Abstract
Herein, we report preparation of a bio-nanohybrid material of homogenously dispersed functionalized multiwall carbon nanotubes (fMWCNTs) in Nafion (Nf) doped with polypyrrole (PPy) and followed by one-step in situ electrochemical polymerization along with glucose oxidase (GOx) on a platinum (Pt) electrode. The bioengineered Nf-GOx-fMWCNTs-PPy/Pt electrode showed excellent electrocatalytic performance to detect glucose with a high sensitivity (54.2 μAmM−1 cm−2) in linear range of up to 4.1 mM as well as a low detection limit of 5 μM (S/N = 3), response time within 4 s, good selectivity, stability, and practical applicability. It is our hope that the comprehensive results will contribute to design an efficient glucose biosensor with practical prospects for biomedical applications.
Introduction
Over the past few decades, electrical stimulus-responsive organic polymers have been investigated, and their composite materials offer novel uses in energy storage, electrochromic displays, information memory, anti-static materials, anti-corrosives, fuel cells, electromechanical devices, biosensors, and biomedical devices1–3. Electroactive conducting polymers (ECPs) have been tailored to obtain superior electrical conductivity, thermal, and chemical stabilities at normal temperature and pressures. Also, ECPs can be synthesized as bioactive and biocompatible materials to employ them as a better alternative than metal and metal-oxide nanoparticles. ECPs are eco-friendly functional materials that can be used to fabricate biosensor devices. To date, various ECPs have been used to construct highly sensitive and selective glucose biosensors4. Among these, polypyrrole (PPy) has been extensively used to provide stable, facile, low-cost, biocompatible, and convenient materials for glucose biosensors5. Oxidized PPy has strong affinity to immobilize negatively-charged enzymes via its electrostatic attraction, and it has shown robust activity both with an electrocatalytic and remarkable sensitivity toward glucose using electrochemical techniques6. A large number of studies have been carried out to develop effective techniques for glucose biosensors application, which is important for early identification, monitoring, and comprehensive treatment of diseases including diabetes, kidney disease and thalassemia7. Taking this into account, biosensors based on electrochemical techniques promise reliability and accuracy in real time detection as well as high sensitivity and specificity to glucose8.
In recent years, the demand for electrochemical glucose biosensors has grown dramatically in biomedical, pharmaceutical, food industry, and biofuel cell applications due to their simplicity, cost effectiveness, widespread availability, and low power requirements and extreme precision9. Nano-engineered metal nanoparticles and their oxides integrated on PPy within carbon nanotubes (CNTs), carbon dots, and graphene oxide are also prominent materials that can be used to fabricate glucose biosensors10–13. The excessive use of metal oxide nanoparticles, while fabricating the sensing devices have many challenges, e.g. limited biocompatibility, time consuming for biofunctionalization, expensive, and also show adverse effects on ecological and biological toxicity. Furthermore, lack of dispersibility of pristine CNTs and their allotropes in aqueous or other organic solvents results in agglomeration and an increase in roughness of the biosensor electrode surface so that loading of biomolecules become less stable during the fabrication process14,15. These disadvantages and obstacles can successfully replace by metal-free and label-free nanohybrid composite materials, i.e. PPy-doped functionalized MWCNTs. The synthesis of high aspect ratio composite materials using ECPs through electrochemical polymerization for glucose biosensor application has been well studied16,17. The anodic polymerized PPy in the form of nanowires, nanoparticles and layered structures at a low applied voltage not only improves enzyme immobilization but also improves the sensing performance of enzymatic electrochemical-based glucose biosensors. Moreover, the inherent electroactive polymeric activities and a tendency to integrate with hydrogel-forming porous composite matrix ensure that PPy retains its benefits in biomedicine, engineering, and highly-sensitive amperometric immunosensors17–19.
Enzymatic glucose biosensors based on MWCNTs and its composite material has been introduced to glucose detection with electrochemical methods20–22. CNTs are ideal elements with a high surface area-to-volume ratio, and they play a pivotal role as biosensor electrode materials that enhance the electron transport rate, increase sensitivity, and electrocatalytic activity towards glucose oxidation during electrochemical analysis23. In addition, Cosnier et. al. described the π-stacking or non-covalent interactions between single-walled CNTs and biotin functionalized PPy composite to demonstrate the synergistic effects for excellent electrical conductivity and biocompatibility of the functional materials24–26. To enhance GOx amount that covalently immobilizes or entraps into intrinsically high aspect interfacial surfaces of nanosized one-dimensional CNTs can also be possible through their surface functionalization in acidic conditions. This attribute suggests that improvement of physicochemical stability of the composites could be improved. Moreover, PPy-decorated fMWCNTs containing carbonyl groups have a tendency to immobilize a high amount of enzymes using an electrochemical polymerization method25,27. It is noteworthy that a breakthrough began when fMWCNTs were uniformly dispersed in aqueous solution in the presence of Nf, resulting in a large active surface area for biosensor electrode fabrication28,29. The hydrophilic anionic polar group side chains in Nf have a high electron density, generating electrostatic repulsive forces and Ven Der Waals repulsion force between Nf and fMWCNTs, resulting homogenous dispersion of fMWCNTs. As a result, oxidized PPy can easily be doped on fMWCNTs with covalent bonding at a low applied voltage during polymerization. In addition, Nf exhibits better selectivity towards glucose in the presence of interfering species due to the electrostatic repulsion to the most negatively-charged electroactive species, and it also supports an increase in the enzyme loading capacity of functional composite films30–32. Therefore, it is highly desirable to develop stable, selective, and sensitive bio-nanohybrid composite-based enzymatic biosensor to overcome dip-coating and casting process methods.
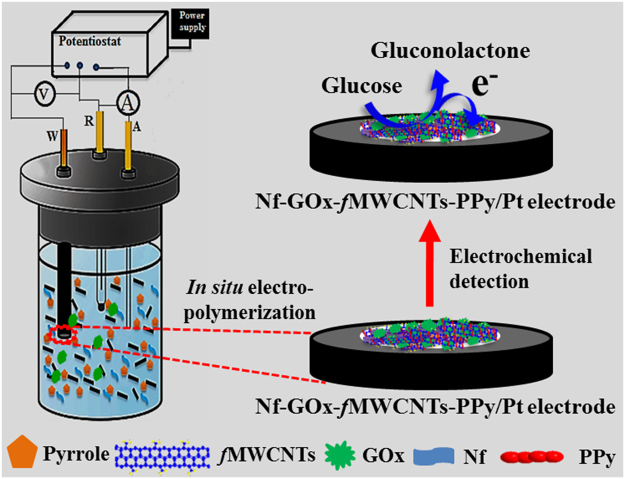
Here, we synthesized high conductance, large aspect ratio and electroactive bio-nanohybrid functional composite (Nf-GOx-fMWCNTs-PPy) materials. The aqueous suspension of the functional composite materials were deposited on a Pt working electrode using a simple one-step in situ electropolymerization technique to achieve a novel microarchitecture with precisely controlled large active surface area (Fig. 1; details are given in experimental section). The modified Pt electrode with new architecture has high electrocatalytic efficiency towards glucose oxidation and renown as the electrochemical based glucose biosensor. The regular shaped globular array of PPy doped on the surface of well-dispersed fMWCNTs in Nafion (Nf) provided a suitable avenue to immobilize or encapsulate large amount of GOx uniformly. Furthermore, Nf has potential ability to migrate protons in an aqueous phase, and this creates proton tunnelling within the sulfonate groups through narrow holes. As a result, the fabricated biosensor electrodes exhibited excellent performance in both ionic and electronic conductivity as well as high sensitivity during electrochemical detection of glucose. In addition, we were able to investigate the practical application of our purposed biosensor through addition of different glucose concentrations in human serum samples.
