Abstract
Objective
Intensity-modulated radiation therapy (IMRT) has largely supplanted three-dimensional conformal radiation (3D-CRT) for definitive anal cancer treatment due to decreased toxicity and potentially improved outcomes. Convincing data demonstrating its advantages, however, remain limited. We compared outcomes and toxicity with concurrent chemotherapy and IMRT vs 3D-CRT for anal cancer.
Methods
We performed a single-institution retrospective review of patients treated with IMRT or 3D-CRT as part of definitive mitomycin-C/5-fluorouricil-based chemoradiation for anal cancer from January 2003 to December 2012.
Results
One hundred sixty-five patients were included, with 61 and 104 receiving IMRT and 3D-CRT, respectively. Overall, 92.7% had squamous cell carcinoma. The mean initial pelvic dose was 48.3 and 44 Gy for IMRT and 3D-CRT, respectively. Complete response, partial response, and disease progression rates were similar (IMRT 83.6, 8.2, 8.2%; 3D-CRT 85.6, 6.7, 7.7%; p = 0.608, p = 0.728, p = 0.729). There was no significant difference in overall survival (p = 0.971), event-free survival (p = 0.900), or local or distant recurrence rates (p = 0.118, p = 0.373). IMRT caused significantly less acute grade 1–2 incontinence (p = 0.035), grade 3–4 pain (p = 0.033), and fatigue (p = 0.030). IMRT patients had significantly fewer chronic post-treatment toxicities (p = 0.008), outperforming 3D-CRT in six of eight toxicities reviewed. Though total treatment length was comparable (43.6 and 44.5 days), IMRT recipients had fewer (27.9 vs 41.3% of patients, p = 0.89), shorter treatment breaks (mean 2.9 vs 4.1 days, p = 0.229).
Conclusion
This report represents the largest series directly comparing concurrent chemotherapy with IMRT vs 3D-CRT for definitive treatment of anal cancer. IMRT significantly reduced acute and post-treatment toxicities and allowed for safe and effective pelvic dose escalation.
Keywords: Anal cancer, Radiation, IMRT, 3D, Toxicity
Introduction
Eight thousand eighty people in the USA are expected to be diagnosed with anal cancer in 2016, resulting in 1080 deaths [1]. Despite comprising only 1.5% of all gastrointestinal tumors, the rates of diagnosis have been increasing in the USA and globally [2, 3]. The current standard of care is definitive chemoradiation, with abdominoperineal resection (APR) reserved for salvage therapy [4, 5].
Advancements in radiation treatment over time have attempted to better optimize its delivery to potentially reduce toxicity and allow for maximal dose escalation. Historically, two-dimensional techniques based on surface anatomy and bony landmarks were used to deliver radiation for anal cancer. Until recently, three-dimensional conformal radiation (3D-CRT) was the most commonly utilized treatment modality, incorporating CT imaging data to better identify the intended target. Intensity-modulated radiation therapy (IMRT) followed, which can be used to design still more conformal radiation fields. By modulating the intensity of each beam delivered, a dose can be designed to target the concavities and convexities of a tumor volume, thereby further reducing dose to adjacent tissues.
Within the last few years, IMRT has been shown to be more efficacious and less toxic than traditional 3D-CRT for multiple disease sites [6–9]. Smaller case series have reported similar advantages of IMRT in the context of anal cancer, but conclusive data remain limited [10–15]. Though these studies present survival and toxicity outcomes, even fewer are comparative in nature [12, 13]. Furthermore, a wide range of results have been reported thus far, from a significant survival benefit with IMRT to more modest results of decreased toxicities and fewer treatment breaks [11, 12, 15, 16]. Since IMRT is both substantially more expensive and technically demanding, it is critical to rigorously evaluate the scope of its benefit. We therefore present a comparison of patients treated for anal squamous cell carcinoma with 3D-CRT vs IMRT, with an emphasis on acute toxicity, chronic post-treatment sequelae, and clinical outcomes.
Methods
Internal Review Board approval was obtained to perform a retrospective review of all anal cancer patients evaluated at our institution between January 2003 and December 2012. Patients had biopsy-confirmed invasive anal cancer treated with definitive chemoradiation using either 3D-CRT or IMRT, including patients with regional nodal involvement. Patients were excluded if they had distant metastases or if treatment intent was palliative. Of the 249 patients initially identified, 165 were eligible. All patients underwent complete staging using contrast-enhanced CT, MRI, or FDG-PET.
Pertinent demographic information and tumor characteristics were collected based on previously identified risk factors for the development of anal cancer [17]. Tumor factors included histological type, grade, AJCC stage (7th edition), and distance from the anal verge measured on imaging and exam. Acute toxicities during treatment were collected from weekly on-treatment and radiation completion notes, while post treatment, late toxicity were collected from radiation and medical oncology follow-up documentation.
Chemotherapy
Patients received 2 cycles of concurrent infusional 5-fluorouracil (5-FU)-based cytotoxic chemotherapy. In the majority of instances, treatment entailed combination 5-FU and bolus mitomycin-C (MMC) per the Nigro protocol [18]. Based on practice variability among medical oncologists, 10.9% of patients received 5-FU alone, 5-FU/cisplatin, oral Xeloda, 5-FU/cisplatin/vinblastine, or an etoposide-based treatment.
Radiation therapy
All patients underwent CT simulation, either prone with a belly board or supine. Non-IMRT radiotherapy planning followed classic two-dimensional radiotherapy borders for mini-pelvic fields, cone downs, and tumor boosts [19]. Electrons were used to supplement dose to the inguinal lymph nodes. IMRT contours and dose constraints were according to RTOG 05-29 [20]. Briefly, contours including the gross tumor, and elective lymph node regions (inguinal, internal iliac, external iliac) were defined using imaging studies with appropriate margin expansions for CTV and PTV. Total dose was 50.4 Gy for T2, 54 Gy for T3–T4, and 45 Gy for elective lymph node regions.
