Abstract
Modern treatment of partial-thickness burns follows the paradigm of less frequent dressing changes to allow for undisturbed reepithelialization of the burn wound. We compared Mepilex Ag (M), a silver-impregnated foam dressing, and Suprathel (S), a DL-lactid acid polymer, in the outpatient treatment of partial-thickness burns in pediatric and adult patients. Patients were enrolled in a randomized, controlled, prospective clinical trial. We monitored time to reepithelialization, wound pain, discomfort during dressing changes, and treatment cost. Objective scar characteristics (elasticity, transepidermal water loss, hydration, and pigmentation) and subjective assessments (Patient and Observer Scar Assessment Scale) were measured at 1 month post burn. Data are presented as mean ± SEM, and significance was accepted at P < 0.05. Sixty-two patients (S n = 32; M n = 30) were enrolled; age, sex, and burn size were comparable between the groups. Time to reepithelialization was not different between the groups (12 days; P = 0.75). Pain ratings were significantly reduced during the first 5 days after burn in the Suprathel group in all patients (P = 0.03) and a pediatric subgroup (P < 0.001). Viscolelasticity of burned skin was elevated compared with unburned skin in the Mepilex Ag group at 1 month post burn. Patients treated with Suprathel reported better overall scar quality (S: 2; M: 4.5; P < 0.001). The cost of treatment per square centimeter for Mepilex Ag was considerably lower than that of Suprathel. Both dressings are feasible and efficacious for the outpatient treatment of minor and selected moderate partial-thickness burns. Reduced pain, especially in a pediatric patient population, may be advantageous, despite increased treatment cost.
Partial-thickness burns typically cover a relatively small percentage of TBSA and are a common occurrence in emergency and trauma as well as in specialized burn care.1 Depending on its duration and intensity, the thermal insult can affect both the epidermal and dermal layers of the skin; it may extend superficially into the papillary dermis or deep into the reticular dermis and characteristically causes severe pain.2,3 Healing of the resulting wounds typically occurs within 2 to 3 weeks after injury, and significant scarring is not expected in superficial partial- thickness burns.4 Standard treatment involves immediate debridement of nonviable tissue and coverage of the wound with dressings that provide favorable conditions for reepithelialization.2 An ideal wound dressing serves as a barrier to prevent transdermal fluid loss, mitigates the risk of infection, allows reepithelialization of the wound surface, is cost-effective, is easy to use, and controls pain. While a wide variety of dressings are currently available, conclusive data on the most favorable treatment option for partial-thickness burns in an outpatient setting are sparse.5
Synthetic dressings, such as Biobrane® (Smith & Nephew, Andover, MA) and Mepilex Ag® (Mölnlycke, Göteborg, Sweden), can serve as an alternative to topical creams, ointments, antimicrobials, or biological dressings and combine the advantage of fewer required dressing changes with accelerated wound healing.6–9 A newer synthetic dressing for partial-thickness burns, Suprathel® (PolyMedics Innovations GmbH, Denkendorf, Germany), consists of a thin polylactic acid membrane that is applied to the debrided burn wound and remains on the site until reepithelialization is complete, while allowing for wound inspection due to its translucent properties. It has been successfully used in superficial, mixed, and deep partial-thickness burns in adult and pediatric patients.10,11 However, all clinical trial data comparing Suprathel® with other dressings have been obtained in the inpatient setting, as partial-thickness burn wounds are typically treated on an inpatient basis in Europe and the United Kingdom.10,11
The current standard of care for partial-thickness burns at our burn center consists of debridement under analgesia, coverage with silver-coated foam dressing (Mepilex Ag®), and outpatient follow-up with dressing changes every 3 to 7 days until wound healing is complete. In this prospective randomized study, we sought to determine whether Suprathel® is a feasible alternative to Mepilex Ag® for the treatment of adult and pediatric partial-thickness burn wounds and to establish recommendations and indications for its use under preestablished outpatient conditions.
