Abstract
Objective
Excess body weight is associated with increased risk of developing hepatocellular cancer (HCC), but its effect on HCC-related mortality remains unclear. We performed a systematic review and meta-analysis to assess the association between premorbid obesity and HCC-related mortality.
Methods
Through a systematic literature search up to March 2016, we identified 9 observational studies (1,599,453 individuals, 5,705 HCC-related deaths) reporting the association between premorbid body mass index (BMI), and HCC-related mortality. We estimated summary adjusted hazard ratio (aHR) with 95% confidence intervals (CI), comparing obese (BMI>30 kg/m2) and overweight (BMI 25–29.9 kg/m2) individuals with normal BMI individuals using random effects model.
Results
On meta-analysis, compared to individuals with normal BMI, obese (aHR, 1.95; 95% CI, 1.46–2.46), but not overweight individuals (aHR, 1.08; 95% CI, 0.97–1.21), had higher HCC-related mortality, with moderate heterogeneity. On subgroup analysis, magnitude of increased mortality was higher in obese men (aHR, 2.50; 95% CI, 2.02–3.09; 3 studies) as compared to obese women (aHR, 1.45; 95% CI, 1.08–1.97; 2 studies). The impact of premorbid obesity on HCC-related mortality was observed only in Western populations (aHR, 2.10; 95% CI, 1.77–2.48; 4 studies), but not Asian populations (aHR, 1.10; 95% CI, 0.63–1.92; 1 study). There was limited assessment of competing risk due to advanced liver disease.
Conclusions
Based on this meta-analysis, premorbid obesity may be independently associated with a 2-fold risk of HCC-related mortality. This association was more pronounced in men and Western populations. Strategies targeting obesity-associated metabolic abnormalities may provide novel pathways for HCC therapy.
Keywords: obesity, overweight, body mass index, premorbid, hepatocellular cancer, mortality, prognosis
INTRODUCTION
Hepatocellular carcinoma (HCC) accounts for approximately 600,000 deaths annually, and is the second most common cause of cancer-related mortality in men worldwide.1,2 Although the reported HCC incidence and mortality is highest in sub-Saharan Africa and East Asia, an estimated 39,230 new HCC cases and 27,170 deaths are expected from HCC in the United States alone in 2016. It is one of few cancers with rising incidence and mortality in the last decade; rates of rising mortality mirror incidence indicating the high fatality rate.2 The epidemiology of HCC varies distinctly with geographic region, with hepatitis B being the leading cause of chronic liver diseases and HCC in South East Asia and sub-Saharan Africa, and non-alcoholic fatty liver disease being increasingly recognized as one the leading causes of HCC in the West, besides hepatitis C.3
Paralleling the rise in HCC incidence is the global epidemic of obesity. Approximately 1.9 billion adults are overweight and 600 million are obese worldwide. Obesity has been identified as an independent risk factor for development of HCC. In a meta-analysis of 21 prospective studies comprising 17,624 cases of primary liver cancer, there was a 39% increased risk of HCC per 5-unit increase in BMI (kg/m2)4. However, there is limited and conflicting data on the impact of obesity on HCC-related mortality. In a landmark study by Calle et al, the liver cancer-related mortality in obese men (body mass index, BMI, 30–34.9 kg/m2) was 1.9 times the number in men with normal BMI (BMI 18.5–24.9 kg/m2).5 A similar observation was reported in another large cohort from the United Kingdom where the liver cancer-related mortality was almost 4 times in obese individuals (BMI≥30 kg/m2) compared to individuals with normal BMI (BMI 18.5–24.9 kg/m2).6 Besides type 2 diabetes mellitus (T2DM) and alcohol consumption, higher body weight may be an independent prognostic factors for HCC-related mortality.7 In a small cohort of 159 patients, an increase in incidence of recurrent disease (16% vs 8%, P<.05) was reported in overweight and obese patients who underwent orthotropic liver transplantation compared to HCC patients with normal BMI.8 In another study, the 5 year survival rate in patients who underwent repeat hepatectomy for recurrent HCC was also found to be lower in overweight and obese compared to patients who had normal BMI (51% vs 92%; p<0.05).9 Taken together, these results highlight that obesity could be an independent poor prognostic factor in HCC patients who receive appropriate treatment for the primary disease condition.
To better understand this association between pre-morbid obesity and HCC-related mortality, we performed a systematic review with meta-analysis of prospective observational studies that investigated the association between premorbid BMI and HCC-related mortality.
MATERIALS AND METHODS
We followed the Meta-analysis of Observational Studies in Epidemiology (MOOSE) statement for performing and reporting the present meta-analysis10, which was conducted following a priori established protocol.
Study Selection
We included studies that reported statistical measures of association (hazard ratio [HR], incidence rate ratio or relative risk, with 95% confidence intervals [CI]) between premorbid (i.e., at least 1 year prior to diagnosis of HCC) categories of BMI and HCC-related mortality. Two types of participant populations were considered: studies performed in patients with established HCC, and those performed in cancer-free individuals at inception and followed for development of HCC-related mortality. The high fatality of HCC can be appreciated from the fact that HCC has an annual incidence of 6.2 cases per 100,000 and annual mortality of 5 cases per 100,000.11,12 Inclusion was otherwise not restricted by study size, or publication type. Only human studies in the English language were considered. When there were multiple publications from the same cohort, only data from the most recent comprehensive report were included. We excluded case-control and cross-sectional studies, studies reporting data on BMI at or after diagnosis of HCC (confounding by underlying disease and therapy) and studies which did not provide a measure of association (precluded statistical analysis).