Toxicity
Acute toxicity was defined as treatment effect occurring between radiation therapy start and 8 weeks after radiation treatment completion [20]. Late-developing effects were defined as occurring from 8 weeks through 1 year post treatment, the longest period of follow-up for the majority of IMRT recipients. Acute toxicities assessed were localized pain, diarrhea, fatigue, dermatitis, hematologic changes, incontinence, enterocolitis, colitis, proctitis, fistula formation, and anal/vaginal stenosis. Late toxicities included intractable diarrhea, anal/vaginal stenosis, enterocolitis, proctitis, anal ulcers, fistula formation, wound dehiscence, and refractory pain. The Common Terminology Criteria for Adverse Events v3.0 (CTCAEv3) quantified toxicity [21].
Treatment response and follow-up
Tumor response was assessed by physical exam and follow-up imaging. Recurrence was determined clinically by physical exam. Biopsy was only performed if the lesion was clinically indeterminate. A complete response (CR) was recorded if there was no evidence of residual tumor, partial response (PR) if there was a response of at least 30% on exam or imaging compared with presentation, or progressive disease (PD), defined as an increase in tumor size on exam or imaging. Follow-up was every 3–4 months for the first year, and every 4–6 months thereafter without evidence of clinical recurrence.
Results
Patient factors
Of 165 patients, 61 (37%) received IMRT and 104 (63%) received 3D-CRT. Since the start of 2010, 76.7% of patients received IMRT, rising to 83.3% after 2012. Both treatment groups were demographically comparable (Table 1), and the majority had a favorable ECOG performance status of 0–1 (85.2 and 94.3%, respectively). Though HIV infection was more common in the IMRT group (13.1 vs 5.8% in 3D-CRT), there was a comparable percentage of patients in each group receiving chronic immunosuppressive therapy (14.8% in the IMRT group and 10.6% in the 3D-CRT group).
Table 1.
Demographic factors (n = 165)
| Value (%) | ||
|---|---|---|
| IMRT (n = 61) | 3D-CRT (n = 104) | |
| Sex | ||
| Male | 21 (34.4) | 37 (35.6) |
| Female | 40 (65.6) | 67 (64.4) |
| Age | ||
| Mean | 58.8 ± 10.9 | 55.9 ± 11.5 |
| Range | 38–89 | 26–85 |
| Performance status | ||
| ECOG 0–1 | 52 (85.2) | 98 (94.3) |
| 2 | 7 (11.5) | 2 (1.9) |
| 3 | 2 (3.3) | 4 (3.8) |
| KPS 100 − 90 | 38 (62.3) | 68 (65.4) |
| 80–70 | 21 (34.4) | 32 (30.8) |
| ≤ 60 | 2 (3.3) | 4 (3.8) |
| BMI | ||
| < 18.5 | 0 (0) | 3 (2.9) |
| 18.5–24.9 | 28 (45.9) | 40 (38.5) |
| 25–29.9 | 22 (36.1) | 26 (25) |
| ≥ 30 | 11 (18) | 35 (33.7) |
| Smoking | ||
| Yes | 36 (59.0) | 69 (66.3) |
| Pack years (average) | 26.1 ± 21.5 | 32.7 ± 22.0 |
| HPV positive | 10 (16.4) | 11 (10.6) |
| HIV positive | 8 (13.1) | 6 (5.8) |
| Immunosuppressive therapy | 9 (14.8) | 11 (10.6) |
| Tumor type | ||
| Squamous cell | 56 (91.8) | 97 (93.3) |
| Adenocarcinoma | 3 (4.9) | 4 (3.8) |
| Other | 2 (3.3) | 3 (2.9) |
| Anal verge distance | ||
| Mean | 0.44 ± 0.99 cm | 0.61 ± 1.22 cm |
| Range | 0–4 cm | 0–6 cm |
| Spanning verge | 25 (41.0) | 49 (47.1) |
| T stage | ||
| 1 | 8 (13.1) | 12 (11.5) |
| 2 | 34 (55.7) | 53 (51.0) |
| 3 | 13 (21.3) | 27 (26.0) |
| 4 | 6 (9.8) | 12 (11.5) |
| N stage | ||
| 0 | 23 (37.7) | 59 (56.7) |
| 1 | 14 (23.0) | 19 (18.3) |
| 2 | 13 (21.3) | 16 (15.4) |
| 3 | 11 (18.0) | 10 (9.6) |
| M stage | ||
| 0 | 54 (88.5) | 96 (92.3) |
| 1 | 7 (11.5) | 8 (7.7) |
| Tumor grade | ||
| 1 | 9 (14.8) | 15 (14.4) |
| 2 | 39 (63.9) | 64 (61.5) |
| 3 | 13 (21.3) | 25 (24.0) |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimentional conventional radiation therapy, ECOG Eastern Cooperative Oncology Group, KPS Karnofsky performance status
Tumor factors
Squamous cell carcinoma was the most common histology in both IMRT and 3D-CRT groups (91.8%, 93.3%). Adenocarcinoma, cloacogenic, small cell, mucinous, and adenosquamous tumors were rarer histologies. Seventy-seven percent of the tumors in both groups were T2–3 (Table 1).
Chemotherapy
One hundred fifty-two of 165 (92.1%) patients received 2 cycles of concurrent infusional 5-FU-based chemotherapy. Of these 152 patients, 135 (88.8%) were treated with combination 5-FU and bolus MMC according to the Nigro protocol, 46 (75.4%) in the IMRT group, and 89 (85.6%) in the 3D-CRT group.