PATIENTS AND METHODS
This study was approved by the University of Texas Medical Branch Institutional Review Board (15-0009). Pediatric and adult patients who sustained partial-thickness flame, scald, or contact burn; who were admitted within 48 hours of injury; and who were not treated with any topical agents, creams, or pharmaceutically active dressings before admission were eligible and enrolled after consenting to participate in this prospective study.
Presence of partial-thickness burn was determined clinically on admission by an experienced burn physician. After initial debridement of the wound under procedural sedation, the affected area was documented photographically. Using a computerized randomization algorithm that was not accessible to the treating physician, we randomly assigned patients to treatment with either Mepilex Ag® (M) or Suprathel® (S). Patients were blinded to the hypothesized effects of either treatment. Mepilex Ag® was cut to size, held in place with woven gauze (Kerlix®, Covidien, Dublin, Ireland), and secured with elastic bandage (ACE®, 3M, St. Paul, MN). Suprathel® was cut to size, applied to the wound as a monolayer, covered with a monolayer of petrolatum gauze (Albahealth LLC, Rockwood, TN), held in place with woven gauze, and wrapped with elastic bandage (ACE®). All patients were discharged into the outpatient setting within 24 hours of their admission and scheduled for regular follow-up visits every 3 to 7 days according to our standard protocol of care. Patients were instructed to keep the dressings in place until their follow-up visits. Patients and/or their caregivers were instructed on how to rate their pain at the wound site once daily according to Visual Analog Scale (children aged 9–17 years and adults) or Wong-Baker Faces (children aged 3–8 years) and how to record whether and which pain medication was taken. Mepilex Ag® was changed during each follow-up visit, and the progress of reepithelialization of the wound bed was noted. The translucent Suprathel® and petrolatum gauze remained on the wound surface, which was inspected after removal of only the outer dressing during each follow-up visit. Time to heal was recorded when reepithelialization was complete with no necessity for further wound dressing and documented both by the attending physician as well as through blinded review of photographs.
One month after burn injury, patients’ healed wounds were objectively compared with normal adjacent or contralateral skin using the Dermalab Combo (Cortex Technology ApS, Hadsund, Denmark). Specifically, pigmentation was measured based on light absorption for melanin and erythema. Hydration was assessed based on skin conductance in μS. Transepidermal water loss (TEWL) was quantified by measuring water evaporation in g/m2/h. Finally, dermal viscoelasticity was measured through negative pressure suction and retraction time. Subjective assessment was performed using the Patient and Observer Scar Assessment Scale.12 Patients or their caregivers were also asked to rank discomfort during dressing changes (none, moderate, severe). The cost of either product per square centimeter of wound area was documented.
Statistical analysis was performed using R (R Core Team, 2016, version 3.3.2, Vienna, Austria) and Graphpad Prism 7 (GraphPad Software, Inc., La Jolla, CA). Time to heal was assessed using a Log Rank (Mantel Cox) survival model. Pain scores were modeled separately for all patients and for children by mixed multiple regression model with relation to treatment group, days from application of dressing, and an interaction between them, while blocking on subject with a continuous AR1 correlation structure. Parametric data were analyzed using Student’s t test or paired t test. Nonparametric data were analyzed using Mann-Whitney U test, and categorical data were analyzed using Fisher’s exact test. Significance was accepted at P < 0.05. Data are presented as mean ± SD or median ± 95% Confidence Interval.
RESULTS
Sixty-two patients were enrolled in the study and randomized to treatment with Mepilex Ag® (n = 30) or Suprathel® (n = 32). Two patients (1 per treatment group) were excluded from further analysis on conversion to full-thickness burns, which required skin grafting. The study groups were comparable in age, sex distribution, and percentage TBSA burned (Table 1). Mean TBSA burned was 5.9 ± 5.8% (range, 1–29%) in the Mepilex Ag® group and 5.5 ± 4.6% (range, 1–20%) in the Suprathel® group. A subset of 33 pediatric patients (M: 17; S: 16) also showed no group differences in age, sex, or percentage TBSA burned (Table 2). In the entire study population, the causes of burn in descending order were scald (58%), flame (35%), and contact burn (7%), with no differences being detected between the groups (Table 3).