Data Sources and Search Strategy
First, we conducted a systematic search of MEDLINE, MEDLINE InProcess, Embase, the Cochrane Database of Systematic Reviews, the Cochrane Database of Reviews of Effect, and the Cochrane Central Register of Controlled Trials through March 2016, using a combination of key words or subject headings for HCC/liver cancer, obesity/BMI, mortality, survival and prognosis. All identified studies were combined in a single reference manager file (EndNote), duplicates were discarded, and the title and abstracts were reviewed by two authors independently (AG and AD) to exclude studies that did not report the association between obesity and mortality in patients with HCC, based on pre-specified inclusion and exclusion criteria. The full text of the remaining articles was examined to determine whether it contained relevant information. Disagreements were harmonized by consensus, in conjunction with the senior investigator (SS). Second, the reference lists from included original articles and recent reviews and meta-analyses on obesity and mortality were hand searched to identify additional studies. Third, conference proceedings of major oncology and gastroenterology/hepatology conferences (Digestive Diseases Week, Gastrointestinal Cancers Symposium, annual meetings of the American Society of Clinical Oncology and European Society of Medical Oncology) from 2010–2015, were reviewed for relevant abstracts. Figure 1 summarizes the study identification and selection process.
Figure 1.
Flow diagram of the study identification and selection process.
Data Abstraction and Quality Assessment
Two reviewers independently abstracted data on the following study- and patient-related characteristics onto a standardized form: (a) study characteristics – last name of primary author, time period of study/year of publication, country/region of the population studied, study design (pooled cohort vs. cohort, prospective vs. retrospective); (b) patient characteristics – population-type (all HCC patients, or cancer-free participants at inception), total number and number of patients with HCC, demographic, clinical and treatment characteristics (age, sex, alcohol use, presence of T2DM, history of cirrhosis, chronic hepatitis B and hepatitis C);(c) exposure status – measure of obesity (BMI), definition and categories of obesity, including reference category for analysis, time period of assessing premorbid obesity in relation to HCC diagnosis, method of assessment (self-reported vs measured); (d) outcome assessment: all-cause and/or cancer-related mortality, attrition rate, information source for exposure ascertainment and outcome assessment; and (e) statistical analysis: HR or relative risk, along with 95% CI, of association between obesity and outcome (using normal category as reference), with adjusted analysis (including variables adjusted for in individual studies), and duration of follow-up. When there were separate data from one study based on sex, we extracted them separately. The risk of bias in these prognostic studies individual studies was assessed by two authors independently using the Quality In Prognosis Studies (QUIPS) tool, which evaluates validity and bias in studies of prognostic factors across six domains: participation, attrition, prognostic factor measurement, confounding measurement and account, outcome measurement, and analysis and reporting.13 Any discrepancies were addressed by a joint re-evaluation of the original article.
Outcomes Assessed
Our primary outcome focused on assessing HCC-related mortality, compared the mortality risk of the obese participants (BMI>30 kg/m2) with the normal participants (BMI<18–25 kg/m2) and summarized these estimates. Similar comparison between overweight participants (BMI >25–30 kg/m2) and normal BMI participants and pooling data to form summary estimates were undertaken. There were minor differences in reported categories of obesity in individual studies, and hence, for standardized interpretation, we calculated mortality risk in obese (BMI ≥30 kg/m2) and overweight participants (BMI 25.0–29.9kg/m2), compared with normal BMI participants. For this analysis, we pooled effects for all BMI categories of BMI ≥30 kg/m2 into a single summary estimate for obese participants, and likewise pooled effects of all BMI categories for BMI 25.0–29.9 kg/m2 into a single summary estimate for overweight participants. For example, where multiple categories of BMI like BMI 25–27.4 and 27.4–29.9 kg/m2 were reported, we combined those categories into an overweight category (BMI 25–29.9 kg/m2) and used them for analyses.14
Subgroup and Sensitivity Analyses
A priori hypotheses to assess robustness of the analysis and explain potential heterogeneity in the direction and magnitude of effect included location of study (Western vs. Asia-Pacific), study design (pooled cohorts vs. individual studies), sex (male vs. female), T2DM (present or absent) and alcohol use (present or absent). We planned sensitivity analyses restricting only to studies reporting the association between obesity and mortality in a cohort of patients with established HCC, and stratified analysis by studies that did and did not adjust for baseline cirrhosis, HCC stage, therapeutic modality and performance status. For subgroup and sensitivity analysis, we used comparisons between overweight and obese individuals, with normal BMI individuals as reference.
Statistical Analysis
We used the random-effects model described by DerSimonian and Laird to calculate summary HR and 95% CI.15 Maximally adjusted HR, when reported in studies, was used for analysis to account for confounding variables. To estimate what proportion of total variation across studies was due to heterogeneity rather than chance, inconsistency index (I2 statistic) was calculated; in this, values of <30%, 30–59%, 60–75% and >75% were suggestive of low, moderate, substantial and considerable heterogeneity, respectively.16 A subgroup analyses was performed by stratifying original estimates per study characteristics (as described above). In this analysis, a p-value for differences between subgroups of <0.10 was considered statistically significant (i.e. a value of P <.10 suggested that stratifying based on that study characteristic partly explained the heterogeneity observed in the analysis). We assessed for publication bias quantitatively using Egger’s regression test (publication bias considered present if P≤.10), and qualitatively, by visual inspection of funnel plots of the logarithm of HRs versus their standard errors.17,18 All p-values were two tailed. All calculations and graphs were performed using Comprehensive Meta-Analysis (CMA) version 2 (Biostat, Englewood, NJ).