Figure 1.
Schematic illustration of glucose biosensor fabrication via one-step in situ electrochemical polymerization method.
Results and Discussions
Morphological characterization of as-synthesized bio-nanohybrid composites
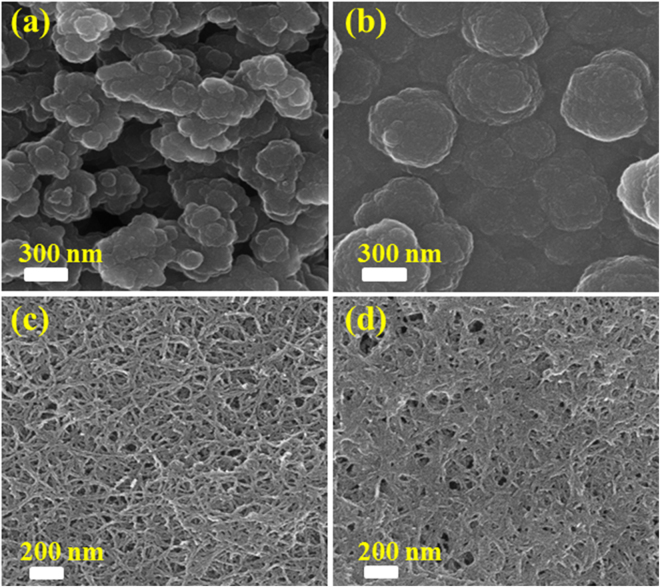
The morphological characteristics of nano-hybrid bio-composite films deposited on Pt disk electrode via electrochemical polymerization were characterized (Fig. 2). The FE-SEM image of oxidized PPy in Fig. 2a shows micro-globular, spherical and relatively high surface roughness. However, the GOx intercalation with the help of electrostatic forces occupies some porous space in the polymeric chain of the PPy film, which reduces a significant decrease in surface roughness, as shown in Fig. 2b. Well-dispersed fMWCNTs in Nf were decorated with globular shape PPy and modified in the form of spiral nanowires (Fig. 2c), where Nf plays a crucial role for the distribution of fMWCNTs. The uniformly-dispersed CNTs decorated with PPy create highly porous and large active surface area. After GOx encapsulation, the porosity of the film was occupied and displayed a homogenous and smooth surface of Nf-GOx-fMWCNTs-PPy composite film (Fig. 2d). We used electrochemical technique to determine the effective surface area of Pt electrode modified with Nf-GOx-fMWCNTs-PPy nanohybrid film (0.22 cm2), which was found to be much higher than effective surface area of unmodified Pt electrode (0.023 cm2). Detail of effective surface area measurements (Fig. S1) are given in the supplementary information.
Figure 2.
FE-SEM images of oxidized PPy (a), PPy-GOx (b), Nf-fMWCNTs-PPy (c), and Nf-GOx-fMWCNTs-PPy (d) after electropoymerization.
Figure 3 shows the TEM images of fMWCNTs (a) and PPy-doped fMWCNTs (b and c). The inset in Fig. 3a shows HRTEM image of the fMWCNTs. The decrease in the external diameter of the nanotubes (~8.01 nm) is due to the destruction occurred on edge of nanotubes during the carboxylic group’s functionalization. The TEM image of the PPy-doped fMWCNTs clearly shows a uniform decoration of PPy (Fig. 3b). In addition, the elemental mapping image shows a green color around fMWCNTs surface, which indicates the electrodeposition of PPy array in Fig. 3c.
Figure 3.
TEM images of functionalized MWCNTs (a), fMWCNTs-PPy (b), and mapping showing PPy doped fMWCNTs (c). Inset a shows HR-TEM image of fMWCNT.
The typical XRD patterns of MWCNTs (curve a), fMWCNTs (curve b), PPy (curve c), and Nf-fMWCNTs-PPy composite matrix (curve d) are shown in Supplementary Fig. S2. The hexagonal graphite-like geometry of MWCNTs was assigned from the diffraction peaks at (002) and (101) planes, which corresponds to 2θ degree values at 25.59° and 44.34°, respectively (curve a). However, there was no distortion in crystal lattice of hexagonal graphite-like structure after acid functionalization of MWCNTs, which reveals that fMWCNTs retain their original geometry (curve b). Even though a less intense and small shift of peak from 43.84° to 42.21° in fMWCNTs indicate the successful functionalization of MWCNTs. A sharp peak appears at 27.6° (2θ) is ascribed to amorphous nature of oxidized PPy having inter-planar d spacing of 3.45 Å, resulting from the repeated unit of regular pyrrole rings that is oriented more regularly in a globular array (curve c)33. It is more interesting that a slight shift in the peak intensity towards a lower value of 2θ has been observed in the Nf-fMWCNTs-PPy composite (curve d), confirms that the fMWCNTs are well decorated with a PPy array which is in agreement with the HR-TEM image (Fig. 3b).
To elucidate the bonding configuration of PPy polymers integrated on fMWCNTs surface, FT-IR reflection spectra were investigated (Supplementary Fig. S3). In Fig. S3a, the spectrum of PPy showed a significant peak at 3171 cm−1 that can be attributed to N-H stretching and the broad bands around at 1400 cm−1 −1627 cm−1 correspond to C=C and C-C stretching34. The absorption peaks at 1347 cm−1 and 1207 cm−1 are assigned to C-C ring stretching and C-N deformation mode, respectively. Furthermore, peaks from 782 cm−1 to 848 cm−1 appeared due to C-C stretching and C-H out of plane deformation in heterocyclic aromatic rings of polymeric conjugation in PPy. As we can observe in Figure S3b, FT-IR spectrum of fMWCNTs shows strong bands intensity at 1740 cm−1 and 3595 cm−1 correspond to the C=O and O-H groups, respectively35. Figure S3c illustrates the conjugation of Nf-PPy-fMWCNTs composites during in situ polymerization, where π-electrons on sidewalls of CNTs have a tendency to form covalent bonds with bipolarons state of PPy which was obviously justified from the shifting of 2θ value in Fig. S2d. Furthermore, electron-rich fMWCNTs are prone to form electrostatic and polar bonds with a highly oxidized PPy. Importantly, absorption band of PPy at 1556 cm−1 shifts to 1578 cm−1 in the nanohybrid composite (Fig. S3c), confirming the perfect interfacial interaction (π-π conjugation) between fMWCNTs and PPy supported by Van Der Waals forces and enhances the charge transfer rate through Nf-fMWCNTs-PPy composite material36. Moreover, a small peak shift from 718 cm−1 to 823 cm−1 assigned to quinonoid bipolaronic structure of PPy and a peak at 3392 cm−1 is attributed to the formation of hydrogen bonds between the N-H stretching of PPy with carbonyl group of fMWCNTs that appeared in composite, indicating the formation of a nanohybrid film and resulting in the disappearance of C=O in fMWCNTs (Fig. S3c). A notable appearance of strong bands at around 1654 cm−1 (amide I) and 1532 cm−1 (amide II) confirms that GOx immobilized perfectly and reserves its activity within the bionanohybrid composite (Nf-GOx-fMWCNTs-PPy) after in situ electrochemical polymerization (Fig. S3d).
