Abstract
Background
The Consortium on the Genetics of Schizophrenia (COGS) collected case-control endophenotype and genetic information from 2457 patients and healthy subjects (HS) across 5 test sites over 3.5 years. Analysis of the first “wave” (W1) of 1400 subjects identified prepulse inhibition (PPI) deficits in patients vs. HS. Data from the second COGS “wave” (W2), and the combined W(1+2), were used to assess: 1) the replicability of PPI deficits in this design; 2) the impact of response criteria on PPI deficits; and 3) PPI in a large cohort of antipsychotic-free patients.
Methods
PPI in W2 HS (n=315) and schizophrenia patients (n=326) was compared to findings from W1; planned analyses assessed the impact of diagnosis, “wave” (1 vs. 2), and startle magnitude criteria. Combining waves allowed us to assess PPI in 120 antipsychotic-free patients, including many in the early course of illness.
Results
ANOVA of all W(1+2) subjects revealed robust PPI deficits in patients across “waves” (p<0.0004). Strict response criteria excluded almost 39% of all subjects, disproportionately impacting specific subgroups; ANOVA in this smaller cohort confirmed no significant effect of “wave” or “wave × diagnosis” interaction, and a significant effect of diagnosis (p<0.002). Antipsychotic-free, early-illness patients had particularly robust PPI deficits.
Discussion
Schizophrenia-linked PPI deficits were replicable across two multi-site “waves” of subjects collected over 3.5 years. Strict response criteria disproportionately excluded older, male, non-Caucasian patients with low-normal hearing acuity. These findings set the stage for genetic analyses of PPI using the combined COGS wave 1 and 2 cohorts.
Keywords: endophenotype, prepulse inhibition, replication, schizophrenia, startle
1. Introduction
Prepulse inhibition (PPI) of startle is a reliable, quantitative operational measure of sensorimotor gating that is deficient in several neuropsychiatric disorders, including schizophrenia (SZ) (Braff et al., 1978; Swerdlow et al., 2008). PPI deficits in SZ patients have been reported from a large number of laboratories in many different countries, using a variety of stimuli to elicit and inhibit startle, both within and across stimulus modalities (Aggernaes et al., 2010; Braff et al., 1978, 1999, 2001; Csomor et al., 2009; Hammer et al., 2011, 2013; Hong et al., 2007; Kishi et al., 2012; Kumari et al., 1999, 2007; Kunugi et al., 2007; Light et al., 2012; Ludewig et al., 2003; Mackeprang et al., 2002; Martinez-Gras et al., 2009; Meincke et al., 2004; Molina et al., 2011; Moriwaki et al., 2009; Oranje and Glenthoj, 2013; Preuss et al., 2011; Quednow et al., 2006; Rabin et al., 2009; Takahashi et al., 2008; Wang et al., 2013; Weike et al., 2000; Xue et al., 2012). PPI has robust heritability (Greenwood et al. 2007), and genes associated with PPI in SZ patients and healthy comparison subjects (HS) have been identified (Hong et al. 2008; Petrovsky et al. 2010; Quednow et al. 2011; Greenwood et al., 2011, 2012; Roussos et al. 2016).
The Consortium on the Genetics of Schizophrenia (COGS) was designed to identify genes associated with SZ endophenotypes, using five geographically dispersed data collection sites. From July, 2010, to February, 2014, neurocognitive and neurophysiological endophenotypes as well as genetic material were collected from 1,405 carefully characterized SZ patients and 1,052 HS. Despite significant efforts in quality control and equipment and procedural standardization, this large, multi-site study presented challenges not faced in smaller, single-site studies of PPI in SZ, including site-based differences in sample demographics, methodologies and test conditions. Our quality assurance plan included an interim (circa January, 2013) analysis of PPI data from the “first wave” of 1400 COGS subjects.
The results of the “first wave” (W1) analysis of PPI (Swerdlow et al., 2014) confirmed significant deficits in PPI in SZ patients. These deficits were sensitive to several moderating variables as previously reported in numerous “single site” PPI studies (e.g. Hong et al., 2008; Kumari et al., 1999, 2004; Swerdlow et al., 2006a; Weike et al., 2000), including prepulse interval (deficits at 60 ms, but not 30 or 120 ms) and medications (deficits blunted by antipsychotics (“APs”)). We discussed opportunities and challenges created by PPI assessment in this multi-site platform. For example, embedded within this multi-site sample was the largest subgroup of AP-free SZ patients in which PPI had been tested, providing the opportunity for potentially novel insights into the nature of SZ-linked PPI deficits independent of drugs that are known to alter PPI. We also reported differences in the magnitude of PPI and SZ-linked PPI deficits across the 5 COGS sites, which created interpretative challenges, and at least in part may have reflected site-specific patterns of racial stratification.
Another challenge emerged from this W1 analysis: the use of strict response inclusion criteria (a “non-responder” defined as reflex magnitude < 10 units (1.31 µV/digital unit)) for either of the two trial blocks during which PPI was analyzed) resulted in the exclusion of over 40% of the test subjects. While PPI deficits were evident with or without the use of these exclusion criteria based on a minimal startle response magnitude, this large attrition rate became important in subsequent COGS analyses, when multiple endophenotypes were integrated across subjects to identify endophenotype “factors” or “pathways” (Seidman et al. 2015; Millard et al. 2016; Thomas et al. 2017). Conceivably, this substantive loss of subjects may also negatively impact the design and interpretation of upcoming COGS genetic analyses, in which PPI data will be used, together with results from all other COGS endophenotypes.
Mutli-site PPI assessment in the COGS “second wave” (W2) was completed in February, 2014. Here, we present the results of the inclusive W1 and W2 PPI assessments, with three goals: 1. To assess the replicability, over time, of SZ-linked PPI deficits within a multi-site study; 2. To assess the impact of reflex response magnitude exclusion criteria on usable sample size and predicted patterns of PPI; 3. Absent evidence of significant W1 vs. W2 differences, to combine W(1+2) samples to achieve adequate power to conduct informative analyses of moderating variables in larger subgroups of potential interest, including patients who were unmedicated and early in their illness.
2. Methods
Other than collection date, methods and procedures for W2 subject ascertainment and collection of W2 data were identical to that for W1. As described previously, COGS participants were recruited and tested at 5 sites: Mount Sinai School of Medicine, University of California Los Angeles, University of California San Diego, University of Pennsylvania and University of Washington. Participants were 18–65 years old and fluent in English. Inclusion and exclusion criteria for W2 subjects were identical to those previously reported for W1 (Swerdlow et al., 2014), designed to exclude participants whose startle data was likely to be confounded by factors that interfere with startle signal acquisition or analysis (e.g. subjects with hearing impairment were not tested; subjects with zero measurable response to startle stimuli, or whose data was entirely missing from one eyeblink side or trial block – generally reflecting electrode removal or failure - were not included in analyses) and those whose PPI might have been altered on the basis of clinical factors unrelated to SZ per se (e.g. subjects with a history of recent substance abuse or electroconvulsive therapy were not tested). Local IRB boards of each testing site approved the study, and all participants provided signed informed consent before study participation (UCSD HRPP #080435). Diagnostic and clinical assessments (Andreasen, 1984a, 1984b; Faraone et al., 1999; First et al., 1995, 1996; Hall, 1995) were identical to those used in W1 (Swerdlow et al., 2014) and in earlier COGS studies (Calkins et al., 2007). As part of the initial structured clinical assessment, a list of all current medications was composed and reviewed with the test subject; it was then confirmed to be correct on the day(s) of testing. For patients whose medications were dispensed via a treatment or structured living facility, medication lists were typically confirmed with that facility. Patients were considered to be “antipsychotic-free” if no antipsychotic agents (including long-acting injectable forms) were included in those confirmed lists.
The full COGS test schedule was described previously (Swerdlow et al., 2015), and in W2 was divided over 2 days in 263 subjects (196 of whom were from test site 2), but the test sequence was maintained. For startle testing, as in W1, the eyeblink component of the acoustic startle response was measured using an EMG system that recorded 250 1-ms epochs, starting with startle stimulus onset. The session lasted 23.5-min, beginning with a 5-min acclimation period with 70-dB(A) SPL noise that continued throughout the session. The acclimation period was followed by 74 active trials, with 18 no stimulation (“nostim”) trials interspersed throughout the session. Startle “pulses” were 40-ms 115-dB(A) SPL noise bursts (near-instantaneous rise time, est. 1 ms). Prepulses were 20 ms noise bursts 15-dB above a 70-dB(A) SPL noise background, initiated 30, 60 or 120 ms prior to pulse onset; using slightly more intense 16 dB prepulses with this startle system, prepulse-associated EMG activity is <0.5% of startle stimulus-induced levels (Swerdlow et al., 2006b). Five startle pulses were presented at the start (Block 1) and end of the session (Block 4) to assess habituation. In Blocks 2–3, pulse presented alone and preceded by each of the 3 prepulse trial types were pseudo-randomly intermixed (9 trials per condition per blocks; inter-trial intervals 11–19 s (mean=15 s)). For “nostim” trials, data were recorded without stimulus presentation, to assess basal EMG activity. Filters, amplification, calibration, scoring and training procedures were described previously (Braff et al., 1992; Calkins et al., 2007; Graham, 1975; Swerdlow et al., 2007).