RESULTS
A total of 1,966 unique studies were identified of which nine studies fulfilled our inclusion criteria (8 reporting individual cohorts and 1 study reporting pooled data from multiple cohorts) and were included in our study (Figure 1).
Characteristics and Quality of Included Studies
The 9 studies included 1,599,453 participants at baseline, and reported a total of 5,705 HCC deaths during follow-up. The minimum follow-up for a study was 1.9 years. For the purposes of data abstraction, all studies were cohort by design (with subjects being followed over time after exposure [BMI], for development of outcome [mortality], though some studies inherently were reported as case-control studies). While a single study included a cohort of patients with established HCC,19 8 studies were large cohorts of cancer-free participants at inception, and followed them for development of HCC-related mortality. The characteristics of the included studies have been shown in Table 1. None of the included studies reported mortality outcomes after adjusting for history of cirrhosis, history of chronic Hepatitis B and Hepatitis C infection. No study adjusted for or reported HCC stage at diagnosis, underlying etiology, baseline performance status or treatment modalities used. Five studies were based in North America or Europe (referred to as Western populations), including two pooled studies, one of which reported data from four cohorts based in Switzerland, and the second study reported data from three cohorts based in Scotland, the island of Tiree and their relatives in the mainland.20,21 Four studies were conducted in the Asia-Pacific region; one was a pooled analysis of 39 cohorts in the Asia-Pacific Cohort Studies Collaboration).20 Study size ranged from 14,758 to 900,053 participants at study inception. Across studies, the number of deaths related to HCC, ranged from 51 to 2171. BMI was assessed as the primary measure of obesity in all studies, with premorbid evaluation performed at least one year prior to HCC diagnosis. Five studies reported data stratified by obese and overweight patients, whereas 4 studies combined obese and overweight patients together. Supplemental Table 1 depicts the methodological quality of all studies using the QUIPS tool. The overall risk of bias was moderate; more studies relied on self-reported BMI (5 studies), rather than measured BMI.
Table 1.
Characteristics of included studies
| First author, Year of publication | Time period; Follow-up | Patients/participants | Deaths; method of outcome assessment | Mean or median BMI; % obese (BMI >30 kg/m2); timing and method of BMI assessment | Association between BMI and mortality (HR, 95% CI) | Covariates adjusted for |
|---|---|---|---|---|---|---|
| Calle, 20035 | 1982–1998, 16y | 900,053 (all cancer-free at inception); unclear what proportion developed HCC), | 620; record linkage with national death index, and personal inquiries by volunteers | NA; NA; BMI assessed based on usual premorbid height and weight (self reported) | Reference: 18.5–24.9kg/m2 Men: 25–29.9 vs. ref: 1.13 (0.94–1.34) 30–34.9 vs. ref: 1.90 (1.46–2.47) 35–39.9 vs. ref: 4.52 (2.94–6.94) Women: 25–29.9 vs. ref: 1.02 (0.80–1.31) 30–34.9 vs. ref: 1.40 (0.97–2.00) 35–39.9 vs. ref: 1.68 (0.93–3.05) |
1,2,3,6,7,9,13,15,16,17,18,24 |
| Parr, 201023 | 1961–1999; 4y | 424,519 (all cancer-free at inception); unclear what proportion developed HCC) | 774; record linkage with registry (ICD coding) | NR; NR; BMI assessed based on usual premorbid height and weight (self reported) | Reference 18.5–24.9 kg/m2 12–18.4 vs. ref: 1.13 (0.78–1.68) 25–29.9 vs. ref: 1.06 (0.87–1.30) 30–60 vs. ref: 1.10 (0.63–1.91) Trend for 5 units rise in BMI >18.5 kg/m2: 1.11 (0.94–1.31) |
1,2,3,7,11,20 |
| Gray, 201122 | 1962–1966; maximum 82 y | 19,593 (all cancer-free at inception); unclear what proportion developed HCC | 69; record linkage with Alumni office and state health department | 24.4; NR; premorbid BMI assessed at inception (measured) | Reference <22.8 kg/m2 22.8 – < 24.3 vs. ref: 1.15 (0.55–2.41) 24.3–<25.8 vs. ref: 0.96 (0.42–2.18) >25.8 vs. ref: 0.91 (0.35–2.40) |
1, 2, 3, 18 |
| Meyer, 201520 | 1997–1993; mean 18.9 years | 35,703 (all cancer-free at inception); unclear what proportion developed HCC | 81; record linkage with Swiss National Cohort | 24.3; 7.9%; premorbid BMI assessed at inception (measured and self reported in different cohorts) | Reference < 25 kg/m2 25–29.9 vs. ref: 1.28 (0.77–2.12) > 30 vs. ref: 2.21 (1.18–4.15) |