The UV-Vis absorption spectra depicted in Supplementary Fig. S4 were investigated to illustrate the incorporation of PPy on dispersed fMWCNTs. A noticeable peak appeared on fMWCNTs at 273 nm, corresponding to the successful functionalization of MWCNTs (Fig. S4, curve b). In addition, a higher peak intensity of fMWCNTs was observed as compared to pure MWCNTs (Fig. S4, curve a), which could be due to the presence of polar functional groups on CNTs surface walls. The band gap adsorption peaks at 230 nm and 320 nm (Fig. S4, curve c) ascribe to π-conjugation and π-π* transition in oxidized PPy originated from pyrrole rings, respectively37. The significance peak arises at 273 nm, confirming the presence of fMWCNTs on nanohybrid film (Fig. S4, curve d). However, similar peaks were highly dominant in Nf-fMWCNTs-PPy (Fig. S4, curve e), indicating that well-dispersed fMWCNTs are uniformly doped by PPy into nanohybrid composite in the presence of Nf. Moreover, higher band intensity of Nf-fMWCNTs-PPy exhibits excellent conductivity and is optically active due to PPy in bipolaron state that gives extra benefit for the fabrication of fluorescence biosensor.
Electrochemical characterizations of surface modified Pt electrodes
To verify the excellent electron transport properties of the Nf-fMWCNTs-PPy nanohybrid composite film, we evaluated EIS obtained from of each modified electrode with different material as the frequency response with respect to electron interception and diffusion at electrode-electrolyte interface (Fig. 4). All impedance spectra consist of a semicircle portion in higher frequency region where the diameter quantifies charge-transfer resistance (Rct), which explains the charge transport kinetic at electrode-electrolyte interface and a linear sloping portion at lower frequencies related to the diffusion control process of the reactive species and conductivity of the materials. The obtained data was fitted using a Randles equivalent circuit, where Q1 represents the interfacial double layer capacitance, Q2 is a Warburg impedance, Rs is the bulk solution resistance, and Rct is charge-transfer resistance of the materials (upper inset of Fig. 4A). From Fig. 4A, all spectra show small semi-circular diameters, indicating the superior electrical conductivity of the materials. Furthermore, the slope of the linear portion of all curves show the diffusion of the redox charged solution on an interfacial electrode surface due to the high aspect ratio. The Rct values for different modified electrodes without GOx immobilization were calculated to be 390.7 Ω, 205.3 Ω, and 129 Ω, assigned to PPy (curve a), PPy-fMWCNTs (curve c), and Nf-fMWCNTs-PPy (curve e), respectively, and obtained Rct values are also presented as histogram (Fig. 4B). The decrease in Rct value of bio-nanohybrid film (Nf-fMWCNTs-PPy) is due to the synergetic effect of a superior conductivity of well-dispersed fMWCNTs that were uniformly doped with an oxidized PPy array in Nf, which makes a highly porous architecture in the film38–40. However, after GOx immobilization sequence of Rct values for each modified electrodes are PPy-GOx (490.16 Ω, curve b) > GOx-fMWCNTs-PPy (262.6 Ω, curve d) >Nf-GOx-fMWCNTs-PPy (186.87 Ω, curve f). The increase in Rct values and a decrease in slope of straight lines in the presence of GOx suggests strong encapsulation of enzymes, which act as a blocking agent for charge transport and electron transfer rate.
Figure 4.
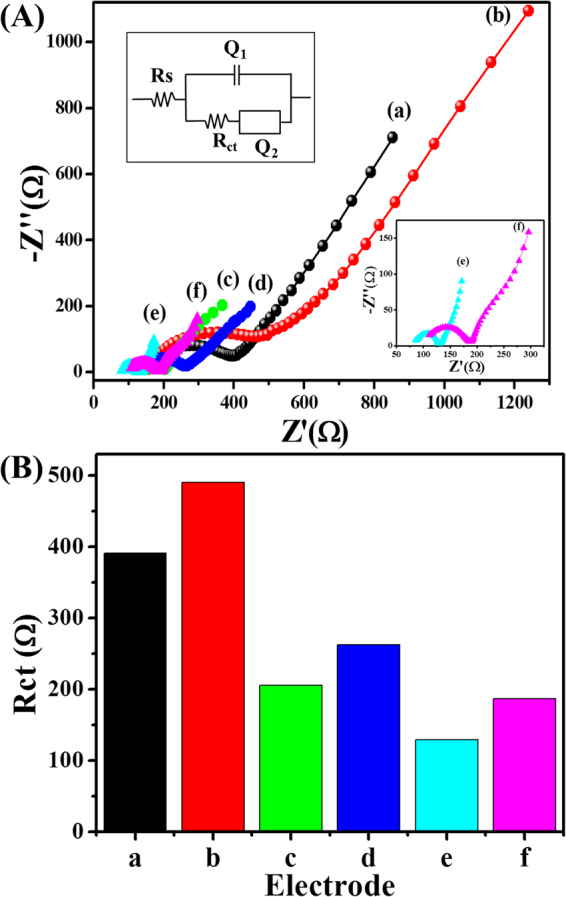
(A) EIS spectra obtained from Pt disk electrodes modified with PPy (a), GOx-PPy (b), fMWCNTs-PPy (c), GOx-fMWCNTs-PPy (d), Nf-fMWCNTs-PPy (e), and Nf-GOx-fMWCNTs-PPy (f) in 5.0 mM K3Fe[CN]6 containing 0.1 M KCl solution in 0.1 M PBS. (B) Histogram of Rct values of different electrodes. In Fig. 4A, the upper inset shows the Randles equivalent circuit, and the lower inset shows the magnified view of the EIS spectra of Nf-fMWCNTs-PPy/Pt (sky blue color) and Nf-GOx-fMWCNTs-PPy/Pt (pink color).
