Abstract
Melanoma is the most aggressive form of skin cancer and incidences continue to rise worldwide. 18F-FDG PET imaging has transformed diagnostic nuclear medicine and become an essential component in the management of melanoma, but still has its drawbacks. With the rapid growth in the field of nuclear medicine and molecular imaging, a variety of promising probes that enable early diagnosis and detection of melanoma have been developed. The substantial preclinical success of melanin- and peptide-based probes has recently resulted in translation of several radiotracers to clinical settings for noninvasive imaging and/or treatment of melanoma in human patients. In this review, we have focused on the latest developments in radiolabelled molecular imaging probes for melanoma in preclinical and clinical settings and discussed the challenges and opportunities for future development.
Keywords: Melanoma, molecular imaging, positron emission tomography (PET), single photon emission computer tomography (SPECT), cancer, theranostics, immunotherapy
1. Introduction
Although melanoma accounts for a small percentage of all skin cancer cases, it is estimated that melanoma accounted for 76,380 new cases and 10,130 deaths in the United States in 2016 [1]. Unsatisfactory 5-year survival rates for patients with distant metastases (less than 10%) render early detection and accurate assessment of metastatic melanoma crucial for improved outcome and disease-free survival [2]. With the gradual increase in understanding of the molecular pathogenesis of melanoma, the treatment landscape for advanced melanoma has changed markedly during the past 10 years. Novel agents such as molecularly-targeted therapies specifically inhibiting carcinogenic pathways and immunotherapies augmenting the antitumor immunity have emerged as new standards of care for patients with melanoma [3].
As elegantly reviewed by Wong et al, 18F-fluorodeoxyglucose (FDG) positron emission tomography (PET) could play a pivotal role in the interpretation of therapeutic response following BRAF inhibition and immunotherapy in patients with melanoma, facilitating early assessment of drug resistance and detection of life-threatening autoimmune side effects [4]. 18F-FDG PET was also recommended for staging and detecting recurrent melanoma [5, 6]. However, since 18F-FDG PET monitors cellular metabolism and immunotherapy elicits a natural inflammatory response, traditional PET imaging using 18F-FDG has proven inadequate in examining responses to immunotherapy in certain cancer types [7]. Moreover, owing to increased glucose metabolism in inflammatory tissues, 18F-FDG displays relatively poor selectivity for distinguishing tumor from inflammatory tissue. Importantly, in one study, 18F-FDG PET failed to detect melanoma in patients showing a positive sentinel lymph node biopsy for cancer, and no recurrent melanoma occurred where the 18F-FDG PET scans were positive or suspicious [8]. 18F-FDG PET scans also failed to detect pulmonary and brain metastases, indicating its limited value when staging patients with more advanced melanoma [9]. Currently, gadolinium-enhanced MRI is the most sensitive and reproducible method available to measure brain metastases or to assess treatment response [10, 11].
In the era of precision medicine and molecular imaging [12], many radiolabeled probes for imaging different molecular targets or biochemical processes have been designed and evaluated for melanoma imaging. Although clinically-available 111In-DOTA-lanreotide and 111In-DOTA-Tyr3-octreotide may image melanoma, the detection rates may not meet the clinical requirement and their mechanisms remain unknown [13]. In the past, we and others organized reviews regarding anatomical and molecular imaging of skin cancer [14, 15]; since then, substantial amounts of molecular imaging probes for melanoma have been developed. Although nanoparticle-based multimodality imaging systems have been intensively applied to melanoma detection and therapy, it is beyond the scope of our current paper. Therefore, with an emphasis on the small molecule- and peptide- based PET imaging probes, we aim to systematically review the recent advances in the field and extend an outlook on future development. Given the multidisciplinary nature of this field, our present review is by no means exhaustive, but we intend to summarize the most recent advances for scientists to further refine these probes and for clinicians to push the clinical transition of those which are promsing, facilitating better management of patients with melanoma in the foreseeable future.
2. Small Molecule-based Probes Targeting Melanin
Melanin, an amorphous, irregular, functional biopolymer and a ubiquitous natural pigment in many organs including human skin, is a source of novel research opportunities in the fields of biomedicine, nanotechnology, and materials science [16]. In malignant melanoma, melanin formation is highly increased because tyrosinase activity is significantly elevated [17, 18], making it a very attractive theranostic target. Many drugs, including methylene blue (MTB), chloroquine, acridine orange, benzamide (BZA) and its analogs, and other aromatic compounds, have been found to bind to melanin both in vivo and in vitro [19–24]. Melanin-targeting prodrugs also have been developed and used for in vivo melanoma imaging [25, 26].
2.1. Radiolabeled benzamide and benzamide derivatives
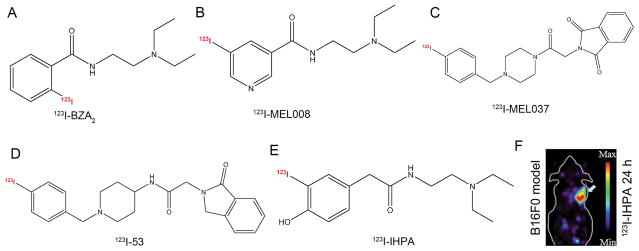
Various versions of radiolabeled benzamide and its analogs have been developed for targeting melanin in clinical practice [27–33]. Specifically, as evidenced by a recent prospective and multicenter phase III clinical study, 123I-BZA2 (Fig. 1A) [30, 34], a benzamide derivative able to bind to melanin pigment in melanoma cells, had statistically higher specificity than 18F-FDG for diagnosis of melanoma metastases in a lesion-based analysis [35]. Katsifis and co-authors substituted the benzamide moiety with a nicotinamide moiety and labeled the compound with no-carrier-added 123I-iodine via iododestannylation reactions. Biodistribution showed that 66% of the injected 123I-1 (123I-MEL008, Fig. 1B) was excreted from the urinary system at 1 h postinjection, also displaying the highest tumor uptake at that time point mainly because of its enhanced hydrophilicity and rapid clearance via renal excretion [36]. Chezal et al. then examined biological properties of aromatic or heteroaromatic BZA analogs in B16 melanoma-bearing mice and found that melanin-specific binding compound ICF01012, when labeled with 131I or 125I, could be applied in radionuclide diagnosis and therapy of disseminated melanoma [20, 37–39]. Furthermore, the authors developed a new multimodal approach by designing iodinated and fluorinated analogs of ICF01012, of which 18F-8 emerged as the most promising compound and demonstrated high tumor uptake, high contrast, and rapid clearance [40]. These above-mentioned efforts also led to the development of 123I-MEL037 and 123I-53 (Fig. 1C, D); while the former demonstrated high and prolonged tumor uptake, the latter was derived from structural modification of 123I-MEL037 and performed even better than 123I-ICF01012, as the tumor-to-background uptake ratios of 123I-53 and 123I-ICF01012 were 31.9 and 18.5, respectively [41, 42]. While the above probes accumulated at high levels in the eyes and thyroid, radioiodinated iochlonicotinamide and radioiodinated phenylacetamides (131I-IHPA and 131I-IHPP) had lower uptake in most normal organs and highly specific uptake in the melanotic tumor (Fig. 1E, F) [43, 44].
Figure 1.
Representative melanin-targeting probes. Chemical structures of (A) 123I-BZA2, (B) 123I-MEL008, (C) 123I-MEL037, (D) 123I-53, and (E) 131I-IHPA. (F) MicroSPECT image of C57BL/6 mice bearing B16/F0 melanotic melanoma at 24 h postinjection of approximately 11.1 MBq of 123I-IHPA, the tumor is indicated by the white arrow. Adapted and modified with permission from references [34, 36, 41, 42, 44].
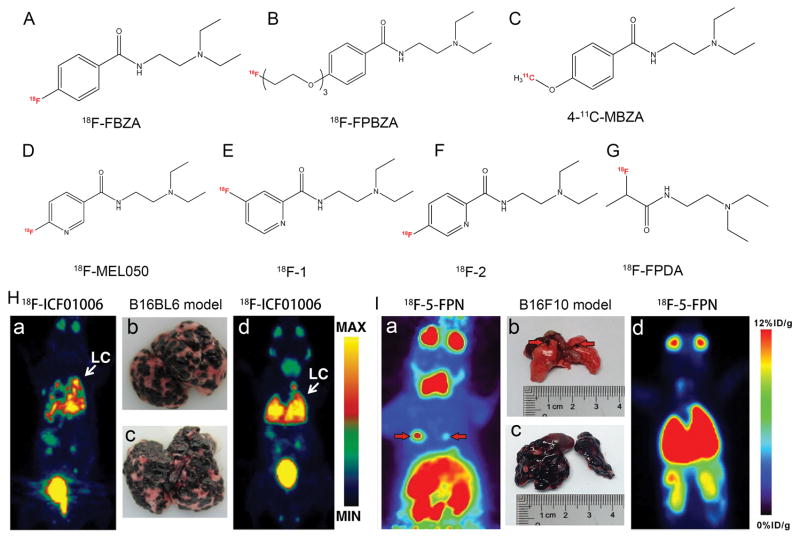
Since 18F-FBZA exhibited high tumor uptake and emerged as a promising candidate for clinical study (Fig. 2A) [45], Wu et al. modified the phenol moiety of benzamide with a short chain PEG and then labeled with 18F to obtain 18F-FPBZA (Fig. 2B). In vivo, it showed excellent tumor-to-background contrast, and this radiotracer was able to distinguish tumor from inflammatory tissue as turpentine-induced inflammation revealed low radioactivity accumulation [46]. Garg and co-authors developed 4-11C-MBZA and reported that this probe displayed advantages over its 18F analogs while delivering a lower radiation dose to the subject (Fig. 2C). In vitro binding studies showed specific binding of 4-11C-MBZA to B16/F1 cells; however, it also accumulated to a high level in the kidneys [47]. Chang et al. developed 18F–NOTA–BZA, and the melanin-specific binding ability, low bone uptake, sustained tumor retention, high hydrophilicity (log P =−1.96), fast normal tissue clearance and low radiation burden indicated that 18F–NOTA–BZA is a promising PET probe for melanin-specific imaging of melanin-positive melanoma [48]. Based on the inspiring and promising preclinical results of 18F-MEL050 (Fig. 2D) [49, 50], Liu et al. successfully synthesized a series of 18F-MEL050 analogs for melanoma imaging (Fig. 2E, F), of which 18F-2 showed superior tumor-targeting efficacy and imaging properties [51]. Interestingly, recent research proposed that N-(2-diethylaminoethyl) rather than the aromatic ring structure in benzamide analogs is a plausible pharmacophore responsible for melanin targeting, as the synthesized probe 18F-FPDA exhibited relatively high B16/F10 tumor-targeting efficacy and favorable in vivo pharmacokinetics (Fig. 2G) [52]. Besides the most commonly used PET radionuclides, 68Ga (t1/2 = 68 min) is an outstanding radioisotope for molecular imaging due to its significant 89% positron yield and its availability without the establishment of expensive cyclotron and synthesis models [53]. Trencsényi et al. conjugated PCA with two different chelators, HBED-CC and NODAGA, then labeled the compounds using Ga-68 and investigated the diagnostic value of 68Ga-HBED-CC-PCA and 68Ga-NODAGA-PCA in vitro and in vivo. The authors found that uptake of 68Ga-NODAGA-PCA by melanin-containing melanoma was significantly higher than the accumulation of the 68Ga-HBED-CC-conjugated PCA [54, 55].
Figure 2.
Representative melanin-targeting probes and in vivo study images. Chemical structures of (A) 18F-FBZA, (B) 18F-FPBZA, (C) 4-11C-MBZA, (D) 18F-MEL050, (E) 18F-1, (F) 18F-2, and (G) 18F-FPDA. (H) Whole-body maximum intensity projection (MIP) images of 18F-ICF01006 and corresponding lung photographs of B16/BL6 melanoma-bearing mice at the early stage (a, b) and late stage (c, d) of tumor development. (I) 18F-5-FPN PET images of two mice with lung metastases from melanoma. Note that this probe was able to detect both micrometastases (a, b) and wide spread lung metastases (c, d) from melanoma. Tumors are indicated by red arrows. Adapted and modified with permission from references [45–47, 49, 51, 52, 66, 67].
2.2. Imaging of melanoma metastases
Considering that the presence of distant metastases, especially brain metastases, confers worse prognosis for patients with melanoma, their early detection is critical [56]. In a study comparing diagnostic values of 18F-FDG PET/CT and MRI in melanoma patients with palpable lymph node metastases, Aukema et al. found that 18F-FDG PET/CT changed the intended regional node dissection in 26 patients (37%) and resulted in a superior diagnostic accuracy of 93%, but missed 5 patients with brain metastases which were detected by MRI [57]. Other study also demonstrated that 18F-FDG PET failed to detect metastatic lesions of less than 1 cm located in the lung, liver or brain [58]. Currently only contrast-enhanced MRI and 18F-FET PET seem to be reliable methods to detect brain metastases from melanoma but still lack specificity [10, 59]. Moreover, in patients with surgically treatable IIIC and IV metastatic melanoma following targeted/immunotherapy, PET/CT can detect unexpected metastases that are missed with conventional imaging, and can be considered as part of preoperative workup [4, 60, 61]. Thus it is of great importance to develop novel radiotracers to identify occult lesions or distant small metastases from melanoma with high specificity and a low false positive rate. Notably, the ability of an imaging agent to cross the blood–brain barrier (BBB) is considered critical to effectively target metastatic lesions in the brain. Of the reported probes, 4-11C-MBZA was able to cross the BBB and the corresponding uptake was moderate in the normal brain [47]. As observed from biodistribution and PET studies, 4-11C-MBZA uptake in normal tissues was noticeably lower than that for several other 18F-benzamides like 18F-FPBZA [46] and 18F-DAFBA [62]. In addition, newly developed radiotracers, such as 18F-FBZA, 18F-5-FPN,18F-MEL050, 18F-FITM and 18F-ICF01006 (Fig. 2H), may have better performance in the delineation of small lymph node and lung metastases from melanoma than that of 18F-FDG PET/CT [45, 46, 63–66]. 18F-5-FPN, a probe identical to 18F-2, successfully detected pigmented B16/F10 tumors as early as 1 min after injection of the tracer. The uptake increased over time and the tracer was rapidly excreted via the kidneys. This and later studies from the same group further validated the potential of 18F-5-FPN PET for the early detection of metastatic melanoma lesions (Fig. 2I) [63, 67].
18F-MEL050 had excellent retention in melanin-containing tumors and rapid background clearance [49]; however it is notable that the route of administration of 18F-MEL050 matters when imaging regional lymph node metastasis from melanoma. While 18F-MEL050 PET correctly identified 100% of the lymph node metastases after subcutaneous administration of the tracer, only 60% of those metastases were found after systemic administration of the tracer in the lateral tail vein [50].
3. Peptide-based imaging probes
Peptides are emerging as potent and selective ligands that can be designed to bind with high affinity and specificity to cell surface receptors on a wide range of tumors [68]. Three major types of peptides, namely α-Melanocyte-stimulating hormone (α-MSH), tumor angiogenesis associated integrins, and peptides targeting both MC1R and integrin, are under intensive development for molecular imaging of melanoma.