Radiation treatment
In the IMRT group, 55.9% of patients were treated supine and 44.1% prone, while 84.8 and 15.2% of 3D-CRT patients were treated supine and prone, respectively. Patients in the IMRT group received a higher mean pelvic dose compared to 3D-CRT (48.3 vs 44 Gy) with a mean tumor boost of 9.6 and 12.7 Gy, respectively. The mean total dose, number of fractions, and overall length of treatment were the same for both groups. 3D-CRT patients exhibited a wider range of treatment length compared to IMRT (25–110 vs 31–71 days) (Table 2).
Table 2.
Radiation treatment regimen
| Mean (SD) | Range | |||
|---|---|---|---|---|
| IMRT | 3D-CRT | IMRT | 3D-CRT | |
| Pelvic dose | 48.3 (6.2) | 44 (6.6) | 59.4–30.6 | 63–30 |
| Boost | 9.6 (4.3) | 12.7 (4.7) | 23.4–3.6 | 24–4 |
| Total Gy | 53.8 (4.9) | 54.1 (7.4) | 62.5–32.4 | 64–30 |
| Fractions | 29.2 (3.1) | 28.4 (4.5) | 34–18 | 35–10 |
| Total length (days) | 43.6 (8.0) | 44.5 (11.8) | 31–71 | 25–110 |
SD standard deviation, Gy Gray
Clinical outcomes
The mean and median follow-up after IMRT and 3D-CRT was 20 months (0.33–76) and 14.7 months, and 47.4 months (1–125) and 46.1 months, respectively. In the IMRT group, rates of CR, PR, and PD were 83.6, 8.2, and 8.2%, respectively, very similar to those among patients treated with 3D-CRT, which were 85.6, 6.7, and 7.7%, respectively (p = 0.608, p = 0.728, p = 0.729). Median overall survival (OS) was not reached for the IMRT group and was 64.2 months for 3D-CRT. There was no statistically significant difference in OS and event-free survival (EFS) (p = 0.971, p = 0.900) (Fig. 1). Local recurrences were found in eight (13.1%) and seven (6.7%) patients in the IMRT and 3D-CRT groups, respectively (p = 0.118). Six (9.8%) and 15 (14.4%) of IMRT and 3D-CRT patients respectively recurred distantly (p = 0.373), the majority of which were to liver, lung, perirectal, and inguinal lymph nodes.
Fig. 1.
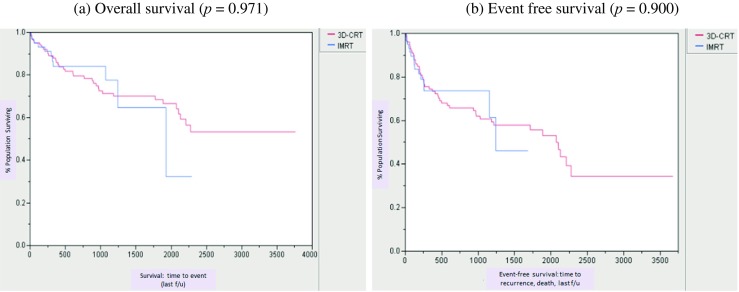
Kaplan-Meier analysis of overall survival and event-free survival by treatment modality
Toxicity
IMRT resulted in less acute high grade (3–4) toxicity, reaching statistical significance for pelvic pain (p = 0.033) and fatigue (p = 0.030). Decreased rates of high-grade dermatitis (p = 0.067), incontinence (p = 0.094), and hematologic abnormalities (p = 0.239) resulted from IMRT as well. There were no reported instances of grade 3 fatigue or incontinence with IMRT. In both groups, grade 4 toxicity was minimal, three instances (4.9%) in the IMRT group and seven instances (6.8%) in the 3D-CRT group. During the 8-week period following treatment, the 3D-CRT group experienced seven instances of grade 3 toxicity, compared to one instance after IMRT. These were all similar in nature to the late-developing toxicities that occurred from 8 weeks post treatment through 1 year. Hematologic toxicity and dermatitis were the most prevalent high-grade acute toxicities for both IMRT and 3D-CRT (Table 3). Both groups experienced a high degree of acute low-grade (1–2) toxicities, the most prevalent being grade 2 localized dermatitis. IMRT resulted in significantly less low-grade bladder/bowel incontinence compared to 3D-CRT (p = 0.035). While not statistically significant, IMRT also resulted in fewer occurrences of grade 2 localized pain, 32.8 vs 45.2% (p = 0.115).
Table 3.
Acute toxicities from chemoradiation treatment
| Value (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
| IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | |
| Localized pain | 25 (41.0) | 28 (26.9) | 20 (32.8) | 47 (45.2) | 2 (3.3) | 12 (11.5) | 0 (0) | 1 (1.0) |
| Diarrhea | 19 (31.1) | 38 (36.5) | 14 (23.0) | 26 (25) | 4 (6.6) | 8 (7.7) | 0 (0) | 1 (1.0) |
| Fatigue | 20 (32.8) | 32 (30.8) | 15 (24.6) | 23 (22.1) | 0 (0) | 5 (4.8) | 0 (0) | 0 (0) |
| Dermatitis | 16 (26.2) | 22 (21.2) | 37 (60.7) | 58 (55.8) | 5 (8.2) | 19 (18.3) | 0 (0) | 0 (0) |
| Hematologic | 19 (31.1) | 43 (41.3) | 14 (23) | 24 (23.1) | 4 (6.6) | 15 (14.4) | 3 (4.9) | 4 (3.8) |
| Incontinence | 12 (19.7) | 30 (28.8) | 5 (8.2) | 16 (15.4) | 0 (0) | 2 (1.9) | 0 (0) | 1 (1.0) |
| Gastroenteritisa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (1.0) | 1 (1.0) | 0 (0) | 0 (0) |
| Colitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 2 (1.9) | 0 (0) | 0 (0) |
| Proctitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (1.0) | 0 (0) | 0 (0) |
| Fistula | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 2 (1.9) | 0 (0) | 0 (0) |
| Stenosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (1.0) | 0 (0) | 0 (0) |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimensional conventional radiation therapy
aFrom treatment completion through 8 weeks post treatment
Overall, there were significantly fewer late-developing post-treatment toxicities caused by IMRT (4 occurrences, 6.6% of patients) within 1 year of treatment completion vs 3D-CRT (18 occurrences, 15.4% of patients) (p = 0.008). This trend was apparent in every toxicity category assessed except for anal ulcers and stenosis, which was reported in one and two instances, respectively, in each of the two treatment groups. Among 3D-CRT patients, five patients reported proctitis and five patients reported localized pain (Table 6).