Patterns of subject “attrition” based on exclusion criteria are seen in Table 1S. Of the 660 W2 subjects for whom startle data were uploaded to the COGS database, 641 had a non-zero startle response, and 621 had sufficient startle data to allow calculation of the key dependent measure (60 ms PPI). Of these 621 subjects, 373 (195 HS, 178 patients) met the strict inclusion criteria for startle magnitude generally applied in single-site PPI studies, and applied with W1 data (“responder” defined as “mean startle magnitude for both PPI blocks ≥ 10 digital units (1.31 uV/unit)”) in addition to other criteria listed in Table 2S. Of the 884 subjects for whom these startle magnitude exclusion criteria were applied in “wave 1” (Swerdlow et al. 2014), 2 subjects were later excluded from analyses based on new clinical (i.e. not experimental) information obtained in long-term follow-up: (psychotropic drug use in 1 HS, and extended loss of consciousness in 1 patient).
Experimental measures (startle magnitude, habituation, latency and PPI) were analyzed using Rm-ANOVAs and post-hoc comparisons with “wave”, diagnosis and sex as between-subject factors for main analyses (the latter based on: 1) known male>female sexual dimorphism of PPI, and 2) known cohort difference in sex ratios among HS (female>male) and patients (male>female)). Where relevant to specific questions, other characteristics (e.g. medication status, illness duration) were included as between-subject factors for planned analyses. While many known PPI-moderating factors were investigated in W1, the current primary analyses were limited to those specifically needed to answer three experimental questions:
-
1)
Replicability: Are the same general patterns of PPI produced in both W1 and W2, as indicated by significant main effects of diagnosis (HS>patients) and sex (male>female), and a lack of significant interactions of “wave” (W1 vs. W2) for these main effects?
-
2)
Impact of inclusion / exclusion criteria: Does the use of more restrictive startle magnitude exclusion criteria for PPI impact the ability to detect reduced PPI in SZ patients, or the demographic or clinical characteristics of the HS and patients involved in those analyses?
-
3)
AP medications: Are there identifiable clinical or demographic differences among AP-free vs. AP-medicated patients, other than the use of APs, that contribute to the relatively blunted PPI deficits among medicated patients?
The key statistical main and interaction effects that address these three questions are reported in the Results. %PPI was calculated as 100×(1-(magnitude of startle to pulse preceded by prepulse)/magnitude of startle to pulse without a preceding prepulse)). As in our W1 report, we first confirmed that PPI deficits were interval-specific; we next focused on the primary dependent variable of % inhibition at 60 ms prepulse intervals, which we have reported to differ significantly between HS vs. samples including both medicated and unmedicated SZ patients (Swerdlow et al., 2006a; 2014), and which has been the primary dependent measure in previous studies of PPI and genetics by COGS (Greenwood et al., 2007, 2011) and individual laboratories (Greenwood et al., 2012; Light et al., 2012; Swerdlow et al., 2006a, 2006b). Secondary analyses also compared measures of startle magnitude during PPI testing, reflex habituation (startle magnitude reduction in trial block 4 vs. 1), peak reflex latency, and latency facilitation (latency reduction on trials with a prepulse followed by pulse vs. pulse alone trials). All variables were compared across testing sites, and for simplicity given the large number of variables and factors, were collapsed across right and left eyes. Alpha for all comparisons was 0.05. Effect sizes (Cohen’s d (1988)) are reported where appropriate.
3. Results
W1 vs. W2 subject characteristics
Demographic and clinical characteristics of COGS W1 vs. W2 subjects are seen in Table 1. W1 and W2 were comparable in proportions of patients vs. HS and men vs. women; subject age, patient age at illness onset and racial distributions were also comparable in W1 and W2. Across a number of metrics (number of hospitalization, SANS and SAPS), W2 patients were slightly but significantly more impaired, compared to W1 patients. The proportions of subjects tested at each of the 5 sites also differed somewhat across W1 and W2 (Table 1).
Table 1.
Subject characteristics based on “wave” and “responder” status (SD)
| W1 | W2 | Responders | Non-Responders | |||||
|---|---|---|---|---|---|---|---|---|
| HS | SZ | HS | SZ | HS | SZ | HS | SZ | |
| N1 | 628 | 771 | 304 | 317 | 633 | 647 | 299 | 441 |
| M:F2 | 314:314 | 542:229 | 143:161 | 217:100 | 286:347 | 433:214 | 171:128 | 326:115 |
| Age (y) | 338.45(13.25) | 46.24(11.07) | 38.83(12.85) | 46.03(11.55) | 37.07(13.09) | 44.69(11.73) | 41.74(12.62) | 48.37(10.01) |
| Race (A:AA:C)4 | 50:129: 375 | 45:292: 350 | 31:78:1 57 | 12:99:1 43 | 44:100: 414 | 19:183: 344 | 37:107: 118 | 18:208: 149 |
| Education (y) | 15.02(2.27) | 12.77(2.14) | 14.98(2.17) | 12.42(2.04) | 15.04(2.18) | 12.77(2.18) | 14.93(2.35) | 12.52(2.03) |
| GAF | 86.82(7.82) | 43.79(8.48) | 85.41(8.72) | 42.35(6.64) | 86.35(7.98) | 43.01(8.11) | 86.38(8.50) | 43.89(7.84) |
| MMSE Score5 | 33.54(1.74) | 31.10(3.20) | 33.57(1.66) | 31.12(3.27) | 33.63(1.63) | 31.27(3.16) | 33.36(1.86) | 30.87(3.30) |
| Age, onset (y) | 22.50(6.96) | 22.15(7.10) | 22.54(7.01) | 22.17(6.99) | ||||
| Duration ill (y)6 | 23.87(11.63) | 23.67(11.59) | 22.02(11.87) | 26.21(10.74) | ||||
| # hospitalizations7 | 7.15(9.15) | 8.51(12.57) | 7.24(10.52) | 7.99(9.91) | ||||
| Global SANS8 | 11.27(5.52) | 12.37(5.48) | 11.74(5.42) | 11.37(5.68) | ||||
| Global SAPS9 | 6.76(4.10) | 7.58(4.04) | 7.15(4.12) | 6.78(4.05) | ||||
| SOF total score | 46.81(6.23) | 45.01(5.28) | 46.11(5.94) | 46.53(6.14) | ||||
| UPSA-B score | 72.36(15.62) | 70.41(14.33) | 71.94(15.38) | 71.61(15.15) | ||||
| Hearing (dB) at 1000Hz10 | 14.03(7.92) | 17.38(7.87) | 13.04(6.87) | 16.70(7.89) | 13.13(7.69) | 16.42(7.99) | 14.91(7.29) | 18.31(7.58) |
| Site (1 : 2 : 3 : 4 : 5)11, 12 | 377 : 257 : 149 : 296 : 320 | 151 : 126 : 146 : 93 : 105 | 314 : 237: 170 251 : 308 | 214 : 146 : 125 : 138 : 117 | ||||
M:F = male:female; GAF = Global Assessment of Function Scale; MMSE = Mini-Mental State Examination; SANS, SAPS, SOF, UPSA: see text
Non-responder (NR) / Responder (R), SZ>HS: χ2=15.45, p<0.0001
M/F, SZ>HS:χ2=90.01, p<0.0001
NR > R: F(1,2016)=55.70, p<0.0001
Asian : African American : Caucasian; NR/R, C<A,AA: χ2=120.89, p<0.0001
R>NR: F(1,1908)=7.09, p<0.008
NR>R: F(1,1076)=35.52, p<0.0001
W2>W1: F(1,1082)=3.94, p<0.05
W2>W1: F(1,1080)=8.78, p<0.004
W2>W1: F(1,1078)=8.98, p<0.003
NR>R: F(1,2006)=25.76, p<0.0001
Site difference in W1 vs. W2 representation (W2 vs. W1, relative increase in Site 3 and decrease in Sites 4 and 5)
Site difference in NR rate: χ2=23.40, p<0.0002 (Site 5 < Site 1–4)
Exclusion rates based on low startle response magnitude were 35.7% in W1 and 38.6% in W2. Characteristics of startle “responders” vs. “non-responders” are seen in Table 1. Across W1 and W2, subjects excluded for low startle magnitude were more likely to be older, male, non-Caucasian, to have less sensitive hearing (though still within established inclusion criteria for auditory acuity) and a diagnosis of SZ. After exclusion for low startle magnitude, HS “responders” were mostly women (55%), and SZ “responders” were mostly men (67%); based solely on these sex distributions, PPI in the HS group was biased towards lower values, and PPI in the SZ group was biased towards higher values.