3.1. α-Melanocyte-stimulating hormone (α-MSH)-based probes
α-MSH, a ligand specific for melanocortin receptor subtype 1 (MC1R), has been reported to be overexpressed in both melanotic and amelanotic human melanoma cases and has been widely used as a vehicle for melanoma-targeted imaging and therapy [69–73]. As native α-MSH (a linear 13 amino acid peptide, Ac-Ser-Tyr-Ser-Met-Glu-His-Phe-Arg-Trp-Gly-Lys-Pro-Val-NH2) has a biological half-life of less than 3 minutes in vivo [74], tremendous work has been done in the past 20 years and several modified analogs and synthesis strategies have been developed in an effort to add biological stability and improve targeting. For example, substitution of Met4 with Nle4 and Phe7 with D-Phe7 yields NDP-α-MSH [75]. Since His6-Phe7-Arg8-Trp9 had been identified as the “essential core” of native α-MSH peptide [76], both linear and transition metal rhenium cyclized α-MSH such as NAPamide [77], DOTA-NAPamide [78], ReCCMSH [77], MTII [79], analogs of MTII [80], DOTA-CycMSH and DOTA-GlyGlu-CycMSH [81], DOTA-Nle-CycMSHhex [82], were constructed. Recently new highly-specific and selective ligands against MC1R for melanomas were also developed and have been explored as platforms for molecular imaging of melanoma [83, 84].
Using the previously synthesized ReCCMSH which has nanomolar affinity for MC1R, McQuade et al. labeled the peptide with PET isotopes 64Cu and 86Y and assessed their diagnostic efficacy in melanoma tumors. Biodistribution studies revealed that the tumor concentration of 86Y-DOTA-ReCCMSH and 64Cu-DOTA-ReCCMSH was two times higher compared to that of the metabolic agent 18F-FDG [85]. Additionally, the use of the chelator CBTE2A provided improved stability, as uptake of 64Cu-CBTE2A-ReCCMSH was significantly lower than that for 64Cu-DOTA-ReCCMSH in normal organs such as liver, lung, heart, and spleen [86]. Wei et al. then successfully labeled DOTA-ReCCMSH (Arg11) with 68Ga and reported that the tumor uptake of 68Ga-DOTA-ReCCMSH reached a maximum after 30 min and remained stable 2 h postinjection. A pretargeting strategy employing D-lysine administration was able to significantly reduce kidney retention of the tracer [87]. 18F-FB-RMSH-1 and 18F-FP-RMSH-1, another two metallopeptide Ac-D-Lys-ReCCMSH(Arg11) probes [88, 89], showed less optimal imaging properties than these three probes illustrated above.
Since 111In-DOTA-GlyGlu-CycMSH was first developed to target MC1 receptors for primary and metastatic melanoma imaging [81, 90], recent studies determined that reduction of the ring size of 111In-DOTA-GlyGlu-CycMSH, L-lysine co-injection, introduction of a -GG-linker, substitution of DOTA with NOTA, and 99mTc radiolabeling via new chelators may further increase high melanoma tumor uptake while reducing nonspecific kidney and liver uptake [82, 91–94].
In addition, MC1-R specific NAPamide analogs have been labeled with various radiometals and non-metallic radionuclides (such as 64Cu, 68Ga, 18F, 111In, 99mTc and 44Sc) and have been intensively used to detect melanomas or to evaluate the expression level of MC1-R [95–101]. While 64Cu–DOTA–NAPamide showed mild tumor uptake in B16/F10 xenografted melanoma (Fig. 3A) [96], 64Cu-NOTA-GGNle-CycMSHhex showed dramatic uptake at 2 h postinjection [93]. The latter study also elucidated that the substitution of DOTA with NOTA dramatically increased the melanoma uptake and decreased the renal and liver uptake of 64Cu-NOTA-GGNle-CycMSHhex.
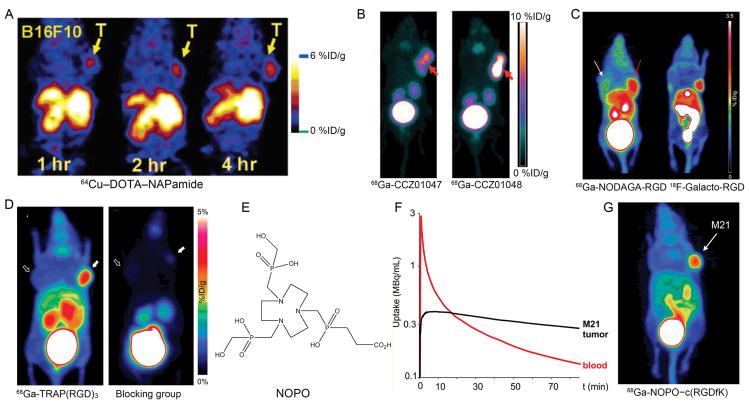
Figure 3.
Examples of peptide-based imaging of melanoma. (A) Coronal microPET images of mice bearing B16/F10 tumors at different time points after tail vein injection of 64Cu–DOTA–NAPamide. (B) PET images of 68Ga-CCZ01047 and 68Ga-CCZ01048 at 1 h postinjection of the corresponding tracer in mice bearing B16/F10 tumors. (C) MIP PET images of mice bearing M21 and M21L tumor xenografts on right and left shoulder, respectively. 68Ga-NODAGA-RGD (left) showed more intense tumor uptake than 18F-Galacto-RGD (right) in αvβ3 positive M21 melanoma models. (D) MIP images of microPET scans of M21 (solid arrows) and M21L (outline arrows) human melanoma models. 68Ga-TRAP(RGD)3 showed high-contrast visualization of the M21 tumor. (E) Chemical structure of bifunctional chelator NOPO. (F) The relative uptake of 68Ga-NOPO–c(RGDfK) in M21 tumor tissue and blood over time. (G) MIP of 68Ga-NOPO–c(RGDfK) PET imaging in M21/M21L xenografted mice. Tumor site was marked with white arrow. Adapted and modified with permission from references [96, 105, 120, 130–132].
Due to its positron emission with a high branching ratio (I =94.27%, Emean (β+) =0.63 MeV), convenient production by the 44Ti/44Sc generator and satisfactory physical half-life (3.97 h), 44Sc is a novel radiometal which has gained significant interest as a potential radioisotope for PET imaging [102–104]. A proof-of-concept study investigated the biological properties of the 44Sc-labeled DOTA-NAPamide and found that this probe showed excellent binding properties to MC1-R positive melanoma cell and tumors, slightly superior to that of 68Ga-DOTA-NAPamide [95].
Most recently, three new MC1R-targeting peptides (CCZ01047, CCZ01048, and CCZ01056) were successfully developed and all the three 68Ga-labeled tracers produced high contrast PET images in B16/F10 tumors, of which 68Ga-CCZ01048 exhibited the most ideal tumor uptake (Fig. 3B). This study indicated that introduction of a cationic Pip linker to Nle-CycMSHhex could improve tumor uptake and tumor-to-normal tissue contrast in detecting melanoma [105].
3.2. Peptide-based probes targeting the integrin family
Integrins are heterodimeric αβ transmembrane receptors that connect the extracellular matrix (ECM) to the cytoskeleton, always forming dimers by combining 1 of 18 α-chains with 1 of 8 β-chains [106]. Integrin αvβ3, and less commonly integrin α5β1, have been attractive molecular targets for developing melanoma imaging probes [107–111]. Clinically, recent studies have validated that 18F-Fluciclatide (formerly known as 18F-AH111585) and 18F-Galacto-RGD were able to measure αvβ3 expression in melanoma and may be useful radiotracers to assess the response to the antiangiogenic therapy in melanoma patients [112–116]. Nevertheless, low concentration of these clinically-available probes in αvβ3 positive tumors and less than optimal PET/CT imaging quality warrants many sensitive and specific probes to be developed.
In the preclinical setting, as we previously reviewed [117], a large variety of imaging strategies have been successfully employed for imaging of integrin expression in various cancer types, including melanoma. One study compared the diagnostic efficacy of 18F-Galacto-RGD with that of 68Ga-DOTA-RGD and 111In-DOTA-RGD and found that 18F-Galacto-RGD remained superior for imaging αvβ3 expression [108]. Even though 68Ga-Oxo-DO3A-RGD and 68Ga-NS3-RGD turned out to have inferior characteristics compared to the already existing 68Ga-labeled RGD peptides [118], 68Ga-NODAGA-RGD possessed improved imaging properties compared to 68Ga-DOTA-RGD [119], even performing similarly to 18F-Galacto-RGD (Fig. 3C) [120]. To achieve an easier and more rapid radiosynthesis, fusarinine C (FSC) and SarAr have been reported to be promising Ga-68 and Zr-89 binding bifunctional chelators [110, 121–124]. The most recent study from Zhai et al. reported that 68Ga-FSC(succ-RGD)3 exhibited improved properties compared to 68Ga-NODAGA-RGD. The half-life of the radionuclide used (68Ga, 68 min) was compatible with the pharmacokinetics of RGD peptides [125].
When compared to DOTA, the bifunctional chelator NODAGA has gained popularity because of its significant advantages in terms of labeling chemistry [119, 126]. However, the TRAP chelator possesses even better 68Ga labeling properties and enables high yields and excellent reproducibility [127–129]. Notni et al. synthesized 68Ga-avebetrin (formerly known as 68Ga-TRAP(RGD)3) and performed a comparison of biodistribution and PET data of 68Ga-TRAP(RGD)3 with those of 68Ga-NODAGA-c(RGDyK) and 18F-Galacto-RGD. Different from 68Ga-NODAGA-RGD and 18F-Galacto-RGD, 68Ga-TRAP(RGD)3 showed a very rapid blood clearance and renal excretion while maintaining activity concentration in tumor tissue, indicating the potential value of 68Ga-TRAP(RGD)3 as a next generation αvβ3 imaging agent (Fig. 3D) [130]. Considering that the TRAP-type chelator NOPO showed excellent 68Ga labeling properties even in the presence of high concentrations of competing metal cations (Fig. 3E) [131], researchers further developed 68Ga-NOPO–c(RGDfK) and found that this αvβ3 targeting probe exhibited a higher degree of hydrophilicity than similar conjugates with other chelators, resulting in rapid and specific uptake in M21 tumor xenografts, very rapid pharmacokinetics and renal clearance (Fig. 3F, G) [132].
There are a few reports on the development of α5β1 specific radiotracers [111, 133–135]. Although there was one candidate peptide with a high specificity and affinity for α5β1 in vitro, in vivo biodistribution studies demonstrated this radiotracer was not suitable for in vivo imaging due to its considerably high and constant radioactivity accumulation in the blood and other major organs [136]. The reported α5β1 specific probes 18F-PR_b and 68Ga-NODAGAFR366 showed specific but low binding to α5β1 positive murine melanoma tumors, and as a result, the poor tumor concentration and high kidney uptake may hinder these probe from further clinical transition [111, 137]. Recently Notni and colleagues obtained 68Ga-aquibeprin by click-chemistry (CuAAC) trimerization of a α5β1 pseudo-peptide on the TRAP chelator, followed by automated 68Ga labeling. Surprisingly, the trimer 68Ga-aquibeprin possessed approximately 16-times-higher α5β1 affinity than the previously reported 68Ga-labeled pseudo-peptide monomer. Although 68Ga-aquibeprin showed lower uptake than 68Ga-avebetrin, low background activity and high target-to-nontarget contrast of the probe may offer great potential for elucidating biologic functions of α5β1 and for detecting melanoma [138, 139].
RGD mimetic integrin inhibitors, like Cilengitide and Cilengitide-like RGD peptidomimetics, have also been investigated as chemical probes for the molecular imaging of angiogenesis in the literature [133, 140, 141]. In the near future, other strategies like sulfonation of tyrosine moieties in RGD peptides, which can modify the hydrophilicity of RGD peptides to increase renal clearance and to improve overall biodistribution [142], can also be applied to design novel probes for mapping αvβ3 expression in melanoma. Notably, RGD can be used as an agent for surface engineering in other imaging systems to enhance melanoma targeting efficacy and therapeutic effects [143–146].
3.3. Dual-targeted peptide-based probes
To improve the in vivo pharmacokinetics and stability of the above mentioned molecular probes, many hybrid peptides targeting both MC1R and integrin αvβ3 have been developed [147–153]. Initial synthesis and evaluation of 99mTc-RGD-Lys-(Arg11)CCMSH showed MC1R-mediated cellular uptake of the tracer, higher tumor uptake, and prolonged tumor retention in B16/F1 melanoma-bearing mice [148]. Substitution of Gly with Ala in the hybrid peptide not only dramatically increased the MC1R binding affinity of RAD-Lys-(Arg11)CCMSH compared to RGD-Lys-(Arg11)CCMSH (0.3 vs. 2.0 nM) but also enhanced the melanoma uptake [149]. Further studies demonstrated that 99mTc-RTD-Lys-(Arg11)CCMSH and 99mTc-RVD-Lys-(Arg11)CCMSH exhibited similar imaging properties to RAD-Lys-(Arg11)CCMSH, but 99mTc-RVD-Lys-(Arg11)CCMSH reached its highest concentration in melanoma lesions at a later time point [150].
Flook et al. replaced the Gly with another four amino acids (Ser, Nle, Phe, and D-Phe) and found that 99mTc-RSD-Lys-(Arg11)CCMSH displayed the strongest MC1R binding affinity in vitro and exhibited the most optimal melanoma uptake in vivo [151]. Importantly, linkers and charge status of the linkers between hybrid peptides may affect the renal uptake of the tracer significantly, as 99mTc-RGD-(Arg11)-CCMSH without a linker dramatically enhanced the tumor-to-kidney uptake ratio [154], and substitution of the Lys linker with Aoc, PEG2, β Ala or Ahx linker reduced the renal uptake of the relevant probe by 58%~63% at 2 h post-injection [155–157].
4. Other PET/SPECT probes for melanoma imaging
Besides radiolabeled melanin, MCR1, and integrin based probes, here we would like to summarize other potential radiolabelled molecular imaging probes that have been found effective in detecting not only melanoma but also other solid tumors. These probes can be divided into several categories as discussed in the following sections.
4.1. Probes targeting the metabotropic glutamate 1 receptor
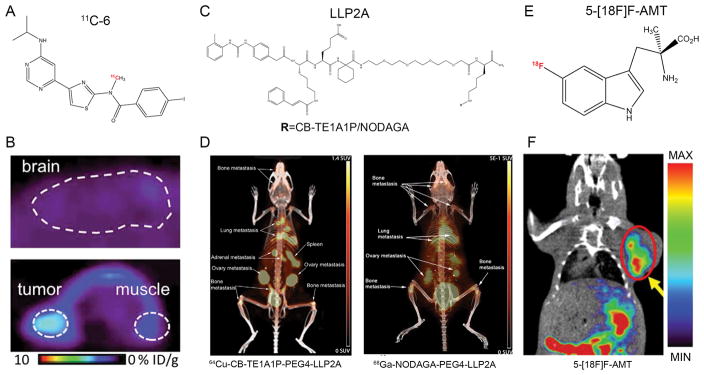
Metabotropic glutamate 1 (mGlu1) receptor is a G protein-coupled receptor normally expressed in the central nervous system, essential for learning and modulating the excitatory synaptic transmission in the central nervous system [158]. Recent reports have elucidated that mGlu1 has oncogenic characteristics in melanoma by driving constitutive activation of mitogen activated protein kinase and phoshatidylinositol-3-kinase/protein kinase B pathways [159–163]. Xie et al. initially developed 18F-FITM for quantifying mGlu1 expression in the brain [164, 165], and then successfully extended 18F-FITM PET imaging in melanoma [65]. However, considerable uptake and slow clearance of radioactivity in the brain undermined its usage in clinical applications. The same group elaborately introduced halogen atoms (chlorine, bromine, or iodine) rather than 18F into 18F-FITM and labeled these compounds using 11C (half-life: 20.2 min). Of the reported compounds, the iodine analogue 11C-6 showed the highest ratio of radioactivity of tumor to brain and may act as a useful PET tracer for imaging mGlu1 in melanoma (Fig. 4A, B) [166]. Notably, future studies can feasibly label 11C-6 using the isotopes of 124I, 123I or 131I for mGlu1-based theranostics of melanoma without altering their chemical structures or pharmacological profiles.