Table 6.
Late toxicities within 1 year of treatment
| Number of occurrences | ||
|---|---|---|
| IMRT | 3D-CRT | |
| Diarrhea | 1 | 2 |
| Stenosis | 2 | 2 |
| Enterocolitis | 0 | 1 |
| Proctitis | 0 | 5 |
| Anal ulcer | 1 | 1 |
| Fistula | 0 | 1 |
| Wound dehiscence | 0 | 1 |
| Pain | 0 | 5 |
| Total occurrences | 4 | 18 |
| % of patients | 6.6 | 15.4 |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimensional conventional radiation therapy
Limiting the comparison of toxicity to patients who received MMC chemotherapy resulted in decreased rates of acute and chronic toxicity in the IMRT group comparable to those from the complete group analysis (Tables 4 and 7). IMRT continued to yield lower acute high-grade pain (2 vs 12 occurrences, 4.3 vs 13.5%, p = 0.078) and fatigue (0 vs 2 occurrences, 0 vs 2.2%, p = 0.034), as well as decreased rates of high-grade dermatitis (5 vs 18 occurrences, 10.9 vs 20.2%, p = 0.158), incontinence (0 vs 3 occurrences, 0 vs 3.3%, p = 0.111), and hematologic toxicity (6 vs 17 occurrences, 13 vs 19.1%, p = 0.366). Grade 4 toxicity remained minimal, with one less occurrence relative to the complete analysis in the 3D-CRT group. IMRT also continued to yield decreased low-grade incontinence (14 vs 42 occurrences, 30.4 vs 47.2%, p = 0.059) and grade 2 pain (16 vs 39 occurrences, 34.8 vs 43.8%, p = 0.309). Long-term toxicity results were similar to the complete analysis as well. One less instance of stenosis after IMRT and pain after 3D-CRT were recorded, totaling 6.5 and 19.1% of patients (3 vs 17 occurrences), respectively (p = 0.078).
Table 4.
Acute toxicities from chemoradiation treatment—patients who received mitomycin-C (IMRT 75.4%; 3D-CRT 85.6%)
| Value (%) | ||||||||
|---|---|---|---|---|---|---|---|---|
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
| IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | |
| Localized pain | 19 (41.3) | 26 (29.2) | 16 (34.8) | 39 (43.8) | 2 (4.3) | 11 (12.4) | 0 (0) | 1 (1.1) |
| Diarrhea | 15 (32.6) | 34 (38.2) | 8 (17.4) | 23 (25.8) | 4 (8.7) | 7 (7.9) | 0 (0) | 0 (1.1) |
| Fatigue | 15 (32.6) | 28 (31.5) | 10 (21.7) | 22 (24.7) | 0 (0) | 2 (2.2) | 0 (0) | 0 (0) |
| Dermatitis | 11 (23.9) | 14 (15.7) | 30 (65.2) | 54 (60.7) | 5 (10.9) | 18 (20.2) | 0 (0) | 0 (0) |
| Hematologic | 15 (32.6) | 36 (40.4) | 10 (21.7) | 22 (24.7) | 3 (6.5) | 13 (14.6) | 3 (6.5) | 4 (4.5) |
| Incontinence | 11 (23.9) | 28 (31.5) | 3 (6.5) | 14 (15.7) | 0 (0) | 2 (2.2) | 0 (0) | 1 (1.1) |
| Gastroenteritisa | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 1 (2.2) | 1 (1.1) | 0 (0) | 0 (0) |
| Colitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 2 (2.2) | 0 (0) | 0 (0) |
| Proctitis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (1.1) | 0 (0) | 0 (0) |
| Fistula | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (1.1) | 0 (0) | 0 (0) |
| Stenosis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | 0 | 1 (1.1) | 0 (0) | 0 (0) |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimensional conventional radiation therapy
aFrom treatment completion through 8 weeks post treatment
Table 7.
Late toxicities within 1 year of treatment—patients who received mitomycin-C (IMRT 75.4%; 3D-CRT 85.6%)
| Number of occurrences | ||
|---|---|---|
| IMRT | 3D-CRT | |
| Diarrhea | 1 | 2 |
| Stenosis | 1 | 2 |
| Enterocolitis | 0 | 1 |
| Proctitis | 0 | 5 |
| Anal ulcer | 1 | 1 |
| Fistula | 0 | 1 |
| Wound dehiscence | 0 | 1 |
| Pain | 0 | 4 |
| Total occurrences | 3 | 17 |
| % of patients | 6.5 | 19.1 |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimensional conventional radiation therapy
Of patients who received IMRT, 27.9% had at least one unplanned treatment break, while 41.3% in the 3D-CRT group required a treatment interruption (p = 0.89). Patients receiving 3D-CRT missed a mean 4.1 days, compared with 2.9 days for IMRT patients (p = 0.229). Of 3D-CRT recipients, 17.3% missed ≥ 10 days, compared to 9.8% of IMRT recipients (p = 0.178).