PPI
PPI results are seen in Figure 1 and Table 2. As reported in W1, patients had decreased PPI compared to HS when data from all subjects with measurable (non-zero) startle response magnitude were analyzed (n=621); ANOVA of W2 data revealed a significant interaction of diagnosis × prepulse interval (F(2,1234) = 3.04, p<0.05); analysis of the key 60 ms interval revealed a significant effect of diagnosis (HS>patient: F(1,617) = 3.99, p<0.05). Among the inclusive group of 2,020 wave 1+2 subjects, ANOVA revealed no significant main or interaction effects of wave, a significant main effect of diagnosis (HS>patient; F(1,2012) = 4.64, p<0.032), and a significant diagnosis × prepulse interval interaction (p<0.0001); ANOVA of 60 ms PPI from these 2,020 subjects revealed no significant main or interaction effects of wave, and a significant main effect of diagnosis (HS>patients: F(1,2012) = 13.03, p<0.0004). Thus, when data were analyzed without restrictive response criteria for startle magnitude, SZ-linked PPI deficits were present and comparable in W1 and W2.
Figure 1.
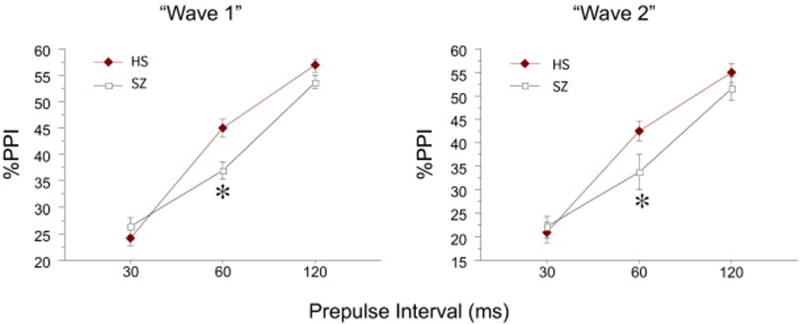
%PPI for the full samples in W1 (n=1399) and W2 (n=621), including all subjects with measureable startle responses from both eye sides across the full test session. Independent analyses of W1 and W2 samples revealed relatively comparable patterns in W1 vs. W2, with significant interactions of diagnosis × prepulse interval, and statistically significant PPI deficits in SZ patients detected for 60 ms prepulse intervals (*). ANOVA of the combined W1+W2 samples confirmed these findings, with no main or interaction effect of “wave”.
Table 2.
PPI results in key groups (60 ms prepulse intervals, mean (SEM)):
| Blocks 2 & 3 | Block 2 alone | |||
|---|---|---|---|---|
| HS | SZ | HS | SZ | |
| All subjects | 44.2 (1.5) | 36.0 (1.8) | 49.2 (1.2) | 37.3 (2.3) |
| All males | 45.8 (2.2) | 37.9 (2.3) | 50.2 (1.8) | 38.2 (3.0) |
| All females | 42.6 (2.0) | 31.5 (2.7) | 48.2 (1.5) | 35.1 (3.4) |
| All “AP-free” | 44.2 (1.5) | 32.9 (3.8) | 49.2 (1.2) | 35.1 (4.2) |
| “Responders”1 | 51.0 (1.0) | 48.0 (1.1) | 53.0 (1.0) | 48.1 (1.1) |
| Male “Responders” | 54.0 (1.5) | 50.9 (1.4) | 56.3 (1.4) | 50.7 (1.3) |
| Female “Responders” | 48.5 (1.4) | 41.9 (2.1) | 50.2 (1.4) | 42.6 (2.0) |
| “AP-free”, “Responders” | 51.0 (1.0) | 42.3 (3.4) | 53.0 (1.0) | 41.7 (3.8) |
| Ill < 10 y, “AP-free” “Responders”2 | 55.5 (3.8) | 40.5 (5.4) | 58.8 (3.1) | 41.2 (5.9) |
mean startle magnitude ≥ 10 units
age-and sex-matched subgroups (see “Results”)
When strict (> 10 units for both block 2 and block 3) startle magnitude criteria were applied, ANOVA of %PPI again revealed no overall significant effect of “wave” (F<1) and a significant interaction of diagnosis × interval (F(2,2476) = 10.17, p<0.0001). ANOVA of %PPI at 60 ms again revealed no significant effect of “wave” (F<1), and a significant effect of diagnosis (HS>patient; F(1,1238) = 6.30, p<0.015). The impact of different startle magnitude inclusion thresholds on the magnitude of SZ-linked PPI deficits is shown in Figure 2A. In general, less restrictive inclusion thresholds resulted in greater arithmetic differences in group mean PPI values, accompanied by greater variability of PPI values within groups, with an end result of modestly larger effect sizes for SZ-linked PPI deficits. Compared to using data from both PPI blocks 2 and 3, limiting data to PPI block 2 (i.e., earlier in the test session, before startle reflex habituation is complete) permits the inclusion of about 22% more subjects whose startle levels are > 10 units (1557 vs. 1280; Figure 2B, right panel, vs. Figure 2A, right panel), with a modestly larger between-group effect size (0.16 vs. 0.11). Subjects whose startle magnitudes fell below response thresholds were disproportionately represented among subjects with the most extreme values of %PPI (Figure 2C). Subjects meeting “non-responder” criteria constituted 32.1% and 40.5% of the full HS and patient samples, respectively; however, among subjects whose PPI values were in the most extreme 5% of each group, “non-responders” constituted 62% and 70.4% of HS and patients, respectively.
Figure 2.

Impact of different exclusion criteria on sample size and effect size for the key 60 ms PPI comparison for the combined W(1+2) subjects. A. Data from both PPI trial blocks (2 and 3) in (from left to right) the full COGS-2 sample (n=2020), criteria set at ≥ 10 units for block 2 and ≥ 5 units for block 3 (middle, n=1506) and criteria set at ≥ 10 units for both blocks 2 and 3 (right, n=1280). Increasingly strict startle magnitude exclusion criteria lead to sample attrition and modest reductions in effect size. B. Data from PPI block 2 only, from all subjects (left; n=2020) and subjects whose block 2 reflex magnitude was > 10 units (n=1557). C. Distribution of 60 ms PPI values for startle “non-responders” vs. “responders” (i.e. those excluded vs. included in “A”, right panel), showing a disproportionate number of startle “non-responders” among the extreme (negative) PPI values.
Numerous strategies were used to attempt to identify a meaningful relationship between startle magnitude and 60 ms PPI in these groups, that might contribute to the observed group differences in PPI. Dividing the sample into quartiles based on startle magnitude, ANOVA confirmed main effects of diagnosis (p<0.006), sex (p<0.003) and quartile (p<0.0001), but found no interactions of quartile with either diagnosis or sex (both F<1). Eliminating the top quartile, the main effect of diagnosis on 60 ms PPI remained significant (p<0.0008; d=0.16). Among all “responders”, there was no significant correlation between startle magnitude and 60 ms PPI (r = 0.026, NS). After eliminating extreme values (the top and bottom 2.5% of the startle distribution), the main effect of diagnosis on 60 ms PPI remained significant (p<0.0002; d=0.15).
Moderating effects of medications
Potential moderating effects of medication on HS vs. patient differences in PPI were assessed in W1 and W2 data (Figure 3). Significant PPI deficits were detected among patients who, by self-report, were not taking AP medications. The combination of wave 1+2 data yielded a total of 120 AP-free patients, 74 (62%) of whom met startle magnitude criteria (Figure 3A). Because a cohort of 74–120 AP-free SZ patients is rare, we assessed PPI in these AP-free patients vs. HS. ANOVAs revealed HS>patient PPI levels among AP-free patients (diagnosis × interval interaction: F(2,136) = 3.09, p<0.05; 60 ms interval: F(1,68) = 5.49, p<0.025), that were independent of both exclusion criteria and “wave” (Figure 3A & B). As previously reported, PPI deficits were blunted among AP-medicated vs. AP-free patients, though the specific impact on PPI of first-generation APs (FGAPs) vs. second-generation APs (SGAPs) and their combination varied somewhat, based on the applied inclusion criteria and test wave. Demographic and clinical characteristics of AP-free vs. medicated patients in W1 and W2 are seen in Table 2. Compared to the 3 groups of medicated patients (FGAP, SGAP and FGAP+SGAP), AP-free patients had fewer hospitalizations and higher SAPS scores, and were disproportionately represented among patients in the first decade of illness. Analyses of 60 ms PPI in AP-free patients revealed expected effects of sex (M>F; F(1,118) = 6.18, p<0.015), but not “wave” (F(1,118) = 1.10, NS); regression analyses revealed no meaningful relationships between PPI and demographic or clinical variables (listed in Table 3) in this large sample of AP-free patients (0.22 > r > −0.22; data not shown) (but see Duncan et al. 2006).
Figure 3.
