner S, Martin R, et al. Inter and intraobserver variability of total skin thickness score (modified Rodnan TSS) in systemic sclerosis. J Rheumatol. 1995;22(7):1281–5. [PubMed] [Google Scholar]
- 3.Khanna D, Denton CP, Jahreis A, van Laar JM, Frech TM, Anderson ME, et al. Safety and efficacy of subcutaneous tocilizumab in adults with systemic sclerosis (faSScinate): a phase 2, randomised, controlled trial. Lancet. 2016 doi: 10.1016/S0140-6736(16)00232-4. [DOI] [PubMed] [Google Scholar]
- 4.Pope JE, Bellamy N, Seibold JR, Baron M, Ellman M, Carette S, et al. A randomized, controlled trial of methotrexate versus placebo in early diffuse scleroderma. Arthritis and rheumatism. 2001;44(6):1351–8. doi: 10.1002/1529-0131(200106)44:6<1351::AID-ART227>3.0.CO;2-I. [DOI] [PubMed] [Google Scholar]
- 5.van den Hoogen FH, Boerbooms AM, Swaak AJ, Rasker JJ, van Lier HJ, van de Putte LB. Comparison of methotrexate with placebo in the treatment of systemic sclerosis: a 24 week randomized double-blind trial, followed by a 24 week observational trial. British journal of rheumatology. 1996;35(4):364–72. doi: 10.1093/rheumatology/35.4.364. [DOI] [PubMed] [Google Scholar]
- 6.Tashkin DP, Elashoff R, Clements PJ, Goldin J, Roth MD, Furst DE, et al. Cyclophosphamide versus placebo in scleroderma lung disease. N Engl J Med. 2006;354(25):2655–66. doi: 10.1056/NEJMoa055120. [DOI] [PubMed] [Google Scholar]
- 7.Hoyles RK, Ellis RW, Wellsbury J, Lees B, Newlands P, Goh NS, et al. A multicenter, prospective, randomized, double-blind, placebo-controlled trial of corticosteroids and intravenous cyclophosphamide followed by oral azathioprine for the treatment of pulmonary fibrosis in scleroderma. Arthritis and rheumatism. 2006;54(12):3962–70. doi: 10.1002/art.22204. [DOI] [PubMed] [Google Scholar]
- 8.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. Lancet Respir Med. 2016 doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Domiciano DS, Bonfa E, Borges CT, Kairalla RA, Capelozzi VL, Parra E, et al. A long-term prospective randomized controlled study of non-specific interstitial pneumonia (NSIP) treatment in scleroderma. Clin Rheumatol. 2011;30(2):223–9. doi: 10.1007/s10067-010-1493-4. [DOI] [PubMed] [Google Scholar]
- 10.Pakas I, Ioannidis JP, Malagari K, Skopouli FN, Moutsopoulos HM, Vlachoyiannopoulos PG. Cyclophosphamide with low or high dose prednisolone for systemic sclerosis lung disease. J Rheumatol. 2002;29(2):298–304. [PubMed] [Google Scholar]
- 11.Nadashkevich O, Davis P, Fritzler M, Kovalenko W. A randomized unblinded trial of cyclophosphamide versus azathioprine in the treatment of systemic sclerosis. Clin Rheumatol. 2006;25(2):205–12. doi: 10.1007/s10067-005-1157-y. [DOI] [PubMed] [Google Scholar]
- 12.Nihtyanova SI, Brough GM, Black CM, Denton CP. Mycophenolate mofetil in diffuse cutaneous systemic sclerosis--a retrospective analysis. Rheumatology (Oxford) 2007;46(3):442–5. doi: 10.1093/rheumatology/kel244. [DOI] [PubMed] [Google Scholar]
- 13.Le EN, Wigley FM, Shah AA, Boin F, Hummers LK. Long-term experience of mycophenolate mofetil for treatment of diffuse cutaneous systemic sclerosis. Ann Rheum Dis. 2011;70(6):1104–7. doi: 10.1136/ard.2010.142000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Tashkin DP, Elashoff R, Clements PJ, Roth MD, Furst DE, Silver RM, et al. Effects of 1-year treatment with cyclophosphamide on outcomes at 2 years in scleroderma lung disease. American journal of respiratory and critical care medicine. 2007;176(10):1026–34. doi: 10.1164/rccm.200702-326OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tashkin DP, Roth MD, Clements PJ, Furst DE, Khanna D, Kleerup EC, et al. Mycophenolate mofetil versus oral cyclophosphamide in scleroderma-related interstitial lung disease (SLS II): a randomised controlled, double-blind, parallel group trial. The Lancet Respiratory medicine. 2016;4(9):708–19. doi: 10.1016/S2213-2600(16)30152-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna D, Furst DE, Hays RD, Park GS, Wong WK, Seibold JR, et al. Minimally important difference in diffuse systemic sclerosis: results from the D-penicillamine study. Annals of the rheumatic diseases. 2006;65(10):1325–9. doi: 10.1136/ard.2005.050187. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Clements PJ, Hurwitz EL, Wong WK, Seibold JR, Mayes M, White B, et al. Skin thickness score as a predictor and correlate of outcome in systemic sclerosis: high-dose versus low-dose penicillamine trial. Arthritis and rheumatism. 2000;43(11):2445–54. doi: 10.1002/1529-0131(200011)43:11<2445::AID-ANR11>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 18.Khanna D, Merkel PA. Outcome measures in systemic sclerosis: an update on instruments and current research. Current rheumatology reports. 2007;9(2):151–7. doi: 10.1007/s11926-007-0010-5. [DOI] [PubMed] [Google Scholar]
- 19.Nagaraja V, Denton CP, Khanna D. Old medications and new targeted therapies in systemic sclerosis. Rheumatology. 2015;54(11):1944–53. doi: 10.1093/rheumatology/keu285. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.