Discussion
We conducted the largest single-institution study directly comparing outcomes and toxicities of patients treated with 3D-CRT vs IMRT and concurrent 5-FU/MMC for biopsy-proven anal cancer. No statistical significance in OS or EFS emerged between the two groups. IMRT, however, resulted in significantly less acute and late toxicity. While the percentage of patients who required treatment breaks was similar, the IMRT group had shorter breaks (4.1 vs 2.9 days), as well as required fewer extensive (≥ 10 days) breaks compared to the 3D-CRT group.
The relatively brief long-term follow-up time of this study likely limited the statistical significance of OS and EFS as clinical outcomes measures. However, although OS and EFS are often used to assess treatment efficacy, they may not be relevant markers in the context of anal cancer as local recurrences can be salvaged with APR, with a 75% 5-year survival when clean margins are achieved [22]. Survival analyses, therefore, are confounded by the bias introduced by a successful salvage treatment and, as such, are largely measures of death by other causes. It is, therefore, difficult to extrapolate the degree to which reported OS and EFS advantages of IMRT are truly indicative of increased efficacy. Other, more specific end points that have been utilized in large prospective trials include toxicity, locoregional control, complete locoregional response, and progression-free survival [23].
The primary advantage of IMRT over 3D-CRT in our series was reduced acute and late toxicity, in particular high-grade toxicity (Table 3). These outcomes were notwithstanding the higher mean pelvic dose delivered with IMRT (48.3 Gy) compared to 3D-CRT (44 Gy). While some reports, including from the RTOG, recommended a pelvic dose of 30.6 Gy for conformal radiotherapy due to concerns for toxicity [24–26], doses of 40–45 Gy are commonly prescribed. The prospective trial RTOG 05–29 revealed a significant reduction in grade ≥ 3 dermatologic and gastrointestinal and grade ≥ 2 hematologic toxicities using IMRT to deliver 42–45 Gy to the pelvis before boost [20].
The most prevalent high-grade acute toxicity in both groups was hematologic (11.5 and 18.2% for IMRT and 3D-CRT, respectively), primarily leukopenia. This may have been due in part to the known immunosuppressant effects of MMC, which 84.8% of patients received, as well as inclusion of the broad pelvic bones in the radiation field [27]. IMRT, which limits the dose delivered to the greatest regions of pelvic bone marrow, had a lower rate compared with 3D-CRT. Of note, controlling for the presence of MMC resulted in nearly identical rates of toxicity compared to the whole group analysis in which fewer IMRT patients received MMC (75.4%) than did 3D-CRT patients (85.6%) (Table 5). Other studies found high rates of grade 3–4 hematologic toxicity, for example, 61% in RTOG 98-11 using conventional techniques [28], and 31% reported by Pepek et al. with IMRT (47 patients, no direct 3D-CRT comparison) [16]. Milano et al. found no benefit to IMRT (17 patients) relating to hematologic toxicity compared to 3D-CRT (7 patients) (rates not reported) [11].
Table 5.
Change in percent (%) acute toxicity: Total group ➔ patients who received mitomycin-C (IMRT 75.4%; 3D-CRT 85.6%)
| Grade 1 | Grade 2 | Grade 3 | Grade 4 | |||||
|---|---|---|---|---|---|---|---|---|
| IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | IMRT | 3D-CRT | |
| Localized pain | 41 ➔ 41.3 | 26.9 ➔ 29.2 | 32.8 ➔ 34.8 | 45.2 ➔ 43.8 | 3.3 ➔ 4.3 | 11.5 ➔ 12.4 | 0 | 1 ➔ 1.1 |
| Diarrhea | 31.1 ➔ 32.6 | 36.5 ➔ 38.2 | 23 ➔ 17.4 | 25 ➔ 25.8 | 6.6 ➔ 8.7 | 7.7 ➔ 7.9 | 0 | 1 ➔ 1.1 |
| Fatigue | 32.8 ➔ 32.6 | 30.8 ➔ 31.5 | 24.6 ➔ 21.7 | 22.1 ➔ 24.7 | 0 | 4.8 ➔ 2.2 | 0 | 0 |
| Dermatitis | 26.6 ➔ 23.9 | 21.2 ➔ 15.7 | 60.7 ➔ 65.2 | 55.8 ➔ 60.7 | 8.2 ➔ 10.9 | 18.3 ➔ 20.2 | 0 | 0 |
| Hematologic | 31.1 ➔ 32.6 | 41.3 ➔ 40.4 | 23 ➔ 21.7 | 23.1 ➔ 24.7 | 6.6 ➔ 6.5 | 14.4 ➔ 14.6 | 4.9 ➔ 6.5 | 3.8 ➔ 4.5 |
| Incontinence | 19.7 ➔ 23.9 | 28.8 ➔ 31.5 | 8.2 ➔ 6.5 | 15.4 ➔ 15.7 | 0 | 1.9 ➔ 2.2 | 0 | 1 ➔ 1.1 |
| Gastroenteritisa | 0 | 0 | 0 | 0 | 1 ➔ 2.2 | 1 ➔ 1.1 | 0 | 0 |
| Colitis | 0 | 0 | 0 | 0 | 0 | 1.9 ➔ 2.2 | 0 | 0 |
| Proctitis | 0 | 0 | 0 | 0 | 0 | 1 ➔ 1.1 | 0 | 0 |
| Fistula | 0 | 0 | 0 | 0 | 0 | 1.9 ➔ 1.1 | 0 | 0 |
| Stenosis | 0 | 0 | 0 | 0 | 0 | 1 ➔ 1.1 | 0 | 0 |
IMRT intensity-modulated radiation therapy, 3D-CRT 3-dimensional conventional radiation therapy
aFrom treatment completion through 8 weeks post treatment
Toxicity rates from both IMRT and 3D-CRT in our study were also lower than those presented in previous studies. In the 2008 report from RTOG 98-11, 48% of patients receiving 3D-CRT and 5-FU/MMC had grade 3–4 dermatologic toxicity based on the CTCAE v2.0 [28], compared with 18.3% grade 3 toxicity in our series. Kachnic et al. reported a 20% rate of grade ≥ 3 dermatologic toxicity, as well as 22% of patients experiencing grade ≥ 3 gastrointestinal toxicity for IMRT [29] (43 patients, no direct 3D-CRT comparison), compared with 8.2 and 6.6% in our study. Pepek et al. reported rates of 9% grade 3 and no grade 4 diarrhea, comparable to our findings [16].