1,2,7,9, 11, 17, 18, 20,21, |
| Batty, 20056 | 1967–1970; median 28.1 years | 17, 102 (all cancer-free at inception); unclear what proportion developed HCC) | 51; record linkage with National Health Service Central Registry (NHSCR) | NR;4.2%; premorbid BMI assessed at inception (measured) | Reference 18.5–25 kg/m2 25–29.9 vs. ref: 0.99 (0.53 – 1.88) > 30 vs. ref: 3.76 (1.36 – 10.40) |
1,2,3,4, 5, 8, 10,17,18, 19, 23, 25 |
| Hart, 201021 | 1965–1976 across 3 subgroup studies; median 26 years | 26,738 (all cancer-free at inception); unclear what proportion developed HCC) | 69; record linkage with NHS | NR;8% men and 14% women; premorbid BMI assessed at inception (measured and self reported in different cohorts) | Reference 18.5–25 kg/m2 Men 25–29.9 vs. ref: 1.01 (0.54 – 1.91) > 30 vs. ref: 3.05 (1.33 – 7.00) Women 25–29.9 vs. ref: 1.06 (0.40 – 2.80) > 30 vs. ref: 1.11 (0.24–5.27) |
1,3,4,5,8,20,25, 26,27,28 |
| Li, 201340 | 1988–1990; median 19 years | 72,473 (all cancer-free at inception); unclear what proportion developed HCC) | 527; review of death certificates | 22.7 in men and 23.0 in women ; NR; premorbid BMI assessed at inception (self-reported) | Reference 21.0–22.9 kg/m2 Men <18.5 vs. ref: 1.42 (0.93 – 2.15) 18.5–20.9 vs. ref: 1.09 (0.81– 1.48) 23.0–24.9 vs. ref: 1.04 (0.76 – 1.42) > 25 vs. ref: 1.15 (0.83 – 1.60) Women <18.5 vs. ref: 0.74 (0.35 – 1.60) 18.5–20.9 vs. ref: 1.08 (0.69– 1.68) 23.0–24.9 vs. ref: 1.16 (0.75 – 1.79) > 25 vs. ref: 1.42 (0.95 – 2.13) |
1,3,4,7,8,11,18,29,30,31,32 |
| Park, 200619 | 1996–2002; median 1.9 years | 14,578 (all had cancer at inception, of which 2,815 had HCC) | 2,171; record linkage with National Statistical Office | NR; 1%; premorbid BMI assessed at inception (measured) | Reference: BMI<23kg/m2 23–24.9 vs. ref: 0.83 (0.75–0.93) ≥25 vs. ref: 1.03 (0.92–1.14) |
1,2,4,5,6,7,10,15,18,19,21 |
| Tseng, 201241 | 1995–1998; 12 years | 88,694 (all cancer-free at inception); unclear what proportion developed HCC) | 1,345; record linkage with national registry | NR; NR; NR | Reference: 18.5–22.9 kg/m2 <18.5 vs. ref: 1.15 (0.84–1.58) 23–24.9 vs. ref: 0.86 (0.76–0.97) ≥25 vs. ref: 0.77 (0.66–0.89) |
1,2,3,4,33 |
NR-not reported, HCC-hepatocellular cancer, BMI-body mass index, HR-hazard ratio, CI-confidence interval 1-Age, 2-Sex, 3-Smoking, 4-Diabetes mellitus/fasting glucose, 5-Hypertension/blood pressure/antihypertensive medication, 6-Race/ethnicity, 7-Alcohol intake, 8-Socioeconomic status/housing status/occupation, 9-Education, 10-Hyperlipidemia/hypercholesterolemia/cholesterol levels, 11-Region/Nation, 12-Reproductive history, 13-Hormone replacement therapy use, 14-Time since menopause, 15-Fat/vegetable consumption, 16-Aspirin use, 17-Marital status, 18-Physical activity/Exercise, 19-Comorbidities, 20-Particular study (in pooled studies), 21-Healthy diet, 22-Family history of malignancy, 23-Skin fold thickness, 24-Study year, 25-Pulmonary function tests, 26-MI/Angina, 27-Bronchitis, 28-Height, 29-Consumption of coffee/fish, 30-History of liver disease, 31-History of disease of gall bladder, 32-History of transfusion, 33-History of insulin use, 34-History of cirrhosis, 35-History of chronic Hepatitis B, 36-History of Hepatitis C
Measures of adiposity and hepatocellular cancer-related mortality
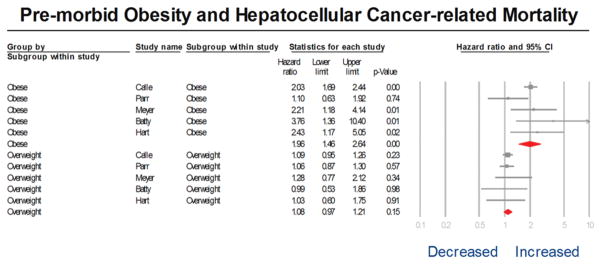
Of 9 studies, five studies observed higher mortality in participants with higher premorbid BMI, and this was statistically significant across all groups of BMI >30 kg/m2 in 4 studies.5,6,21–23 On meta-analysis, comparing HCC-related mortality in participants in the obese category of BMI with the normal category of BMI, we observed that obese HCC participants almost 2-fold higher HCC-related mortality than participants with normal BMI (aHR, 1.96; 95% CI, 1.46–2.46) with moderate heterogeneity (I2=37%) (Figure 2). In contrast, on meta-analysis comparing HCC-related mortality in overweight and normal BMI participants, there was no significant association between being overweight and HCC-related mortality, (aHR, 1.08; 95%CI, 0.97–1.21) with minimal heterogeneity (I2=0%) (Figure 2). On meta-analysis of 4 studies, which compared the combination of obese and overweight participants with the normal BMI participants, pre-morbid BMI was not associated with increased HCC-related mortality. However, it is unclear what proportion of patients in these studies were obese (BMI >30kg/m2) vs. overweight (BMI 25.0–29.9kg/m2).