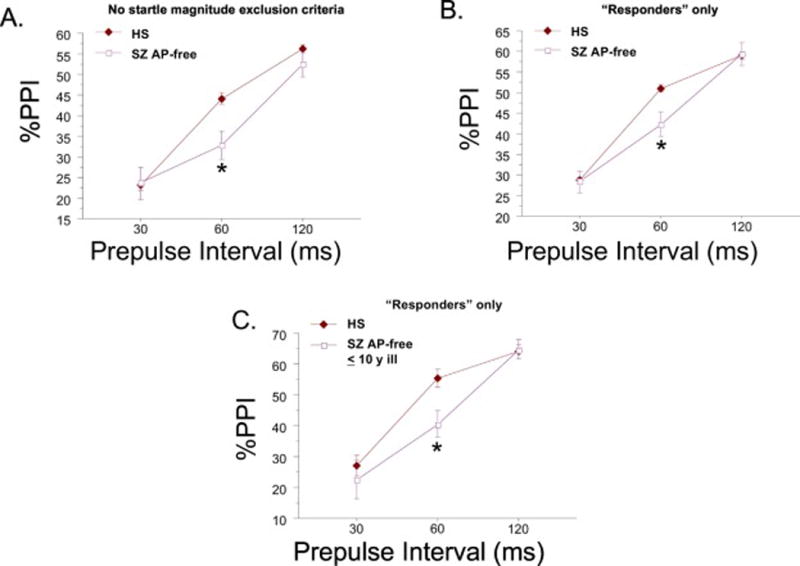
A. PPI in all AP-free patients (n=120) vs. all HS (d=0.26), and B. the same comparison as “A”, with strict exclusion criteria applied for minimal startle magnitude (n=78 AP-free patients) (d=0.32). C. PPI in AP-free SZ patients in the first decade of their illness (n=24) vs. sex- and age-matched HS (case-matched 2:1; n=48, all “responders”) (d=0.57) (* significant main effect of diagnosis for 60 ms prepulse intervals, after significant interaction of diagnosis × interval).
Table 3.
Patient characteristics based on antipsychotic use (SD)
| No AP | FGAP | SGAP | FGAP+SGAP | |
|---|---|---|---|---|
| N | 120 | 79 | 786 | 103 |
| M:F | 79:41 | 56:23 | 548:238 | 76:27 |
| Age (y)1 | 45.73 (12.72) | 49.43 (10.19) | 45.75 (11.17) | 47.49 (9.95) |
| Race (A:AA:C) | 4 : 44 : 56 | 3 : 39 : 31 | 29 : 275 : 351 | 1 : 33 : 55 |
| Education (y) | 12.79 (2.24) | 12.68 (2.61) | 12.70 (2.08) | 12.27 (1.83) |
| GAF2 | 42.00 (7.88) | 43.69 (8.59) | 43.91 (8.07) | 40.54 (6.43) |
| MMSE score3 | 31.69 (3.13) | 31.10 (3.39) | 31.12 (3.14) | 30.36 (3.60) |
| Age, onset (y)4 | 23.01 (8.36) | 23.58 (6.73) | 22.37 (6.97) | 20.85 (5.27) |
| Duration ill (y)5 | 22.59 (13.55) | 25.85 (10.46) | 23.31 (11.47) | 26.64 (10.41) |
| % in 1st decade of illness6 | 25.4 | 10.1 | 16.5 | 9.0 |
| # hospitalizations7 | 5.73 (9.90) | 9.13 (12.18) | 7.45 (9.87) | 9.24 (11.79) |
| Global SANS8 | 11.70 (4.93) | 10.87 (5.20) | 11.34 (5.63) | 13.93 (5.16) |
| Global SAPS9 | 8.59 (4.24) | 6.89 (4.07) | 6.67 (4.01) | 7.73 (4.09) |
| SOF total score | 45.33 (5.83) | 46.30 (5.51) | 46.61 (6.02) | 44.85 (6.43) |
| UPSA-B score10 | 72.90 (14.84) | 72.22 (15.84) | 72.34 (14.94) | 66.19 (16.85) |
| Hearing (dB) at 1000Hz11 | 17.48 (8.45) | 18.10 (7.67) | 16.82 (7.77) | 18.93 (7.94) |
| Site (1 : 2 : 3 : 4 : 5) | 12:18:18:26:30 | 14:16:12:20:17 | 232:149:110:137:172 | 43:13:6:25:15 |
M:F = male:female; A:AA:C = Asian : African American : Caucasian; GAF = Global Assessment of Function Scale; MMSE = Mini-Mental State Examination; SANS, SAPS, SOF, UPSA: see text
FGAP > No AP (p<0.025); FGAP > SGAP (p<0.006)
FGAP+SGAP > SGAP (p<0.0001); FGAP+SGAP > FGAP (p<0.009); SGAP > No AP (p<0.016)
No AP > FGAP+SGAP (p<0.003); SGAP > SGAP+FGAP (p<0.03)
FGAP+SGAP < No AP (p<0.025); FGAP+SGAP < SGAP (p<0.04); FGAP+SGAP < FGAP (p<0.01)
FGAP+SGAP > No AP (p=0.01); FGAP+SGAP > SGAP (p<0.007)
χ2=13.34, p<0.004
FGAP+SGAP > No AP (p<0.015)
FGAP+SGAP > No AP (p<0.003); FGAP+SGAP > SGAP (p<0.0001); FGAP+SGAP > FGAP (p<0.0003)
No AP > SGAP (p<0.0001); No AP > FGAP (p<0.004); FGAP+SGAP > SGAP (p<0.02)
FGAP+SGAP < No AP (p<0.002); FGAP+SGAP < SGAP (p<0.0001); FGAP+SGAP <FGAP (p<0.001)
FGAP+SGAP > SGAP (p=0.01)
Site difference in AP distribution: χ2=39.95, p<0.0001 (No AP: Site 1 < Sites 2–5)
A small number of AP-free patients (n=24; M:F = 2:1) met “responder” criteria, and were also within the first decade of illness onset; arguably, data from this patient cohort was the “purest” in this study, i.e. least impacted by potential influences of low reflex magnitude, medications or illness chronicity. Compared to a case-control age- and sex-matched cohort of HS (two HS matched to each patient case), analyses of %PPI revealed the same patterns as described for the overall sample: a significant interaction of diagnosis × prepulse interval (F(2,136) = 3.09, p<0.05), with post-hoc analyses for 60 ms PPI revealing a significant main effect of group (F(1,68) = 5.49, p<0.025; d = 0.57) (Figure 3C).
Analyses of many other potential moderators of the PPI “phenotype” in SZ patients were reported in analyses of W2 data (Swerdlow et al. 2014); those patterns were generally consistent with the combined (W1+W2) sample. For example, among “responders”, 60 ms PPI deficits in SZ patients were evident regardless of whether analyses were limited to patients and HS who had never smoked (p<0.01) or to those who were currently smoking (p<0.007).
Startle magnitude
Startle magnitude and latency data are seen in Figure 4. As in W1, startle magnitude in W2 was reduced in patients vs. HS (Figure 4A). ANOVA of startle magnitude revealed no significant effect of “wave” (F<1), a significant effect of diagnosis (HS>SZ; F(1,1276) = 6.32, p<0.02) and trial block (F(1,1276) = 800.04, p<0.0001), and a significant interaction of diagnosis × block (F(1,1276) = 7.44, p<0.007), but no other significant main or interaction effects. Analysis of reflex habituation (reduction in reflex magnitude in block 4 vs. block 1) revealed no significant main or interaction effects of “wave” or diagnosis (all F’s<1) (Figure 4B).
Figure 4.
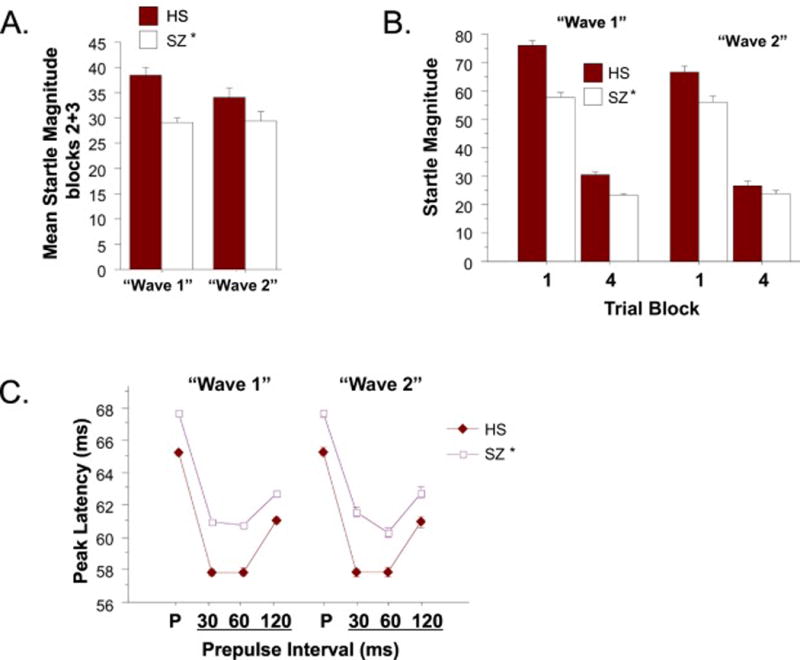
Startle reflex magnitude (A), habituation (B) and reflex latency (C) for W1 and W2. (* significant main effect of diagnosis for startle magnitude and peak reflex latency across trials, including pulse alone (P) and 30, 60 and 120 ms prepulse conditions).