IMRT reduced toxicity up to 1 year following the end of chemoradiation (Table 6). While few studies have investigated post-treatment toxicity, even fewer have reported on specific categories of long-term sequelae [13]. In our series, notable differences emerged for proctitis (0 vs 5) and pain (0 vs 5), with IMRT demonstrating fewer chronic toxicities in six of eight categories analyzed. Such chronic sequelae are debilitating and substantially contribute to the cost of disease management. While analyzing influences on treatment breaks and toxicity from radiation for gastrointestinal malignancies, Hill et al. reported a strong correlation between toxicity and unplanned hospital admissions [30], which inevitably entails added resources and services. In contrast, Hodges et al., based on a novel Markov decision model designed to assess both cost and quality of life discrepancies between patients with anal cancer treated with IMRT vs 3D-CRT, concluded a cost-ineffectiveness of IMRT despite its lower overall risk of acute toxicity [31].
In our analysis, there was a trend towards fewer and shorter treatment breaks for IMRT patients compared with 3D-CRT. Recent studies have suggested that treatment breaks are associated with poorer clinical outcomes: RTOG 92-08, which included a pre-specified 2-week treatment break, resulted in decreased overall, disease-free, and colostomy-free survival compared to trials with no treatment breaks [32]. Similarly, Roohipour et al. and investigators of the ACT II trial both reported that treatment breaks contributed to worse OS, relapse-free survival, and local control [33, 34]. Other series, however, failed to show any correlation between treatment duration, breaks and outcome [12, 35]. In our series, IMRT patients experienced significantly fewer instances of high-grade toxicities, likely resulting in their tendency to need shorter interruptions. Rates of low-grade toxicity were only slightly reduced with IMRT, perhaps resulting in the comparable need for short treatment breaks (Table 7).
Studies that found a positive correlation between treatment breaks and worse outcomes have concluded that interruptions can be used as a quantitative surrogate for toxicity, based on the assumption that unplanned breaks are an opportunity to recover from an acute radiation-induced toxicity. Differences in physicians’ willingness to continue treatment despite toxicities, unique institutional protocols, and vigilance in toxicity prevention, however, all contribute variability. Additionally, some institutions elect to treat over weekends, or may lack the ability to continue treatment in spite of a hospital admission or other need. These considerations weaken the ability to directly equate toxicity with treatment interruption both between and within institutions.
Possible limitations of our analysis include heterogeneity in the exact chemotherapy and radiation given, as well as inter-rater variability of physician-reported toxicity. Both potential biases, however, should be equally present in both groups. Furthermore, IMRT was gradually adopted as the primary treatment modality for anal cancer at our institution (2006–2012) while 3D-CRT was being utilized (2003–2012), which should have limited differences in the learning curve or level of caregiver training between treatment types. Another limitation of our study is the relatively short follow-up time of IMRT (median 14.7 months) limiting the reporting of late toxicity to 1 year post treatment. In order to perform the most robust toxicity analysis possible with the greatest number of patients, we were limited to long-term follow-up of 1 year. In the context of a comparison of chronic toxicity, however, this is a relatively short time. While a clear trend of decreased post-treatment toxicity was established, a longer follow-up comparison would likely reveal more definitive results. Additional prospective studies with longer follow-up time following IMRT would further elucidate its benefits.
Conclusions
In conclusion, IMRT for anal cancer treatment results in reduced acute and late toxicity compared to 3D-CRT. IMRT also allows for safe dose escalation with survival and recurrence outcomes similar to 3D-CRT. Patients who received IMRT required less extensive treatment breaks. A decreased rate of grade 3–4 toxicity may help reduce the need for costly therapies and services to address side effects.