Figure 4.
Other PET probes for melanoma imaging. (A) Chemical structure of 11C-6 for imaging of mGlu1 receptor. (B) Representative PET images of mice bearing B16/F10 after injection of 11C-6. Upper: sagittal image of the brain. Lower: Axial image of tumor and muscle. (C) Chemical structures of NODAGA-PEG4-LLP2A and CB-TE1A1P-PEG4-LLP2A. (D) Small animal PET/CT imaging at 2 h after injection of the radiotracers (7.4 MBq). Both 64Cu-CB-TE1A1P-PEG4-LLP2A and 68Ga-NODAGA-PEG4-LLP2A were able to image melanoma lung metastases with high contrast and minimal lung background. (E) Chemical structure of 5-[18F]F-AMT. (F) Coronal PET/CT image of B16/F10 melanoma 30 min after injection of 5-[18F]F-AMT. Adapted and modified with permission from references [166, 172, 180].
4.2. Probes targeting the very late antigen-4
In the past several years, very late antigen-4 (VLA-4; also called integrin α4β1) has been found in cancers including melanoma [167]. LLP2A, a high-affinity peptidomimetic ligand for VLA-4, was identified from a 1-bead 1-compound library and has been used for cancer imaging and therapy [168–170]. Specifically, Jiang and co-authors conjugated LLP2A with two different chelators and assessed their imaging properties in melanoma models after labeling the conjugated compounds with 64Cu. From the biodistribution and in vivo imaging data, both 64Cu-CB-TE1A1P-LLP2A and 64Cu-CB-TE2A-LLP2A clearly visualized the tumors with better contrast than was observed for 64Cu-CB-TE1A1P-LLP2A [171]. When compared to 68Ga-labeled NODAGA-LLP2A, 64Cu-CB-TE1A1P-PEG4-LLP2A trended toward higher uptake and better tumor–to–nontarget tissue ratios (Fig. 4C, D) [172].
Recently Gai et al. designed a new probe 64Cu-NE3TA-PEG4-LLP2A using the newly discovered chelator p-SCN-PhPr-NE3TA and assessed the diagnostic efficacy of the probe in melanoma xenografts. Small animal PET/CT imaging with 64Cu-NE3TA-PEG4-LLP2A demonstrated high uptake with a superior tumor-to-muscle ratio at 4 h postinjection [173]. In addition, a study from Beaino et al. reported that 177Lu-DOTA-PEG4-LLP2A could accumulate not only in primary melanoma but also in the metastatic lesions in the lung and brain, demonstrating the potential of 177Lu-DOTA-PEG4-LLP2A as an alternative treatment strategy for metastatic VLA-4 expressing melanoma [174].
4.3. Probes targeting indoleamine 2,3-dioxygenase (IDO)
In recent years, immunotherapy has dramatically changed the landscape of melanoma treatment [175]. However, recent findings revealed that indoleamine 2,3-dioxygenase (IDO) can be triggered by innate responses during tumorigenesis, and also by attempted T cell activation (either spontaneous or due to immunotherapy), contributing to restrain immunity and establish immunotolerance. IDO inhibitors act as a novel class of immunomodulators with broad application in the treatment of advanced human cancer [176, 177]. In an effort to map tryptophan (Trp, a substrate of IDO) metabolism [178, 179], one group found that radionuclide labelled IDO inhibitor, 5-[18F]F-AMT, may hold great potential for melanoma imaging [180]. By using B16/F10 melanoma model, the authors found that tumors were clearly visible and this probe was possibly excreted through the urinary system as the kidneys showed initial high uptake (Fig. 4E, F). These preliminary results implied potential usage of this series of probes because the superior tumor-to-background ratio may precisely delineate primary and metastatic melanomas, but these probes are not melanoma-specific because other tumor types like breast cancer concentrate this kind of probe as well [178].
4.4. Probes targeting the immune checkpoints
With the field of cancer immunotherapy has undergone tremendous growth during the past decade, as we recently pointed out, noninvasive molecular imaging strategies have been used to map the biodistribution of immune checkpoint molecules, monitor the efficacy and potential toxicities of the treatments, and identify potential patients who will benefit from immunotherapies [181]. Studies have shown that PET may be used for the noninvasive imaging of PD-1/PD-L1 expression in melanoma and for determining the extent of tumor-infiltration of lymphocytes [182, 183]. Recently Nedrow et al. developed a new PD-L1-targeted imaging agent, 111In-DTPA-anti-PD-L1-BC and demonstrated that tumors had the greatest uptake at 24 h p.i. with a tumor-to-muscle ratio of 4.6 in mice bearing B16/F10 tumors. Whole body SPECT images demonstrated that the tumor as well as the spleen and liver were clearly defined at the later time points [184].
4.5. Probes targeting the human copper transporter 1 (CTR1)
Human copper transporter 1 (CTR1), a 190-amino acid protein of 28 kDa with three transmembrane domains [185], has been proven to be overexpressed in melanoma. The usefulness of 64Cu2+ ions as PET probes is based on the fact that Cu is an essential element which plays an important role in cell proliferation and angiogenesis [186]. Therefore, copper radionuclide-based imaging of cancers have been investigated by several studies [187, 188]. 64CuCl2 has been reported to be a novel and promising PET probe for imaging melanoma [189]. However, there have been reports that CTR1 is the specific influx copper transporter for 64Cu(I) rather than 64Cu(II). Using antioxidants, Jiang et al. prepared 64Cu(I) and evaluated cellular uptake of 64Cu(I) and 64Cu(II) by melanoma cells in vitro and in vivo. The authors demonstrated that although 64Cu(I) exhibited higher cellular uptake, no significant difference between 64Cu(I) and 64Cu(II) was observed through in vivo PET images and biodistribution [190]. However, future studies are still needed to evaluate whether or not 64Cu(I) can act as a feasible PET imaging radiotracer for melanoma detection.
4.6. Antibody-based imaging probes
Radiolabeled monoclonal antibodies (mAb), antibody fragments, and engineered antibody derivatives are increasingly utilized as agents in diagnosis and therapy because of developments in antibody engineering and in vivo stability and high specificity of these probes [191, 192]. Twenty years have passed since the initial attempts to detect melanoma using radiolabeled antibodies [193, 194]. Besides melanin-specific antibodies [195–199], GD2 is highly expressed on the cell surface of a broad spectrum of human cancers including melanoma and has been successfully exploited as a molecular target for therapy and molecular imaging [200]. Voss et al. successfully developed a SarAr-conjugated, 64Cu-labeled, anti-GD2 antibody construct, 64Cu-SarAr-GD2 mAb ch14.18, and performed in vivo studies using GD2-expressing melanoma xenografts. Their results showed that about 20.5% of the injected dose accumulated in the M21 melanoma tumors [201], and further studies from the same group confirmed this finding [202, 203]. These preliminary results may indicate the feasibility of the 64Cu-SarAr antibody as a platform for imaging melanoma.
4.7. Probes targeting CXCR4
C-X-C chemokine receptor type 4 (CXCR4, also called fusin, CD184) is a 7-transmembrane G-coupled receptor belonging to the chemokine receptor family and has been found to be overexpressed in various human cancers including lymphoma, neuroendocrine tumors, malignant glioma, lung cancer and multiple myeloma [204–208]. CXCR4-based imaging has also been investigated to detect melanoma recently [209]. In a clinical trial dedicated to estimate CXCR4 overexpression by using the novel CXCR4-specific probe 68Ga-Pentixafor, Vag et al. included 21 patients (2 of them melanoma patients) and demonstrated the feasibility of 68Ga-Pentixafor for PET imaging of solid malignancies, although the detectability of solid cancers by 68Ga-Pentixafor seemed to be lower than with 18F-FDG PET [210].
5. Image-guided therapy of melanoma
In addition to melanoma imaging, molecular imaging-guided therapy of melanoma has long been a hot topic in the field, as recently reviewed by Norain et al. [211]. Radionuclide-based therapy of melanoma can be realized through radiolabeled antibodies, peptides or small molecules.
5.1. Radiolabeled antibodies and peptides
Lutetium-177 (177Lu) is a low energy β-emitter (497 keV, 90%) with a half-life of 6.7 days and a maximum tissue penetration of 1.6 mm. 177Lu also emits γ-rays (113 and 208 keV, 6% and 11%) suitable for image-guided drug delivery using SPECT. Antibodies and peptides radiolabeled with 177Lu are attractive therapeutic agents due to localized deposition of beta decay energy and a radioactive half-life which matches in vivo pharmacokinetics of targeting antibodies quite well [212–214]. Vascular endothelial growth factor (VEGF) has been extensively studied as one of the most important proteins involved in the development of physiological and pathological angiogenesis [215, 216]. Recently, 177Lu-DOTA-bevacizumab (a recombinant humanized monoclonal antibody that binds to all VEGF isoforms) has shown preliminary potential as a novel radioimmunotherapy and molecular imaging agent for melanoma [217]. Building upon the success of the lactam bridge-cyclized a-MSH peptides for melanoma imaging discussed above, Guo et al. assessed the image-guided therapeutic effect of 177Lu-DOTAGGNle-CycMSHhex in B16/F1 melanoma-bearing mice and found high melanoma uptake and fast urinary clearance of the probe, underscoring its potential as a theranostic agent for metastatic melanoma [218].
188Re, a high-energy β-emitter (maximal energy: 2.12 MeV), has considerable range (several millimeters) in tissue and and relatively short half-life of 16.9 h. 188Re-labeled melanin-specific antibodies for melanoma therapy have also been studied. 188Re-labeled 6D2 [197], a melanin-binding IgM antibody, has been validated to be effective in treating pigmented human melanoma tumors and in augmenting the efficacy of the chemotherapeutic agent dacarbazine (DTIC) [198]. Notably, by administering the targeting vector and radioisotope separately, in vivo pretargeting seems to be a promising approach to enhance tumor-targeting properties of radiolabeled antibodies while simultaneously skirting their pharmacokinetic limitations.
5.2. Radiolabeled small molecules
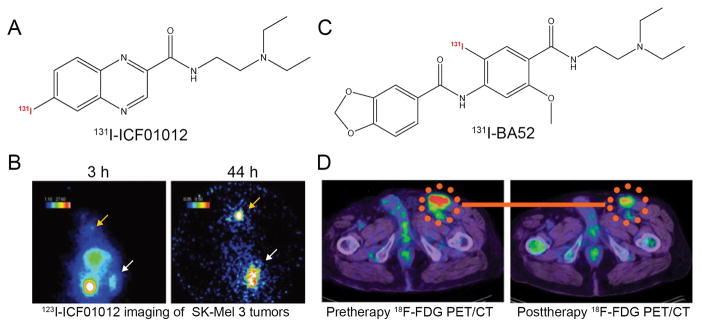
For melanin-targeted imaging-guided radionuclide therapy, many radiolabeled benzamide derivatives such as 131I-MIP-1145 and 131I-ICF01012 exhibited strong efficacy in murine and human melanoma xenografts [17, 219, 220]. ICF01012 was labeled with 123I for melanoma imaging and with 131I for melanoma treatment (Fig. 5A,B). The preliminary results not only showed a correlation between radiotracer uptake and melanin content but also demonstrated significantly reduced tumor growth and prolonged the median survival of the melanoma-bearing mice after administration of 131I-ICF01012 [221]. The combination of 131I-ICF01012 and coDbait, a DNA repair inhibitor, could overcome melanoma radioresistance and increase the efficacy of targeted radionuclide therapy (TRT) without increasing side effects [222]. Clinically, in a study which enrolled 26 patients with metastatic melanoma, Mier et al. reported that, out of five patients who received higher doses of treatment by administration of the melanin-binding 131I-BA52 (Fig. 5C,D), three of them survived more than two years after therapy. In contrast, the mean overall survival of the untreated and insufficiently dosed patients was approximately three months [223]. Interestingly, iodinated and fluorinated radiotracers targeting melanin and offering potential for both diagnosis (SPECT and PET imaging) and therapy (iodine-131) have also been developed [224–226].
Figure 5.
Representative radionuclide-labeled therapeutic agents for melanoma. (A) Chemical structure of 131I-ICF01012. (B) Gamma-camera imaging of SK-Mel 3 melanoma-bearing mice after injection of 3.7 MBq 123I-ICF01012. A clear concentration of 123I-ICF01012 occurred 3 hours after radiotracer administration and a rapid elimination of 123I-ICF01012 from non-specific organs was observed at 44 h postinjection. Tumor and thyroid were indicated by white and orange arrows, respectively. (C) Chemical structure of 131I-BA52. (D) 18F-FDG PET/CT examinations in a melanoma patient before and after 131I-BA52 treatment. After treatment using 131I-BA52, post-therapeutic 18F-FDG PET/CT examination demonstrated that SUV of the inguinal lymph node metastasis decreased from 9.02 to 5.81. Adapted and modified with permission from references [221, 223].
6. Conclusion and future persepectives
In this review, as summarized in Fig. 6, we provided an informative survey of the recent progress on radiolabelled molecular imaging probes for imaging different molecular targets or processes in malignant melanoma. While melanin, MCR1 and integrins are the traditional targets extensively used for melanoma detection and therapy, tumor metabolism and immune microenvironment-based molecular imaging probes have also been developed in recent years. In the era of precision medicine, devotion and efforts on radiolabelled molecular probes for mapping and treating melanoma is extremely important, and new imaging probes with optimal imaging properties are still highly demanded.
Figure 6.
Pictorial abstract showing PET and SPECT imaging probes for malignant melanoma. Molecular targets discussed above can be divided into two groups, melanoma specific targets which are indicated by grey/black models and melanoma nonspecific targets which are indicated by colorful models.
Looking into the future, we would like to put forward three proposals from our perspective. First, with the rapid development of the molecular imaging field, novel molecular imaging probes with high sensitivity and specificity enable us to characterize melanoma at the molecular level. These new probes will provide powerful platforms for early diagnosis of both primary and metastatic melanoma, monitoring of therapeutic response, accurate staging and restaging of melanoma, stratification of patients for antiangiogenesis therapy, immunotherapy and/or radionuclide therapy, and facilitation of new drug discovery for melanoma treatment. Second, melanin, MC1R, and integrins have been intensively investigated as targets for selective imaging and therapeutic agents against melanoma, and though the potential of many of these probes could be effectively demonstrated in preclinical settings, very few of them could actually be translated to the clinics. Future studies should be dedicated to push the clinical transition of some of these most promising probes. Third, usage of humanized mice, rather than cultured cell lines and mouse xenografts [227], could examine the molecular imaging probes in the context of the human immune system and tumor microenvironment and accelerate the clinical transition of these molecular imaging probes.