To investigate the electrochemical behavior of each surface modified electrode, cyclic voltammetric (CV) responses were recorded in 5.0 mM K3Fe[CN]6 containing 0.1 M KCl prepared in 0.1 M PBS (pH 7.4) at a scan rate of 25 mV/s (Fig. 5A). Each modified electrode exhibited a pair of reversible redox peak currents that could be attributed to direct electron transfer, but quasi-reversible electrochemical behavior. The CV response of the Nf-fMWCNTs-PPy/Pt electrode shows a significantly higher redox peak current with an anodic peak potential (Epa) at 0.21 V and cathodic peak potential (Eca) at 0.14 V (curve e). However, Nf-fMWCNTs-PPy/Pt electrode showed a lower peak-to-peak potential separation (ΔEp = 67 mV) compared to fMWCNTs-PPy/Pt (~70 mV, curve c) and PPy/Pt (~74 mV, curve a) electrodes. The larger background current of Nf-fMWCNTs-PPy/Pt is due to uniform, highly dispersed CNTs that were well-decorated with PPy, which results in a larger electroactive and catalytic active surface area with sufficiently defective sidewalls of fMWCNTs. In addition, smooth film of Nf-fMWCNTs-PPy offers a high mechanical stability and a favorable microenvironment for GOx immobilization, which employs synergistic effects for glucose oxidation. Each modified electrode along with GOx shows decrease in redox peak currents (curves b, d, and f) indicating that a thin blocking layer of GOx was formed between electrode-electrolyte phases that hinders the diffusion process of ferricyanide ions on electrode surface. Furthermore, electrochemical behavior of Nf-GOx-fMWCNTs-PPy/Pt electrode was tested using 5.0 mM [Fe(CN)6]3−/4− as a redox marker containing 0.1 M KCl prepared in 0.1 M PBS (pH 7.4) at different scan rates (25–110 mV/s), presented in Fig. 5B. The redox probe [Fe (CN)6]3−/4− shows an average peak-to-peak separation (ΔEpa) of about 78 mV, suggesting a quasi-reversible electron transport process attributed to the presence of GOx. Moreover, both curves of anodic and cathodic peak currents vs. square root of scan rate (inset Fig. 5B) were linearly proportional with correlation coefficient (R2) values of 0.9979 and 0.9973 for anodic and cathodic peaks, respectively, suggesting a surface-confined electrochemical redox process by Nf-GOx-fMWCNTs-PPy/Pt electrode41.
Figure 5.
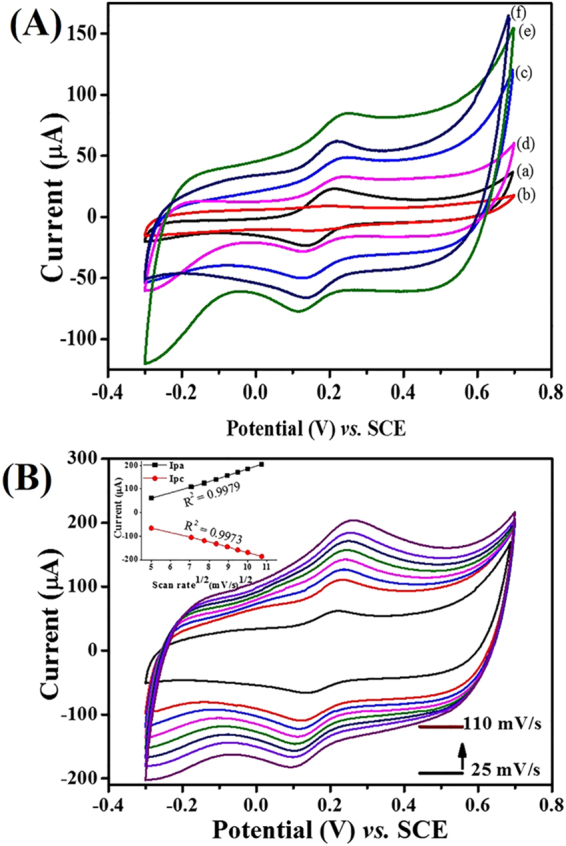
(A) CV response curves of different modified Pt electrodes, i.e., PPy/Pt (a), GOx-PPy/Pt (b), fMWCNTs-PPy/Pt (c), GOx-fMWCNTs-PPy/Pt (d), Nf-fMWCNTs-PPy/Pt (e), and Nf-GOx-fMWCNTs-PPy/Pt (f) in 5.0 mM K3Fe[CN]6 containing 0.1 M KCl in 0.1 M PBS. (B) CV curves of Nf-GOx-fMWCNTs-PPy/Pt electrode at different scan rates (25–110 mV/s) in 5.0 mM K3Fe[CN]6 containing 0.1 M KCl in 0.1 M PBS. Inset B shows the calibrated curve of Ipa and Ipc vs. square roots of scan rate.
Figure 6A illustrates the CV responses describing redox reaction behavior of GOx immobilized on electrodes, i.e. GOx-PPy/Pt, GOx-fMWCNTs-PPy/Pt, Nf-fMWCNTs-PPy (without GOx) and Nf-GOx-fMWCNTs-PPy/Pt in N2-saturated PBS (pH 7.4) solution. The redox peak current recorded for Nf-GOx-fMWCNTs-PPy/Pt electrode was higher than that for GOx-PPy/Pt and GOx-fMWCNTs-PPy/Pt electrodes. In addition, Nf-GOx-fMWCNTs-PPy/Pt electrode showed two well-defined redox peaks of reduction and oxidation at −0.45 V and −0.39 V, respectively. These average redox peaks were centered at −0.42 V and were taken as the surface formal potential (E°′) assigned to FAD-GOx/FADH2-GOx conversion, which is in consistent with the potential range reported in the literatures42,43. It is well known that a pair of redox peaks appeared in these potential ranges confirms the direct electron transfer from GOx, which illustrate the electrons transfer process between electrode surface and redox active center of FAD in GOx indicating quasi-reversible process44–46. Also, redox peak-to-peak separation of Nf-GOx-fMWCNTs-PPy/Pt electrode equal to 61 mV which is the characteristic of reversible electron transfer phenomenon on active center of GOx and surface-confined process. Importantly, a small value of redox peak-to-peak separation of Nf-GOx-fMWCNTs-PPy/Pt electrode as compared to GOx-fMWCNTs-PPy/Pt (97 mV) and GOx-PPy/Pt (121 mV) indicates a fast electron transfer process. The obtained results confirm that the architecture and bipolaron state of PPy forming Nf-fMWCNTs-PPy nanohybrid composite film has a tendency to immobilize large amounts of enzyme and has potential advantage of direct electron transfer capacity as compared to other electrodes, which makes it preferable to fabricate high-performance bio-electrocatalytic sensing devices47. The CVs of Nf-GOx-fMWCNTs-PPy/Pt electrodes at different scan rates (50–140 mV/s) were also recorded (Fig. 6B). The anodic (I pa) and cathodic (I pc) redox peak currents showed linear proportionality to the scan rates with a linear regression of 0.9979 and 0.9972 for I pa and I pc respectively (Fig. 6B, inset). Furthermore, the redox process of GOx in bio-nanohybrid composite film exhibited the reversible and surface-confined electrochemical process.
Figure 6.