Table 1.
Patient demographics*
| Characteristics | Mepilex Ag® (n = 30) | Suprathel® (n = 32) | P |
|---|---|---|---|
| Percent TBSA burn | 5.9 ± 5.8 | 5.5 ± 4.6 | 0.8 |
| Age at burn (yr) | 20.0 ± 20.0 | 24.0 ± 23.0 | 0.4 |
| Sex, F:M | 11:19 | 11:22 | 0.8 |
*Values reported as mean ± SD unless noted otherwise.
Table 2.
Demographics of the pediatric patient subgroup*
| Characteristics | Mepilex Ag® (n = 17) | Suprathel® (n = 16) | P |
|---|---|---|---|
| Percent TBSA burn | 9.5 ± 4.3 | 11.9 ± 3.5 | 0.1 |
| Age at burn (yr) | 6 ± 1 | 6 ± 1 | 0.7 |
| Sex, F:M | 7:9 | 7:8 | 0.8 |
*Values reported as mean ± SD unless noted otherwise.
Table 3.
Causes of burn
| Cause | Mepilex Ag® (n = 30; %) | Suprathel® (n = 32; %) | P |
|---|---|---|---|
| Scald | 19 (63) | 17 (53) | 0.45 |
| Flame | 9 (30) | 13 (41) | 0.43 |
| Contact | 2 (7) | 2 (6) | 0.99 |
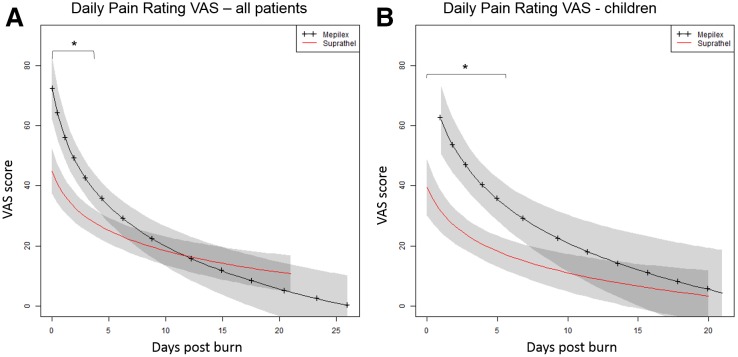
Regression modeling showed that pain ratings were significantly lower in patients treated with Suprathel® than in those treated with Mepilex Ag® during the first 5 days after burn injury (P < 0.05), with ratings converging at a common lower level after this time (Fig. 1A). Subgroup analysis showed that in pediatric patients pain ratings were initially lower in the Suprathel® group than in the Mepilex Ag® group (P = 0.001), with a steady decline in pain being seen in both groups (Fig. 1B). In the adult patient population, no differences were noted between groups in the incidence of moderate or severe discomfort during dressing changes. No severe discomfort occurred in pediatric patients treated with Suprathel®. There was no difference in the type, dosing, and duration of pain medication administered to either group (days under comparable pain medication S: 9 ± 6; M: 11 ± 8; P = 0.3).
Figure 1.
Regression modeling showing adjusted mean pain scores for all patients (A) and children (B) over time. Shaded regions indicate SEM. *P < 0.05.
The median time to complete reepithelialization was 12 days in both groups (P = 0.75). Twenty percentage (6/30) of patients had a reepithelialization time greater than 21 days in the Mepilex Ag® group, compared with 7% (2/30) in the Suprathel® group (P = 0.25). Wound infection was confirmed in 2 patients (8%) in the Suprathel® group but was not observed in patients in the Mepilex Ag® group (P = 0.5). No additional complications or adverse events were noted in either group.
