Abstract
Objective
Our ability to speak is complex, and in the field of Otolaryngology, the role of the central nervous system in controlling speech production is often overlooked. In this brief review, we present an integrated overview of speech production with a focus on the role of central nervous system. The role of central control of voice production is then further discussed in relation to the potential pathophysiology of spasmodic dysphonia (SD).
Data Sources
Peer-review articles on central laryngeal control and SD were identified from PUBMED search. Selected articles were augmented with designated relevant publications.
Review Methods
Publications that discussed central and peripheral nervous system control of voice production and the central pathophysiology of laryngeal dystonia were chosen.
Results
Our ability to speak is regulated by specialized complex mechanisms coordinated by high-level cortical signaling, brainstem reflexes, peripheral nerves, muscles and mucosal actions. Recent studies suggest that SD results from a primary central disturbance associated with dysfunction at our highest levels of central voice control. The efficacy of botulinum toxin in treating SD may not be limited solely to its local effect on laryngeal muscles and may also modulate the disorder at the level of the central nervous system.
Conclusion
Future therapeutic options that target the central nervous system may help modulate the underlying disorder in SD and allow clinicians to better understand the principal pathophysiology.
Keywords: laryngeal motor cortex, phonation, voice, spasmodic dysphonia, laryngeal dystonia, botulinum toxin
Introduction
Our ability to speak is regulated by a number of complex specialized mechanisms that coordinate high-level cortical processing, brainstem reflexes and peripheral nerves. While vocalization was mapped to the motor cortex by Penfield in 1930s,1 our current understanding of neural control of voice and speech production is based on studies conducted in the past 5 years. However, in the field of Otolaryngology, the role of the central nervous system (CNS) in controlling speech production has often been overlooked. In this review, we present an overview of central control of voice production and discuss the potential neuro-pathophysiology of spasmodic dysphonia (SD). Although SD was characterized in 1980s and 1990s, much of what we currently know regarding the detailed clinical phenomenology, neural correlates and genetics of SD is through contemporary studies conducted within the past decade. A better understanding SD has helped us appreciate the importance of CNS regulation in voice production.
Development
Voice production in humans can be voluntary like speaking and singing, or involuntary as is occasionally observed in response to pain, fright, or emotions. Voluntary and involuntary voice production is coordinated under the control of brain stem, midbrain and cortical structures. The intricate neural circuitry involved in human voice production develops gradually over time from initial involuntary shrieks and cries in infants to clearly articulated vocal communication later in life. Vocalization is not acquired through explicit instruction, but rather it is implicitly acquired through a gradual process of increased adaptation and development resulting in more complex behaviors, such as speaking and singing. As a child gradually acquires control over orofacial and laryngeal muscles, early signs of speech develop which are often heard as babbling. As development continues, speech motor control becomes increasingly more skilled and voice onset and offset come to be timed to differentiate between different sounds.2
Voice, Speech and Language
The distinction between voice, speech and language is important. Voice is usually used for speech, and speech conveys meaning. Language involves the formulation of meaningful phrases in grammatically articular relationships. Examination of voice control without the confounding effects of language would more accurately characterize voice production without meaning. Non-language voice production involves voice changes alone and does not require the use of lips, tongue and jaw movements for speech or articulation. Although vocalization requires precise control of the larynx and utilizes skilled laryngeal motor patterns necessary for speech production, it does not necessarily convey language or meaning.2 Different brain levels control vocalizations of different degrees of complexity, and the CNS control over voice production can be perceived as somewhat hierarchical.3 As one moves up the hierarchical ladder, increasingly more complex vocalizations begin to incorporate voice with speech and language.
Central Control of Voice Production
The lowest level in this hierarchical system is under the control of the reticular formation and phonatory sensory and motor nuclei within the brainstem, with motoneurons to the intrinsic laryngeal muscles located in the nucleus ambiguus and motoneurons of extrinsic laryngeal muscles located near the hypoglossal nucleus.3-5 (Figure 1) This level of central control is responsible for production of innate vocalizations, which include nonverbal vocalization such as the cry or laugh of an infant. The structure of innate vocalizations is genetically preprogrammed.6 This means that nonverbal emotional vocalizations are not learned actions and are not under the control of the forebrain. For example, anencephalic infants with intact brain stems and no forebrain are still capable of vocal utterances and verbal reactions to painful stimuli.7
Figure 1.