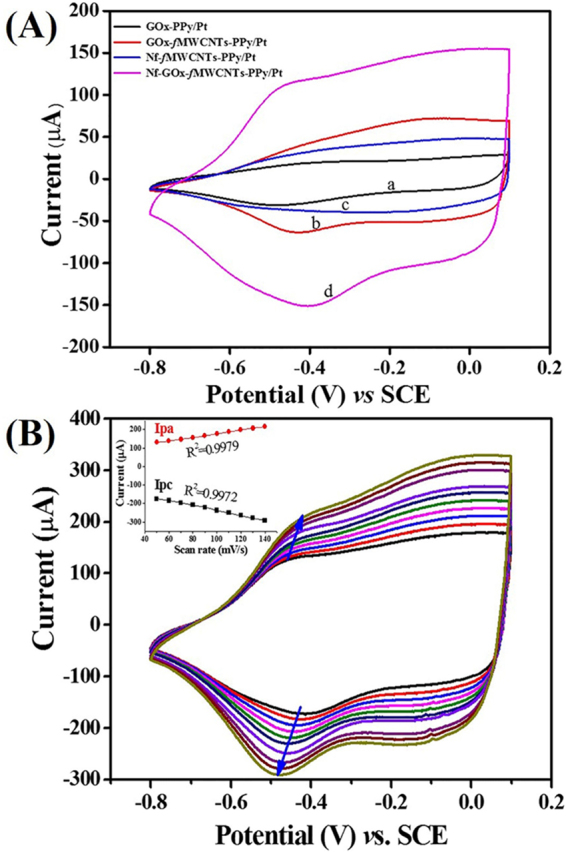
(A) CV response curves of GOx-PPy/Pt (a), (GOx-fMWCNTs-PPy/Pt (b), Nf-fMWCNTs-PPy/Pt (c), and Nf-GOx-fMWCNTs-PPy/Pt (d) in 0.1 M PBS at an applied potential range of −0.8 – + 0.1 V. (B) CVs of Nf-GOx-fMWCNTs-PPy/Pt electrode in 0.1 M PBS at different scan rate (50–140 mV/s). Inset B shows plot of redox peak currents vs. different scan rate.
CV measurements of glucose biosensor electrodes
The electrocatalytic performance of composites materials toward glucose detection were measured through CV technique using different electrodes, as shown in Fig. 7. The CVs obtained from different electrodes i.e. without GOx (Fig. 7A) and with (Fig. 7B) GOx immobilization were measured in 0.1 M PBS (pH 7.4) in the presence and absence of glucose. The CVs from different modified Pt-disk electrodes without GOx immobilization were expressed as solid lines (presence of 0.05 mM glucose) and short dash lines (absence of glucose) in Fig. 7A. From these response curves, there were no significant changes in I pa and I pc currents from the PPy film in the absence (Fig. 7A, curve a) and presence (Fig. 7A, curve a1) of 0.05 mM glucose. However, CVs obtained from the Nf-fMWCNTs-PPy/Pt electrode showed a higher background current (Fig. 7A, curve c1) compared to the fMWCNTs-PPy/Pt (Fig. 7A), curve b1) due to presence of Nf, where Nf has tendency to create a large active surface area of modified electrode after uniform dispersion of fMWCNTs. Even though, these nanohybrids could not exhibit a pronounced electrochemical and electrocaltalytic response for glucose oxidation.
Figure 7.
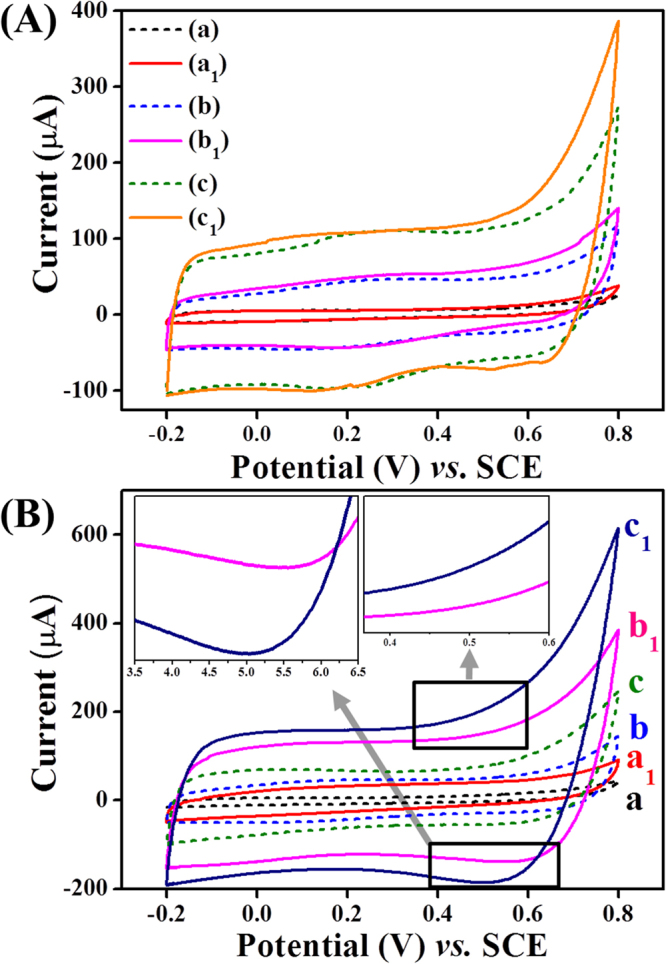
(A) CV response of PPy/Pt (curve a and a1), fMWCNTs-PPy/Pt (curve b and b1), and Nf-fMWCNTs-PPy/Pt (curve c and c1) electrodes measured in the presence of 0.05 mM glucose (solid lines) and in only 0.1 M PBS (pH 7.4) at 50 mV/s scan rate (short dash lines). (B) CVs of GOx immobilized electrodes i.e. GOx-PPy/Pt (curve a and a1), GOx-fMWCNTs-PPy/Pt (curves b and b1)), and Nf-GOx-fMWCNTs-PPy/Pt (curve c and c1) in the absence (short dash lines) and presence (solid lines) of 0.05 mM glucose in 0.1 M PBS (pH 7.4) at 50 mV/s scan rate.
Further, we recorded the CV responses from different electrodes after GOx immobilization on the composite materials in the presence and absence of glucose in 0.1 M PBS (pH 7.4), as shown in Fig. 7B. From the CV response curves, the redox current from GOx-PPy/Pt (Fig. 7B, curve a1) and GOx-fMWCNTs-PPy/Pt (Fig. 7B, curve b1) electrodes were smaller than current obtained from Nf-GOx-fMWCNTs-PPy/Pt (Fig. 7B, curve c1) electrode in the presence of 0.05 mM glucose. Interestingly, Nf provided a uniform and large effective surface area on modified electrode that enhances the capacity to integrate a higher amount of GOx. Also, the architecture of Nf-GOx-fMWCNTs-PPy nanohybrid material has abundant active sites due to the high aspect ratio of the fMWCNTs and well-dispersion of PPy decorated fMWCNTs forms an interfacial electronic configuration that employs the capacitive behaviour of the nanohybrid materials. The nanohybrid composite not only exhibits a higher electrical conductivity but it also has the ability to hold large capacitance currents. An enhanced capacitance current shows a dominant current response over the Faradic and redox currents during glucose oxidation. More importantly, we obtained a lower redox potential at 0.52 V (insets of Fig. 7B). The anodic current (264 µA) obtained during the oxidation of glucose by Nf-GOx-fMWCNTs-PPy/Pt electrode is ~3.17 times higher than current (80.8 µA) recorded in only 0.1 M PBS. The current increase can be attributed to the synergetic effect of electrical conductivity of completely-oxidized PPy-doped fMWCNTs in Nf and higher electrocatalytic function of large amount of GOx encapsulated into functional matrix. The possible mechanism for electrocatalytic oxidation of glucose can be expressed as follows48.