Assessment through Patient and Observer Scar Assessment Scale was performed at 1 month post burn. Observer scores of the healed wound at this time did not show significant differences for vascularity, pigmentation, thickness, relief, pliability, surface, or overall appearance. Patient ratings for pain, itch, color, stiffness, thickness, and irregularity also did not differ between the groups. Patients rated the overall appearance of their healed wound better after treatment with Suprathel® (S: 2; Confidence Interval, 1.4–3.5; M: 4.5; Confidence Interval, 3.8–6.2; P = 0.002). Observer score for pliability (M: 5; S: 2; P = 0.08) and patient score for irregularity (M: 3.5; S: 2; P = 0.075) approached significance.
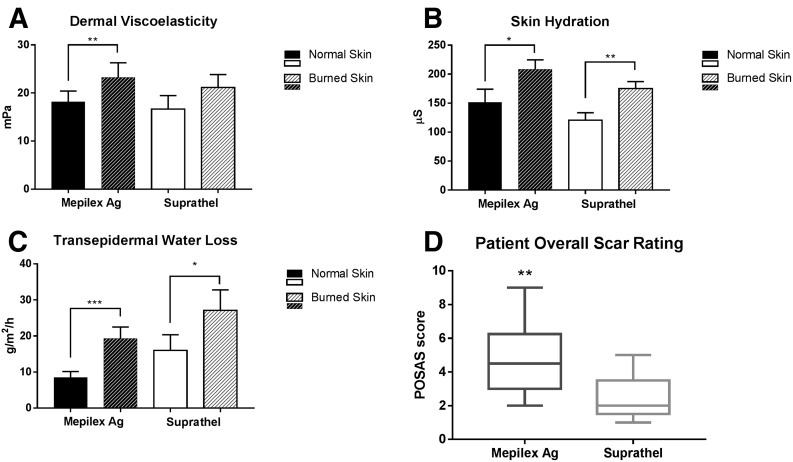
In the Mepilex Ag® group, viscoelasticity (ie, stiffness) of burned skin was elevated compared with baseline (unburned skin) at 1 month post burn (P = 0.004; Fig. 2). In the Suprathel® group, burned skin did not differ from baseline (P = 0.3). Hydration and TEWL of the burned skin area were elevated compared with baseline in both groups, with no difference being seen between the groups (Fig. 2). No statistically significant differences in pigmentation (erythema and melanin) were noted between normal skin and burned skin for either group or between the groups.
Figure 2.
Skin characteristics at 1 month post burn. A, Viscoelasticity. B, Skin hydration. C, Transepidermal water loss. D, POSAS. The POSAS score ranges from 1 (indistinguishable from normal skin) to 10 (worst imaginable scar). *P < 0.05, **P < 0.01, and ***P < 0.001. POSAS, Patient-Rated Overall Scar Appearance Scale.
The cost per square centimeter of Mepilex Ag® was $0.08, and on average, each patient required 2 dressing changes. Suprathel® cost $0.56 per square centimeter and was applied once per patient, resulting in direct product costs of $0.16/cm2 per patient for Mepilex Ag® and $0.56/cm2 per patient for Suprathel®.
DISCUSSION
This is the first prospective randomized comparison of Suprathel® to the widely established standard of care dressing, Mepilex Ag®, for the treatment of partial-thickness burns in a strict outpatient setting. While the overall principles of treating partial-thickness burns in specialized centers are commonly agreed on across the industrialized world, evidence supporting whether and which burns should be treated in outpatient or inpatient settings remains inconclusive. Available evidence on the use of Suprathel® as a dressing for partial-thickness burns mainly originates from Europe and the United Kingdom. There, the majority of pediatric and adult patients presenting to a burn center with any second-degree burn are treated as inpatients.6,10,11,13,14 However, in the United States, large burn centers increasingly lean toward outpatient treatment of partial-thickness burns. In fact, recent evidence suggests that up to 90% of burn patients are treatable as outpatients, and in a large retrospective review, Brown et al.15 showed that doing so in pediatric patient populations is safe and efficacious.16 Potentially unnecessary inpatient treatment of burn injury is associated with potential exposure to multidrug-resistant microorganisms,17,18 increased economic burden for the patient and healthcare system,19 delayed return to work or school,20,21 and a less psychologically comfortable environment for the patient.22 Here, we provide evidence that outpatient treatment of all minor and some moderate partial-thickness burns with either Suprathel® or Mepilex Ag® is feasible and efficacious.