Compliance with ethical standards
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
IRB approval was obtained to perform this retrospective analysis. This article does not contain any studies with human participants or animals performed by any of the authors.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
References
- 1.SEER Cancer Statistics Review, 1975–2013: National Cancer Institute (2015). http://seer.cancer.gov/csr/1975_2013/. Accessed 17 Apr 2017
- 2.Siegel R, Ward E, Brawley O, Jemal A. Cancer statistics, 2011: the impact of eliminating socioeconomic and racial disparities on premature cancer deaths. CA: A Cancer J Clin. 2011;61:212–236. doi: 10.3322/caac.20121. [DOI] [PubMed] [Google Scholar]
- 3.Martin FT, Kavanagh D, Waldron R. Squamous cell carcinoma of the anal canal. Surgeon. 2009;7:232–237. doi: 10.1016/S1479-666X(09)80091-7. [DOI] [PubMed] [Google Scholar]
- 4.Klotz RG, Jr, Pamukcoglu T, Souilliard DH. Transitional cloacogenic carcinoma of the anal canal: clinicopathologic study of three hundred seventy-three cases. Cancer. 1967;20:1727–1745. doi: 10.1002/1097-0142(196710)20:10<1727::AID-CNCR2820201024>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 5.Ryan DP, Compton CC, Mayer RJ. Carcinoma of the anal canal. N Engl J Med. 2000;342:792–800. doi: 10.1056/NEJM200003163421107. [DOI] [PubMed] [Google Scholar]
- 6.Mundt AJ, Lujan AE, Rotmensch J, Waggoner SE, Yamada SD, Fleming G, Roeske JC. Intensity-modulated whole pelvic radiotherapy in women with gynecologic malignancies. Int J Radiat Oncol Biol Phys. 2002;52:1330–1337. doi: 10.1016/S0360-3016(01)02785-7. [DOI] [PubMed] [Google Scholar]
- 7.Mundt AJ, Mell LK, Roeske JC. Preliminary analysis of chronic gastrointestinal toxicity in gynecology patients treated with intensity-modulated whole pelvic radiation therapy. Int J Radiat Oncol Biol Phys. 2003;56:1354–1360. doi: 10.1016/S0360-3016(03)00325-0. [DOI] [PubMed] [Google Scholar]
- 8.Ashman JB, Zelefsky MJ, Hunt MS, Leibel SA, Fuks Z. Whole pelvic radiotherapy for prostate cancer using 3D conformal and intensity-modulated radiotherapy. Int J Radiat Oncol Biol Phys. 2005;63:765–771. doi: 10.1016/j.ijrobp.2005.02.050. [DOI] [PubMed] [Google Scholar]
- 9.Nutting C, Dearnaley DP, Webb S. Intensity modulated radiation therapy: a clinical review. Br J Radiol. 2000;73:459–469. doi: 10.1259/bjr.73.869.10884741. [DOI] [PubMed] [Google Scholar]
- 10.Bazan JG, Hara W, Hsu A, Kunz PA, Ford J, Fisher GA, Welton ML, Shelton A, Kapp DS, Koong AC, Goodman KA, Chang DT. Intensity-modulated radiation therapy versus conventional radiation therapy for squamous cell carcinoma of the anal canal. Cancer. 2011;117:3342–3351. doi: 10.1002/cncr.25901. [DOI] [PubMed] [Google Scholar]
- 11.Milano MT, Jani AB, Farrey KJ, Rash C, Heimann R, Chmura SJ. Intensity-modulated radiation therapy (IMRT) in the treatment of anal cancer: toxicity and clinical outcome. Int J Radiat Oncol Biol Phys. 2005;63:354–361. doi: 10.1016/j.ijrobp.2005.02.030. [DOI] [PubMed] [Google Scholar]
- 12.Dewas CV, Maingon P, Dalban C, Petitfils A, Peignaux K, Turc G, Martin E, Khoury C, Dewas S, Crehange G. Does gap-free intensity modulated chemoradiation therapy provide a greater clinical benefit than 3D conformal chemoradiation in patients with anal cancer? Radiat Oncol. 2012;7:201. doi: 10.1186/1748-717X-7-201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong MD, Freilich JM, Hoffe SE, Fulp W, Weber JM, Almhanna K, Dinwoodie W, Rao N, Meredith KL, Shridhar R. Intensity-modulated radiation therapy vs. 3D conformal radiation therapy for squamous cell carcinoma of the anal canal. Gastrointest Cancer Res. 2013;6:39–45. [PMC free article] [PubMed] [Google Scholar]
- 14.Koerber SA, Slynko A, Haefner MF, Krug D, Schoneweg C, Kessel K, Kopp-Schneider A, Herfarth K, Debus J, Sterzing F. Efficacy and toxicity of chemoradiation in patients with anal cancer—a retrospective analysis. Radiat Oncol. 2014;9:113. doi: 10.1186/1748-717X-9-113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.De Francesco I, Thomas K, Wedlake L, Tate D. Intensity-modulated radiotherapy and anal cancer: clinical outcome and late toxicity assessment. Clin Oncol. 2016;28:604–610. doi: 10.1016/j.clon.2016.04.039. [DOI] [PubMed] [Google Scholar]
- 16.Pepek JM, Willett CG, Wu QJ, Yoo S, Clough RW, Czito BG. Intensity-modulated radiation therapy for anal malignancies: a preliminary toxicity and disease outcomes analysis. Int J Radiat Oncol Biol Phys. 2010;78:1413–1419. doi: 10.1016/j.ijrobp.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 17.Salati SA, Kadi AA. Anal cancer—a review. Int J Health Sci. 2012;6:206–230. doi: 10.12816/0006000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nigro ND, Vaitkevicius VK, Buroker T, Bradley GT, Considine B (1981) Combined therapy for cancer of the anal canal. Dis Colon Rectum 24:73–75. doi:10.1007/BF02604287 [DOI] [PubMed]
- 19.Burdick MJ, Stephans KL. Gastrointestinal (non-esophageal) radiotherapy. In: Videtic G, Vassil A, editors. Handbook of treatment planning in radiation oncology. 1st. New York: Demos Medical Publishing; 2011. pp. 67–84. [Google Scholar]