| 1 |
| 2 |
| 3 |
Amperometric response of the fabricated biosensor
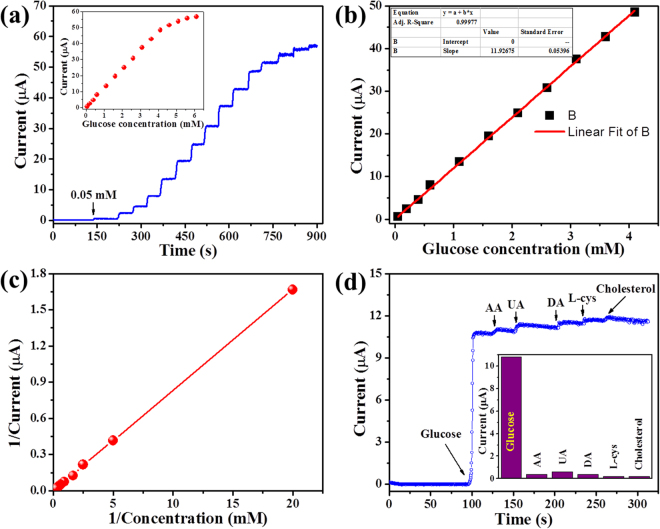
To evaluate the sensing performance of the fabricated glucose biosensor, we measured the amperometric response of Nf-GOx-fMWCNTs-PPy/Pt electrode with a successive addition of different concentrations of glucose (0.05–6.6 mM) in 0.1 M PBS (pH 7.4) at an applied potential of +0.52 V vs. SCE under a stirring condition (Fig. 8). A rapid response was observed with every addition and as a result a stable step curve was obtained, which takes less than 4 s to reach 94% of the steady-state current (Fig. 8a). The corresponding calibration graph of the biosensor response shows a linear increase in the current response with an increase in the glucose concentration (inset, Fig. 8a). However, at a higher concentration a non-linear slope was obtained, indicating a saturation of the active site of the enzymes. In Fig. 8b, the linear plot (steady-state current vs. glucose concentration) obtained from Nf-GOx-fMWCNTs-PPy/Pt biosensor electrode showed a high sensitivity (54.2 µA cm−2 mM−1, calculated using effective surface area of modified electrode (0.22 cm2)) in the linear range of 0.05 to 4.1 mM with a high R2 of 0.9997). The lower limit of detection (LOD) was calculated to be 5.0 μM using a signal to noise ratio (S/N) of 3. Due to the excellent biocompatibility and electrocatalytic activity of Nf-GOx-fMWCNTs-PPy, the biosensor electrode holds high sensing performance compared to the previously-reported PPy based biosensors (Table 1). The apparent Michaelis-Menten constant () was calculated from the Lineweaver-Burk equation49:
where i, imax and C correspond to the steady-state catalytic current after adding the substrate, maximum current measured under saturation of the substrate-enzyme reaction, and concentration of glucose, respectively. From the Lineweaver-Burk plot (Fig. 8c), the lower value of (0.083 mM) was calculated, which is lower than mostly used ECPs based-biosensors50,51. The lower value of ensures a high affinity of the enzyme to the substrate, which is essential for enzymatic-based biosensors and confirm the homogenous integration of the enzyme on the nanohybrid film.
Figure 8.
(a) Amperometric responses of the Nf-GOx-fMWCNTs-PPy/Pt electrode to successive addition of different concentration of glucose at constant applied potential of +0.52 vs. SCE in 0.1 M PBS (pH 7.4), (b) calibration plot i.e. current response vs. glucose concentration, (c) the Lineweaver-Burk plot, and (d) anti-inference test of biosensor with successive addition of 1 mM glucose and 0.1 mM of each interfering species i.e. AA, UA, DA, L-cys, and cholesterol. Inset (a) and (d) show the overall calibrated curve and histogram of anti-interference test, respectively.
Table 1.
Comparison of our biosensor performance with other PPy-based biosensors.
| Working electrode | Sensitivity (µAcm−2 mM−1) | (mM) | LOD (μM) | Linear range (mM) | Ref. |
|---|---|---|---|---|---|
| GOD/PPY-HRP-FCA/CCE | 0.33 | 0.026 | 10 | 0.08–1.3 | 58 |
| Al2O3/Pt/PPy/GOx | 7.4 | 7.01 | 10 | 0.5–10 | 59 |
| GOx/Pt-PAMAM-PPy | 164 | — | 10 | 0.002–0.6 | 60 |
| PPY/GOx/SWCNTs-PhSO3 −1/PB | 6 | — | 10 | 0.02–6 | 61 |
| Pt/PPy/GOx | 0.007 | 37.6 | — | 0–10 | 62 |
| Nf-GOx-fMWCNTs-PPy/Pt | 54.2 | 0.083 | 5 | 0.05–4.1 | This work |
Anti-interference test, reproducibility and stability study
The selectivity of the fabricated Nf-GOx-fMWCNTs-PPy/Pt glucose biosensor electrode was tested in the presence of common interfering species such as ascorbic acid (AA), uric acid (UA), dopamine (DA), L-cysteine (L-cys), and cholesterol. The amperometric response of the biosensor was measured in 0.1 M PBS (pH 7.4) after addition of 1.0 mM glucose and 0.1 mM of each interfering species. As shown in Fig. 8d, addition of 1.0 mM glucose showed a rapid response. However, 0.1 mM addition of each interfering species showed a negligible response compared to that of glucose (inset, Fig. 8d). Moreover, we checked the effect of 0.01 mM of DA on CVs responses along with 0.05 mM glucose but no significant change occurred on the current response as compared to CV response from only glucose prepared in PBS (Supplementary Fig. S5). Thus, a good selectivity may enlist this glucose biosensor electrode to exhibit a high reliability in detecting glucose in blood serum samples.
The reproducibility and repeatability of the biosensor were examined by measuring the response current from six similarly fabricated biosensor electrodes. The relative standard deviation (RSD) obtained from an amperometric determination of 1.0 mM glucose was 3.2% at an applied potential of +0.52 V. Furthermore, stability of biosensor electrodes was investigated by the amperometric method after storing at 4 °C in 0.1 M PBS (pH 7.4) and measuring the current response every 3 days. The biosensor electrode showed almost no change for 15 days, and it retains ~96% of its original response over a storage period of 45 days (Supplementary Fig. S6).
Real sample analysis
The applicability of the proposed glucose biosensor (Nf-GOx-fMWCNTs-PPy/Pt) electrode was investigated. The glucose concentration was determined in human serum samples after diluting it in different concentrations in 0.1 M PBS (pH 7.4). Then, the recovery of glucose was measured using standard addition of a known concentration of pure glucose in the serum samples (Table 2). From Table, the obtained glucose concentrations were in good agreement with known concentrations of glucose (4.89 mM) in human serum (Sigma-Aldrich, H4522)52. Therefore, the proposed biosensor showed excellent reliability and accuracy for glucose detection in a real sample. Additionally, we checked long term stability of our sensor electrode in the serum (Supplementary Fig. S7). For long term stability measurement using amperometric method, we took 9.5 mL PBS in electrochemical cell and added 0.5 mL serum. The amperometric response of Nf-GOx-fMWCNTs-PPy/Pt electrode remains unchanged over a continuous 10 min period, indicating long term stability of our electrode for glucose detection in serum.