To conclude, malignant melanoma represents a serious public health problem and is a deadly disease when diagnosed at late stage. With the elucidation of many important oncogenic signaling pathways and biomarkers involved in malignant melanoma pathogenesis, we belive that PET and SPECT imaging as well as image-guided therapy can undoubtedly provide better visualization and management of malignant melanoma in the forthcoming future.
Acknowledgments
This work was sponsored by Ph.D. Innovation Fund of Shanghai Jiao Tong University School of Medicine to Weijun Wei (No. BXJ201736), National Institutes of Health (1R01CA205101, P30CA014520, and T32GM008505) and the American Cancer Society (125246-RSG-13-099-01-CCE).
Footnotes
Conflict of interest
The authors have declared no competing interest.
References
- 1.American Cancer Society. [Internet] http://wwwcancerorg/cancer/skincancer-melanoma/detailedguide/melanoma-skin-cancer-key-statistics.
- 2.Trinh VA. Current management of metastatic melanoma. American journal of health-system pharmacy : AJHP : official journal of the American Society of Health-System Pharmacists. 2008;65:S3–8. doi: 10.2146/ajhp080460. [DOI] [PubMed] [Google Scholar]
- 3.Luke JJ, Flaherty KT, Ribas A, Long GV. Targeted agents and immunotherapies: optimizing outcomes in melanoma. Nature reviews Clinical oncology. 2017 doi: 10.1038/nrclinonc.2017.43. [DOI] [PubMed] [Google Scholar]
- 4.Wong AN, McArthur GA, Hofman MS, Hicks RJ. The Advantages and Challenges of Using FDG PET/CT for Response Assessment in Melanoma in the Era of Targeted Agents and Immunotherapy. European journal of nuclear medicine and molecular imaging. 2017 doi: 10.1007/s00259-017-3691-7. [DOI] [PubMed] [Google Scholar]
- 5.Krug B, Crott R, Lonneux M, Baurain JF, Pirson AS, Vander Borght T. Role of PET in the initial staging of cutaneous malignant melanoma: systematic review. Radiology. 2008;249:836–44. doi: 10.1148/radiol.2493080240. [DOI] [PubMed] [Google Scholar]
- 6.Danielsen M, Kjaer A, Wu M, Martineau L, Nosrati M, Leong SP, et al. Prediction of positron emission tomography/computed tomography (PET/CT) positivity in patients with high-risk primary melanoma. American journal of nuclear medicine and molecular imaging. 2016;6:277–85. [PMC free article] [PubMed] [Google Scholar]
- 7.Gilles R, de Geus-Oei LF, Mulders PF, Oyen WJ. Immunotherapy response evaluation with (18)F-FDG-PET in patients with advanced stage renal cell carcinoma. World journal of urology. 2013;31:841–6. doi: 10.1007/s00345-011-0723-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Acland KM, Healy C, Calonje E, O’Doherty M, Nunan T, Page C, et al. Comparison of positron emission tomography scanning and sentinel node biopsy in the detection of micrometastases of primary cutaneous malignant melanoma. Journal of clinical oncology : official journal of the American Society of Clinical Oncology. 2001;19:2674–8. doi: 10.1200/JCO.2001.19.10.2674. [DOI] [PubMed] [Google Scholar]
- 9.Fletcher JW, Djulbegovic B, Soares HP, Siegel BA, Lowe VJ, Lyman GH, et al. Recommendations on the use of 18F-FDG PET in oncology. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49:480–508. doi: 10.2967/jnumed.107.047787. [DOI] [PubMed] [Google Scholar]
- 10.Lin NU, Lee EQ, Aoyama H, Barani IJ, Barboriak DP, Baumert BG, et al. Response assessment criteria for brain metastases: proposal from the RANO group. The Lancet Oncology. 2015;16:e270–8. doi: 10.1016/S1470-2045(15)70057-4. [DOI] [PubMed] [Google Scholar]
- 11.Schellinger PD, Meinck HM, Thron A. Diagnostic accuracy of MRI compared to CCT in patients with brain metastases. Journal of neuro-oncology. 1999;44:275–81. doi: 10.1023/a:1006308808769. [DOI] [PubMed] [Google Scholar]
- 12.Ghasemi M, Nabipour I, Omrani A, Alipour Z, Assadi M. Precision medicine and molecular imaging: new targeted approaches toward cancer therapeutic and diagnosis. American journal of nuclear medicine and molecular imaging. 2016;6:310–27. [PMC free article] [PubMed] [Google Scholar]
- 13.Valencak J, Heere-Ress E, Traub-Weidinger T, Raderer M, Schneeberger A, Thalhammer T, et al. Somatostatin receptor scintigraphy with 111In-DOTA-lanreotide and 111In-DOTA-Tyr3-octreotide in patients with stage IV melanoma: in-vitro and in-vivo results. Melanoma research. 2005;15:523–9. doi: 10.1097/00008390-200512000-00007. [DOI] [PubMed] [Google Scholar]
- 14.Hong H, Sun J, Cai W. Anatomical and molecular imaging of skin cancer. Clinical, cosmetic and investigational dermatology. 2008;1:1–17. doi: 10.2147/ccid.s4249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ren G, Pan Y, Cheng Z. Molecular probes for malignant melanoma imaging. Current pharmaceutical biotechnology. 2010;11:590–602. doi: 10.2174/138920110792246465. [DOI] [PubMed] [Google Scholar]
- 16.d’Ischia M, Wakamatsu K, Cicoira F, Di Mauro E, Garcia-Borron JC, Commo S, et al. Melanins and melanogenesis: from pigment cells to human health and technological applications. Pigment cell & melanoma research. 2015;28:520–44. doi: 10.1111/pcmr.12393. [DOI] [PubMed] [Google Scholar]
- 17.Dadachova E, Casadevall A. Melanin as a potential target for radionuclide therapy of metastatic melanoma. Future oncology. 2005;1:541–9. doi: 10.2217/14796694.1.4.541. [DOI] [PubMed] [Google Scholar]
- 18.Thompson JF, Scolyer RA, Kefford RF. Cutaneous melanoma. Lancet. 2005;365:687–701. doi: 10.1016/S0140-6736(05)17951-3. [DOI] [PubMed] [Google Scholar]
- 19.Ings RM. The melanin binding of drugs and its implications. Drug metabolism reviews. 1984;15:1183–212. doi: 10.3109/03602538409033561. [DOI] [PubMed] [Google Scholar]
- 20.Chezal JM, Papon J, Labarre P, Lartigue C, Galmier MJ, Decombat C, et al. Evaluation of radiolabeled (hetero)aromatic analogues of N-(2-diethylaminoethyl)-4-iodobenzamide for imaging and targeted radionuclide therapy of melanoma. Journal of medicinal chemistry. 2008;51:3133–44. doi: 10.1021/jm701424g. [DOI] [PubMed] [Google Scholar]
- 21.Link E, Lukiewicz S. A new radioactive drug selectively accumulating in Melanoma cells. European journal of nuclear medicine. 1982;7:469–73. doi: 10.1007/BF00253084. [DOI] [PubMed] [Google Scholar]
- 22.Link EM, Blower PJ, Costa DC, Lane DM, Lui D, Brown RS, et al. Early detection of melanoma metastases with radioiodinated methylene blue. European journal of nuclear medicine. 1998;25:1322–9. doi: 10.1007/s002590050302. [DOI] [PubMed] [Google Scholar]
- 23.Link EM, Carpenter RN. 211At-methylene blue for targeted radiotherapy of human melanoma xenografts: treatment of cutaneous tumors and lymph node metastases. Cancer research. 1992;52:4385–90. [PubMed] [Google Scholar]
- 24.Michelot JM, Moreau MF, Labarre PG, Madelmont JC, Veyre AJ, Papon JM, et al. Synthesis and evaluation of new iodine-125 radiopharmaceuticals as potential tracers for malignant melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1991;32:1573–80. [PubMed] [Google Scholar]
- 25.El Aissi R, Chezal JM, Tarrit S, Chavignon O, Moreau E. Melanoma-targeted delivery system (part 1): design, synthesis and evaluation of releasable disulfide drug by glutathione. European journal of medicinal chemistry. 2015;101:668–80. doi: 10.1016/j.ejmech.2015.06.055. [DOI] [PubMed] [Google Scholar]
- 26.El Aissi R, Miladi I, Chezal JM, Chavignon O, Miot-Noirault E, Moreau E. Melanoma-targeted delivery system (part 2): Synthesis, radioiodination and biological evaluation in B16F0 bearing mice. European journal of medicinal chemistry. 2016;120:304–12. doi: 10.1016/j.ejmech.2016.05.019. [DOI] [PubMed] [Google Scholar]
- 27.Sillaire-Houtmann I, Bonafous J, Veyre A, Mestas D, D’Incan M, Moins N, et al. Phase 2 clinical study of 123I-N-(2-diethylaminoethyl)-2-iodobenzamide in the diagnostic of primary and metastatic ocular melanoma. Journal francais d’ophtalmologie. 2004;27:34–9. doi: 10.1016/s0181-5512(04)96089-5. [DOI] [PubMed] [Google Scholar]
- 28.Michelot JM, Moreau MF, Veyre AJ, Bonafous JF, Bacin FJ, Madelmont JC, et al. Phase II scintigraphic clinical trial of malignant melanoma and metastases with iodine-123-N-(2-diethylaminoethyl 4-iodobenzamide) Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1993;34:1260–6. [PubMed] [Google Scholar]
- 29.Brandau W, Niehoff T, Pulawski P, Jonas M, Dutschka K, Sciuk J, et al. Structure distribution relationship of iodine-123-iodobenzamides as tracers for the detection of melanotic melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1996;37:1865–71. [PubMed] [Google Scholar]
- 30.Moins N, D’Incan M, Bonafous J, Bacin F, Labarre P, Moreau MF, et al. 123I-N-(2-diethylaminoethyl)-2-iodobenzamide: a potential imaging agent for cutaneous melanoma staging. European journal of nuclear medicine and molecular imaging. 2002;29:1478–84. doi: 10.1007/s00259-002-0971-6. [DOI] [PubMed] [Google Scholar]
- 31.Dittmann H, Coenen HH, Zolzer F, Dutschka K, Brandau W, Streffer C. In vitro studies on the cellular uptake of melanoma imaging aminoalkyl-iodobenzamide derivatives (ABA) Nuclear medicine and biology. 1999;26:51–6. doi: 10.1016/s0969-8051(98)00046-8. [DOI] [PubMed] [Google Scholar]
- 32.Bacin F, Michelot J, Bonafous J, Veyre A, Moreau MF, Kemeny JL, et al. Clinical study of [123I] N-(2-diethylaminoethyl)-4-iodobenzamide in the diagnosis of primary and metastatic ocular melanoma. Acta ophthalmologica Scandinavica. 1998;76:56–61. doi: 10.1034/j.1600-0420.1998.760110.x. [DOI] [PubMed] [Google Scholar]
- 33.Larisch R, Schulte KW, Vosberg H, Ruzicka T, Muller-Gartner HW. Differential accumulation of iodine-123-iodobenzamide in melanotic and amelanotic melanoma metastases in vivo. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 1998;39:996–1001. [PubMed] [Google Scholar]
- 34.Labarre P, Papon J, Moreau MF, Moins N, Veyre A, Madelmont JC. Evaluation in mice of some iodinated melanoma imaging agents using cryosectioning and multi-wire proportional counting. European journal of nuclear medicine. 1999;26:494–8. doi: 10.1007/s002590050416. [DOI] [PubMed] [Google Scholar]
- 35.Cachin F, Miot-Noirault E, Gillet B, Isnardi V, Labeille B, Payoux P, et al. (123)I-BZA2 as a melanin-targeted radiotracer for the identification of melanoma metastases: results and perspectives of a multicenter phase III clinical trial. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:15–22. doi: 10.2967/jnumed.113.123554. [DOI] [PubMed] [Google Scholar]
- 36.Liu X, Pham TQ, Berghofer P, Chapman J, Greguric I, Mitchell P, et al. Synthesis and evaluation of novel radioiodinated nicotinamides for malignant melanoma. Nuclear medicine and biology. 2008;35:769–81. doi: 10.1016/j.nucmedbio.2008.05.011. [DOI] [PubMed] [Google Scholar]
- 37.Bonnet-Duquennoy M, Papon J, Mishellany F, Labarre P, Guerquin-Kern JL, Wu TD, et al. Targeted radionuclide therapy of melanoma: anti-tumoural efficacy studies of a new 131I labelled potential agent. International journal of cancer Journal international du cancer. 2009;125:708–16. doi: 10.1002/ijc.24413. [DOI] [PubMed] [Google Scholar]
- 38.Billaud EM, Maisonial-Besset A, Rbah-Vidal L, Vidal A, Besse S, Bequignat JB, et al. Synthesis, radiolabeling and preliminary in vivo evaluation of multimodal radiotracers for PET imaging and targeted radionuclide therapy of pigmented melanoma. European journal of medicinal chemistry. 2015;92:818–38. doi: 10.1016/j.ejmech.2015.01.034. [DOI] [PubMed] [Google Scholar]
- 39.Degoul F, Borel M, Jacquemot N, Besse S, Communal Y, Mishellany F, et al. In vivo efficacy of melanoma internal radionuclide therapy with a 131I-labelled melanin-targeting heteroarylcarboxamide molecule. International journal of cancer Journal international du cancer. 2013;133:1042–53. doi: 10.1002/ijc.28103. [DOI] [PubMed] [Google Scholar]
- 40.Billaud EM, Rbah-Vidal L, Vidal A, Besse S, Tarrit S, Askienazy S, et al. Synthesis, radiofluorination, and in vivo evaluation of novel fluorinated and iodinated radiotracers for PET imaging and targeted radionuclide therapy of melanoma. Journal of medicinal chemistry. 2013;56:8455–67. doi: 10.1021/jm400877v. [DOI] [PubMed] [Google Scholar]
- 41.Pham TQ, Berghofer P, Liu X, Greguric I, Dikic B, Ballantyne P, et al. Preparation and biologic evaluation of a novel radioiodinated benzylpiperazine, 123I-MEL037, for malignant melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:1348–56. doi: 10.2967/jnumed.107.041673. [DOI] [PubMed] [Google Scholar]
- 42.Roberts MP, Nguyen V, Ashford ME, Berghofer P, Wyatt NA, Krause-Heuer AM, et al. Synthesis and in Vivo Evaluation of [123I]Melanin-Targeted Agents. Journal of medicinal chemistry. 2015;58:6214–24. doi: 10.1021/acs.jmedchem.5b00777. [DOI] [PubMed] [Google Scholar]
- 43.Chang CC, Chang CH, Shen CC, Chen CL, Liu RS, Lin MH, et al. Synthesis and evaluation of (1)(2)(3)/(1)(3)(1)I-Iochlonicotinamide as a novel SPECT probe for malignant melanoma. Bioorganic & medicinal chemistry. 2015;23:2261–9. doi: 10.1016/j.bmc.2015.02.017. [DOI] [PubMed] [Google Scholar]
- 44.Chang CC, Chang CH, Shen CC, Chen CL, Liu RS, Lin MH, et al. Synthesis and characterization of a novel radioiodinated phenylacetamide and its homolog as theranostic agents for malignant melanoma. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2016;81:201–9. doi: 10.1016/j.ejps.2015.10.019. [DOI] [PubMed] [Google Scholar]