- 20.Kachnic LA, Winter K, Myerson RJ, Goodyear MD, Willins J, Esthappan J, Haddock MG, Rotman M, Parikh PJ, Safran H, Willett CG. RTOG 0529: a phase 2 evaluation of dose-painted intensity modulated radiation therapy in combination with 5-fluorouracil and mitomycin-C for the reduction of acute morbidity in carcinoma of the anal canal. Int J Radiat Oncol Biol Phys. 2013;86:27–33. doi: 10.1016/j.ijrobp.2012.09.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Trotti A, Colevas AD, Setser A, Rusch V, Jaques D, Budach V, Langer C, Murphy B, Cumberlin R, Coleman CN, Rubin P. CTCAE v3.0: development of a comprehensive grading system for the adverse effects of cancer treatment. Semin Radiat Oncol. 2003;13:176–181. doi: 10.1016/S1053-4296(03)00031-6. [DOI] [PubMed] [Google Scholar]
- 22.Mullen JT, Rodriguez-Bigas MA, Chang GJ, Barcenas CH, Crane CH, Skibber JM, Feig BW. Results of surgical salvage after failed chemoradiation therapy for epidermoid carcinoma of the anal canal. Ann Surg Oncol. 2007;14:478–483. doi: 10.1245/s10434-006-9221-7. [DOI] [PubMed] [Google Scholar]
- 23.James RD, Glynne-Jones R, Meadows HM, Cunningham D, Myint AS, Saunders MP, Maughan T, McDonald A, Essapen S, Leslie M, Falk S, Wilson C, Gollins S, Begum R, Ledermann J, Kadalayil L, Sebag-Montefiore D. Mitomycin or cisplatin chemoradiation with or without maintenance chemotherapy for treatment of squamous-cell carcinoma of the anus (ACT II): a randomized, phase 3, open-label, 2 x 2 factorial trial. Lancet Oncol. 2013;14:516–524. doi: 10.1016/S1470-2045(13)70086-X. [DOI] [PubMed] [Google Scholar]
- 24.John M, Pajak T, Flam M, Hoffman J, Markoe A, Wolkov H, Paris K. Dose escalation in chemoradiation for anal cancer: preliminary results of RTOG 92-08. Cancer J Sci Am. 1996;2:205–211. [PubMed] [Google Scholar]
- 25.Constantinou EC, Daly W, Fung CY, Willett CG, Kaufman DS, DeLaney TF. Time-dose considerations in the treatment of anal cancer. Int J Radiat Oncol Biol Phys. 1997;39:651–657. doi: 10.1016/S0360-3016(97)00329-5. [DOI] [PubMed] [Google Scholar]
- 26.Martenson JA, Lipsitz SR, Wagner H, Jr, Kaplan EH, Otteman LA, Schuchter LM, Mansour EG, Talamonti MS, Benson AB., III Initial results of a phase II trial of high dose radiation therapy, 5-fluorouracil, and cisplatin for patients with anal cancer (E4292): an Eastern Cooperative Oncology Group study. Int J Radiat Oncol Biol Phys. 1996;35:745–749. doi: 10.1016/0360-3016(96)00146-0. [DOI] [PubMed] [Google Scholar]
- 27.Hayman JA, Callahan JW, Herschtal A, Everitt S, Binns DS, Hicks RJ, Manus MM. Distribution of proliferating bone marrow in adult cancer patients determined using FLT-PET imaging. Int J Radiat Oncol Biol Phys. 2011;79:847–852. doi: 10.1016/j.ijrobp.2009.11.040. [DOI] [PubMed] [Google Scholar]
- 28.Ajani JA, Winter KA, Gunderson LL, Pedersen J, Benson AB, III, Thomas CR, Jr, Mayer RJ, Haddock MG, Rich TA, Willett C. Fluorouracil, mitomycin, and radiotherapy vs fluorouracil, cisplatin, and radiotherapy for carcinoma of the anal canal: a randomized controlled trial. JAMA. 2008;299:1914–1921. doi: 10.1001/jama.299.16.1914. [DOI] [PubMed] [Google Scholar]
- 29.Kachnic LA, Tsai HK, Coen JJ, Blaszkowsky LS, Hartshorn K, Kwak EL, Willins JD, Ryan DP, Hong TS. Dose-painted intensity-modulated radiation therapy for anal cancer: a multi-institutional report of acute toxicity in response to therapy. Int J Radiat Oncol Biol Phys. 2012;82:153–158. doi: 10.1016/j.ijrobp.2010.09.030. [DOI] [PubMed] [Google Scholar]
- 30.Hill A, Kiss N, Hodgson B, Crowe TC, Walsh AD. Associations between nutritional status, weight loss, radiotherapy treatment toxicity and treatment outcomes in gastrointestinal cancer patients. Clin Nutr. 2011;30:92–98. doi: 10.1016/j.clnu.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 31.Hodges JC, Beg MS, Das P, Meyer J. Cost-effectiveness analysis of intensity modulated radiation therapy versus 3-dimensional conformal radiation therapy for anal cancer. Int J Radiat Oncol Biol Phys. 2014;89:773–783. doi: 10.1016/j.ijrobp.2014.02.012. [DOI] [PubMed] [Google Scholar]
- 32.Konski A, Garcia M, Jr, John M, Krieg R, Pinover W, Myerson R, Willett C. Evaluation of planned treatment breaks during radiation therapy for anal cancer: update of RTOG 92-08. Int J Radiat Oncol Biol Phys. 2008;72:114–118. doi: 10.1016/j.ijrobp.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Roohipour R, Patil S, Goodman KA, Minsky BD, Wong WD, Guillem JG, Paty PB, Weiser MR, Neuman HB, Shia J, Schrab D, Temple LK (2008) Squamous-cell carcinoma of the anal canal: predictors of treatment outcome. Dis Colon Rectum 51:147–153. doi:10.1007/s10350-007-9125-z [DOI] [PubMed]
- 34.James R, Wan S, Glynne-Jones R, Sebag-Montefiore D, Kadalayil L, Northover J, Cunningham D, Meadows H, Ledermann J. A randomized trial of chemoradiation using mitomycin or cisplatin, with or without maintenance cisplatin/5FU in squamous cell carcinoma of the anus (ACT II) J Clin Oncol. 2009;27:LBA4009. doi: 10.1200/jco.2009.27.18_suppl.lba4009. [DOI] [Google Scholar]
- 35.Meyer A, Meier Zu Eissen J, Karstens JH, Bremer M. Chemoradiotherapy in patients with anal cancer: impact of length of unplanned treatment interruption on outcome. Acta Oncol. 2006;45:728–735. doi: 10.1080/02841860600726729. [DOI] [PubMed] [Google Scholar]