Figure 2.
Forest plot of adjusted relative risks of obese and overweight categories of body mass index (BMI) category vs. normal weight, with HCC-related mortality.
The size of each box indicates the relative weight of each study in the meta-analysis. Horizontal lines represent the 95% confidence intervals (CIs).
Subgroup analysis
Due to significant differences in outcomes in obese and overweight individuals, further subgroup analyses were limited to five studies which stratified results by obese and overweight categories. An increased mortality in obese participants was observed in studies conducted both in Western (aHR, 2.10; 95%CI, 1.77–2.48), but not Asia-Pacific regions (aHR, 1.10; 95% CI, 0.63–1.92) (p-interaction=0.03). A stronger effect size of obesity on HCC-related mortality was also seen in men (aHR, 2.50; 95% CI, 2.02–3.09) compared to women (aHR, 1.45; 95% CI, 1.08–1.97) (p interaction <0.01). Results were not different in studies that did and did not adjust for T2DM or alcohol use (Table 2). We were unable to perform stratified analysis of studies that did and did not adjust for cirrhosis, HCC stage, underlying etiology, treatment modality or performance status, since no studies reported or adjusted for these factors.
Table 2.
Subgroup analysis of obese participants and normal-BMI participants with hepatocellular cancer (HCC)
| Subgroups | Categories | No. of studies | Obese vs Normal | |
|---|---|---|---|---|
| HR (95% CI) | Pinteraction | |||
| Location | Asia-Pacific | 1 | 2.10 (1.77–2.48) | .03 |
| Western | 4 | 1.10 (0.63–1.92) | ||
| Sex | Males | 3 | 2.50 (2.02–3.09) | .004 |
| Females | 2 | 1.45 (1.08–1.97) | ||
| Adjusted for alcohol | Yes | 3 | 1.77 (1.21–2.57) | .19 |
| No | 2 | 2.82 (1.56–5.11) | ||
| Adjusted for diabetes mellitus | Yes | 2 | 2.82 (1.56–5.11) | .19 |
| No | 3 | 1.77 (1.21–2.57) | ||
Publication Bias
Based on the visual inspection of the funnel plot as well as on quantitative measurement using the Egger’s regression test, there was no evidence of publication bias (P=.90).
DISCUSSION
In this systematic review based on 9 studies in over 1.5 million participants with 5,705 HCC-related deaths, we observed that obese, but not overweight, individuals have a 2-fold higher risk of HCC-related mortality compared to individuals with normal BMI. This effect is observed primarily in Western populations, where hepatitis C and non-alcoholic fatty liver disease (NAFLD) are predominant causes of HCC, but not in Asian populations. Additionally, the effect is stronger in men, as compared to women.
There exists a cache of evidence demonstrating the meteoric rise in the incidence of obesity around the globe.24,25 There has been a parallel rise in the incidence of NAFLD-related HCC worldwide.26,27 Several pooled studies have observed an increased risk of developing HCC with increasing adiposity, as measured by BMI.28,29 Although the exact pathophysiology of the development of NAFLD-related HCC remains unclear, there has been several mechanistic links which points towards the chronic inflammatory response to obesity, hyperglycemia and insulin resistance which contribute significantly in the development of HCC. These pro-inflammatory responses along with decrease in anti-inflammatory pathways and decrease in adipokines shifts the internal milieu of hepatocytes in favor of chronic inflammation without adequate repair mechanisms that facilitates tumor growth.30 Cellular mechanisms involving aberrant proliferation of hepatic progenitor cells and modification of the hedgehog signaling pathways facilitating such uncontrolled proliferation have been implicated in NAFLD-related HCC.30
The potential reasons for the negative prognostic effect of obesity on HCC-related mortality are likely multifactorial. First, obesity predisposes to metabolic syndrome, and subsequent chronic liver diseases, which increase the risk of HCC and cirrhosis. Unfortunately, none of the studies adjusted for presence or severity of cirrhosis, so it is difficult to tease out the independent effect of obesity on HCC-related mortality. Second, obese patients are significantly more likely to have suboptimal HCC surveillance, due to poor ultrasound quality. Surveillance is recommended in high risk patients with the intention to identify early lesions (within Milan criteria) which may be amenable to curative resection or liver transplantation.31 In a cohort study of 941 patients, obese patients had 3–8 fold higher risk of having an inadequate surveillance exam, with increasing risk of failure with increasing BMI; over 45% of patients with BMI >30 kg/m2, NAFLD or alcoholic liver disease as underlying etiology and Child B or C cirrhosis had inadequate exams.32 Hence, due to delay in detection, obese patients with HCC may be at an advanced stage at diagnosis, resulting in poor prognosis. Included studies did not adjust for underlying stage and treatment for HCC. Third, obese patients are also at higher risk of post-surgical complications, including risk of hepatic decompensation, bile leakage and wound infections, which can contribute to postoperative mortality.33 We have previously demonstrated increased mortality in pancreatic cancer patients with elevated premorbid BMI,34 and a paracrine effect of abdominal adiposity on increased locoregional cancer risk cannot be excluded. However, none of the studies reported the relationship between abdominal obesity (waist hip ratio, or image-estimated visceral adipose tissue) and HCC-related mortality. In contrast to the detrimental effect of excess body weight in HCC and pancreatic cancer, a prior study demonstrated improved outcomes in obese lung cancer patients.35 It is unclear if this reflects unmeasured confounding, or a true representation of the ‘obesity paradox’, a phenomenon where overweight patients with certain chronic medical conditions have lower mortality than normal weight counterparts. These discrepant findings in site-specific cancer mortality point toward the tremendous heterogeneity in cancer biology and pathophysiology by site, and highlight the need for further study.36–38
The strengths of this systematic review include: (a) comprehensive and systematic literature search with well-defined inclusion criteria, carefully excluding redundant studies and studies in which BMI was assessed at the time of or after HCC diagnosis; (b) rigorous evaluation of study quality using a validated tool for prognostic studies; (c) sub-group and sensitivity analyses to evaluate the stability of findings, regardless of presence or absence of heterogeneity; and (d) assessment of a dose-response relationship using obese vs overweight approach, adding biological credibility to findings.