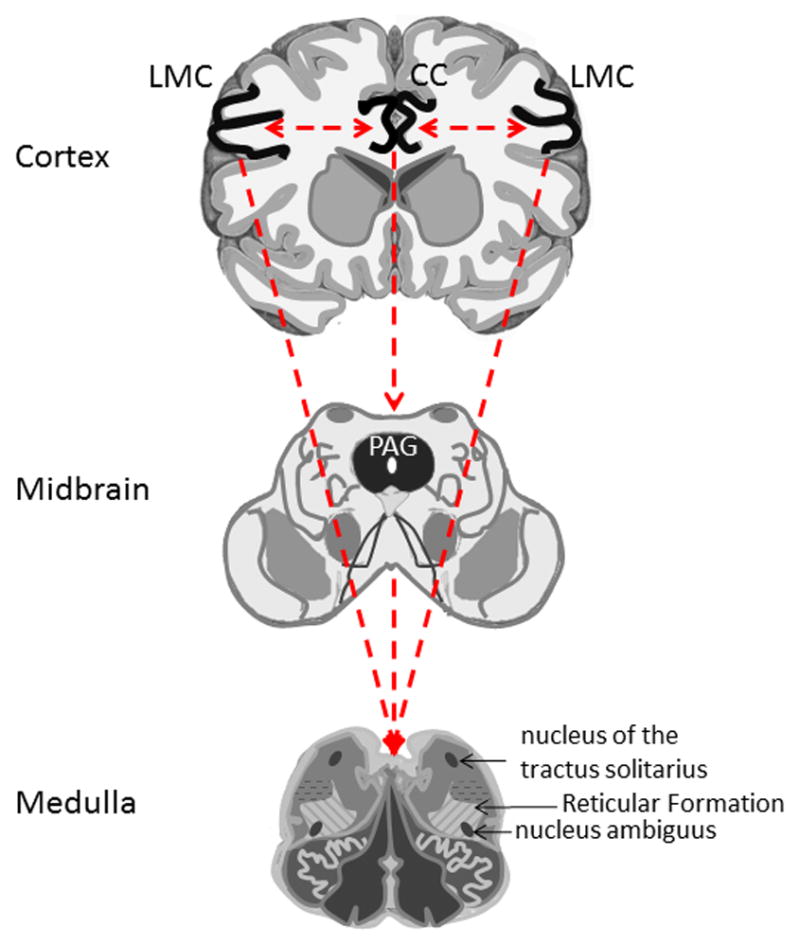
Hierarchical organization of central voice control depicting different interconnected levels of the voice control. The lowest level represented by the brain stem and spinal cord. Higher level of voice control is represented by the periaqueductal gray (PAG) and cingulate cortex (CC). The highest level is represented by the laryngeal/orofacial motor cortex (LMC). The dotted lines represent interconnections between regions.
As children develop and become capable of learning and mimicking vocal utterances, innate vocalizations become increasingly more voluntary. At this stage of development, a cry can be produced without the presence of an emotional stimulus or suppressed despite the presence of discomfort. Although still part of the innate vocalization system, this level of vocal control is more advanced and requires input from higher brain regions like the cingulate cortex (CC) and the periaqueductal gray (PAG). (Figure 1) The CC and PAG are responsible for the control of emotional vocalizations, voice initiation and modulation of its intensity.8 The PAG appears to act as a gateway between the CC and the brainstem linking the external stimulus with the motivational vocal reactions. The role of the PAG and CC in voice production can be further understood in their absence. Destruction of the CC does not interfere with voice that is initiated in the PAG. Thus, the ability to speak and vocalize is preserved with the loss of emotional intonation. By contrast, destruction of the PAG abolishes all vocalizations that originate from the CC resulting in mutism.8,9
The above innate emotional voice system differs from the cortically based system which supports the development of learned voice productions necessary for speech.2,9,10 This highest level of voice production is under the control of the speech motor cortex, including laryngeal and orofacial motor cortex (LMC) which coordinate more than 100 muscles used in phonation, swallowing, and breathing. (Figure 1) The LMC is responsible for highly skilled learned laryngeal movements, such as speaking and singing. Almost all laryngeal muscles receive bilateral innervation from the left and right LMC, thus patients with unilateral injury to the LMC still maintain the ability of voluntarily voice control.9 Neuroimaging and electrical stimulation studies have localized the LMC in humans to area 4 of the primary motor cortex.11-16 (Figure 2) Interestingly, the motocortical location of the larynx in non-human primates is different than in humans, where it is located far more rostrally and ventrally in area 6 of the premotor cortex. This difference likely represents an evolutionary adaptation towards enhanced verbal communication in humans when compared to non-human primates. The LMC in area 4 of the motor cortex is thought to enable direct connection between the LMC and laryngeal motoneurons in the nucleus ambiguus of the brainstem.16,17 The importance of the LMC in human voice production is highlighted when juxtaposed with its more limited role in the non-human primate who have markedly limited capacity for complex speech and voice production. Faster more direct coordination of complex laryngeal, orofacial, and respiratory movements in humans likely facilitates learning and voluntary vocal control for the purposes of speech and singing. In non-human primates, this connection is made indirectly,17,18 which may explain why these species are less capable of learning new vocal tasks. Bilateral lesions to the LMC in non-human primates result in a very limited deficit without a profound effect on vocalizations. By contrast, bilateral lesion to the LMC in humans result in speech loss preserving only the nonverbal emotional vocalizations such as grunting, crying and laughing controlled through the cingulate-PAG circuitry.9
Figure 2.