Peak reflex latency and latency facilitation
As in W1, peak reflex latency on pulse alone trials was longer (slower) in patients vs. HS (Figure 4C). Because of the substantial loss of reflex latency data when startle magnitude is near-zero, latencies were analyzed only using data that met minimum startle magnitude criteria (i.e. ≥ 10 units). ANOVA of peak reflex latency revealed no significant effect of “wave” (F<1) and a significant effect of diagnosis (HS<SZ; F(1,1268) = 57.82, p<0.0001) and sex (women>men: F(1,1268) = 6.43, p<0.015) and no 2- or 3-way interactions. Latency facilitation (reduction in reflex latency on prepulse+pulse vs. pulse alone trials) was also comparable in W1 vs. W2.
4. Discussion
The relative loss of sensorimotor gating in SZ patients vs. HS, as reflected by deficient PPI, has been detected in several dozen studies since the original observation by Braff et al. (1978) in Callaway’s research group (e.g. Aggernaes et al., 2010; Braff et al., 1999, 2001, 2005; Csomor et al., 2009; Hammer et al., 2011, 2013; Hong et al., 2007; Kishi et al., 2012; Kumari et al., 1999, 2007; Kunugi et al., 2007; Light et al., 2012; Ludewig et al., 2003; Mackeprang et al., 2002; Martinez-Gras et al., 2009; Meincke et al., 2004; Molina et al., 2011; Moriwaki et al., 2009; Oranje and Glenthoj, 2013; Preuss et al., 2011; Quednow et al., 2006; Rabin et al., 2009; Swerdlow et al. 2006a; Takahashi et al., 2008; Wang et al., 2013; Weike et al., 2000; Xue et al., 2012). Since this first report, nearly 3000 PubMed-listed “PPI papers” have illuminated much about PPI, its neurobiological substrates and its abnormalities in a number of different brain disorders. Some of this information is reviewed and extended in the reports within this special issue of Schizophrenia Research. In particular, the robust heritability of PPI (Anokhin et al. 2003; Greenwood et al. 2007), its consistent deficiency in SZ patients and its reported deficiency in asymptomatic first-degree relatives of schizophrenia patients (Cadenhead et al. 2000) led the COGS to study PPI as a quantitative endophenotype of SZ in its five-site study of SZ genetics. Since PPI is a quantitative measure, COGS investigators reasoned that its use in gene localization should provide significantly more power, compared to a “fuzzy” qualitative diagnosis in a case-control design (Blangero et al., 2003). Subsequent to the onset of these COGS studies, several (but not all) studies have reported reduced PPI among individuals at clinical high-risk for psychosis, including some identified as “prodromal” (Cadenhead 2011; Quednow et al. 2008; Ziermans et al. 2011, 1012).
While results from a planned interim COGS analysis confirmed the predicted phenotype of significantly reduced PPI in SZ subjects, they also highlighted several potential limitations to the use of this measure as an endophenotype for multi-site genetic analyses. In particular, the use of minimal startle magnitude exclusion criteria led to a loss of almost 39% of the test sample, who – compared to the “included” subjects - were more likely to be older, male, non-Caucasian, to have less sensitive hearing and a diagnosis of SZ. This attrition not only reduced power for identifying genes that regulate PPI, but it also limited the use of PPI in factor- and path-analyses that incorporated many other COGS endophenotypes with much lower subject attrition rates (Seidman et al. 2015; Millard et al. 2016; Thomas et al. 2017). Furthermore, when the sample was pared to include only subjects with startle reflex magnitudes in excess of 10 units, the overall effect size for 60 ms PPI deficits in SZ patients was small (d≈0.15) compared to reported effect sizes in single-site studies. A number of factors may have contributed to this small effect size difference, including the predominance of (lower PPI) women among HS and of (higher PPI) males among patients, as well as the use among patients of PPI-enhancing nicotine and APs. While these known effects of sex, smoking and medications on PPI can be incorporated statistically into models that test group differences, it is important that they cannot easily be extricated from an individual subject’s PPI value, and thereby complicate the genomic and neurobiological signal provided by this endophenotype.
With the completion of the second COGS “wave” of subjects, analyses of PPI within this full COGS sample generally confirmed the internal “replicability” of several startle-related SZ phenotypes, including reduced 60 ms PPI, reduced startle magnitude and increased (slower) reflex latency within this multi-site sample. The potential importance of inhibitory processes active 60 ms after a lead stimulus, to the flow of preconscious information into conscious awareness (Grobstein, 2005; Kanabus et al., 2002; Libet et al., 1979; Libet 1985) has been discussed in our previous reports (e.g. Swerdlow et al. 2006a). The basic characteristics of PPI, startle magnitude, habituation and latency in the W2 and combined “W1+W2” samples closely reproduced those detected in the W1 sample; while this report did not focus on several known moderators of PPI (e.g. nicotine) and startle magnitude (e.g. ethnicity), the combined “W1+W2” sample exhibited all of these expected patterns. We also used the unique opportunities presented by this sample – which to our knowledge reflects the largest single reported study of PPI in HS and SZ patients – to examine two issues of relevance to the design and interpretation of PPI studies in SZ cohorts: the impact of more or less restrictive reflex magnitude inclusion criteria on SZ-linked patterns of PPI, and the demographic, clinical and response characteristics of AP-free SZ patients, who manifest the greatest deficits in PPI.
Subjects excluded based on very low startle response magnitudes were more likely to be patients vs. HS, male vs. female, non-Caucasian vs. Caucasian, older vs. younger, and – perhaps most importantly – to have less sensitive hearing (despite the fact that all subjects met inclusion criteria based on a hearing threshold of 40 dB for 1000 Hz tones) - compared to higher startle “responders”. Startle magnitude is a complex phenotype that reflects many physiological characteristics, from stimulus detection to muscle effector properties; it is thus not surprising that startle responders vs. non-responders would differ both in hearing thresholds and in the distribution of ethnicities with known genetically-based differences in startle musculature that generate the startle response (Swerdlow et al. 2005; Hasenkamp et al. 2008; Nelson et al. 2014). In addition to these trans-diagnostic characteristics, among patients, “non-responders” had been ill significantly longer – a relationship that cannot be dissociated fully from the effects of aging or longer AP exposure on startle magnitude - but otherwise were not easily distinguished from responders based on demographic or clinical features. Perhaps the most succinct summary of this set of analyses is that restrictive startle magnitude criteria result in the disproportionate exclusion of specific groups of subjects that might introduce systematic bias into genetic or other analyses that rely on startle phenotypes, including PPI.
As for quantitative outcomes, inclusion of subjects with lower reflex magnitudes resulted in modestly bigger differences in group mean PPI values, and modestly larger variability within groups; these two effects were offsetting in their impact on group PPI effect sizes. On the one hand, it is reassuring that the ability to detect PPI deficits in SZ patients is relatively impervious to the decision to use, or not use, reflex magnitude criteria for inclusion/exclusion decisions in PPI analyses. On the other hand, PPI values from the more inclusive cohort of subjects – which includes extreme values introduced by greater noise (both in signal acquisition and in PPI calculations) associated with very small startle magnitudes – may not be as useful for analyses in which individual subject values are used to identify factors, pathways or genes. One “low tech” approach to enhancing the yield of meaningful PPI values from this type of data set is to limit analyses to trials in the initial portions of the test session, before reflex habituation has maximally suppressed startle magnitude (Figure 2B). Applying this strategy to the present data increased both the number of “included” test subjects, and the effect size of HS vs. patient group differences in PPI analyses. From a practical standpoint, this finding underscores the value of utilizing brief test sessions, and potentially a restricted number of total trials and different stimulus conditions, when employing PPI as a quantitative phenotype for genetic analyses.
The moderating impact of APs on PPI deficits in SZ patients was evident in the combined W1+2 sample, with the lowest (most impaired) PPI levels detected among AP-free SZ patients, and the highest PPI levels detected among patients medicated with both first- and second-generation APs. While greater PPI deficits among AP-free vs. medicated SZ patients have been reported in other studies (including the interim W1 analysis (Swerdlow et al. 2014)), such evidence comes almost exclusively from cross-sectional studies; because the clinical and sociological algorithms resulting in AP use (or non-use) in SZ are complex, it is conceivable that cross-sectional differences in PPI among AP-free vs. medicated patients might reflect factors other than the pharmacological impact of APs. The large number of AP-free patients accumulated in this 5-site, 3.5-year study allowed us to begin to assess demographic or clinical features among these patients – other than the lack of AP use – that might contribute to the most substantive loss of PPI in this group.