- 45.Ren G, Miao Z, Liu H, Jiang L, Limpa-Amara N, Mahmood A, et al. Melanin-targeted preclinical PET imaging of melanoma metastasis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1692–9. doi: 10.2967/jnumed.109.066175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Wu SY, Huang SP, Lo YC, Liu RS, Wang SJ, Lin WJ, et al. Synthesis and preclinical characterization of [18F]FPBZA: a novel PET probe for melanoma. BioMed research international. 2014;2014:912498. doi: 10.1155/2014/912498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Garg PK, Nazih R, Wu Y, Singh R, Garg S. 4-11C-Methoxy N-(2-Diethylaminoethyl) Benzamide: A Novel Probe to Selectively Target Melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58:827–32. doi: 10.2967/jnumed.116.184564. [DOI] [PubMed] [Google Scholar]
- 48.Chang CC, Chang CH, Lo YH, Lin MH, Shen CC, Liu RS, et al. Preparation and characterization of a novel Al(18)F-NOTA-BZA conjugate for melanin-targeted imaging of malignant melanoma. Bioorganic & medicinal chemistry letters. 2016;26:4133–9. doi: 10.1016/j.bmcl.2016.06.022. [DOI] [PubMed] [Google Scholar]
- 49.Greguric I, Taylor SR, Denoyer D, Ballantyne P, Berghofer P, Roselt P, et al. Discovery of [18F]N-(2-(diethylamino)ethyl)-6-fluoronicotinamide: a melanoma positron emission tomography imaging radiotracer with high tumor to body contrast ratio and rapid renal clearance. Journal of medicinal chemistry. 2009;52:5299–302. doi: 10.1021/jm9008423. [DOI] [PubMed] [Google Scholar]
- 50.Denoyer D, Potdevin T, Roselt P, Neels OC, Kirby L, Greguric I, et al. Improved detection of regional melanoma metastasis using 18F-6-fluoro-N-[2-(diethylamino)ethyl] pyridine-3-carboxamide, a melanin-specific PET probe, by perilesional administration. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2011;52:115–22. doi: 10.2967/jnumed.110.078154. [DOI] [PubMed] [Google Scholar]
- 51.Liu H, Liu S, Miao Z, Deng Z, Shen B, Hong X, et al. Development of 18F-labeled picolinamide probes for PET imaging of malignant melanoma. Journal of medicinal chemistry. 2013;56:895–901. doi: 10.1021/jm301740k. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Liu H, Liu S, Miao Z, Jiang H, Deng Z, Hong X, et al. A novel aliphatic 18F-labeled probe for PET imaging of melanoma. Molecular pharmaceutics. 2013;10:3384–91. doi: 10.1021/mp400225s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Zhernosekov KP, Filosofov DV, Baum RP, Aschoff P, Bihl H, Razbash AA, et al. Processing of generator-produced 68Ga for medical application. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:1741–8. doi: 10.2967/jnumed.107.040378. [DOI] [PubMed] [Google Scholar]
- 54.Trencsenyi G, Denes N, Nagy G, Kis A, Vida A, Farkas F, et al. Comparative preclinical evaluation of 68Ga-NODAGA and 68Ga-HBED-CC conjugated procainamide in melanoma imaging. Journal of pharmaceutical and biomedical analysis. 2017;139:54–64. doi: 10.1016/j.jpba.2017.02.049. [DOI] [PubMed] [Google Scholar]
- 55.Kertesz I, Vida A, Nagy G, Emri M, Farkas A, Kis A, et al. In Vivo Imaging of Experimental Melanoma Tumors using the Novel Radiotracer 68Ga-NODAGA-Procainamide (PCA) Journal of Cancer. 2017;8:774–85. doi: 10.7150/jca.17550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Wang J, Wei C, Noor R, Burke A, McIntyre S, Bedikian AY. Surveillance for brain metastases in patients receiving systemic therapy for advanced melanoma. Melanoma research. 2014;24:54–60. doi: 10.1097/CMR.0000000000000022. [DOI] [PubMed] [Google Scholar]
- 57.Aukema TS, Valdes Olmos RA, Wouters MW, Klop WM, Kroon BB, Vogel WV, et al. Utility of preoperative 18F-FDG PET/CT and brain MRI in melanoma patients with palpable lymph node metastases. Annals of surgical oncology. 2010;17:2773–8. doi: 10.1245/s10434-010-1088-y. [DOI] [PubMed] [Google Scholar]
- 58.Belhocine TZ, Scott AM, Even-Sapir E, Urbain JL, Essner R. Role of nuclear medicine in the management of cutaneous malignant melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:957–67. [PubMed] [Google Scholar]
- 59.Unterrainer M, Galldiks N, Suchorska B, Kowalew LC, Wenter V, Schmid-Tannwald C, et al. 18F-FET PET Uptake Characteristics in Patients with Newly Diagnosed and Untreated Brain Metastasis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017;58:584–9. doi: 10.2967/jnumed.116.180075. [DOI] [PubMed] [Google Scholar]
- 60.Valdes Olmos RA, Vidal-Sicart S, Manca G, Mariani G, Leon-Ramirez LF, Rubello D, et al. Advances in radioguided surgery in oncology. The quarterly journal of nuclear medicine and molecular imaging : official publication of the Italian Association of Nuclear Medicine (AIMN) [and] the International Association of Radiopharmacology (IAR), [and] Section of the So. 2017;61:247–70. doi: 10.23736/S1824-4785.17.02995-8. [DOI] [PubMed] [Google Scholar]
- 61.Bronstein Y, Ng CS, Rohren E, Ross MI, Lee JE, Cormier J, et al. PET/CT in the management of patients with stage IIIC and IV metastatic melanoma considered candidates for surgery: evaluation of the additive value after conventional imaging. AJR American journal of roentgenology. 2012;198:902–8. doi: 10.2214/AJR.11.7280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Garg S, Kothari K, Thopate SR, Doke AK, Garg PK. Design, synthesis, and preliminary in vitro and in vivo evaluation of N-(2-diethylaminoethyl)-4-[18F]fluorobenzamide ([18F]-DAFBA): a novel potential PET probe to image melanoma tumors. Bioconjugate chemistry. 2009;20:583–90. doi: 10.1021/bc8005094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Wang Y, Li M, Zhang Y, Zhang F, Liu C, Song Y, et al. Detection of melanoma metastases with PET-Comparison of 18F-5-FPN with 18F-FDG. Nuclear medicine and biology. 2017;50:33–8. doi: 10.1016/j.nucmedbio.2017.03.005. [DOI] [PubMed] [Google Scholar]
- 64.Denoyer D, Greguric I, Roselt P, Neels OC, Aide N, Taylor SR, et al. High-contrast PET of melanoma using (18)F-MEL050, a selective probe for melanin with predominantly renal clearance. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:441–7. doi: 10.2967/jnumed.109.070060. [DOI] [PubMed] [Google Scholar]
- 65.Xie L, Yui J, Fujinaga M, Hatori A, Yamasaki T, Kumata K, et al. Molecular imaging of ectopic metabotropic glutamate 1 receptor in melanoma with a positron emission tomography radioprobe (18) F-FITM. International journal of cancer Journal international du cancer. 2014;135:1852–9. doi: 10.1002/ijc.28842. [DOI] [PubMed] [Google Scholar]
- 66.Rbah-Vidal L, Vidal A, Besse S, Cachin F, Bonnet M, Audin L, et al. Early detection and longitudinal monitoring of experimental primary and disseminated melanoma using [(1)(0)F]ICF01006, a highly promising melanoma PET tracer. European journal of nuclear medicine and molecular imaging. 2012;39:1449–61. doi: 10.1007/s00259-012-2168-y. [DOI] [PubMed] [Google Scholar]
- 67.Feng H, Xia X, Li C, Song Y, Qin C, Liu Q, et al. Imaging malignant melanoma with (18)F-5-FPN. European journal of nuclear medicine and molecular imaging. 2016;43:113–22. doi: 10.1007/s00259-015-3134-2. [DOI] [PubMed] [Google Scholar]
- 68.Sun X, Li Y, Liu T, Li Z, Zhang X, Chen X. Peptide-based imaging agents for cancer detection. Advanced drug delivery reviews. 2017;110–111:38–51. doi: 10.1016/j.addr.2016.06.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Singh M, Mukhopadhyay K. Alpha-melanocyte stimulating hormone: an emerging anti-inflammatory antimicrobial peptide. BioMed research international. 2014;2014:874610. doi: 10.1155/2014/874610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Chen J, Cheng Z, Hoffman TJ, Jurisson SS, Quinn TP. Melanoma-targeting properties of (99m)technetium-labeled cyclic alpha-melanocyte-stimulating hormone peptide analogues. Cancer research. 2000;60:5649–58. [PubMed] [Google Scholar]
- 71.Siegrist W, Oestreicher M, Stutz S, Girard J, Eberle AN. Radioreceptor assay for alpha-MSH using mouse B16 melanoma cells+ Journal of receptor research. 1988;8:323–43. doi: 10.3109/10799898809048996. [DOI] [PubMed] [Google Scholar]
- 72.Garg PK, Alston KL, Welsh PC, Zalutsky MR. Enhanced binding and inertness to dehalogenation of alpha-melanotropic peptides labeled using N-succinimidyl 3-iodobenzoate. Bioconjugate chemistry. 1996;7:233–9. doi: 10.1021/bc960001+. [DOI] [PubMed] [Google Scholar]
- 73.Vaidyanathan G, Zalutsky MR. Fluorine-18-labeled [Nle4,D-Phe7]-alpha-MSH, an alpha-melanocyte stimulating hormone analogue. Nuclear medicine and biology. 1997;24:171–8. doi: 10.1016/s0969-8051(96)00211-9. [DOI] [PubMed] [Google Scholar]
- 74.Cowell SM, Balse-Srinivasan PM, Ahn JM, Hruby VJ. Design and synthesis of peptide antagonists and inverse agonists for G protein-coupled receptors. Methods in enzymology. 2002;343:49–72. doi: 10.1016/s0076-6879(02)43127-8. [DOI] [PubMed] [Google Scholar]
- 75.Sawyer TK, Sanfilippo PJ, Hruby VJ, Engel MH, Heward CB, Burnett JB, et al. 4-Norleucine, 7-D-phenylalanine-alpha-melanocyte-stimulating hormone: a highly potent alpha-melanotropin with ultralong biological activity. Proceedings of the National Academy of Sciences of the United States of America. 1980;77:5754–8. doi: 10.1073/pnas.77.10.5754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hruby VJ, Wilkes BC, Hadley ME, Al-Obeidi F, Sawyer TK, Staples DJ, et al. alpha-Melanotropin: the minimal active sequence in the frog skin bioassay. Journal of medicinal chemistry. 1987;30:2126–30. doi: 10.1021/jm00394a033. [DOI] [PubMed] [Google Scholar]
- 77.Giblin MF, Wang N, Hoffman TJ, Jurisson SS, Quinn TP. Design and characterization of alpha-melanotropin peptide analogs cyclized through rhenium and technetium metal coordination. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:12814–8. doi: 10.1073/pnas.95.22.12814. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Froidevaux S, Calame-Christe M, Tanner H, Eberle AN. Melanoma targeting with DOTA-alpha-melanocyte-stimulating hormone analogs: structural parameters affecting tumor uptake and kidney uptake. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:887–95. [PubMed] [Google Scholar]
- 79.Bednarek MA, Silva MV, Arison B, MacNeil T, Kalyani RN, Huang RR, et al. Structure-function studies on the cyclic peptide MT-II, lactam derivative of alpha-melanotropin. Peptides. 1999;20:401–9. doi: 10.1016/s0196-9781(99)00048-0. [DOI] [PubMed] [Google Scholar]
- 80.Grieco P, Cai M, Liu L, Mayorov A, Chandler K, Trivedi D, et al. Design and microwave-assisted synthesis of novel macrocyclic peptides active at melanocortin receptors: discovery of potent and selective hMC5R receptor antagonists. Journal of medicinal chemistry. 2008;51:2701–7. doi: 10.1021/jm701181n. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Miao Y, Gallazzi F, Guo H, Quinn TP. 111In-labeled lactam bridge-cyclized alpha-melanocyte stimulating hormone peptide analogues for melanoma imaging. Bioconjugate chemistry. 2008;19:539–47. doi: 10.1021/bc700317w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Guo H, Yang J, Gallazzi F, Miao Y. Reduction of the ring size of radiolabeled lactam bridge-cyclized alpha-MSH peptide, resulting in enhanced melanoma uptake. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2010;51:418–26. doi: 10.2967/jnumed.109.071787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Barkey NM, Tafreshi NK, Josan JS, De Silva CR, Sill KN, Hruby VJ, et al. Development of melanoma-targeted polymer micelles by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. Journal of medicinal chemistry. 2011;54:8078–84. doi: 10.1021/jm201226w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Tafreshi NK, Huang X, Moberg VE, Barkey NM, Sondak VK, Tian H, et al. Synthesis and characterization of a melanoma-targeted fluorescence imaging probe by conjugation of a melanocortin 1 receptor (MC1R) specific ligand. Bioconjugate chemistry. 2012;23:2451–9. doi: 10.1021/bc300549s. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.McQuade P, Miao Y, Yoo J, Quinn TP, Welch MJ, Lewis JS. Imaging of melanoma using 64Cu- and 86Y-DOTA-ReCCMSH(Arg11), a cyclized peptide analogue of alpha-MSH. Journal of medicinal chemistry. 2005;48:2985–92. doi: 10.1021/jm0490282. [DOI] [PubMed] [Google Scholar]
- 86.Wei L, Butcher C, Miao Y, Gallazzi F, Quinn TP, Welch MJ, et al. Synthesis and biologic evaluation of 64Cu-labeled rhenium-cyclized alpha-MSH peptide analog using a cross-bridged cyclam chelator. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:64–72. [PubMed] [Google Scholar]