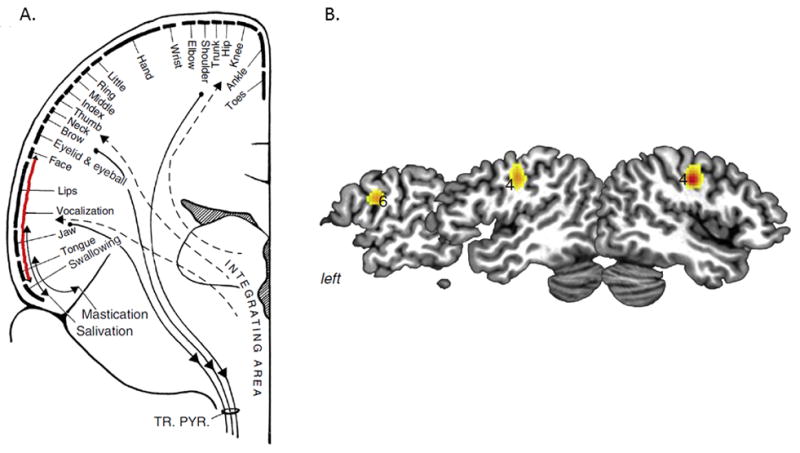
A) Motor sequence within the primary motor cortex with the vocalization region in the inferior portion of the precentral gyrus. B) Functional Magnetic Resonance Imaging studies of 19 patients during voice production. Bilateral peaks of laryngeal/orofacial motor cortex (LMC) activation were found in the area 4 with an additional peak of activation in the left area 6. TR. PYR. = tractus pyramidalis [With permission from Simonyan K. The laryngeal motor cortex: its organization and connectivity. Current Opinion in Neurobiology 2013; 28:15–2]
Somatosensory Feedback
The exact receptors type and its role in laryngeal somatosensory feedback during speech are less well known and are still debated.19 Some authors have suggested that stretch reflexes in the laryngeal muscles may provide proprioceptive feedback assisting in voice control.20-22 Thus far no studies have demonstrated the physiological of effect stretched human laryngeal muscle. Recent findings suggest that spindle fibers only occur within the interarytenoid muscle and are sparse or absent in the thyroarytenoid, lateral cricoarytenoid, cricothyroid and posterior crioarytenoid muscles.23-25 Furthermore, animal models show that sensory afferent fibers from the internal branch of the superior laryngeal nerve (iSLN) are more sensitive to mucosal deflection than to muscle stretch. Bhabu et al. demonstrated initiation of the laryngeal adductor response in human by an air stimulus to the laryngeal mucosa supporting the role that mucosal mechanoreceptors provide dynamic sensory feedback to the central nervous system.26-31 The laryngeal adductor reflex can also be elicited by a direct electrical stimulus to the iSLN. Both electrical stimulation and mechanical air stimulation result in an early ipsilateral response designated as R1and a later bilateral response designated as R2. The R1/R2 response is comparable to the blink reflex.26,29,32 Like the blink reflex, repeated laryngeal stimulation leads to reduction of frequency and amplitude to the R2 response and is likely a result of central inhibition.32-34 Phonation causes repeated mechanical perturbation to the vocal folds, and central suppression likely plays an integral role in in facilitating fluidity of sound during vocalization and speech by controlling the adductor reflex responses.
Spasmodic Dysphonia
The importance of the role of the CNS in controlling speech production can be further understood within the context of centrally derived speech disorders such as spasmodic dysphonia. Laryngeal dystonia is a clinical syndrome characterized by task-specific involuntary contractions of the internal laryngeal muscles. Respiratory laryngeal dystonia, results in laryngeal contraction of varied degrees during breathing that usually disappears with sleep, and spares patients’ fluency during speech.35 Patients with “singers dystonia” have laryngeal hyperkinesias only while singing without affecting conversational speech.36,37 Spasmodic dysphonia (SD) is a focal dystonia affecting fluency of voice during speech and is the most commonly affected task of the laryngeal dystonias. Focal dystonias were believed to result from dysfunction at the level of the basal ganglia, although this view has recently been expanded to include the pathophysiological network to the cerebellum and sensorimotor cortical regions.38
Although the exact pathophysiology of SD is unknown, recently, structural alterations in brain organization were demonstrated in patients with SD, including focal reduction of axonal density and myelin along the corticobulbar/corticospinal tract.39-43 Functional magnetic resonance imaging (fMRI) identified brain abnormalities in patients with SD and demonstrated a greater extent of brain activation in the cortical brain regions responsible for the control of voice production during both symptomatic driven and asymptomatic tasks.44-46 The extent of activation within the subcortical structures (basal ganglia, thalamus, and cerebellum) was also increased, but only during symptomatic speech but was decreased during asymptomatic laryngeal tasks. These changes were noted both in patients with adductor and abductor SD and suggest that the primary disturbance in SD is associated with dysfunction of the sensorimotor cortex as well as the basal ganglia–thalamo-cortical circuitry. (Figure 3)
Figure 3.
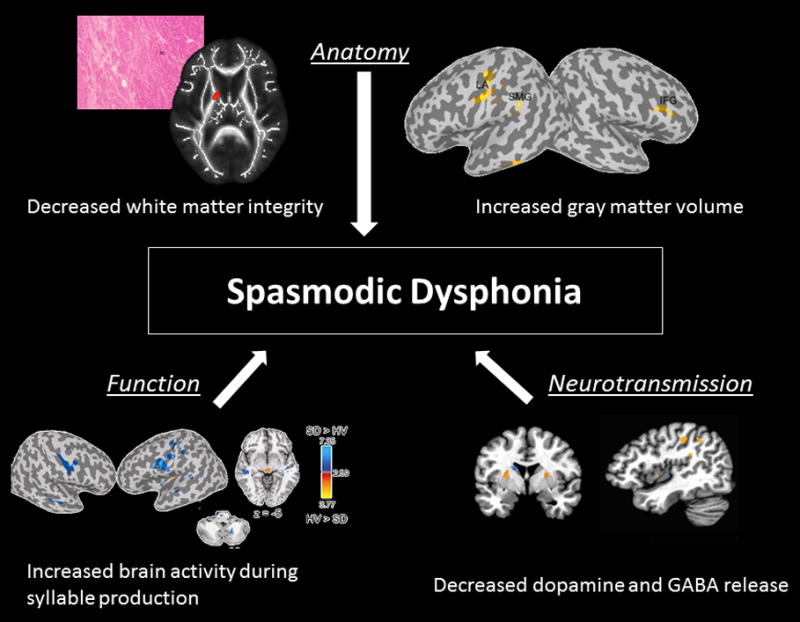
Interplay between structural, functional and neurochemical alterations. Microstructural changes of the basal ganglia and sensorimotor cortex noted by Functional Magnetic Resonance Imaging have a global effect on brain sensorimotor network, organization and function.