The average time for complete reepithelialization of a partial-thickness burn wound ranges from 7 to 15 days according to various study groups and treatments applied.23–26 Here, we found that the median time to heal was 12 days for both groups, a value that falls well within this range and confirms the efficacy of both therapy options.
Pain at the wound site is a hallmark of and chief complaint associated with partial-thickness burn injury.2 We found that patient-reported pain scores in the Suprathel® group were significantly lower than those in the Mepilex Ag® group during the first 5 days post burn, after which time these ratings converged at a lower level with those of the Mepilex Ag® group. Pain medication prescribed and administered to patients was comparable between study groups, suggesting that the observed early decrease in pain scores was at least partly attributable to the applied dressing. This finding is consistent with reports that Suprathel® has an analgesic effect on burn wounds and donor sites.14,27 This effect has been hypothesized to be related to favorable adherence to the wound surface throughout reepithelialization, translucent properties that allow for wound inspection without dressing removal, as well as to the material’s elasticity and easy detachment once wound healing is complete.6,14. Suprathel® detaches by itself from completely reepithelialized sections of a burn wound and can thus be trimmed selectively during follow-up visits. During this procedure, incompletely healed areas of the wound remained undisturbed under intact dressing in contrast to the complete removal and reapplication of Mepilex Ag®. This effect, although not relevant for healing time in this trial, may have affected patients’ significantly reduced perception of pain and contributed to pediatric patients’ lack of severe discomfort during dressing changes, as has been reported by others.11,14,27 While favorable for all patients, pain reduction can be particularly useful in the treatment of children with burn injuries. This patient population is less capable of effectively communicating their level of discomfort, at increased risk for inadequate pain control, and more susceptible to the side effects of common pain medication.28
Various groups have attributed a prophylactic antimicrobial effect of Mepilex Ag® to the bactericidal properties of ionic silver contained in its foam.24,26,29 Indeed, in the study group treated with Mepilex Ag®, only 1 wound infection was suspected, though it was ruled out through microbiological follow-up assessment. On the other hand, 2 cases of infection (7%) were confirmed in the Suprathel® group: culture-positive staphylococcal wound infections were successfully treated with topical and oral antibiotics for 1 week and healed without complication. The difference in infection incidence between the groups did not reach statistical significance; overall, the observed incidence of infection is consistent with reported rates of 6 to 9% in comparable studies.24
Mepilex Ag® is a well-established dressing in the United States, while Suprathel® is relatively new and less widely used. During this trial, we experienced a learning curve with Suprathel® that can be summarized by the following recommendations. The application of Suprathel® is simple, and the instantly self-adhering properties are ideal for the quick coverage of large burned areas such as those on the chest, back, forearm, or skin. Although the dressing and overlaying petrolatum gauze can easily be molded to any contour, some areas that are prone to fine movement and wrinkling such as the web spaces of fingers and toes or the sole of the foot seemed to benefit more from the foam structure of Mepilex Ag®, which is less dependent on total adherence and tends to reposition itself after intermittent dislocation. Furthermore, we found it important to apply only a monolayer of petrolatum gauze onto Suprathel® to minimize fluid entrapment underneath. We attribute the 2 bacterial wound infections in the Suprathel® group to nonadherence to this protocol step. The outer dressing should furthermore be nonocclusive to allow for an optimal wound healing environment, especially in geographical regions with high temperatures and humidity. Patients should be instructed to refrain from extensive movement of the burned body part during the first 24 to 48 hours following its application. Thereafter, the combination of Suprathel® and petrolatum gauze was securely adherent to the wound bed in all cases, and no unwanted detachment occurred until completion of reepithelialization. For this reason, we recommend that the first outer dressing change occur at 2 days after application of Suprathel® rather than at 3 to 5 days, as done in this trial.