In fact, our cross-sectional comparisons revealed very few differences between AP-free and – medicated patients; overall, compared to medicated patients, AP-free patients were slightly younger, earlier in their illness, and exhibited somewhat more intact scores on cognitive measures. Among the 4 patient groups – AP-free, FGAP, SGAP and FGAP+SGAP – patients who were AP-free exhibited arithmetically-lower values for 30, 60 and 120 ms PPI compared to the other 3 groups, though these differences did not reach statistical significance. Such was not the case for other startle measures – reflex magnitude, latency or habituation – where values among AP-free patients were intermediate among the 4 groups. This relatively large group of AP-free patients enabled us to identify a modest number who were relatively early in the course of their illness; compared to age- and sex-matched HS, these AP-free, early illness SZ patients exhibited particularly robust deficits in PPI, supporting the view that the “low PPI phenotype” reflects processes that may be independent of both medication use and illness chronicity.
In summary, deficient PPI is a replicable finding in large, complex, multi-site genetic studies. Subject attrition due to factors unique to startle measures is non-trivial. Relaxing inclusion criteria to reduce attrition produces off-setting effects of arithmetically greater group differences and greater within-group variability, and HS > SZ group differences in PPI are evident with, or without, the use of these inclusion criteria. Excluding startle “non-responders” systematically favors the loss of specific subgroups – i.e. older, male, non-Caucasians SZ patients, with low-normal hearing sensitivity. We present evidence for robust deficits in PPI among patients who are AP-free and relatively early in their illness, that are not easily attributable to identifiable clinical or demographic factors, including medications and illness chronicity. Overall, these findings support the value of deficient PPI as a replicable SZ phenotype that, on a group level, is relatively immune to potentially complicating effects of startle response criteria and medications. For the definitive evidence for, or against, the utility of this quantitative endophenotype in genetic analyses of both PPI and SZ, we must await results from genomic analyses currently in progress.
Supplementary Material
Supplemental Figure S1. Scatterplot of %60 ms PPI in all “responders”, divided by diagnosis and sex. Small horizontal lines mark the group mean values. For “subgroups” exhibiting greater diagnosis-based differences in PPI, see Table 2 and Figure 2A.
Acknowledgments
This study was supported by grants R01-MH065571, R01-MH065588, R01-MH065562, R01-MH065707, R01-MH065554, R01-MH065578, R01-MH065558, R01 MH86135, and K01-MH087889 from the National Institute of Mental Health.
Role of funding source: Other than providing support, the NIH had no further role in this manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest: Drs. Braff, Calkins, Greenwood, RE Gur, RC Gur, Lazzeroni, Light, Radant, Seidman, Siever, Silverman, Stone, Sugar, Swerdlow, DW Tsuang, MT Tsuang and Turetsky report no financial relationships with commercial interests. Dr. Green reports having been a consultant to Abbott laboratories (AbbVie), Biogen, and Roche, he is a member of the scientific board for Mnemosyne, and he has received research funds from Amgen. Dr. Nuectherlein has received unrelated research support from Janssen Scientific Affairs, Genentech, and Brain Plasticity, Inc., and has consulted to Genentech, Otsuka, and Brain Plasticity, Inc.
Contributors: Dr. Swerdlow supervised 5-site quality assurance for PPI measures, completed all statistical analyses and wrote the first draft of this manuscript. Joyce Sprock performed quality assurance for all data and coordinated database activities. All other authors participated in aspects of study design, including subject recruitment, phenotyping, and validation of the clinical and endophenotype data. All authors were responsible for reviewing and approving the final manuscript.
References
- Aggernaes B, Glenthoj BY, Ebdrup BH, Rasmussen H, Lublin H, Oranje B. Sensorimotor gating and habituation in antipsychotic-naive, first-episode schizophrenia patients before and after 6 months’ treatment with quetiapine. Int. J. Neuropsychopharmacol. 2010;13(10):1383–1395. doi: 10.1017/S1461145710000787. [DOI] [PubMed] [Google Scholar]
- Andreasen NC. The scale for the assessment of negative symptoms (SANS) The University of Iowa; Iowa City, IA: 1984a. [Google Scholar]
- Andreasen NC. The scale for the assessment of positive symptoms (SAPS) The University of Iowa; Iowa City, IA: 1984b. [Google Scholar]
- Anokhin AP, Heath AC, Myers E, Ralano A, Wood S. Genetic influences on prepulse inhibition of startle reflex in humans. Neurosci. Lett. 2003;353(1):45–48. doi: 10.1016/j.neulet.2003.09.014. [DOI] [PubMed] [Google Scholar]
- Blangero J, Williams JT, Almasy L. Novel family-based approaches to genetic risk in thrombosis. J. Thromb. Haemost. 2003;1(7):1391–1397. doi: 10.1046/j.1538-7836.2003.00310.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Geyer MA, Light GA, Sprock J, Perry W, Cadenhead KS, Swerdlow NR. Impact of prepulse characteristics on the detection of sensorimotor gating deficits in schizophrenia. Schizophr. Res. 2001;49(1–2):171–178. doi: 10.1016/s0920-9964(00)00139-0. [DOI] [PubMed] [Google Scholar]
- Braff DL, Grillon C, Geyer MA. Gating and habituation of the startle reflex in schizophrenic patients. Arch. Gen. Psychiatry. 1992;49(3):206–215. doi: 10.1001/archpsyc.1992.01820030038005. [DOI] [PubMed] [Google Scholar]
- Braff DL, Light GA, Ellwanger J, Sprock J, Swerdlow NR. Female schizophrenia patients have prepulse inhibition deficits. Biol. Psychiatry. 2005;57(7):817–820. doi: 10.1016/j.biopsych.2004.12.030. [DOI] [PubMed] [Google Scholar]
- Braff D, Stone C, Callaway E, Geyer M, Glick I, Bali L. Prestimulus effects on human startle reflex in normals and schizophrenics. Psychophysiology. 1978;15(4):339–343. doi: 10.1111/j.1469-8986.1978.tb01390.x. [DOI] [PubMed] [Google Scholar]
- Braff DL, Swerdlow NR, Geyer MA. Symptom correlates of prepulse inhibition deficits in male schizophrenic patients. Am. J. Psychiatry. 1999;156(4):596–602. doi: 10.1176/ajp.156.4.596. [DOI] [PubMed] [Google Scholar]
- Cadenhead KS. Startle reactivity and prepulse inhibition in prodromal and early psychosis: effects of age, antipsychotics, tobacco and cannabis in a vulnerable population. Psychiatry Res. 2011;188(2):208–216. doi: 10.1016/j.psychres.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cadenhead KS, Swerdlow NR, Shafer KM, Diaz M, Braff DL. Modulation of the startle response and startle laterality in relatives of schizophrenic patients and in subjects with schizotypal personality disorder: evidence of inhibitory deficits. Am. J. Psychiatry. 2000;157(10):1660–1668. doi: 10.1176/appi.ajp.157.10.1660. [DOI] [PubMed] [Google Scholar]
- Calkins ME, Dobie DJ, Cadenhead KS, Olincy A, Freedman R, Green MF, Greenwood TA, Gur RE, Gur RC, Light GA, Mintz J, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. The consortium on the genetics of endophenotypes in schizophrenia: model recruitment, assessment, and endophenotyping methods for a multisite collaboration. Schizophr. Bull. 2007;33(1):33–48. doi: 10.1093/schbul/sbl044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cohen J. Statistical Power Analysis for the Behavioral Sciences. 2. Lawrence Erlbaum Associates, Inc; Hillsdale, NJ: 1988. [Google Scholar]
- Csomor PA, Yee BK, Feldon J, Theodoridou A, Studerus E, Vollenweider FX. Impaired prepulse inhibition and prepulse-elicited reactivity but intact reflex circuit excitability in unmedicated schizophrenia patients: a comparison with healthy subjects and medicated schizophrenia patients. Schizophr. Bull. 2009;35(1):244–255. doi: 10.1093/schbul/sbm146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duncan EJ, Bollini AM, Lewison B, Keyes M, Jovanovic T, Gaytan O, Egan G, Szilagyi S, Schwartz M, Parwani A, Chakravorty S, Rotrosen J. Medication status affects the relationship of symptoms to prepulse inhibition of acoustic startle in schizophrenia. Psychiatry Res. 2006;145(2–3):137–145. doi: 10.1016/j.psychres.2006.04.006. [DOI] [PubMed] [Google Scholar]