A recent study also found neurochemical alterations in the basal ganglia in patients with SD.47 Positron emission tomography with the radioligand [11C] raclopride (RAC) was used to explore striatal dopaminergic neurotransmission during symptomatic speech and was compared to healthy controls. Finger tapping was used as an internally controlled task and was designated as an asymptomatic task. Patients with SD had fewer available striatal dopamine D2 /D3 receptors as well as decreased levels of dopamine release during symptomatic speech. Interestingly, patients with SD demonstrated increased dopamine release during the asymptomatic tasks (finger tapping) when compared to healthy controls. It is possible that decreased dopaminergic transmission is responsible for the generation of symptoms in patients with SD, and the observed increased striatal dopaminergic function compensatory adaptation of the nigrostriatal dopaminergic system to decreased dopamine D2 /D3 receptor availability. Also patients who were more symptomatic had greater RAC displacement and those with longer duration of spasmodic dysphonia had decreased task-induced RAC displacement.
Neural abnormalities described in spasmodic dysphonia explain which brain regions and their connections or neurochemical makeup that is implicated in the pathophysiology of this disorder. Further identification of these alterations in spasmodic dysphonia and other voice disorders can not only help physician better understand the disorder itself but could simultaneously help define the contribution of specific brain regions in normal voice control.
Treatment of Spasmodic Dysphonia
The role of the CNS in voice production can be further elucidated in the context of the evolution of the treatment of SD. Although SD is now recognized as a CNS disorder, initial attempts to restore voice focused at alterations to the laryngeal framework, muscles or peripheral nerves.48 Initial studies reported an 85-90% success rate following recurrent laryngeal nerve (RLN) transection; however, a follow-up study showed that 64% of patients had return of pathologic voice quality.49-51 Previous explanations for the return of the disorder have been proposed, including reinnervation by proximal RLN axons.52 In an attempt to provide permanent selective denervation, Berke et al. performed selective laryngeal adduction denervation and reinnervation (SLAD/R).53 This procedure incorporates bilateral RLN transection to branches responsible for innervating only the adductor laryngeal muscles. To prevent unwanted reinnervation from the RLN and to maintain muscle tone, the cut branches were anastomosed to a branch of the ansa cervicalis. Initial results with SLAD/R were promising and showed improved success when compared to RLN transection alone. However, long-term results demonstrated return of voice breaks in 26% and a persistent breathy voice quality in 30%.54
Disappointing results with any surgical alterations, weather to the end-organ or peripheral nerves, to provide long-term symptomatic relief are likely due to the failure of surgery to address the CNS. Surgery is permanent and fixed alterations do not account for the possibility of CNS plasticity and adaption.55 In addition, RLN transection does not address the numerous interconnections within the larynx and interconnections between the superior laryngeal nerve SLN and RLN (Figure 4). Theoretically these interconnections could allow persistent CNS access to alter normal laryngeal muscles physiology.56-61
Figure 4.
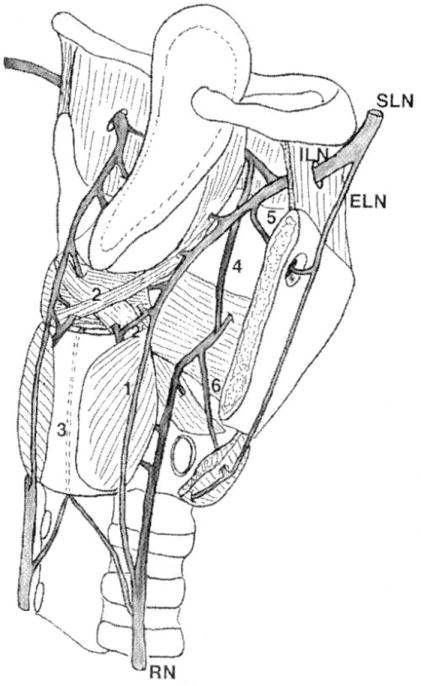
Anastomoses between the laryngeal nerves. SLN = superior laryngeal nerve; ILN = internal laryngeal nerve; ELN = external laryngeal nerve; RN = recurrent nerve; 1 = Galen’s anastomosis; 2 = deep arytenoid plexus; 2’ = superficial arytenoid plexus; 3 = cricoid anastomosis; 4 = thyroarytenoid anastomosis; 5 = foramen thyroideum anastomosis; 6 = cricothyroid anastomosis. [With permission from Sanudo, JR, Maranillo E, Leon, X, Mirapeix RM, Orus C, Quer M. An Anatomical Study of Anastomoses Between the Laryngeal Nerves. The Laryngoscope. 1999; 109(6): 983-987]
Botulinum Toxin
In 1986, Blitzer found dramatic improvement of voice quality following direct injection of botulinum toxin (BoNT) to the affected laryngeal muscles.62 BoNT is a 150-Kilodalton exotoxin produced from clostridium botulinum, whose action is medicated through the cleavage of docking proteins responsible for membrane fusion of pre-synaptic vesicles and is now the gold standard for treatment for laryngeal dystonia. Cleavage of these docking proteins inhibits release of acetylcholine (Ach) at the neuromuscular junction and results in muscle weakness. Unlike surgery, BoNT is continuously metabolized, and the ever-changing effect does not allow for central adaptation. In addition to weakening laryngeal muscle activity, BoNT decreases the activation of muscle fibers directly through its effect on the intrafusal sensory fibers. However, local chemodenervation does not fully explain the clinical effects of BoNT. If BoNT interfered solely with muscle action and sensory fiber tone, then BoNT injections should have no effect on central efferent pathways. However, it is well known that unilateral BoNT injections reduce involuntary aberrant contractions to the contralateral untreated laryngeal muscle groups.63,64 A possible explanation for this finding is that modulation of the laryngeal muscles has an affect on the sensory feedback loop.