- 87.Wei L, Miao Y, Gallazzi F, Quinn TP, Welch MJ, Vavere AL, et al. Gallium-68-labeled DOTA-rhenium-cyclized alpha-melanocyte-stimulating hormone analog for imaging of malignant melanoma. Nuclear medicine and biology. 2007;34:945–53. doi: 10.1016/j.nucmedbio.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Ren G, Liu Z, Miao Z, Liu H, Subbarayan M, Chin FT, et al. PET of malignant melanoma using 18F-labeled metallopeptides. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2009;50:1865–72. doi: 10.2967/jnumed.109.062877. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Ren G, Liu S, Liu H, Miao Z, Cheng Z. Radiofluorinated rhenium cyclized alpha-MSH analogues for PET imaging of melanocortin receptor 1. Bioconjugate chemistry. 2010;21:2355–60. doi: 10.1021/bc100391a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Guo H, Shenoy N, Gershman BM, Yang J, Sklar LA, Miao Y. Metastatic melanoma imaging with an (111)In-labeled lactam bridge-cyclized alpha-melanocyte-stimulating hormone peptide. Nuclear medicine and biology. 2009;36:267–76. doi: 10.1016/j.nucmedbio.2009.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Guo H, Gallazzi F, Miao Y. Gallium-67-labeled lactam bridge-cyclized alpha-MSH peptides with enhanced melanoma uptake and reduced renal uptake. Bioconjugate chemistry. 2012;23:1341–8. doi: 10.1021/bc300191z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Guo H, Miao Y. Introduction of an 8-aminooctanoic acid linker enhances uptake of 99mTc-labeled lactam bridge-cyclized alpha-MSH peptide in melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:2057–63. doi: 10.2967/jnumed.114.145896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Guo H, Miao Y. Cu-64-labeled lactam bridge-cyclized alpha-MSH peptides for PET imaging of melanoma. Molecular pharmaceutics. 2012;9:2322–30. doi: 10.1021/mp300246j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Guo H, Gallazzi F, Miao Y. Design and evaluation of new Tc-99m-labeled lactam bridge-cyclized alpha-MSH peptides for melanoma imaging. Molecular pharmaceutics. 2013;10:1400–8. doi: 10.1021/mp3006984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Nagy G, Denes N, Kis A, Szabo JP, Berenyi E, Garai I, et al. Preclinical evaluation of melanocortin-1 receptor (MC1-R) specific 68Ga- and 44Sc-labeled DOTA-NAPamide in melanoma imaging. European journal of pharmaceutical sciences : official journal of the European Federation for Pharmaceutical Sciences. 2017;106:336–44. doi: 10.1016/j.ejps.2017.06.026. [DOI] [PubMed] [Google Scholar]
- 96.Cheng Z, Xiong Z, Subbarayan M, Chen X, Gambhir SS. 64Cu-labeled alpha-melanocyte-stimulating hormone analog for microPET imaging of melanocortin 1 receptor expression. Bioconjugate chemistry. 2007;18:765–72. doi: 10.1021/bc060306g. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Froidevaux S, Calame-Christe M, Schuhmacher J, Tanner H, Saffrich R, Henze M, et al. A gallium-labeled DOTA-alpha-melanocyte- stimulating hormone analog for PET imaging of melanoma metastases. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2004;45:116–23. [PubMed] [Google Scholar]
- 98.Cheng Z, Zhang L, Graves E, Xiong Z, Dandekar M, Chen X, et al. Small-animal PET of melanocortin 1 receptor expression using a 18F-labeled alpha-melanocyte-stimulating hormone analog. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:987–94. doi: 10.2967/jnumed.107.039602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Cheng Z, Chen J, Miao Y, Owen NK, Quinn TP, Jurisson SS. Modification of the structure of a metallopeptide: synthesis and biological evaluation of (111)In-labeled DOTA-conjugated rhenium-cyclized alpha-MSH analogues. Journal of medicinal chemistry. 2002;45:3048–56. doi: 10.1021/jm010408m. [DOI] [PubMed] [Google Scholar]
- 100.Miao Y, Benwell K, Quinn TP. 99mTc- and 111In-labeled alpha-melanocyte-stimulating hormone peptides as imaging probes for primary and pulmonary metastatic melanoma detection. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2007;48:73–80. [PubMed] [Google Scholar]
- 101.Quinn T, Zhang X, Miao Y. Targeted melanoma imaging and therapy with radiolabeled alpha-melanocyte stimulating hormone peptide analogues. Giornale italiano di dermatologia e venereologia : organo ufficiale, Societa italiana di dermatologia e sifilografia. 2010;145:245–58. [PMC free article] [PubMed] [Google Scholar]
- 102.Price TW, Greenman J, Stasiuk GJ. Current advances in ligand design for inorganic positron emission tomography tracers 68Ga, 64Cu, 89Zr and 44Sc. Dalton transactions. 2016;45:15702–24. doi: 10.1039/c5dt04706d. [DOI] [PubMed] [Google Scholar]
- 103.Chakravarty R, Goel S, Valdovinos HF, Hernandez R, Hong H, Nickles RJ, et al. Matching the decay half-life with the biological half-life: ImmunoPET imaging with (44)Sc-labeled cetuximab Fab fragment. Bioconjugate chemistry. 2014;25:2197–204. doi: 10.1021/bc500415x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Hernandez R, Valdovinos HF, Yang Y, Chakravarty R, Hong H, Barnhart TE, et al. (44)Sc: an attractive isotope for peptide-based PET imaging. Molecular pharmaceutics. 2014;11:2954–61. doi: 10.1021/mp500343j. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Zhang C, Zhang Z, Lin KS, Pan J, Dude I, Hundal-Jabal N, et al. Preclinical Melanoma Imaging with 68Ga-Labeled alpha-Melanocyte-Stimulating Hormone Derivatives Using PET. Theranostics. 2017;7:805–13. doi: 10.7150/thno.17117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Margadant C, Monsuur HN, Norman JC, Sonnenberg A. Mechanisms of integrin activation and trafficking. Current opinion in cell biology. 2011;23:607–14. doi: 10.1016/j.ceb.2011.08.005. [DOI] [PubMed] [Google Scholar]
- 107.Decristoforo C, Faintuch-Linkowski B, Rey A, von Guggenberg E, Rupprich M, Hernandez-Gonzales I, et al. [99mTc]HYNIC-RGD for imaging integrin alphavbeta3 expression. Nuclear medicine and biology. 2006;33:945–52. doi: 10.1016/j.nucmedbio.2006.09.001. [DOI] [PubMed] [Google Scholar]
- 108.Decristoforo C, Hernandez Gonzalez I, Carlsen J, Rupprich M, Huisman M, Virgolini I, et al. 68Ga- and 111In-labelled DOTA-RGD peptides for imaging of alphavbeta3 integrin expression. European journal of nuclear medicine and molecular imaging. 2008;35:1507–15. doi: 10.1007/s00259-008-0757-6. [DOI] [PubMed] [Google Scholar]
- 109.Hultsch C, Schottelius M, Auernheimer J, Alke A, Wester HJ. (18)F-Fluoroglucosylation of peptides, exemplified on cyclo(RGDfK) European journal of nuclear medicine and molecular imaging. 2009;36:1469–74. doi: 10.1007/s00259-009-1122-0. [DOI] [PubMed] [Google Scholar]
- 110.Wei L, Ye Y, Wadas TJ, Lewis JS, Welch MJ, Achilefu S, et al. (64)Cu-labeled CB-TE2A and diamsar-conjugated RGD peptide analogs for targeting angiogenesis: comparison of their biological activity. Nuclear medicine and biology. 2009;36:277–85. doi: 10.1016/j.nucmedbio.2008.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Jin ZH, Furukawa T, Kumata K, Xie L, Yui J, Wakizaka H, et al. Development of the Fibronectin-Mimetic Peptide KSSPHSRN(SG)5RGDSP as a Novel Radioprobe for Molecular Imaging of the Cancer Biomarker alpha5beta1 Integrin. Biological & pharmaceutical bulletin. 2015;38:1722–31. doi: 10.1248/bpb.b15-00344. [DOI] [PubMed] [Google Scholar]
- 112.Mena E, Owenius R, Turkbey B, Sherry R, Bratslavsky G, Macholl S, et al. [(1)(8)F]fluciclatide in the in vivo evaluation of human melanoma and renal tumors expressing alphavbeta 3 and alpha vbeta 5 integrins. European journal of nuclear medicine and molecular imaging. 2014;41:1879–88. doi: 10.1007/s00259-014-2791-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Sharma R, Kallur KG, Ryu JS, Parameswaran RV, Lindman H, Avril N, et al. Multicenter Reproducibility of 18F-Fluciclatide PET Imaging in Subjects with Solid Tumors. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2015;56:1855–61. doi: 10.2967/jnumed.115.158253. [DOI] [PubMed] [Google Scholar]
- 114.Beer AJ, Haubner R, Goebel M, Luderschmidt S, Spilker ME, Wester HJ, et al. Biodistribution and pharmacokinetics of the alphavbeta3-selective tracer 18F-galacto-RGD in cancer patients. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2005;46:1333–41. [PubMed] [Google Scholar]
- 115.Beer AJ, Haubner R, Sarbia M, Goebel M, Luderschmidt S, Grosu AL, et al. Positron emission tomography using [18F]Galacto-RGD identifies the level of integrin alpha(v)beta3 expression in man. Clinical cancer research : an official journal of the American Association for Cancer Research. 2006;12:3942–9. doi: 10.1158/1078-0432.CCR-06-0266. [DOI] [PubMed] [Google Scholar]
- 116.Gaertner FC, Kessler H, Wester HJ, Schwaiger M, Beer AJ. Radiolabelled RGD peptides for imaging and therapy. European journal of nuclear medicine and molecular imaging. 2012;39(Suppl 1):S126–38. doi: 10.1007/s00259-011-2028-1. [DOI] [PubMed] [Google Scholar]
- 117.Cai W, Chen X. Multimodality molecular imaging of tumor angiogenesis. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2008;49(Suppl 2):113S–28S. doi: 10.2967/jnumed.107.045922. [DOI] [PubMed] [Google Scholar]
- 118.Knetsch PA, Petrik M, Rangger C, Seidel G, Pietzsch HJ, Virgolini I, et al. [(6)(8)Ga]NS(3)-RGD and [(6)(8)Ga] Oxo-DO3A-RGD for imaging alpha(v)beta(3) integrin expression: synthesis, evaluation, and comparison. Nuclear medicine and biology. 2013;40:65–72. doi: 10.1016/j.nucmedbio.2012.09.006. [DOI] [PubMed] [Google Scholar]
- 119.Knetsch PA, Petrik M, Griessinger CM, Rangger C, Fani M, Kesenheimer C, et al. [68Ga]NODAGA-RGD for imaging alphavbeta3 integrin expression. European journal of nuclear medicine and molecular imaging. 2011;38:1303–12. doi: 10.1007/s00259-011-1778-0. [DOI] [PubMed] [Google Scholar]
- 120.Pohle K, Notni J, Bussemer J, Kessler H, Schwaiger M, Beer AJ. 68Ga-NODAGA-RGD is a suitable substitute for (18)F-Galacto-RGD and can be produced with high specific activity in a cGMP/GRP compliant automated process. Nuclear medicine and biology. 2012;39:777–84. doi: 10.1016/j.nucmedbio.2012.02.006. [DOI] [PubMed] [Google Scholar]
- 121.Knetsch PA, Zhai C, Rangger C, Blatzer M, Haas H, Kaeopookum P, et al. [(68)Ga]FSC-(RGD)3 a trimeric RGD peptide for imaging alphavbeta3 integrin expression based on a novel siderophore derived chelating scaffold-synthesis and evaluation. Nuclear medicine and biology. 2015;42:115–22. doi: 10.1016/j.nucmedbio.2014.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Zhai C, Summer D, Rangger C, Haas H, Haubner R, Decristoforo C. Fusarinine C, a novel siderophore-based bifunctional chelator for radiolabeling with Gallium-68. Journal of labelled compounds & radiopharmaceuticals. 2015;58:209–14. doi: 10.1002/jlcr.3286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Zhai C, Summer D, Rangger C, Franssen GM, Laverman P, Haas H, et al. Novel Bifunctional Cyclic Chelator for (89)Zr Labeling-Radiolabeling and Targeting Properties of RGD Conjugates. Molecular pharmaceutics. 2015;12:2142–50. doi: 10.1021/acs.molpharmaceut.5b00128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Petrik M, Zhai C, Novy Z, Urbanek L, Haas H, Decristoforo C. In Vitro and In Vivo Comparison of Selected Ga-68 and Zr-89 Labelled Siderophores. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016;18:344–52. doi: 10.1007/s11307-015-0897-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Zhai C, Franssen GM, Petrik M, Laverman P, Summer D, Rangger C, et al. Comparison of Ga-68-Labeled Fusarinine C-Based Multivalent RGD Conjugates and [(68)Ga]NODAGA-RGD-In Vivo Imaging Studies in Human Xenograft Tumors. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016;18:758–67. doi: 10.1007/s11307-016-0931-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Eisenwiener KP, Prata MI, Buschmann I, Zhang HW, Santos AC, Wenger S, et al. NODAGATOC, a new chelator-coupled somatostatin analogue labeled with [67/68Ga] and [111In] for SPECT, PET, and targeted therapeutic applications of somatostatin receptor (hsst2) expressing tumors. Bioconjugate chemistry. 2002;13:530–41. doi: 10.1021/bc010074f. [DOI] [PubMed] [Google Scholar]
- 127.Simecek J, Schulz M, Notni J, Plutnar J, Kubicek V, Havlickova J, et al. Complexation of metal ions with TRAP (1,4,7-triazacyclononane phosphinic acid) ligands and 1,4,7-triazacyclononane-1,4,7-triacetic acid: phosphinate-containing ligands as unique chelators for trivalent gallium. Inorganic chemistry. 2012;51:577–90. doi: 10.1021/ic202103v. [DOI] [PubMed] [Google Scholar]
- 128.Notni J, Simecek J, Hermann P, Wester HJ. TRAP, a powerful and versatile framework for gallium-68 radiopharmaceuticals. Chemistry (Weinheim an der Bergstrasse, Germany) 2011;17:14718–22. doi: 10.1002/chem.201103503. [DOI] [PubMed] [Google Scholar]
- 129.Notni J, Pohle K, Wester HJ. Comparative gallium-68 labeling of TRAP-, NOTA-, and DOTA-peptides: practical consequences for the future of gallium-68-PET. EJNMMI research. 2012;2:28. doi: 10.1186/2191-219X-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Notni J, Pohle K, Wester HJ. Be spoilt for choice with radiolabelled RGD peptides: preclinical evaluation of (6)(8)Ga-TRAP(RGD)(3) Nuclear medicine and biology. 2013;40:33–41. doi: 10.1016/j.nucmedbio.2012.08.006. [DOI] [PubMed] [Google Scholar]
- 131.Simecek J, Hermann P, Wester HJ, Notni J. How is (68)Ga labeling of macrocyclic chelators influenced by metal ion contaminants in (68)Ge/(68)Ga generator eluates? ChemMedChem. 2013;8:95–103. doi: 10.1002/cmdc.201200471. [DOI] [PubMed] [Google Scholar]
- 132.Simecek J, Notni J, Kapp TG, Kessler H, Wester HJ. Benefits of NOPO as chelator in gallium-68 peptides, exemplified by preclinical characterization of (68)Ga-NOPO-c(RGDfK) Molecular pharmaceutics. 2014;11:1687–95. doi: 10.1021/mp5000746. [DOI] [PubMed] [Google Scholar]
- 133.Neubauer S, Rechenmacher F, Beer AJ, Curnis F, Pohle K, D’Alessandria C, et al. Selective imaging of the angiogenic relevant integrins alpha5beta1 and alphavbeta3. Angewandte Chemie (International ed in English) 2013;52:11656–9. doi: 10.1002/anie.201306376. [DOI] [PubMed] [Google Scholar]
- 134.Heckmann D, Meyer A, Laufer B, Zahn G, Stragies R, Kessler H. Rational design of highly active and selective ligands for the alpha5beta1 integrin receptor. Chembiochem : a European journal of chemical biology. 2008;9:1397–407. doi: 10.1002/cbic.200800045. [DOI] [PubMed] [Google Scholar]