The subjective and objective wound assessments at 1 month post burn showed that hydration and TEWL were elevated above baseline in both groups. This finding is consistent with that of Gardien et al30, who recently described this elevation as a characteristic trait of the restoration of the dermal barrier function during midterm burn wound healing and showed that differences with normal skin fade over time until 12 months after thermal trauma. Various groups have claimed that scar properties of donor sites and partial-thickness burns are improved following therapy with Suprathel®.11,13 Indeed, we observed that Mepilex Ag® significantly increased stiffness of the burned skin compared with baseline, showing that the affected area is less elastic than normal skin at 1 month post burn. This effect was not seen following treatment with Suprathel® and was paralleled by improved subjective observer ratings for scar pliability, which approached significance. However, larger sample sizes and longer follow-up durations are warranted to determine whether the mid- and long-term properties of healed skin treated with Suprathel® are in fact improved in comparison with Mepilex Ag® and how long lasting any improvements may be. Along the same lines, the statistically significant superiority of the patients’ overall scar appearance rating after treatment with Suprathel® needs to be interpreted with caution, as it is not paralleled by a corresponding improvement in the overall observer rating.
Most studies evaluating Suprathel® have been conducted in European hospitals, where individuals with partial-thickness burn injuries are treated as inpatients. This creates a more controlled treatment environment and allows for effective immobilization to prevent dressing dislocation over joints as well as more frequent wound inspection. The vast majority of the patients in this study were discharged within a few hours of their initial treatment and returned for the first follow-up visit 3 to 5 days later. The reduced surveillance associated with this regimen makes it necessary to provide clear instructions to the patient, regardless of treatment group, so that favorable results can be achieved: keep the wound elevated, keep the dressing intact and dry, and refrain from physical activity or work during the first 1 to 2 days after injury.
Cost efficiency is an important factor in implementing a treatment regimen for second-degree burns. Our data suggest that Suprathel® is considerably more expensive per square centimeter than Mepilex Ag, given that retention dressings are the same for both groups. On one hand, this difference is not significantly offset by the need to apply Mepilex Ag® twice as often as Suprathel®, as observed in this study. Treatment of larger burns, on the other hand, which inevitably involves waste of excess material during multiple dressing changes, would decrease relative costs of Suprathel®.
Certain factors, which can significantly impact overall cost, such as time for and ease of dressing changes, were not assessed quantitatively in this trial. While we did not observe any qualitative differences in nursing effort during application and dressing changes, these limited observations warrant more detailed assessment in the future. This leaves the question as to whether the benefits of Suprathel® in terms of patient comfort and pain control can be outweighed by the noted price premium. Perhaps, globally, this seems more unlikely for middle- and low-income countries, which are more bound by treatment cost-related constraints.
There are limitations to this study. Burn depth was assessed clinically only by experienced burn physicians. While our observed healing times in general support the overall accuracy of our clinical judgment, future studies should involve objective assessment tools, such as laser Doppler, to further improve diagnostic precision and group comparability. One limitation of this study is the relatively large number of patients who were lost to effective follow-up at later time points of the study. One explanation for this phenomenon is the large geographical draw of our outpatient population, with a mean distance of 83 miles between the patients’ homes and our burn center. As partial-thickness burn wounds are almost always completely healed at 1 month after injury, motivating patients to participate at this timepoint is difficult. This observation also influenced the decision to evaluate healed wounds at 1 month post burn, despite knowing that scar maturation is not complete at this time point. Incentivizing outpatients to return for research may enable us to obtain more definitive data at later time points.
Finally, blinding of treatment personnel which may have introduced bias into the trial, was not possible.