- Faraone SV, Tsuang D, Tsuang MT. Genetics of Mental Disorders: A Guide for Students, Clinicians, and Researchers. Guilford, New York: 1999. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders -- Patient Edition. New York State Psychiatric Institute; New York: 1995. SCID-I/P, Version 2.0. [Google Scholar]
- First MB, Spitzer RL, Gibbon M, Williams JB. Structured Clinical Interview for DSM-IV Axis I Disorders -- Non-Patient Edition. New York State Psychiatric Institute; New York: 1996. SCID-I/NP, Version 2.0. [Google Scholar]
- Graham F. The more or less startling effects of weak prestimuli. Psychophysiology. 1975;12(3):238–248. doi: 10.1111/j.1469-8986.1975.tb01284.x. [DOI] [PubMed] [Google Scholar]
- Greenwood TA, Braff DL, Light GA, Cadenhead KS, Calkins ME, Dobie DJ, Freedman R, Green MF, Gur RE, Gur RC, Mintz J, Nuechterlein KH, Olincy A, Radant AD, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Schork NJ. The Consortium on the Genetics of Schizophrenia (COGS): Initial Heritability Analyses of Endophenotypic Measures for Schizophrenia. Arch. Gen. Psychiatry. 2007;64(11):1242–1250. doi: 10.1001/archpsyc.64.11.1242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Lazzeroni LC, Murray SS, Cadenhead KS, Calkins ME, Dobie DJ, Green MF, Gur RE, Gur RC, Hardiman G, Kelsoe JR, Leonard S, Light GA, Nuechterlein KH, Olincy A, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Freedman R, Braff DL. Analysis of 94 candidate genes and twelve endophenotypes for schizophrenia from the Consortium on the Genetics of Schizophrenia. Am. J. Psychiatry. 2011;168(9):930–946. doi: 10.1176/appi.ajp.2011.10050723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Greenwood TA, Light GA, Swerdlow NR, Radant AD, Braff DL. Association analysis of 94 candidate genes and schizophrenia-related endophenotypes. PLoS One. 2012;7(1):e1002134. doi: 10.1371/journal.pone.0029630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grobstein P. rMaking the unconscious conscious: a bi-directional bridge between neuroscience/cognitive science and psychotherapy? Cortex. 2005;41:663–668. doi: 10.1016/s0010-9452(08)70283-1. [DOI] [PubMed] [Google Scholar]
- Hall RC. Global assessment of functioning. A modified scale. Psychosomatics. 1995;36(3):267–275. doi: 10.1016/S0033-3182(95)71666-8. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Fagerlund B, Bro H, Glenthøj BY. Stability of prepulse inhibition and habituation of the startle reflex in schizophrenia: a 6-year follow-up study of initially antipsychotic-naive, first-episode schizophrenia patients. Int. J. Neuropsychopharmacol. 2011;14(7):913–925. doi: 10.1017/S1461145711000034. [DOI] [PubMed] [Google Scholar]
- Hammer TB, Oranje B, Skimminge A, Aggernæs B, Ebdrup BH, Glenthøj B, Baaré W. Structural brain correlates of sensorimotor gating in antipsychotic-naive men with first-episode schizophrenia. J. Psychiatry Neurosci. 2013;38(1):34–42. doi: 10.1503/jpn.110129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasenkamp W, Norrholm SD, Green A, Lewison B, Boshoven W, Keyes M, Duncan E. Differences in startle reflex and prepulse inhibition in European-Americans and African-Americans. Psychophysiology. 2008;45(5):876–882. doi: 10.1111/j.1469-8986.2008.00680.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Summerfelt A, Wonodi I, Adami H, Buchanan RW, Thaker GK. Independent domains of inhibitory gating in schizophrenia and the effect of stimulus interval. Am. J. Psychiatry. 2007;164(1):61–65. doi: 10.1176/ajp.2007.164.1.61. [DOI] [PubMed] [Google Scholar]
- Hong LE, Wonodi I, Lewis J, Thaker GK. Nicotine effect on prepulse inhibition and prepulse facilitation in schizophrenia patients. Neuropsychopharmacology. 2008;33(9):2167–2174. doi: 10.1038/sj.npp.1301601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hong LE, Wonodi I, Stine OC, Mitchell BD, Thaker GK. Evidence of missense mutations on the neuregulin 1 gene affecting function of prepulse inhibition. Biol. Psychiatry. 2008;63(1):17–23. doi: 10.1016/j.biopsych.2007.05.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanabus M, Szelag E, Rojek E, Poppel E. Temporal order judgment for auditory and visual stimuli. Acta Neurobiol. Exp. (Wars) 2002;62:263–270. doi: 10.55782/ane-2002-1443. [DOI] [PubMed] [Google Scholar]
- Kishi T, Fukuo Y, Okochi T, Kawashima K, Moriwaki M, Furukawa O, Fujita K, Musso GM, Correll CU, Kane JM, Iwata N. The relationship between acoustic startle response measures and cognitive functions in Japanese patients with schizophrenia. Neuromolecular Med. 2012;14(2):131–138. doi: 10.1007/s12017-012-8177-y. [DOI] [PubMed] [Google Scholar]
- Kumari V, Aasen I, Sharma T. Sex differences in prepulse inhibition deficits in chronic schizophrenia. Schizophr. Res. 2004;69(2–3):219–235. doi: 10.1016/j.schres.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Kumari V, Fannon D, Sumich AL, Sharma T. Startle gating in antipsychotic-naïve first episode schizophrenia patients: one ear is better than two. Psychiatry Res. 2007;151(1–2):21–28. doi: 10.1016/j.psychres.2006.09.013. [DOI] [PubMed] [Google Scholar]
- Kumari V, Soni W, Sharma T. Normalization of information processing deficits in schizophrenia with clozapine. Am. J. Psychiatry. 1999;156(7):1046–1051. doi: 10.1176/ajp.156.7.1046. [DOI] [PubMed] [Google Scholar]
- Kunugi H, Tanaka M, Hori H, Hashimoto R, Saitoh O, Hironaka N. Prepulse inhibition of acoustic startle in Japanese patients with chronic schizophrenia. Neurosci. Res. 2007;59(1):23–28. doi: 10.1016/j.neures.2007.05.006. [DOI] [PubMed] [Google Scholar]
- Libet B. Unconscious cerebral initiative and the role of conscious will in voluntary action. Behav. Brain Sci. 1985;8:529–566. [Google Scholar]
- Libet B, Wright EW, Jr, Feinstein B, Pearl DK. Subjective referral of the timing for a conscious sensory experience. Brain. 1979;102:193–224. doi: 10.1093/brain/102.1.193. [DOI] [PubMed] [Google Scholar]
- Light GA, Swerdlow NR, Rissling AJ, Radant A, Sugar CA, Sprock J, Pela M, Geyer MA, Braff DL. Characterization of neurophysiologic and neurocognitive biomarkers for use in genomic and clinical outcome studies of schizophrenia. PLoS One. 2012;7(7):e39434. doi: 10.1371/journal.pone.0039434. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ludewig K, Geyer MA, Vollenweider FX. Deficits in prepulse inhibition and habituation in never-medicated, first-episode schizophrenia. Biol. Psychiatry. 2003;54(2):121–128. doi: 10.1016/s0006-3223(02)01925-x. [DOI] [PubMed] [Google Scholar]
- Mackeprang T, Kristiansen KT, Glenthoj BY. Effects of antipsychotics on prepulse inhibition of the startle response in drug-naive schizophrenic patients. Biol. Psychiatry. 2002;52(9):863–873. doi: 10.1016/s0006-3223(02)01409-9. [DOI] [PubMed] [Google Scholar]
- Martinez-Gras I, Rubio G, del Manzano BA, Rodriguez-Jimenez R, Garcia-Sanchez F, Bagney A, Leza JC, Borrell J. The relationship between prepulse inhibition and general psychopathology in patients with schizophrenia treated with long-acting risperidone. Schizophr. Res. 2009;115(2–3):215–221. doi: 10.1016/j.schres.2009.09.035. [DOI] [PubMed] [Google Scholar]
- Meincke U, Mörth D, Voss T, Thelen B, Geyer MA, Gouzoulis-Mayfrank E. Prepulse inhibition of the acoustically evoked startle reflex in patients with an acute schizophrenic psychosis--a longitudinal study. Eur. Arch. Psychiatry Clin. Neurosci. 2004;254(6):415–421. doi: 10.1007/s00406-004-0523-0. [DOI] [PubMed] [Google Scholar]
- Millard SP, Shofer J, Braff D, Calkins M, Cadenhead K, Freedman R, Green MF, Greenwood TA, Gur R, Gur R, Lazzeroni LC, Light GA, Olincy A, Nuechterlein K, Seidman L, Siever L, Silverman J, Stone W, Sprock J, Sugar CA, Swerdlow NR, Tsuang M, Turetsky B, Radant A, Tsuang D. Prioritizing Schizophrenia Endophenotypes for Future Genetic Studies: An Example Using Data from the COGS-1 Family Study. Schizophr. Res. 2016;174(1–3):1–9. doi: 10.1016/j.schres.2016.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molina V, López DE, Villa R, Pérez J, Martín C, Ballesteros A, Cardoso A, Sancho C. Prepulse inhibition of the startle reflex in schizophrenia remains stable with short-term quetiapine. Eur. Psychiatry. 2011;26(5):271–275. doi: 10.1016/j.eurpsy.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Moriwaki M, Kishi T, Takahashi H, Hashimoto R, Kawashima K, Okochi T, Kitajima T, Furukawa O, Fujita K, Takeda M, Iwata N. Prepulse inhibition of the startle response with chronic schizophrenia: a replication study. Neurosci. Res. 2009;65(3):259–262. doi: 10.1016/j.neures.2009.07.009. [DOI] [PubMed] [Google Scholar]