- 135.Koivunen E, Wang B, Ruoslahti E. Isolation of a highly specific ligand for the alpha 5 beta 1 integrin from a phage display library. The Journal of cell biology. 1994;124:373–80. doi: 10.1083/jcb.124.3.373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Haubner R, Maschauer S, Prante O. PET radiopharmaceuticals for imaging integrin expression: tracers in clinical studies and recent developments. BioMed research international. 2014;2014:871609. doi: 10.1155/2014/871609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.D’Alessandria C, Pohle K, Rechenmacher F, Neubauer S, Notni J, Wester HJ, et al. In vivo biokinetic and metabolic characterization of the (6)(8)Ga-labelled alpha5beta1-selective peptidomimetic FR366. European journal of nuclear medicine and molecular imaging. 2016;43:953–63. doi: 10.1007/s00259-015-3218-z. [DOI] [PubMed] [Google Scholar]
- 138.Notni J, Steiger K, Hoffmann F, Reich D, Kapp TG, Rechenmacher F, et al. Complementary, Selective PET Imaging of Integrin Subtypes alpha5beta1 and alphavbeta3 Using 68Ga-Aquibeprin and 68Ga-Avebetrin. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:460–6. doi: 10.2967/jnumed.115.165720. [DOI] [PubMed] [Google Scholar]
- 139.Notni J, Steiger K, Hoffmann F, Reich D, Schwaiger M, Kessler H, et al. Variation of Specific Activities of 68Ga-Aquibeprin and 68Ga-Avebetrin Enables Selective PET Imaging of Different Expression Levels of Integrins alpha5beta1 and alphavbeta3. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1618–24. doi: 10.2967/jnumed.116.173948. [DOI] [PubMed] [Google Scholar]
- 140.Bianchini F, Fabbrizzi P, Menchi G, Raspanti S, Bottoncetti A, Passeri A, et al. Radiosynthesis and micro-SPECT analysis of triazole-based RGD integrin ligands as non-peptide molecular imaging probes for angiogenesis. Bioorganic & medicinal chemistry. 2015;23:1112–22. doi: 10.1016/j.bmc.2014.12.065. [DOI] [PubMed] [Google Scholar]
- 141.Mas-Moruno C, Fraioli R, Rechenmacher F, Neubauer S, Kapp TG, Kessler H. alphavbeta3- or alpha5beta1-Integrin-Selective Peptidomimetics for Surface Coating. Angewandte Chemie (International ed in English) 2016;55:7048–67. doi: 10.1002/anie.201509782. [DOI] [PubMed] [Google Scholar]
- 142.Haskali MB, Denoyer D, Noonan W, Culinane C, Rangger C, Pouliot N, et al. Sulfonation of Tyrosine as a Method To Improve Biodistribution of Peptide-Based Radiotracers: Novel 18F-Labeled Cyclic RGD Analogues. Molecular pharmaceutics. 2017;14:1169–80. doi: 10.1021/acs.molpharmaceut.6b01062. [DOI] [PubMed] [Google Scholar]
- 143.Park SH, Zheng JH, Nguyen VH, Jiang SN, Kim DY, Szardenings M, et al. RGD Peptide Cell-Surface Display Enhances the Targeting and Therapeutic Efficacy of Attenuated Salmonella-mediated Cancer Therapy. Theranostics. 2016;6:1672–82. doi: 10.7150/thno.16135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Melemenidis S, Jefferson A, Ruparelia N, Akhtar AM, Xie J, Allen D, et al. Molecular magnetic resonance imaging of angiogenesis in vivo using polyvalent cyclic RGD-iron oxide microparticle conjugates. Theranostics. 2015;5:515–29. doi: 10.7150/thno.10319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 145.Fan Q, Cheng K, Hu X, Ma X, Zhang R, Yang M, et al. Transferring biomarker into molecular probe: melanin nanoparticle as a naturally active platform for multimodality imaging. Journal of the American Chemical Society. 2014;136:15185–94. doi: 10.1021/ja505412p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Li C, Wang W, Wu Q, Ke S, Houston J, Sevick-Muraca E, et al. Dual optical and nuclear imaging in human melanoma xenografts using a single targeted imaging probe. Nuclear medicine and biology. 2006;33:349–58. doi: 10.1016/j.nucmedbio.2006.01.001. [DOI] [PubMed] [Google Scholar]
- 147.Yang J, Guo H, Gallazzi F, Berwick M, Padilla RS, Miao Y. Evaluation of a novel Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptide for potential melanoma therapy. Bioconjugate chemistry. 2009;20:1634–42. doi: 10.1021/bc9001954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Yang J, Guo H, Miao Y. Technetium-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptides for human melanoma imaging. Nuclear medicine and biology. 2010;37:873–83. doi: 10.1016/j.nucmedbio.2010.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.Yang J, Miao Y. Substitution of Gly with Ala enhanced the melanoma uptake of technetium-99m-labeled Arg-Ala-Asp-conjugated alpha-melanocyte stimulating hormone peptide. Bioorganic & medicinal chemistry letters. 2012;22:1541–5. doi: 10.1016/j.bmcl.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Flook AM, Yang J, Miao Y. Evaluation of new Tc-99m-labeled Arg-X-Asp-conjugated alpha-melanocyte stimulating hormone peptides for melanoma imaging. Molecular pharmaceutics. 2013;10:3417–24. doi: 10.1021/mp400248f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 151.Flook AM, Yang J, Miao Y. Effects of amino acids on melanoma targeting and clearance properties of Tc-99m-labeled Arg-X-Asp-conjugated alpha-melanocyte stimulating hormone peptides. Journal of medicinal chemistry. 2013;56:8793–802. doi: 10.1021/jm4012356. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Yang J, Flook AM, Feng C, Miao Y. Linker modification reduced the renal uptake of technetium-99m-labeled Arg-Ala-Asp-conjugated alpha-melanocyte stimulating hormone peptide. Bioorganic & medicinal chemistry letters. 2014;24:195–8. doi: 10.1016/j.bmcl.2013.11.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 153.Yang J, Guo H, Padilla RS, Berwick M, Miao Y. Replacement of the Lys linker with an Arg linker resulting in improved melanoma uptake and reduced renal uptake of Tc-99m-labeled Arg-Gly-Asp-conjugated alpha-melanocyte stimulating hormone hybrid peptide. Bioorganic & medicinal chemistry. 2010;18:6695–700. doi: 10.1016/j.bmc.2010.07.061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Yang J, Lu J, Miao Y. Structural modification on the Lys linker enhanced tumor to kidney uptake ratios of 99mTc-labeled RGD-conjugated alpha-MSH hybrid peptides. Molecular pharmaceutics. 2012;9:1418–24. doi: 10.1021/mp2006642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 155.Xu J, Yang J, Miao Y. Dual receptor-targeting (9)(9)mTc-labeled Arg-Gly-Asp-conjugated Alpha-Melanocyte stimulating hormone hybrid peptides for human melanoma imaging. Nuclear medicine and biology. 2015;42:369–74. doi: 10.1016/j.nucmedbio.2014.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Yang J, Hu CA, Miao Y. Tc-99m-labeled RGD-conjugated alpha-melanocyte stimulating hormone hybrid peptides with reduced renal uptake. Amino acids. 2015;47:813–23. doi: 10.1007/s00726-014-1911-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 157.Flook AM, Yang J, Miao Y. Substitution of the Lys linker with the beta-Ala linker dramatically decreased the renal uptake of 99mTc-labeled Arg-X-Asp-conjugated and X-Ala-Asp-conjugated alpha-melanocyte stimulating hormone peptides. Journal of medicinal chemistry. 2014;57:9010–8. doi: 10.1021/jm501114v. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Kunishima N, Shimada Y, Tsuji Y, Sato T, Yamamoto M, Kumasaka T, et al. Structural basis of glutamate recognition by a dimeric metabotropic glutamate receptor. Nature. 2000;407:971–7. doi: 10.1038/35039564. [DOI] [PubMed] [Google Scholar]
- 159.Namkoong J, Shin SS, Lee HJ, Marin YE, Wall BA, Goydos JS, et al. Metabotropic glutamate receptor 1 and glutamate signaling in human melanoma. Cancer research. 2007;67:2298–305. doi: 10.1158/0008-5472.CAN-06-3665. [DOI] [PubMed] [Google Scholar]
- 160.Pollock PM, Cohen-Solal K, Sood R, Namkoong J, Martino JJ, Koganti A, et al. Melanoma mouse model implicates metabotropic glutamate signaling in melanocytic neoplasia. Nat Genet. 2003;34:108–12. doi: 10.1038/ng1148. [DOI] [PubMed] [Google Scholar]
- 161.Ohtani Y, Harada T, Funasaka Y, Nakao K, Takahara C, Abdel-Daim M, et al. Metabotropic glutamate receptor subtype-1 is essential for in vivo growth of melanoma. Oncogene. 2008;27:7162–70. doi: 10.1038/onc.2008.329. [DOI] [PubMed] [Google Scholar]
- 162.Shin SS, Namkoong J, Wall BA, Gleason R, Lee HJ, Chen S. Oncogenic activities of metabotropic glutamate receptor 1 (Grm1) in melanocyte transformation. Pigment cell & melanoma research. 2008;21:368–78. doi: 10.1111/j.1755-148X.2008.00452.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 163.Gelb T, Pshenichkin S, Rodriguez OC, Hathaway HA, Grajkowska E, DiRaddo JO, et al. Metabotropic glutamate receptor 1 acts as a dependence receptor creating a requirement for glutamate to sustain the viability and growth of human melanomas. Oncogene. 2015;34:2711–20. doi: 10.1038/onc.2014.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Yamasaki T, Fujinaga M, Maeda J, Kawamura K, Yui J, Hatori A, et al. Imaging for metabotropic glutamate receptor subtype 1 in rat and monkey brains using PET with [18F]FITM. European journal of nuclear medicine and molecular imaging. 2012;39:632–41. doi: 10.1007/s00259-011-1995-6. [DOI] [PubMed] [Google Scholar]
- 165.Yamasaki T, Fujinaga M, Kawamura K, Yui J, Hatori A, Ohya T, et al. In vivo measurement of the affinity and density of metabotropic glutamate receptor subtype 1 in rat brain using 18F-FITM in small-animal PET. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:1601–7. doi: 10.2967/jnumed.112.105908. [DOI] [PubMed] [Google Scholar]
- 166.Fujinaga M, Xie L, Yamasaki T, Yui J, Shimoda Y, Hatori A, et al. Synthesis and evaluation of 4-halogeno-N-[4-[6-(isopropylamino)pyrimidin-4-yl]-1,3-thiazol-2-yl]-N-[11C]methy lbenzamide for imaging of metabotropic glutamate 1 receptor in melanoma. Journal of medicinal chemistry. 2015;58:1513–23. doi: 10.1021/jm501845n. [DOI] [PubMed] [Google Scholar]
- 167.Kuphal S, Bauer R, Bosserhoff AK. Integrin signaling in malignant melanoma. Cancer metastasis reviews. 2005;24:195–222. doi: 10.1007/s10555-005-1572-1. [DOI] [PubMed] [Google Scholar]
- 168.Peng L, Liu R, Marik J, Wang X, Takada Y, Lam KS. Combinatorial chemistry identifies high-affinity peptidomimetics against alpha4beta1 integrin for in vivo tumor imaging. Nature chemical biology. 2006;2:381–9. doi: 10.1038/nchembio798. [DOI] [PubMed] [Google Scholar]
- 169.Walker D, Li Y, Roxin A, Schaffer P, Adam MJ, Perrin DM. Facile synthesis and 18F-radiolabeling of alpha4beta1-specific LLP2A-aryltrifluoroborate peptidomimetic conjugates. Bioorganic & medicinal chemistry letters. 2016;26:5126–31. doi: 10.1016/j.bmcl.2016.08.011. [DOI] [PubMed] [Google Scholar]
- 170.Shokeen M, Zheleznyak A, Wilson JM, Jiang M, Liu R, Ferdani R, et al. Molecular imaging of very late antigen-4 (alpha4beta1 integrin) in the premetastatic niche. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:779–86. doi: 10.2967/jnumed.111.100073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 171.Jiang M, Ferdani R, Shokeen M, Anderson CJ. Comparison of two cross-bridged macrocyclic chelators for the evaluation of 64Cu-labeled-LLP2A, a peptidomimetic ligand targeting VLA-4-positive tumors. Nuclear medicine and biology. 2013;40:245–51. doi: 10.1016/j.nucmedbio.2012.10.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Beaino W, Anderson CJ. PET imaging of very late antigen-4 in melanoma: comparison of 68Ga- and 64Cu-labeled NODAGA and CB-TE1A1P-LLP2A conjugates. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:1856–63. doi: 10.2967/jnumed.114.144881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Gai Y, Sun L, Hui W, Ouyang Q, Anderson CJ, Xiang G, et al. New Bifunctional Chelator p-SCN-PhPr-NE3TA for Copper-64: Synthesis, Peptidomimetic Conjugation, Radiolabeling, and Evaluation for PET Imaging. Inorganic chemistry. 2016;55:6892–901. doi: 10.1021/acs.inorgchem.6b00395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Beaino W, Nedrow JR, Anderson CJ. Evaluation of (68)Ga- and (177)Lu-DOTA-PEG4-LLP2A for VLA-4-Targeted PET Imaging and Treatment of Metastatic Melanoma. Molecular pharmaceutics. 2015;12:1929–38. doi: 10.1021/mp5006917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Redman JM, Gibney GT, Atkins MB. Advances in immunotherapy for melanoma. BMC medicine. 2016;14:20. doi: 10.1186/s12916-016-0571-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.Mondanelli G, Ugel S, Grohmann U, Bronte V. The immune regulation in cancer by the amino acid metabolizing enzymes ARG and IDO. Current opinion in pharmacology. 2017;35:30–9. doi: 10.1016/j.coph.2017.05.002. [DOI] [PubMed] [Google Scholar]
- 177.Munn DH, Mellor AL. IDO in the Tumor Microenvironment: Inflammation, Counter-Regulation, and Tolerance. Trends in immunology. 2016;37:193–207. doi: 10.1016/j.it.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 178.Xin Y, Cai H. Improved Radiosynthesis and Biological Evaluations of L- and D-1-[18F]Fluoroethyl-Tryptophan for PET Imaging of IDO-Mediated Kynurenine Pathway of Tryptophan Metabolism. Molecular imaging and biology : MIB : the official publication of the Academy of Molecular Imaging. 2016 doi: 10.1007/s11307-016-1024-z. [DOI] [PubMed] [Google Scholar]
- 179.Huang X, Xiao X, Gillies RJ, Tian H. Design and automated production of 11C-alpha-methyl-l-tryptophan (11C-AMT) Nuclear medicine and biology. 2016;43:303–8. doi: 10.1016/j.nucmedbio.2016.02.001. [DOI] [PubMed] [Google Scholar]
- 180.Giglio BC, Fei H, Wang M, Wang H, He L, Feng H, et al. Synthesis of 5-[18F]Fluoro-alpha-methyl Tryptophan: New Trp Based PET Agents. Theranostics. 2017;7:1524–30. doi: 10.7150/thno.19371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Ehlerding EB, England CG, McNeel DG, Cai W. Molecular Imaging of Immunotherapy Targets in Cancer. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:1487–92. doi: 10.2967/jnumed.116.177493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Natarajan A, Mayer AT, Xu L, Reeves RE, Gano J, Gambhir SS. Novel Radiotracer for ImmunoPET Imaging of PD-1 Checkpoint Expression on Tumor Infiltrating Lymphocytes. Bioconjugate chemistry. 2015;26:2062–9. doi: 10.1021/acs.bioconjchem.5b00318. [DOI] [PubMed] [Google Scholar]