CONCLUSIONS
Outpatient treatment of partial-thickness burns requires specific considerations, regardless of the dressing used, but is both safe and efficacious for minor burns and certain moderate burns. Suprathel® can be used as an alternative dressing to Mepilex Ag® for the treatment of partial-thickness burn wounds in an outpatient setting. Both dressings have comparable healing times. Reduced disturbance of the wound bed and decreased pain associated with Suprathel® can be advantageous, especially in the pediatric patient population.
ACKNOWLEDGMENTS
This study was funded by NIH (R01 GM112936, R01 GM056687, P50 GM060338, T32 GM008256) and Shriners of North America (71000, 71008, 71006, 79141, 80100, 84080). It was also supported, in part, by a Clinical and Translational Science Award (UL1 TR001439) from the National Center for Advancing Translational Sciences, National Institutes of Health.
Notes
Gabriel Hundeshagen and Vanessa Collins contributed equally to this study.
The authors have no conflicts of interest to disclose.
REFERENCES
- 1. American Burn Association. 2016 ABA Annual Burn Repository [Internet]. Available from http://www.ameriburn.org/NBR.php. Accessed July 5, 2016.
- 2. Lewis GM, Heimbach DM, Gibran NS. Evaluation of the burn wound: management decisions. In: Herndon DN, editor. Total Burn Care. 4th ed.Philadelphia: Elsevier; 2012. p. 125–30. [Google Scholar]
- 3. Gee Kee E, Kimble RM, Cuttle L, Stockton K. Comparison of three different dressings for partial thickness burns in children: study protocol for a randomised controlled trial. Trials 2013;14:403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Cubison TC, Pape SA, Parkhouse N. Evidence for the link between healing time and the development of hypertrophic scars (HTS) in paediatric burns due to scald injury. Burns 2006;32:992–9. [DOI] [PubMed] [Google Scholar]
- 5. Wasiak J, Cleland H, Campbell F. Dressings for superficial and partial thickness burns. Cochrane Libr [Internet]. 2008. Available from http://onlinelibrary.wiley.com/doi/10.1002/14651858.CD002106.pub3/full. Accessed January 02, 2017. [DOI] [PubMed]
- 6. Rahmanian-Schwarz A, Beiderwieden A, Willkomm LM, Amr A, Schaller HE, Lotter O. A clinical evaluation of Biobrane(®) and Suprathel(®) in acute burns and reconstructive surgery. Burns 2011;37:1343–8. [DOI] [PubMed] [Google Scholar]
- 7. Lesher AP, Curry RH, Evans J, et al. Effectiveness of Biobrane for treatment of partial-thickness burns in children. J Pediatr Surg 2011;46:1759–63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lal S, Barrow RE, Wolf SE, et al. Biobrane improves wound healing in burned children without increased risk of infection. Shock 2000;14:314–8; discussion 318–9. [DOI] [PubMed] [Google Scholar]
- 9. Sheckter CC, Van Vliet MM, Krishnan NM, Garner WL. Cost-effectiveness comparison between topical silver sulfadiazine and enclosed silver dressing for partial-thickness burn treatment. J Burn Care Res 2014;35:284–90. [DOI] [PubMed] [Google Scholar]
- 10. Highton L, Wallace C, Shah M. Use of Suprathel® for partial thickness burns in children. Burns 2013;39:136–41. [DOI] [PubMed] [Google Scholar]
- 11. Keck M, Selig HF, Lumenta DB, Kamolz LP, Mittlböck M, Frey M. The use of Suprathel(®) in deep dermal burns: first results of a prospective study. Burns 2012;38:388–95. [DOI] [PubMed] [Google Scholar]
- 12. Posas. The Patient and Observer Scar Assessment Scale [Internet]. Available from http://www.posas.org/. Accessed January 03, 2017.