There are several limitations in our study. The meta-analysis included only observational studies, with inherent biases and suboptimal control of confounders. Most studies were large cohort studies, which enrolled cancer-free participants at baseline and followed them for development of several outcomes including HCC-related mortality; these studies did not adjust for competing risk such as presence, etiology and severity of cirrhosis, HCC stage and treatment. To address this, we performed subgroup analyses based on history of alcohol use and past medical history of T2DM, and observed a stable association. Our subgroup analyses were not driven by a desire to explore sources of heterogeneity, but to verify stability of findings in different conditions. We did not specifically analyze all-cause and cancer-related mortality due to paucity of such data in the included studies. The included studies had inherent limitations - the categories for BMI were not uniformly reported. Exposure assessment in studies was at variable time points, but all consistently prior to diagnosis of HCC, to minimize confounding by severity. Only one of the studies in this meta-analysis used self-reported BMI; however, the effect of this is likely minimal since self-reported BMI has been shown to be highly correlated with measured BMI.39 Outcomes assessment was based primarily on record linkage with death certificates for population-based studies; however, these databases have been studied extensively in the past with high validity. Only English language articles were considered. Several studies did not report data on physical activity; studies have shown that fitness more than fatness may correlate with prognosis in cardiovascular disease.42 We were unable to analyze other measures of adiposity such as waist circumference, visceral versus subcutaneous fat, and percent body fat.
CONCLUSION
We observed that pre-morbid obesity is associated with a 2-fold higher risk of HCC-related mortality, particularly in men, and in Western populations. In current treatment schemes, premorbid obesity should be considered as a marker of poor prognosis, and may be used as a stratification variable in interventional studies. Additionally, alternative screening modalities may be considered for obese patients to increase diagnostic yield and early detection of HCC.
Supplementary Material
Acknowledgments
Funding Sources: None
Footnotes
Disclosures: Dr. S. Singh is partly supported by the National Library of Medicine/NIH training grant T15LM011271, and the American College of Gastroenterology Junior Faculty Development Award.
Conflicts of Interest: None
The paper will be presented in abstract form at the Gastrointestinal Cancers Symposium, 2017 to be held in San Francisco, CA in January 2017
References
- 1. [Accessed November 6, 2016];Cancer Incidence in Five Continents. IX http://www.iarc.fr/en/publications/pdfs-online/epi/sp160/ [Google Scholar]
- 2.Siegel RL, Miller KD, Jemal A. Cancer statistics, 2016. CA Cancer J Clin. 2016;66(1):7–30. doi: 10.3322/caac.21332. [DOI] [PubMed] [Google Scholar]
- 3.Parkin DM, Bray F, Ferlay J, Pisani P. Estimating the world cancer burden: Globocan 2000. Int J Cancer. 2001;94(2):153–156. doi: 10.1002/ijc.1440. [DOI] [PubMed] [Google Scholar]
- 4.Wang Y, Wang B, Shen F, Fan J, Cao H. Body mass index and risk of primary liver cancer: a meta-analysis of prospective studies. The Oncologist. 2012;17(11):1461–1468. doi: 10.1634/theoncologist.2012-0066. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Calle EE, Rodriguez C, Walker-Thurmond K, Thun MJ. Overweight, obesity, and mortality from cancer in a prospectively studied cohort of U.S. adults. N Engl J Med. 2003;348(17):1625–1638. doi: 10.1056/NEJMoa021423. [DOI] [PubMed] [Google Scholar]
- 6.Batty GD, Shipley MJ, Jarrett RJ, Breeze E, Marmot MG, Smith GD. Obesity and overweight in relation to organ-specific cancer mortality in London (UK): findings from the original Whitehall study. Int J Obes 2005. 2005;29(10):1267–1274. doi: 10.1038/sj.ijo.0803020. [DOI] [PubMed] [Google Scholar]
- 7.El-Serag HB, Rudolph KL. Hepatocellular carcinoma: epidemiology and molecular carcinogenesis. Gastroenterology. 2007;132(7):2557–2576. doi: 10.1053/j.gastro.2007.04.061. [DOI] [PubMed] [Google Scholar]