- 183.Hettich M, Braun F, Bartholoma MD, Schirmbeck R, Niedermann G. High-Resolution PET Imaging with Therapeutic Antibody-based PD-1/PD-L1 Checkpoint Tracers. Theranostics. 2016;6:1629–40. doi: 10.7150/thno.15253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 184.Nedrow JR, Josefsson A, Park S, Ranka S, Roy S, Sgouros G. Imaging of programmed death ligand-1 (PD-L1): impact of protein concentration on distribution of anti-PD-L1 SPECT agent in an immunocompetent melanoma murine model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2017 doi: 10.2967/jnumed.117.193268. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 185.Sharp PA. Ctr1 and its role in body copper homeostasis. The international journal of biochemistry & cell biology. 2003;35:288–91. doi: 10.1016/s1357-2725(02)00134-6. [DOI] [PubMed] [Google Scholar]
- 186.Chakravarty R, Chakraborty S, Dash A. 64Cu2+ Ions as PET Probe: An Emerging Paradigm in Molecular Imaging of Cancer. Molecular pharmaceutics. 2016;13:3601–12. doi: 10.1021/acs.molpharmaceut.6b00582. [DOI] [PubMed] [Google Scholar]
- 187.Kim KI, Jang SJ, Park JH, Lee YJ, Lee TS, Woo KS, et al. Detection of increased 64Cu uptake by human copper transporter 1 gene overexpression using PET with 64CuCl2 in human breast cancer xenograft model. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:1692–8. doi: 10.2967/jnumed.114.141127. [DOI] [PubMed] [Google Scholar]
- 188.Peng F, Lu X, Janisse J, Muzik O, Shields AF. PET of human prostate cancer xenografts in mice with increased uptake of 64CuCl2. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2006;47:1649–52. [PubMed] [Google Scholar]
- 189.Qin C, Liu H, Chen K, Hu X, Ma X, Lan X, et al. Theranostics of malignant melanoma with 64CuCl2. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:812–7. doi: 10.2967/jnumed.113.133850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Jiang L, Tu Y, Hu X, Bao A, Chen H, Ma X, et al. Pilot Study of 64Cu(I) for PET Imaging of Melanoma. Scientific reports. 2017;7:2574. doi: 10.1038/s41598-017-02691-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.England CG, Rui L, Cai W. Lymphoma: current status of clinical and preclinical imaging with radiolabeled antibodies. European journal of nuclear medicine and molecular imaging. 2017;44:517–32. doi: 10.1007/s00259-016-3560-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Jauw YW, Menke-van der Houven van Oordt CW, Hoekstra OS, Hendrikse NH, Vugts DJ, Zijlstra JM, et al. Immuno-Positron Emission Tomography with Zirconium-89-Labeled Monoclonal Antibodies in Oncology: What Can We Learn from Initial Clinical Trials? Frontiers in pharmacology. 2016;7:131. doi: 10.3389/fphar.2016.00131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 193.Halpern SE, Dillman RO, Witztum KF, Shega JF, Hagan PL, Burrows WM, et al. Radioimmunodetection of melanoma utilizing In-111 96.5 monoclonal antibody: a preliminary report. Radiology. 1985;155:493–9. doi: 10.1148/radiology.155.2.3983401. [DOI] [PubMed] [Google Scholar]
- 194.Rosenblum MG, Murray JL, Haynie TP, Glenn HJ, Jahns MF, Benjamin RS, et al. Pharmacokinetics of 111In-labeled anti-p97 monoclonal antibody in patients with metastatic malignant melanoma. Cancer research. 1985;45:2382–6. [PubMed] [Google Scholar]
- 195.Dadachova E, Moadel T, Schweitzer AD, Bryan RA, Zhang T, Mints L, et al. Radiolabeled melanin-binding peptides are safe and effective in treatment of human pigmented melanoma in a mouse model of disease. Cancer biotherapy & radiopharmaceuticals. 2006;21:117–29. doi: 10.1089/cbr.2006.21.117. [DOI] [PubMed] [Google Scholar]
- 196.Dadachova E, Revskaya E, Sesay MA, Damania H, Boucher R, Sellers RS, et al. Pre-clinical evaluation and efficacy studies of a melanin-binding IgM antibody labeled with 188Re against experimental human metastatic melanoma in nude mice. Cancer biology & therapy. 2008;7:1116–27. doi: 10.4161/cbt.7.7.6197. [DOI] [PubMed] [Google Scholar]
- 197.Dadachova E, Nosanchuk JD, Shi L, Schweitzer AD, Frenkel A, Nosanchuk JS, et al. Dead cells in melanoma tumors provide abundant antigen for targeted delivery of ionizing radiation by a mAb to melanin. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:14865–70. doi: 10.1073/pnas.0406180101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 198.Revskaya E, Jongco AM, Sellers RS, Howell RC, Koba W, Guimaraes AJ, et al. Radioimmunotherapy of experimental human metastatic melanoma with melanin-binding antibodies and in combination with dacarbazine. Clinical cancer research : an official journal of the American Association for Cancer Research. 2009;15:2373–9. doi: 10.1158/1078-0432.CCR-08-2376. [DOI] [PubMed] [Google Scholar]
- 199.Jandl T, Revskaya E, Jiang Z, Bryan RA, Casadevall A, Dadachova E. Complement-dependent cytotoxicity of an antibody to melanin in radioimmunotherapy of metastatic melanoma. Immunotherapy. 2013;5:357–64. doi: 10.2217/imt.13.16. [DOI] [PubMed] [Google Scholar]
- 200.Suzuki M, Cheung NK. Disialoganglioside GD2 as a therapeutic target for human diseases. Expert Opin Ther Targets. 2015;19:349–62. doi: 10.1517/14728222.2014.986459. [DOI] [PubMed] [Google Scholar]
- 201.Voss SD, Smith SV, DiBartolo N, McIntosh LJ, Cyr EM, Bonab AA, et al. Positron emission tomography (PET) imaging of neuroblastoma and melanoma with 64Cu-SarAr immunoconjugates. Proceedings of the National Academy of Sciences of the United States of America. 2007;104:17489–93. doi: 10.1073/pnas.0708436104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 202.Dearling JL, Voss SD, Dunning P, Snay E, Fahey F, Smith SV, et al. Imaging cancer using PET--the effect of the bifunctional chelator on the biodistribution of a (64)Cu-labeled antibody. Nuclear medicine and biology. 2011;38:29–38. doi: 10.1016/j.nucmedbio.2010.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 203.Vavere AL, Butch ER, Dearling JL, Packard AB, Navid F, Shulkin BL, et al. 64Cu-p-NH2-Bn-DOTA-hu14.18K322A, a PET radiotracer targeting neuroblastoma and melanoma. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2012;53:1772–8. doi: 10.2967/jnumed.112.104208. [DOI] [PubMed] [Google Scholar]
- 204.Herhaus P, Habringer S, Vag T, Steiger K, Slotta-Huspenina J, Gerngross C, et al. Response assessment with the CXCR4-directed positron emission tomography tracer [68Ga]Pentixafor in a patient with extranodal marginal zone lymphoma of the orbital cavities. EJNMMI research. 2017;7:51. doi: 10.1186/s13550-017-0294-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Werner RA, Weich A, Higuchi T, Schmid JS, Schirbel A, Lassmann M, et al. Imaging of Chemokine Receptor 4 Expression in Neuroendocrine Tumors - a Triple Tracer Comparative Approach. Theranostics. 2017;7:1489–98. doi: 10.7150/thno.18754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Lapa C, Schreder M, Schirbel A, Samnick S, Kortum KM, Herrmann K, et al. [68Ga]Pentixafor-PET/CT for imaging of chemokine receptor CXCR4 expression in multiple myeloma - Comparison to [18F]FDG and laboratory values. Theranostics. 2017;7:205–12. doi: 10.7150/thno.16576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Lapa C, Luckerath K, Kleinlein I, Monoranu CM, Linsenmann T, Kessler AF, et al. (68)Ga-Pentixafor-PET/CT for Imaging of Chemokine Receptor 4 Expression in Glioblastoma. Theranostics. 2016;6:428–34. doi: 10.7150/thno.13986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Derlin T, Jonigk D, Bauersachs J, Bengel FM. Molecular Imaging of Chemokine Receptor CXCR4 in Non-Small Cell Lung Cancer Using 68Ga-Pentixafor PET/CT: Comparison With 18F-FDG. Clinical nuclear medicine. 2016;41:e204–5. doi: 10.1097/RLU.0000000000001092. [DOI] [PubMed] [Google Scholar]
- 209.Liang Z, Zhan W, Zhu A, Yoon Y, Lin S, Sasaki M, et al. Development of a unique small molecule modulator of CXCR4. PloS one. 2012;7:e34038. doi: 10.1371/journal.pone.0034038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Vag T, Gerngross C, Herhaus P, Eiber M, Philipp-Abbrederis K, Graner FP, et al. First Experience with Chemokine Receptor CXCR4-Targeted PET Imaging of Patients with Solid Cancers. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2016;57:741–6. doi: 10.2967/jnumed.115.161034. [DOI] [PubMed] [Google Scholar]
- 211.Norain A, Dadachova E. Targeted Radionuclide Therapy of Melanoma. Seminars in nuclear medicine. 2016;46:250–9. doi: 10.1053/j.semnuclmed.2015.12.005. [DOI] [PubMed] [Google Scholar]
- 212.Kam BL, Teunissen JJ, Krenning EP, de Herder WW, Khan S, van Vliet EI, et al. Lutetium-labelled peptides for therapy of neuroendocrine tumours. European journal of nuclear medicine and molecular imaging. 2012;39(Suppl 1):S103–12. doi: 10.1007/s00259-011-2039-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 213.Strosberg J, El-Haddad G, Wolin E, Hendifar A, Yao J, Chasen B, et al. Phase 3 Trial of 177Lu-Dotatate for Midgut Neuroendocrine Tumors. The New England journal of medicine. 2017;376:125–35. doi: 10.1056/NEJMoa1607427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 214.Horsch D, Ezziddin S, Haug A, Gratz KF, Dunkelmann S, Miederer M, et al. Effectiveness and side-effects of peptide receptor radionuclide therapy for neuroendocrine neoplasms in Germany: A multi-institutional registry study with prospective follow-up. Eur J Cancer. 2016;58:41–51. doi: 10.1016/j.ejca.2016.01.009. [DOI] [PubMed] [Google Scholar]
- 215.Shibuya M. Vascular endothelial growth factor and its receptor system: physiological functions in angiogenesis and pathological roles in various diseases. Journal of biochemistry. 2013;153:13–9. doi: 10.1093/jb/mvs136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 216.Ferrara N, Adamis AP. Ten years of anti-vascular endothelial growth factor therapy. Nature reviews Drug discovery. 2016;15:385–403. doi: 10.1038/nrd.2015.17. [DOI] [PubMed] [Google Scholar]
- 217.Camacho X, Calzada V, Fernandez M, Alonso O, Chammas R, Riva E, et al. 177Lu-DOTA-Bevacizumab: Radioimmunotherapy agent for melanoma. Current radiopharmaceuticals. 2016 doi: 10.2174/1874471009666161010155246. [DOI] [PubMed] [Google Scholar]
- 218.Guo H, Miao Y. Melanoma targeting property of a Lu-177-labeled lactam bridge-cyclized alpha-MSH peptide. Bioorganic & medicinal chemistry letters. 2013;23:2319–23. doi: 10.1016/j.bmcl.2013.02.069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 219.Joyal JL, Barrett JA, Marquis JC, Chen J, Hillier SM, Maresca KP, et al. Preclinical evaluation of an 131I-labeled benzamide for targeted radiotherapy of metastatic melanoma. Cancer research. 2010;70:4045–53. doi: 10.1158/0008-5472.CAN-09-4414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Bonnet M, Mishellany F, Papon J, Cayre A, Penault-Llorca F, Madelmont JC, et al. Anti-melanoma efficacy of internal radionuclide therapy in relation to melanin target distribution. Pigment cell & melanoma research. 2010;23:e1–11. doi: 10.1111/j.1755-148X.2010.00716.x. [DOI] [PubMed] [Google Scholar]
- 221.Viallard C, Perrot Y, Boudhraa Z, Jouberton E, Miot-Noirault E, Bonnet M, et al. [(1)(2)(3)I]ICF01012 melanoma imaging and [(1)(3)(1)I]ICF01012 dosimetry allow adapted internal targeted radiotherapy in preclinical melanoma models. European journal of dermatology : EJD. 2015;25:29–35. doi: 10.1684/ejd.2014.2481. [DOI] [PubMed] [Google Scholar]
- 222.Viallard C, Chezal JM, Mishellany F, Ranchon-Cole I, Pereira B, Herbette A, et al. Targeting DNA repair by coDbait enhances melanoma targeted radionuclide therapy. Oncotarget. 2016;7:12927–36. doi: 10.18632/oncotarget.7340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Mier W, Kratochwil C, Hassel JC, Giesel FL, Beijer B, Babich JW, et al. Radiopharmaceutical therapy of patients with metastasized melanoma with the melanin-binding benzamide 131I-BA52. Journal of nuclear medicine : official publication, Society of Nuclear Medicine. 2014;55:9–14. doi: 10.2967/jnumed.112.112789. [DOI] [PubMed] [Google Scholar]
- 224.Maisonial A, Kuhnast B, Papon J, Boisgard R, Bayle M, Vidal A, et al. Single photon emission computed tomography/positron emission tomography imaging and targeted radionuclide therapy of melanoma: new multimodal fluorinated and iodinated radiotracers. Journal of medicinal chemistry. 2011;54:2745–66. doi: 10.1021/jm101574q. [DOI] [PubMed] [Google Scholar]
- 225.Maisonial A, Billaud EM, Besse S, Rbah-Vidal L, Papon J, Audin L, et al. Synthesis, radioiodination and in vivo screening of novel potent iodinated and fluorinated radiotracers as melanoma imaging and therapeutic probes. European journal of medicinal chemistry. 2013;63:840–53. doi: 10.1016/j.ejmech.2012.11.047. [DOI] [PubMed] [Google Scholar]
- 226.Rbah-Vidal L, Vidal A, Billaud EM, Besse S, Ranchon-Cole I, Mishellany F, et al. Theranostic Approach for Metastatic Pigmented Melanoma Using ICF15002, a Multimodal Radiotracer for Both PET Imaging and Targeted Radionuclide Therapy. Neoplasia. 2017;19:17–27. doi: 10.1016/j.neo.2016.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Morton JJ, Bird G, Refaeli Y, Jimeno A. Humanized Mouse Xenograft Models: Narrowing the Tumor-Microenvironment Gap. Cancer research. 2016;76:6153–8. doi: 10.1158/0008-5472.CAN-16-1260. [DOI] [PMC free article] [PubMed] [Google Scholar]