Table 2.
Glucose detection in real serum samples.
| Samples | Concentration (mM) | RSD (%) (n = 3) | Added glucose (mM) | Recovery (%) |
|---|---|---|---|---|
| 1 | 0.61 | 2.6 | 1.0 | 101 |
| 2 | 1.22 | 1.3 | 1.0 | 97 |
| 3 | 2.45 | 3.2 | 1.0 | 99 |
Conclusions
In summary, a bionanohybrid material with controllable morphology was successfully synthesized via in situ electrochemical polymerization on the Pt electrode to obtain Nf-GOx-fMWCNTs-PPy/Pt modified electrodes and used as a glucose biosensor electrode. Nf in the composite material was used to facilitate the uniform dispersion of fMWCNTs. Also, the oxidized PPy grown in the defect sites of the fMWCNTs ensures a large number of active sites of fMWCNTs and PPy, which provides sufficient space for GOx immobilization. The optimal thickness of Nf-GOx-fMWCNTs-PPy serves as a novel, highly efficient and durable bio-functional electrocalalytic active material for glucose oxidation. Moreover, the Nf prevents GOx leaching and improves the physicochemical stability and preserves the bioactivity under the long-term storage of the biosensor electrode. In addition, the bioengineered electrode exhibits a spatially-biocompatible environment and excellent electrocatalytic activity to enable the direct electron transfer from GOx to the electrode surface. The fabricated biosensor electrode showed excellent performance, including a high sensitivity (54.2 μAmM−1cm−2) in a linear range of up to 4.1 mM, LOD of ~5.0 μM, fast response time (within 4s), good selectivity, excellent stability, and reproducibility for glucose detection. On the basic of experimental results and analysis, our proposed biosensor showed good reliability for glucose detection in a real serum sample. Thus, suggesting a promising applicability for glucose monitoring in real samples, which would pave the way for impressive performance in a routine analysis.
Methods
Materials
Pyrrole above 99% purity was obtained from Daejung-Korea. MWCNTs (Ca. ~10 nm in external diameter) synthesized via chemical vapor deposition (CVD) were purchased from Nanosolutions Co. Ltd., Korea. Glucose oxidase (GOx, EC 1.1.3.4, Type X-S 127 unit/mg) lyophilized powder, from Aspergillus niger, human blood serum (H4522), Nafion (Nf, 5 wt. % in lower aliphatic alcohol), L-cysteine (L-cys), and cholesterol were purchased from Sigma-Aldrich, Korea. β-D-Glucose and ascorbic acid (AA) were purchased from Tokyo Chemical Industry Co., Ltd. Dopamine (DA) and uric acid (UA) were obtained from Bioshop Canada Inc. Disodium hydrogen phosphate (Na2HPO4), monobasic potassium phosphate (KH2PO4), sodium chloride (NaCl), potassium chloride (KCl), sulphuric acid (H2SO4), nitric acid (HNO3), and acetonitrile (CH3CN) were obtained from Samchun Pure Co. Ltd., Korea. Phosphate buffer solution (PBS, 0.1 M, pH 7.4) was prepared in ultra-pure water purified by Millipore-Q system (18 MΩ cm). All chemicals and reagents were of analytical grade and were used as received without further purification.
Fabrication of bio-nanohybrid composite based glucose biosensor
To fabricate the glucose biosensor electrodes, bare Pt electrodes having geometric area of 0.02 cm2 were consecutively polished with alumina slurries (0.3 µm and 0.05 µm), followed by diamond suspensions (0.25 µm) on a Rayon polishing pad. All polishing steps required extensive rinsing before treatment with sonication in ethanol for 15 min. The electrodes were washed and treated using cyclic voltammetry (CV) in an applied potential range of −0.2 to 1.0 V (vs. SCE) till constant CV curves were obtained in 0.5 M H2SO4 electrolytes, and the electrodes were dried under nitrogen (N2) atmosphere. Before making the bio-nanohybrid composite, pure MWCNTs were treated to generate more carbonyl and hydroxyl groups on the surface walls of CNTs. 0.5 g pristine MWCNTs were dispersed with a 3:1 wt % mixture of conc. H2SO4 (90 mL) and conc. HNO3 (30 mL) for 15 min via sonication. Then, the solution was transferred into a reflux condenser and was heated at 70 °C for 12 h to complete the surface functionalization53. After completing reaction, the mixture was allowed to cool down at room temperature, followed by filtration and continuous washing with double-distilled water to get fMWCNTs as a residue having a pH of 7.4.
In the next step, 0.05 M pyrrole in aqueous acetonitrile (1 M) solution containing 0.5 mg/mL GOx, 1.0 mg/mL fMWCNTs, and 50 μL of 0.5% Nf were electrochemically polymerized on the Pt electrode in a single-step using a three-electrode electrochemical system (CV technique), as shown in schematic (Fig. 1). The bio-nanohybrid (Nf-GOx-fMWCNTs-PPy) composite was obtained after 15 cycles (an optimized number of cycles) of CV scans in a fixed potential range from −0.15 to +0.8 V (vs. SCE) at a 25 mV/s scan rate, as shown in Supplementary Fig. S8a. The thickness of the polymeric composite is proportional to the number scan cycles. The increase in scan number may cause longer inter-facial diffusion distance between biomarkers and transducer. As a result transfer of electrons produce from the electrocatalytic reaction through electrode was hindered and the redox current response decreased. In contrast, the lower number of scan cycles were not able to entrap/attach sufficient polymeric nanomaterial/enzyme. Also, the enzyme may easily percolate or leach through the porous film during electrochemical measurements and may cause loss in catalytic behaviour of biosensor. Thus, we have optimized the scan number to obtain the maximum oxidizing current using CV technique in the presence of 0.05 mM glucose (Supplementary Fig. S8b). The facile polymerization encapsulates GOx within the PPy decorated Nf-fMWCNTs film during the anodic oxidation of pyrrole54–57. The fabricated Nf-GOx-fMWCNTs-PPy/Pt electrodes were rinsed with distilled water to remove loosely attached GOx, PPy, pyrrole and other materials. The biosensor electrodes were stored at 4 °C in 0.1 M PBS (pH 7.4) for further utilization. Other electrodes modified with different composite materials were also prepared using the same process.