It has also been shown that the aberrant central activity at the primary sensorimotor cortex (areas 3,1,2) is normalized after peripheral BoNT injections, demonstrating BoNT’s effect on modulating the central nervous system. 65 Although, the exact mechanism is unknown, it is feasible that elements of BoTN are transmitted through the peripheral nerves in a retrograde fashion and modulate the central interneurons directly.66,67 Whatever the cause, BoNT is effective and its actvity does not appear to be limited to local alterations of the laryngeal muscles.
Future therapeutic options targeting the central nervous system in SD are currently being investigated. A recent survey showed that 55.9 % of patients with SD had improvement in voice quality following ingestion of alcohol. This observation is likely related to alcohol’s effect on the central nervous system (via GABA function).68 This finding led researchers to investigate the effects of drugs that could potentially improve SD voice symptoms by acting similar to alcohol on GABAergic transmission. Thus far a metabolite of sodium oxybate which behaves as a GABA receptor agonist has found to improve SD symptoms in 82.2% of SD patients who had previously had symptomatic improvement following alcohol ingestion.69-73
Conclusion
Our ability to produce purposeful vocalizations and speak fluently is regulated by a complex network of mechanisms originating at the level of the CNS. Central regulation in voice production and speech is crucial. Spasmodic dysphonia is a disorder of the CNS and is an example of selectively disordered central voice regulation. Recent studies suggest that SD results from a primary central disturbance in the LMC and its circuitry. BoNT has shown great efficacy in treating patients’ symptoms. Injection of BoNT to the laryngeal muscles is not limited to its effect locally and also conveys an effect to the CNS. Nevertheless, it is important to highlight that BoNT is effective at only temporarily treating the symptoms related to SD and does not ultimately treat the underlying central disturbance. Future therapeutic options that target the central nervous system may help modulate the disorder and allow clinicians to better understand the pathophysiology of SD. Lastly, a better understanding of the types of receptors and the role they play in laryngeal somatosensory feedback during voice production and speech may provide us with additional understandings of the exact therapeutic role of BoNT both locally and centrally in treating the vocal symptoms related to SD.
Acknowledgments
Funding/ Disclosure/ Conflict of Interests:
K. Simonyan was supported by the grants from National Institute on Deafness and Other Communication Disorders (R01DC011805, R01DC012545) and National Institute of Neurological Disorders and Stroke (R01NS088160).
References
- 1.Penfield W, Boldrey E. Somatic motor and sensory representation in the cerebral cortex of man as studied by electrical stimulation. Brain. 1937;60:389–443. [Google Scholar]
- 2.Ludlow C. Central Nervous System Control of Voice and Swallowing. J Clin Neurophysiol. 2015 Aug;32(4):294–303. doi: 10.1097/WNP.0000000000000186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Simonyan K, Horwitz B. Laryngeal Motor Cortex and Control of Speech in Humans. Neuroscientist. 2011 Apr;17(2):197–208. doi: 10.1177/1073858410386727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lalwani AK. Current Diagnosis & Treatment Otolaryngology: Head and Neck Surgery. 3. New York: McGraw-Hill Medical; 2011. [Google Scholar]
- 5.Flint PW, Haughey BH, Lund VJ, Niparko JK, Richardson MA, Robbins KT, et al. Cummings Otolaryngology – Head and Neck Surgery. 5. Philadelphia: Mosby; 2010. [Google Scholar]
- 6.Scheiner E, Hammerschmidt K, Jurgens U, Zwirner P. The influence of hearing impairment on preverbal emotional vocalizations of infants. Folia Phoniatr Logop. 2004;56:27–40. doi: 10.1159/000075326. [DOI] [PubMed] [Google Scholar]
- 7.Monnier M, Willi H. The integrative activity of the nervous system of a meso-rhombencephalic anencephalus. II. Anatomical part. Monatsschr Psychiatr Neurol. 1953;126:259–73. [PubMed] [Google Scholar]
- 8.Demeurisse G, Alberti Fabbro F. Complete mutism after midbrain periaqueductal gray lesion. Antonio Esposito, NeuroReport. 1999;10:681–685. doi: 10.1097/00001756-199903170-00004. [DOI] [PubMed] [Google Scholar]
- 9.Jurgens U. Neural pathways underlying vocal control. Neurosci Biobehav Rev. 2002;26:235–58. doi: 10.1016/s0149-7634(01)00068-9. [DOI] [PubMed] [Google Scholar]
- 10.Jurgens U. The neural control of vocalization in mammals: a review. J Voice. 2009;23:1–10. doi: 10.1016/j.jvoice.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 11.Brown S, Ngan E, Liotti M. A larynx area in the human motor cortex. Cereb Cortex. 2008;18:837–45. doi: 10.1093/cercor/bhm131. [DOI] [PubMed] [Google Scholar]