- 13. Kaartinen IS, Kuokkanen HO. Suprathel(®) causes less bleeding and scarring than Mepilex(®) transfer in the treatment of donor sites of split-thickness skin grafts. J Plast Surg Hand Surg 2011;45:200–3. [DOI] [PubMed] [Google Scholar]
- 14. Schwarze H, Küntscher M, Uhlig C, et al. Suprathel, a new skin substitute, in the management of donor sites of split-thickness skin grafts: results of a clinical study. Burns 2007;33:850–4. [DOI] [PubMed] [Google Scholar]
- 15. Brown M, Coffee T, Adenuga P, Yowler CJ. Outcomes of outpatient management of pediatric burns. J Burn Care Res 2014;35:388–94. [DOI] [PubMed] [Google Scholar]
- 16. Warner PM, Coffee TL, Yowler CJ. Outpatient burn management. Surg Clin North Am 2014;94:879–92. [DOI] [PubMed] [Google Scholar]
- 17. Agnihotri N, Gupta V, Joshi RM. Aerobic bacterial isolates from burn wound infections and their antibiograms—a five-year study. Burns 2004;30:241–3. [DOI] [PubMed] [Google Scholar]
- 18. Keen EF 3rd, Robinson BJ, Hospenthal DR, et al. Prevalence of multidrug-resistant organisms recovered at a military burn center. Burns 2010;36:819–25. [DOI] [PubMed] [Google Scholar]
- 19. Shields BJ, Comstock RD, Fernandez SA, Xiang H, Smith GA. Healthcare resource utilization and epidemiology of pediatric burn-associated hospitalizations, United States, 2000. J Burn Care Res 2007;28:811–26. [DOI] [PubMed] [Google Scholar]
- 20. Esselman PC, Askay SW, Carrougher GJ, et al. Barriers to return to work after burn injuries. Arch Phys Med Rehabil 2007;88(12 Suppl 2):S50–6. [DOI] [PubMed] [Google Scholar]
- 21. Mason ST, Esselman P, Fraser R, Schomer K, Truitt A, Johnson K. Return to work after burn injury: a systematic review. J Burn Care Res 2012;33:101–9. [DOI] [PubMed] [Google Scholar]
- 22. Muller M, Gahankari D, Herndon DN. Operative wound management. Total Burn Care. 2007;3:177–195. [Google Scholar]
- 23. Gee Kee EL, Kimble RM, Cuttle L, Khan A, Stockton KA. Randomized controlled trial of three burns dressings for partial thickness burns in children. Burns 2015;41:946–55. [DOI] [PubMed] [Google Scholar]
- 24. Tang H, Lv G, Fu J, et al. An open, parallel, randomized, comparative, multicenter investigation evaluating the efficacy and tolerability of Mepilex Ag versus silver sulfadiazine in the treatment of deep partial-thickness burn injuries. J Trauma Acute Care Surg 2015;78:1000–7. [DOI] [PubMed] [Google Scholar]
- 25. Hartford EC. Care of outpatient burns. In: Herndon DN, editor. Total burn care. 4th ed.Philadelphia: Elsevier; 2012. p. 81–92. [Google Scholar]
- 26. Brown M, Dalziel SR, Herd E, Johnson K, Wong She R, Shepherd M. A randomized controlled study of silver-based burns dressing in a pediatric emergency department. J Burn Care Res 2016;37:e340–7. [DOI] [PubMed] [Google Scholar]
- 27. Uhlig C, Rapp M, Hartmann B, Hierlemann H, Planck H, Dittel KK. Suprathel-an innovative, resorbable skin substitute for the treatment of burn victims. Burns 2007;33:221–9. [DOI] [PubMed] [Google Scholar]
- 28. Berde CB, Sethna NF. Analgesics for the treatment of pain in children. N Engl J Med 2002;347:1094–103. [DOI] [PubMed] [Google Scholar]
- 29. Barrett S. Mepilex Ag: an antimicrobial, absorbent foam dressing with Safetac technology. Br J Nurs 2009;18:S28, S30–6. [DOI] [PubMed] [Google Scholar]
- 30. Gardien KL, Baas DC, de Vet HC, Middelkoop E. Transepidermal water loss measured with the Tewameter TM300 in burn scars. Burns 2016;42:1455–62. [DOI] [PubMed] [Google Scholar]