- Nelson BD, Bishop JR, Sarapas C, Kittles RA, Shankman SA. Asians demonstrate reduced sensitivity to unpredictable threat: a preliminary startle investigation using genetic ancestry in a multiethnic sample. Emotion. 2014;14(3):615–623. doi: 10.1037/a0035776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oranje B, Glenthøj BY. Clonidine normalizes sensorimotor gating deficits in patients with schizophrenia on stable medication. Schizophr. Bull. 2013;39(3):684–691. doi: 10.1093/schbul/sbs071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovsky N, Quednow BB, Ettinger U, Schmechtig A, Mössner R, Collier DA, Kühn KU, Maier W, Wagner M, Kumari V. Sensorimotor gating is associated with CHRNA3 polymorphisms in schizophrenia and healthy volunteers. Neuropsychopharmacology. 2010;35(7):1429–1439. doi: 10.1038/npp.2010.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Preuss UW, Zimmermann J, Watzke S, Langosch J, Siafarikas N, Wong JW, Hamm A, Weike A. Short-term prospective comparison of prepulse inhibition between schizophrenic patients and healthy controls. PharmacoPsychiatry. 2011;44(3):102–108. doi: 10.1055/s-0031-1271687. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Ettinger U, Mössner R, Rujescu D, Giegling I, Collier DA, Schmechtig A, Kühn KU, Möller HJ, Maier W, Wagner M, Kumari V. The schizophrenia risk allele C of the TCF4 rs9960767 polymorphism disrupts sensorimotor gating in schizophrenia spectrum and healthy volunteers. J Neurosci. 2011;31(18):6684–6691. doi: 10.1523/JNEUROSCI.0526-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quednow BB, Frommann I, Berning J, Kühn KU, Maier W, Wagner M. Impaired sensorimotor gating of the acoustic startle response in the prodrome of schizophrenia. Biol. Psychiatry. 2008;64(9):766–773. doi: 10.1016/j.biopsych.2008.04.019. [DOI] [PubMed] [Google Scholar]
- Quednow BB, Wagner M, Westheide J, Beckmann K, Bliesener N, Maier W, Kuhn KU. Sensorimotor gating and habituation of the startle response in schizophrenic patients randomly treated with amisulpride or olanzapine. Biol. Psychiatry. 2006;59(6):536–545. doi: 10.1016/j.biopsych.2005.07.012. [DOI] [PubMed] [Google Scholar]
- Rabin RA, Sacco KA, George TP. Correlation of prepulse inhibition and Wisconsin Card Sorting Test in schizophrenia and controls: effects of smoking status. Schizophr. Res. 2009;114(1–3):91–97. doi: 10.1016/j.schres.2009.07.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussos P, Giakoumaki SG, Zouraraki C, Fullard JF, Karagiorga VE, Tsapakis EM, Petraki Z, Siever LJ, Lencz T, Malhotra A, Spanaki C, Bitsios P. The Relationship of Common Risk Variants and Polygenic Risk for Schizophrenia to Sensorimotor Gating. Biol. Psychiatry. 2016;79(12):988–996. doi: 10.1016/j.biopsych.2015.06.019. [DOI] [PubMed] [Google Scholar]
- Seidman LJ, Hellemann G, Nuechterlein KH, Greenwood TA, Braff DL, Cadenhead KS, Calkins ME, Freedman R, Gur RE, Gur RC, Lazzeroni LC, Light GA, Olincy A, Radant AD, Siever LJ, Silverman JM, Sprock J, Stone WS, Sugar C, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Green MF. Factor structure and heritability of endophenotypes in schizophrenia: Findings from the Consortium on the Genetics of Schizophrenia (COGS-1) Schizophr. Res. 2015;163(1–3):73–79. doi: 10.1016/j.schres.2015.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Cadenhead KC, Sprock J, Hsieh MH, Braff DL. Startle gating deficits in a large cohort of patients with schizophrenia: Relationship to medications, symptoms, neurocognition and level of function. Arch. Gen. Psychiatry. 2006a;63(12):1325–1335. doi: 10.1001/archpsyc.63.12.1325. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Braff DL. Prepulse-elicited motor reactions do not differ between schizophrenia patients and control subjects. Behav. Neurosci. 2006b;120(1):224–227. doi: 10.1037/0735-7044.120.1.224. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Sprock J, Light GA, Cadenhead K, Calkins ME, Dobie DJ, Freedman R, Green MF, Greenwood TA, Gur RE, Mintz J, Olincy A, Nuechterlein KH, Radant AD, Schork NJ, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Multi-site studies of acoustic startle and prepulse inhibition in humans: Initial experience and methodological considerations based on studies by the Consortium on the Genetics of Schizophrenia. Schizophr. Res. 2007;92(1–3):237–251. doi: 10.1016/j.schres.2007.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Talledo JA, Braff DL. Startle modulation in Caucasian-Americans and Asian-Americans: a prelude to genetic/endophenotypic studies across the “Pacific Rim”. Psychiatr. Genet. 2005;15(1):61–65. doi: 10.1097/00041444-200503000-00010. [DOI] [PubMed] [Google Scholar]
- Swerdlow NR, Weber M, Qu Y, Light GA, Braff DL. Realistic expectations of prepulse inhibition in translational models for schizophrenia research. Psychopharmacology. 2008;199(3):331–388. doi: 10.1007/s00213-008-1072-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Light GA, Sprock J, Calkins ME, Greene MF, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Ray A, Seidman LJ, Siever LJ, Silverman JM, Stone WS, Sugar CA, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL. Deficient prepulse inhibition in schizophrenia detected by the multi-site COGS. Schizophr. Res. 2014;152(2–3):503–512. doi: 10.1016/j.schres.2013.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swerdlow NR, Gur RE, Braff DL. Consortium on the Genetics of Schizophrenia (COGS) Assessment of Endophenotypes for Schizophrenia: An Introduction to this Special Issue of Schizophrenia Research. Schizophr. Res. 2015;163(1–3):9–16. doi: 10.1016/j.schres.2014.09.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takahashi H, Iwase M, Ishii R, Ohi K, Fukumoto M, Azechi M, Ikezawa K, Kurimoto R, Canuet L, Nakahachi T, Iike N, Tagami S, Morihara T, Okochi M, Tanaka T, Kazui H, Yoshida T, Tanimukai H, Yasuda Y, Kudo T, Hashimoto R, Takeda M. Impaired prepulse inhibition and habituation of acoustic startle response in Japanese patients with schizophrenia. Neurosci. Res. 2008;62(3):187–194. doi: 10.1016/j.neures.2008.08.006. [DOI] [PubMed] [Google Scholar]
- Thomas ML, Green MF, Hellemann G, Sugar CA, Tarasenko M, Calkins ME, Greenwood TA, Gur RE, Gur RC, Lazzeroni LC, Nuechterlein KH, Radant AD, Seidman LJ, Shiluk AL, Siever LJ, Silverman JM, Sprock J, Stone WS, Swerdlow NR, Tsuang DW, Tsuang MT, Turetsky BI, Braff DL, Light GA. Modeling the Cascade of Deficits from Early Auditory Information Processing to Psychosocial Functioning in Schizophrenia. JAMA Psychiatry. 2017;74(1):37–46. doi: 10.1001/jamapsychiatry.2016.2980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang ZR, Tan YL, Yang FD, Zhang WF, Zou YZ, Tan SP, Song CS, Li YL, Zhang WH, Zhou DF. Impaired prepulse inhibition of acoustic startle in Chinese patients with first-episode, medication-naïve schizophrenia. Chin. Med. J. (Engl.) 2013;126(3):526–531. [PubMed] [Google Scholar]
- Weike AI, Bauer U, Hamm AO. Effective neuroleptic medication removes prepulse inhibition deficits in schizophrenia patients. Biol. Psychiatry. 2000;47(1):61–70. doi: 10.1016/s0006-3223(99)00229-2. [DOI] [PubMed] [Google Scholar]
- Xue YY, Wang HN, Xue F, Tan QR. Atypical antipsychotics do not reverse prepulse inhibition deficits in acutely psychotic schizophrenia. J. Int. Med. Res. 2012;40(4):1467–1475. doi: 10.1177/147323001204000425. [DOI] [PubMed] [Google Scholar]
- Ziermans T, Schothorst P, Magnée M, van Engeland H, Kemner C. Reduced prepulse inhibition in adolescents at risk for psychosis: a 2-year follow-up study. J. Psychiatry Neurosci. 2011;36(2):127–134. doi: 10.1503/jpn.100063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ziermans TB, Schothorst PF, Sprong M, Magnée MJ, van Engeland H, Kemner C. Reduced prepulse inhibition as an early vulnerability marker of the psychosis prodrome in adolescence. Schizophr. Res. 2012;134(1):10–15. doi: 10.1016/j.schres.2011.10.009. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure S1. Scatterplot of %60 ms PPI in all “responders”, divided by diagnosis and sex. Small horizontal lines mark the group mean values. For “subgroups” exhibiting greater diagnosis-based differences in PPI, see Table 2 and Figure 2A.