- 8.Mathur A, Franco ES, Leone JP, Osman-Mohamed H, Rojas H, Kemmer N, Neff GW, Rosemurgy AS, Alsina AE. Obesity portends increased morbidity and earlier recurrence following liver transplantation for hepatocellular carcinoma. HPB. 2013;15(7):504–510. doi: 10.1111/j.1477-2574.2012.00602.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Utsunomiya T, Okamoto M, Kameyama T, Matsuyama A, Yamamoto M, Fujiwara M, Mori M, Aimitsu S, Ishida T. Impact of obesity on the surgical outcome following repeat hepatic resection in Japanese patients with recurrent hepatocellular carcinoma. World J Gastroenterol WJG. 2008;14(10):1553–1558. doi: 10.3748/wjg.14.1553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stroup DF, Berlin JA, Morton SC, Olkin I, Williamson GD, Rennie D, Moher D, Becker BJ, Sipe TA, Thacker SB. Meta-analysis of observational studies in epidemiology: a proposal for reporting. Meta-analysis Of Observational Studies in Epidemiology (MOOSE) group. JAMA. 2000;283(15):2008–2012. doi: 10.1001/jama.283.15.2008. [DOI] [PubMed] [Google Scholar]
- 11.Wang C, Wang X, Gong G, Ben Q, Qiu W, Chen Y, Li G, Wang L. Increased risk of hepatocellular carcinoma in patients with diabetes mellitus: a systematic review and meta-analysis of cohort studies. Int J Cancer. 2012;130(7):1639–1648. doi: 10.1002/ijc.26165. [DOI] [PubMed] [Google Scholar]
- 12.Abe H, Aida Y, Ishiguro H, Yoshizawa K, Miyazaki T, Itagaki M, Sutoh S, Aizawa Y. Alcohol, postprandial plasma glucose, and prognosis of hepatocellular carcinoma. World J Gastroenterol. 2013;19(1):78–85. doi: 10.3748/wjg.v19.i1.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hayden JA, van der Windt DA, Cartwright JL, Côté P, Bombardier C. Assessing bias in studies of prognostic factors. Ann Intern Med. 2013;158(4):280–286. doi: 10.7326/0003-4819-158-4-201302190-00009. [DOI] [PubMed] [Google Scholar]
- 14.Reeves GK, Pirie K, Beral V, Green J, Spencer E, Bull D Million Women Study Collaboration. Cancer incidence and mortality in relation to body mass index in the Million Women Study: cohort study. BMJ. 2007;335(7630):1134. doi: 10.1136/bmj.39367.495995.AE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7(3):177–188. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 16.Higgins JPT, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 18.Egger M, Davey Smith G, Schneider M, Minder C. Bias in meta-analysis detected by a simple, graphical test. BMJ. 1997;315(7109):629–634. doi: 10.1136/bmj.315.7109.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park SM, Lim MK, Shin SA, Yun YH. Impact of prediagnosis smoking, alcohol, obesity, and insulin resistance on survival in male cancer patients: National Health Insurance Corporation Study. J Clin Oncol Off J Am Soc Clin Oncol. 2006;24(31):5017–5024. doi: 10.1200/JCO.2006.07.0243. [DOI] [PubMed] [Google Scholar]
- 20.Meyer J, Rohrmann S, Bopp M, Faeh D Swiss National Cohort Study Group. Impact of Smoking and Excess Body Weight on Overall and Site-Specific Cancer Mortality Risk. Cancer Epidemiol Biomark Prev Publ Am Assoc Cancer Res Cosponsored Am Soc Prev Oncol. 2015;24(10):1516–1522. doi: 10.1158/1055-9965.EPI-15-0415. [DOI] [PubMed] [Google Scholar]
- 21.Hart CL, Batty GD, Morrison DS, Mitchell RJ, Smith GD. Obesity, overweight and liver disease in the Midspan prospective cohort studies. Int J Obes 2005. 2010;34(6):1051–1059. doi: 10.1038/ijo.2010.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gray L, Lee I-M, Sesso HD, Batty GD. Association of body mass index in early adulthood and middle age with future site-specific cancer mortality: the Harvard Alumni Health Study. Ann Oncol Off J Eur Soc Med Oncol. 2012;23(3):754–759. doi: 10.1093/annonc/mdr270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Parr CL, Batty GD, Lam TH, Barzi F, Fang X, Ho SC, Jee SH, Ansary-Moghaddam A, Jamrozik K, Ueshima H, Woodward M, Huxley RR Asia-Pacific Cohort Studies Collaboration. Body-mass index and cancer mortality in the Asia-Pacific Cohort Studies Collaboration: pooled analyses of 424,519 participants. Lancet Oncol. 2010;11(8):741–752. doi: 10.1016/S1470-2045(10)70141-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ogden CL, Flegal KM, Carroll MD, Johnson CL. Prevalence and trends in overweight among US children and adolescents, 1999–2000. JAMA. 2002;288(14):1728–1732. doi: 10.1001/jama.288.14.1728. [DOI] [PubMed] [Google Scholar]
- 25.Hedley AA, Ogden CL, Johnson CL, Carroll MD, Curtin LR, Flegal KM. Prevalence of overweight and obesity among US children, adolescents, and adults, 1999–2002. JAMA. 2004;291(23):2847–2850. doi: 10.1001/jama.291.23.2847. [DOI] [PubMed] [Google Scholar]
- 26.Alexander J, Torbenson M, Wu T-T, Yeh MM. Non-alcoholic fatty liver disease contributes to hepatocarcinogenesis in non-cirrhotic liver: a clinical and pathological study. J Gastroenterol Hepatol. 2013;28(5):848–854. doi: 10.1111/jgh.12116. [DOI] [PubMed] [Google Scholar]