- 12.Loucks TM, Poletto CJ, Simonyan K, Reynolds CL, Ludlow CL. Human brain activation during phonation and exhalation: common volitional control for two upper airway functions. Neuroimage. 2007;36:131–43. doi: 10.1016/j.neuroimage.2007.01.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Rodel RM, Olthoff A, Tergau F, et al. Human cortical motor representation of the larynx as assessed by transcranial magnetic stimulation (TMS) Laryngoscope. 2004;114:918–22. doi: 10.1097/00005537-200405000-00026. [DOI] [PubMed] [Google Scholar]
- 14.Simonyan K, Ostuni J, Ludlow CL, Horwitz B. Functional but not structural networks of the human laryngeal motor cortex show left hemispheric lateralization during syllable but not breathing production. J Neurosci. 2009;29:14912–23. doi: 10.1523/JNEUROSCI.4897-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wilson SM, Saygin AP, Sereno MI, Iacoboni M. Listening to speech activates motor areas involved in speech production. Nat Neurosci. 2004;7:701–2. doi: 10.1038/nn1263. [DOI] [PubMed] [Google Scholar]
- 16.Simonyan K. The laryngeal motor cortex: its organization and connectivity. Current Opinion in Neurobiology. 2013;28:15–2. doi: 10.1016/j.conb.2014.05.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Simonyan K, Jurgens U. Efferent subcortical projections of the laryngeal motorcortex in the rhesus monkey. Brain Res. 2003;974:43–59. doi: 10.1016/s0006-8993(03)02548-4. [DOI] [PubMed] [Google Scholar]
- 18.Jurgens U. Projections from the cortical larynx area in the squirrel monkey. Exp Brain Res. 1976;25:401–411. doi: 10.1007/BF00241730. [DOI] [PubMed] [Google Scholar]
- 19.Ludlow C. Central nervous system control of the laryngeal muscles in humans. Respir Physiol Neurobiol. 2005 Jul 28;147(2-3):205–222. doi: 10.1016/j.resp.2005.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kirchner JA, Wyke B. Articular reflex mechanisms in the larynx. Ann Otol Rhinol Laryngol. 1965;74:749–769. doi: 10.1177/000348946507400315. [DOI] [PubMed] [Google Scholar]
- 21.Abo-El-Enein MA, Wyke B. Laryngeal myotactic reflexes. Nature. 1966;209:682–686. doi: 10.1038/209682a0. [DOI] [PubMed] [Google Scholar]
- 22.Wyke BD. Laryngeal myotactic reflexes and phonation. Folia Phoniat. 1974;26:249–264. doi: 10.1159/000263784. [DOI] [PubMed] [Google Scholar]
- 23.Ibrahim MK, Abd El Rahman S, Mahran ZY. Experimental studies on the afferent innervation of the cricothyroid muscle in dogs. Acta Anat (Basel) 1980;106:171–179. doi: 10.1159/000145179. [DOI] [PubMed] [Google Scholar]
- 24.Brandon CA, Rosen C, Georgelis G, Horton MJ, Mooney MP, Sciote JJ. Staining of human thyroarytenoid muscle with myosin antibodies reveals some unique extrafusal fibers, but no muscle spindles. J Voice. 2003;17:245–254. doi: 10.1016/s0892-1997(03)00013-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tellis CM, Rosen C, Thekdi A, Sciote JJ. Anatomy and fiber type composition of human interarytenoid muscle. Ann Otol Rhinol Laryngol. 2004;113:97–107. doi: 10.1177/000348940411300203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Bhabu P, Poletto C, Mann E, Bielamowicz S, Ludlow CL. Thyroarytenoid muscle responses to air pressure stimulation of the laryngeal mucosa in humans. Ann Otol Rhinol Laryngol. 2003;112:834–840. doi: 10.1177/000348940311201002. [DOI] [PubMed] [Google Scholar]
- 27.Aviv JE, Martin JH, Kim T, Sacco RL, Thomson JE, Diamond B, Close LG. Laryngopharyngeal sensory discrimination testing and the laryngeal adductor reflex. Ann Otol Rhinol Laryngol. 1999;108:725–730. doi: 10.1177/000348949910800802. [DOI] [PubMed] [Google Scholar]
- 28.Ambalavanar R, Tanaka Y, Selbie WS, Ludlow CL. Neuronal activation in the medulla oblongata during selective elicitation of the laryngeal adductor response. J Neurophysiol. 2004;92:2920–2932. doi: 10.1152/jn.00064.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ludlow CL, VanPelt F, Koda J. Characteristics of late responses to superior laryngeal nerve stimulation in humans. Ann Otol Rhinol Laryngol. 1992;101:127–134. doi: 10.1177/000348949210100204. [DOI] [PubMed] [Google Scholar]
- 30.Sasaki CT, Suzuki M. Laryngeal reflexes in cat, dog and man. Arch Otolaryngol. 1976;102:400–402. doi: 10.1001/archotol.1976.00780120048004. [DOI] [PubMed] [Google Scholar]
- 31.Suzuki M, Sasaki CT. Laryngeal spasm: a neurophysiologic redefinition. Ann Otol Rhinol Laryngol. 1977;86:150–158. doi: 10.1177/000348947708600203. [DOI] [PubMed] [Google Scholar]
- 32.Sanes JN, Ison JR. Habituation and sensitization of components of the human eyeblink reflex. Behav Neurosci. 1983;97:833–836. doi: 10.1037//0735-7044.97.5.833. [DOI] [PubMed] [Google Scholar]