- 27.Adams J, Kuchibhatla M, Christopher EJ, Alexander JD, Clary GL, Cuffe MS, Califf RM, Krishnan RR, O’Connor CM, Jiang W. Association of depression and survival in patients with chronic heart failure over 12 Years. Psychosomatics. 2012;53(4):339–346. doi: 10.1016/j.psym.2011.12.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Saunders D, Seidel D, Allison M, Lyratzopoulos G. Systematic review: the association between obesity and hepatocellular carcinoma – epidemiological evidence. Aliment Pharmacol Ther. 2010;31(10):1051–1063. doi: 10.1111/j.1365-2036.2010.04271.x. [DOI] [PubMed] [Google Scholar]
- 29.Chen Y, Wang X, Wang J, Yan Z, Luo J. Excess body weight and the risk of primary liver cancer: an updated meta-analysis of prospective studies. Eur J Cancer Oxf Engl 1990. 2012;48(14):2137–2145. doi: 10.1016/j.ejca.2012.02.063. [DOI] [PubMed] [Google Scholar]
- 30.Wong CR, Nguyen MH, Lim JK. Hepatocellular carcinoma in patients with non-alcoholic fatty liver disease. World J Gastroenterol. 2016;22(37):8294–8303. doi: 10.3748/wjg.v22.i37.8294. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.El-Serag HB, Davila JA. Surveillance for hepatocellular carcinoma: in whom and how? Ther Adv Gastroenterol. 2011;4(1):5–10. doi: 10.1177/1756283X10385964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Simmons O, Fetzer DT, Yokoo T, Marrero JA, Yopp A, Kono Y, Parikh ND, Browning T, Singal AG. Predictors of adequate ultrasound quality for hepatocellular carcinoma surveillance in patients with cirrhosis. Aliment Pharmacol Ther. 2017;45(1):169–177. doi: 10.1111/apt.13841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rong X, Wei F, Geng Q, Ruan J, Shen H, Li A, Luo R. The Association Between Body Mass Index and the Prognosis and Postoperative Complications of Hepatocellular Carcinoma: A Meta-Analysis. Medicine (Baltimore) 2015;94(31):e1269. doi: 10.1097/MD.0000000000001269. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Majumder K, Gupta A, Arora N, Singh PP, Singh S. Premorbid Obesity and Mortality in Patients With Pancreatic Cancer: A Systematic Review and Meta-analysis. Clin Gastroenterol Hepatol Off Clin Pract J Am Gastroenterol Assoc. 2016;14(3):355–368e. doi: 10.1016/j.cgh.2015.09.036. quiz e32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Gupta A, Majumder K, Arora N, Mayo HG, Singh PP, Beg MS, Hughes R, Singh S, Johnson DH. Premorbid body mass index and mortality in patients with lung cancer: A systematic review and meta-analysis. Lung Cancer Amst Neth. 2016;102:49–59. doi: 10.1016/j.lungcan.2016.10.017. [DOI] [PubMed] [Google Scholar]
- 36.Lavie CJ, De Schutter A, Milani RV. Body composition and the obesity paradox in coronary heart disease: can heavier really be healthier? Heart Br Card Soc. 2015;101(20):1610–1611. doi: 10.1136/heartjnl-2015-307966. [DOI] [PubMed] [Google Scholar]
- 37.Lavie CJ, De Schutter A, Parto P, Jahangir E, Kokkinos P, Ortega FB, Arena R, Milani RV. Obesity and Prevalence of Cardiovascular Diseases and Prognosis-The Obesity Paradox Updated. Prog Cardiovasc Dis. 2016;58(5):537–547. doi: 10.1016/j.pcad.2016.01.008. [DOI] [PubMed] [Google Scholar]
- 38.Lavie CJ, Sharma A, Alpert MA, De Schutter A, Lopez-Jimenez F, Milani RV, Ventura HO. Update on Obesity and Obesity Paradox in Heart Failure. Prog Cardiovasc Dis. 2016;58(4):393–400. doi: 10.1016/j.pcad.2015.12.003. [DOI] [PubMed] [Google Scholar]
- 39.Palta M, Prineas RJ, Berman R, Hannan P. Comparison of self-reported and measured height and weight. Am J Epidemiol. 1982;115(2):223–230. doi: 10.1093/oxfordjournals.aje.a113294. [DOI] [PubMed] [Google Scholar]
- 40.Li Y, Yatsuya H, Yamagishi K, Wakai K, Tamakoshi A, Iso H, Mori M, Sakauchi F, Motohashi Y, Tsuji I, Nakamura Y, Mikami H, Kurosawa M, Hoshiyama Y, Tanabe N, Tamakoshi K, Tokudome S, Suzuki K, Hashimoto S, Kikuchi S, Wada Y, Kawamura T, Watanabe Y, Ozasa K, Miki T, Date C, Sakata K, Kurozawa Y, Yoshimura T, Fujino Y, Shibata A, Okamoto N, Shio H. Body mass index and weight change during adulthood are associated with increased mortality from liver cancer: the JACC Study. J Epidemiol. 2013;23(3):219–226. doi: 10.2188/jea.JE20120199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tseng C-H. Type 2 diabetes, smoking, insulin use, and mortality from hepatocellular carcinoma: a 12-year follow-up of a national cohort in Taiwan. Hepatol Int. 2013;7(2):693–702. doi: 10.1007/s12072-012-9405-0. [DOI] [PubMed] [Google Scholar]
- 42.Ortega FB, Lavie CJ, Blair SN. Obesity and Cardiovascular Disease. Circ Res. 2016;118(11):1752–1770. doi: 10.1161/CIRCRESAHA.115.306883. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.