- 33.Ludlow CL, Schulz GM, Yamashita T, Deleyiannis FW. Abnormalities in long latency responses to superior laryngeal nerve stimulation in adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1995;104:928–935. doi: 10.1177/000348949510401203. [DOI] [PubMed] [Google Scholar]
- 34.Kearney P, Poletto C, Mann EA, Ludlow CL. Suppression of thyroarytenoid muscle responses during repeated air pressure stimulation of the laryngeal mucosa in awake humans. Ann Otol Rhinol Laryngol. 2005;114:264–270. doi: 10.1177/000348940511400403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Blitzer A, Brin MF, Stewart CF. Botulinum toxin management of spasmodic dysphonia (laryngeal dystonia): a 12-year experience in more than 900 patients. Laryngoscope. 1998;108(10):1435–41. doi: 10.1097/00005537-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 36.Chitkara A, Meyer T, Keidar A, Blitzer A. Singer’s dystonia: first report of a variant of spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2006;115(2):89–92. doi: 10.1177/000348940611500201. [DOI] [PubMed] [Google Scholar]
- 37.Grillone GA, Blitzer A, Brin MF, Annino DJ, Jr, Saint-Hilaire MH. Treatment of adductor laryngeal breathing dystonia with botulinum toxin type A. Laryngoscope. 1994;104:30–32. doi: 10.1288/00005537-199401000-00007. [DOI] [PubMed] [Google Scholar]
- 38.Battistella G, Termsarasab P, Ramdhani RA, Fuertinger S, Simonyan K. Isolated Focal Dystonia as a Disorder of Large-Scale Functional Networks. Cereb Cortex. 2015:pii. doi: 10.1093/cercor/bhv313. bhv313. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Simonyan K, Berman BD, Herscovitch P, Hallett M. Abnormal Striatal Dopaminergic Neurotransmission during Rest and Task Production in Spasmodic Dysphonia. The Journal of Neuroscience. 2013;33(37):14705–14714. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Berardelli A, Rothwell JC, Hallett M, Thompson PD, Manfredi M, Marsden CD. The pathophysiology of primary dystonia. Brain. 1998;121:1195–1212. doi: 10.1093/brain/121.7.1195. [DOI] [PubMed] [Google Scholar]
- 41.Hallett M. The neurophysiology of dystonia. Arch Neurol. 1998;55:601–603. doi: 10.1001/archneur.55.5.601. [DOI] [PubMed] [Google Scholar]
- 42.Defazio G, Berardelli A, Hallett M. Do primary adult-onset focal dystonias share aetiological factors? Brain. 2007;130:1183–1193. doi: 10.1093/brain/awl355. [DOI] [PubMed] [Google Scholar]
- 43.Neychev VK, Gross RE, Lehericy S, Hess EJ, Jinnah HA. The functional neuroanatomy of dystonia. Neurobiol Dis. 2011;42:185–201. doi: 10.1016/j.nbd.2011.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Simonyan K, Ludlow CL. Abnormal Structure Function Relationship in Spasmodic Dysphonia. Cerebral Cortex (New York, NY) 2012;22(2):417–425. doi: 10.1093/cercor/bhr120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Simonyan K, Ludlow CL. Abnormal activation of the primary somatosensory cortex in spasmodic dysphonia: an FMRI study. Cereb Cortex. 2010;20:2749–2759. doi: 10.1093/cercor/bhq023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Simonyan K, Tovar-Moll F, Ostuni J, Hallett M, Kalasinsky VF, Lewin-Smith MR, Rushing EJ, Vortmeyer AO, Ludlow CL. Focal white matter changes in spasmodic dysphonia: a combined DTI and neuropathological study. Brain. 2008;131(Pt 2):447–459. doi: 10.1093/brain/awm303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Simonyan K, Berman BD, Herscovitch P, Hallett M. Neurobiology of Disease Abnormal Striatal Dopaminergic Neurotransmission during Rest and Task Production in Spasmodic Dysphonia. The Journal of Neuroscience. 2013;33(37):14705–14714. doi: 10.1523/JNEUROSCI.0407-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Dedo HH. Recurrent laryngeal nerve section for spastic dysphonia. Ann Otol Rhinol Laryngol. 1976;85(4 Pt 1):451–459. doi: 10.1177/000348947608500405. [DOI] [PubMed] [Google Scholar]
- 49.Dedo HL, Izdebski K. Problems with surgical (RLN section) treatment of spastic dysphonia. Laryngoscope. 1983;93:268–271. [PubMed] [Google Scholar]
- 50.Aronson AE, De Santo LW. Adductor spastic dysphonia: three years after recurrent laryngeal nerve resection. Laryngoscope. 1983;93(1):1–8. doi: 10.1288/00005537-198301000-00001. [DOI] [PubMed] [Google Scholar]
- 51.Weed DT, Jewett BS, Rainey C, et al. Long-term follow-up of recurrent laryngeal nerve avulsion for the treatment of spastic dysphonia. Ann Otol Rhinol Laryngol. 1996;105(8):592–601. doi: 10.1177/000348949610500802. [DOI] [PubMed] [Google Scholar]
- 52.Fritzell B, Feuer E, Haglund S, Knutsson E, Schiratzki H. Experiences with recurrent laryngeal nerve section for spastic dysphonia. Folia Phoniatr (Basel) 1982;34:160–7. doi: 10.1159/000265645. [DOI] [PubMed] [Google Scholar]
- 53.Berke GS, Blackwell KE, Gerratt BR, Verneil A, Jackson KS, Sercarz JA. Selective laryngeal adductor denervation-reinnervation: a new surgical treatment for adductor spasmodic dysphonia. Ann Otol Rhinol Laryngol. 1999;108(3):227–231. doi: 10.1177/000348949910800302. [DOI] [PubMed] [Google Scholar]
- 54.Chhetri DK, Mendelsohn AH, Blumin JH, Berke GS. Long-term follow-up results of selective laryngeal adductor denervation-reinnervation surgery for adductor spasmodic dysphonia. Laryngoscope. 2006;116(4):635–642. doi: 10.1097/01.MLG.0000201990.97955.E4. [DOI] [PubMed] [Google Scholar]
- 55.Wissel J, Muller J, Ebersbach G, Poewe W. Trick maneuvers in cervical dystonia: investigation of movement- and touch-related changes in polymyographic activity. Mov Disord. 1999;14:994–999. doi: 10.1002/1531-8257(199911)14:6<994::aid-mds1013>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 56.Sunderland S, Swaney WE. The intraneural topography of the recurrent laryngeal nerve in man. Anat Rec. 1952 Nov;114(3):411–26. doi: 10.1002/ar.1091140303. [DOI] [PubMed] [Google Scholar]
- 57.Chitkara AE. Neuroanatomy of the larynx. In: Sulica L, Blitzer A, editors. Berlin ed Vocal Fold Paralysis. Heidelberg, Germany: Springer-Verlag; 2006. pp. 3–14. [Google Scholar]
- 58.Sanders I, Rai S, Han Y, Biller HF. Human vocalis contains distinct superiorand inferior subcompartments: possible candidates for the two masses of vocal fold vibration. Ann Otol Rhinol Laryngol. 1998;107:826–833. doi: 10.1177/000348949810701003. [DOI] [PubMed] [Google Scholar]
- 59.Sander I, Han Y, Wang J, Biller H. Muscle spindles are concentrated in the superior vocalis subcompartemtn of the human thyroarytenoid muscle. J Voice. 1998;12:7–16. doi: 10.1016/s0892-1997(98)80070-2. [DOI] [PubMed] [Google Scholar]
- 60.Sanduo JR, Maranillo E, Leon X, Mirapeix RM, Orus C, Quer M. An anatomical study of anastomosis between laryngeal nerves. Laryngoscope. 1999;109:983–987. doi: 10.1097/00005537-199906000-00026. [DOI] [PubMed] [Google Scholar]
- 61.Dedo HH, Ogura JH. Vocal cord electromyography in the dog. Laryngoscopy. 1965;75:201–211. doi: 10.1288/00005537-196502000-00001. [DOI] [PubMed] [Google Scholar]
- 62.Blitzer A, Lovelace RE, Brin MF, Fahn S, Fink ME. Electromyographic findings in focal laryngeal dystonia (spastic dysphonia) Ann Otol Rhinol Laryngol. 1985;94(6 Pt 1):591–594. doi: 10.1177/000348948509400614. [DOI] [PubMed] [Google Scholar]
- 63.Ludlow C. Spasmodic Dysphonia: a Laryngeal Control Disorder Specific to Speech. The Journal of Neuroscience. 2011;31(3):793–797. doi: 10.1523/JNEUROSCI.2758-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Bielamowicz S, Ludlow CL. Effects of botulinum toxin on pathophysiology in spasmodic dysphonia. Ann Otol Rhinol Laryngol. 2000;109:194–203. doi: 10.1177/000348940010900215. [DOI] [PubMed] [Google Scholar]
- 65.Ali SO, Thomassen M, Schulz GM, et al. Alterations in CNS activity induced by botulinum toxin treatment in spasmodic dysphonia: an H215O PET study. J Speech Lang Hear Res. 2006;49:1127–1146. doi: 10.1044/1092-4388(2006/081). [DOI] [PubMed] [Google Scholar]
- 66.Moreno-Lopez B, Pastor AM, de la Cruz RR, Delgado-Garcia JM. Dose-dependent, central effects of botulinum neurotoxin type A: a pilot study in the alert behaving cat. Neurology. 1997;48:456–464. doi: 10.1212/wnl.48.2.456. [DOI] [PubMed] [Google Scholar]
- 67.Antonucci F, Rossi C, Gianfranceschi L, Rossetto O, Caleo M. Long-distance retrograde effects of botulinum neurotoxin A. J Neurosci. 2008;28:3689–3696. doi: 10.1523/JNEUROSCI.0375-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kirke DN, Frucht SJ, Simonyan K. Alcohol responsiveness in laryngeal dystonia: a survey study. J Neurol. 2015;262(6):1548–1556. doi: 10.1007/s00415-015-7751-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.FDA Approval Letter for Xyrem; Indication: EDS (Excessive Daytime Sleepiness) associated with narcolepsy. US Food and Drug Administration; 2005. Nov 18, Retrieved 2010-10-03. [Google Scholar]
- 70.FDA Approval Letter for Xyrem; Indication: Cataplexy associated with narcolepsy. US Food and Drug Administration; 2002. Jul 17, Retrieved 2010-10-03. [Google Scholar]
- 71.Summary Basis of Decision (SBD): Xyrem. Health Canada. 2006 Mar 22; Retrieved 2012-11-02. [Google Scholar]
- 72.Simonyan K, Frucht SJ. Long-term Effect of Sodium Oxybate (XyremH) in Spasmodic Dysphonia with Vocal Tremor. In: Louis ED, editor. Tremor Other Hyperkinet Mov. Vol. 3. 2013. tre-03-206-4731-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Rumbach AF, Blitzer A, Frucht SJ, Simonyan K. An open-label study of sodium oxybate (Xyrem®) in spasmodic dysphonia. The Laryngoscope. 2016 doi: 10.1002/lary.26381. [In Press] [DOI] [PMC free article] [PubMed] [Google Scholar]