Physicochemical characterizations
The morphological properties of the as-prepared functional materials were characterized using field-emission scanning electron microscopy (FE-SEM, Carl Zeiss SUPRA 40VP, Germany) and transmission electron microscopy (HR-TEM, JEOL 2010, Japan). X-ray diffraction (XRD) patterns were obtained using an X-ray diffractometer (Rigaku, Japan) with high-intensity monochromatic Cu-Kα radiation as an incident beam (λ = 1.54 Å) over a Bragg’s angle range from 10° to 90°. The formation of nanohybrid composites and a bonding configuration for each of the composite samples was recorded using Fourier transform infrared spectrometry (FT-IR Perkin Elmer, Spectrum GX, USA). The UV-vis absorption spectra were measured using a UV/Vis//NIR spectrophotometer (Jasco V-670, Japan). Each electrochemical analysis was performed on an electrochemical workstation ZIVE SP1 Potentiostat/Galvanostat/EIS from WonATech Co. Ltd. Seoul, Korea. The conventional three-electrode configuration was adjusted using platinum (Pt) as a working bio-electrode modified with different nanohybrid materials, the Pt wire was used as a counter electrode, and saturated calomel electrode (SCE) was used as reference electrode. The potentiostatic electrochemical impedance spectroscopy (EIS) was recorded from each bionanohybrid composite based biosensor electrode using 5.0 mM K3Fe[CN]6 as redox probe containing 0.1 M KCl prepared in 0.1 M PBS (pH 7.4) at an amplitude of 10 mV and zero bias potential in a frequency range from 1 MHz to 1 Hz. All analytical solutions were purged with high-purity N2 for 30 min prior to each measurement.
Electronic supplementary material
Acknowledgements
This work was supported by grant from the Basic Science Research Program through National Research Foundation of Korea (NRF) by Ministry of Education, Science and Technology (Project no NRF-2015R1C1A1A02036404). Partially supported by the Human Resource Training Program for Regional Innovation and Creativity through the Ministry of Education and National Research Foundation of Korea (NRF-2015H1C1A1035635), 2016 R & D Special Zone Development Project Technology Transfer Project. Also, this research work was supported by the Materials and Components Technology Development Program of MOTIE/KEIT, Republic of Korea [10076464], Development of lightweight and high heat dissipating bio-inspired composites for printed circuit board with thermal conductivity of 20 W/mK.
Author Contributions
B.K.S. designed and performed the experiments and measurements. R.A. and S.S. helped in measurements, data acquisition, analysis and analysis tools. B.K.S., C.H.P. and C.S.K. co-wrote the paper. C.H.P. and C.S.K. were responsible for project planning and funding.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16541-9.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Contributor Information
Chan Hee Park, Email: biochan@jbnu.ac.kr.
Cheol Sang Kim, Email: chskim@jbnu.ac.kr.
References
- 1.Shi Y, Peng L, Ding Y, Zhao Y, Yu G. Nanostructured Conductive Polymers for Advanced Energy Storage. Chem. Soc. Rev. 2015;44:6684–6696. doi: 10.1039/C5CS00362H. [DOI] [PubMed] [Google Scholar]
- 2.Balint R, Cassidy NJ, Cartmell SH. Conductive Polymers: Towards a Smart Biomaterial for Tissue Engineering. Acta Biomaterialia. 2014;10:2341–2353. doi: 10.1016/j.actbio.2014.02.015. [DOI] [PubMed] [Google Scholar]
- 3.Gerard M, Chaubey A, Malhotra BD. Application of Conducting Polymers to Biosensors. Biosens. Bioelectron. 2002;17:345–359. doi: 10.1016/S0956-5663(01)00312-8. [DOI] [PubMed] [Google Scholar]
- 4.Aydemir N, Malmstrom J, Travas-Sejdic J. Conducting Polymer Based Electrochemical Biosensors. Phys. Chem.Chem. Phyc. 2016;18:8264–8277. doi: 10.1039/C5CP06830D. [DOI] [PubMed] [Google Scholar]
- 5.Singh M, Kathuroju PK, Jampana N. Polypyrrole Based Amperometric Glucose Biosensors. Sens. Actuators B. 2009;143:430–443. doi: 10.1016/j.snb.2009.09.005. [DOI] [Google Scholar]
- 6.Shrestha BK, et al. High-performance Glucose Biosensor Based on Chitosan-Glucose Oxidase Immobilized Polypyrrole/Nafion/Functionalized Multi-Walled Carbon Nanotubes Bio-Nanohybrid Film. J. Colloid Interface Sci. 2016;482:39–47. doi: 10.1016/j.jcis.2016.07.067. [DOI] [PubMed] [Google Scholar]
- 7.Inzucchi SE. Diagnosis of diabetes. N. Engl. J. Med. 2012;367:542–550. doi: 10.1056/NEJMcp1103643. [DOI] [PubMed] [Google Scholar]
- 8.Li S-J, et al. Electrodeposition of Cobalt Oxide Nanoparticles on Reduced Graphene Oxide: a Two-dimensional Hybrid for Enzyme-free Glucose Sensing. J. Solid State Electrochem. 2014;18:1049–1056. doi: 10.1007/s10008-013-2354-2. [DOI] [Google Scholar]
- 9.Wang J. Electrochemical Glucose Biosensors. Chem. Rev. 2008;108:814–825. doi: 10.1021/cr068123a. [DOI] [PubMed] [Google Scholar]
- 10.Ahuja T, Mir IA, Kumar D. & Rajesh. Biomolecular Immobilization on Conducting Polymers for Biosensing Applications. Biomaterials. 2007;28:791–805. doi: 10.1016/j.biomaterials.2006.09.046. [DOI] [PubMed] [Google Scholar]
- 11.Şenel M. Simple Method for Preparing Glucose Biosensor Based on In-situ Polypyrrole Cross-linked Chitosan/Glucose Oxidase/Gold Bionanocomposite Film. Mater. Sci. Eng. C. 2015;48:287–293. doi: 10.1016/j.msec.2014.12.020. [DOI] [PubMed] [Google Scholar]
- 12.Li, et al. Fluorescence Turn-on Detection of Glucose via the Ag Nanoparticle Mediated Release of a Perylene Probe. Chem. Commun. 2015;51:6354–6356. doi: 10.1039/C4CC10381E. [DOI] [PubMed] [Google Scholar]
- 13.Lee H, Yoon SW, Kim EJ, Park J. In-situ Growth of Copper Sulfide Nanocrystals on Multiwalled Carbon Nanotubes and Their Application as Novel Solar Cell and Amperometric Glucose Sensor Materials. Nano Lett. 2007;7:778–784. doi: 10.1021/nl0630539. [DOI] [PubMed] [Google Scholar]
- 14.Huang W, et al. 3D NiO Hollow Sphere/ Reduced Graphene Oxide Composite for High Performance Glucose Biosensor. Sci. Rep. 2017;7:5220. doi: 10.1038/s41598-017-05528-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen Y-S, Huang J-H, Chuang C-C. Glucose Biosensor Based on Multiwalled. Carbon Nanotubes Grown Directly on Si. Carbon. 2009;47:3106–3112. [Google Scholar]
- 16.Gupta P, Yadav SK, Agrawal B, Goyal RN. A Novel Graphene and Conductive Polymer Modified Pyrolytic Graphite Sensor for Determination of Propranolol in Biological Fluids. Sens. Actuators B. 2014;204:791–798. doi: 10.1016/j.snb.2014.08.040. [DOI] [Google Scholar]
- 17.Suneesh PV, Chandhini K, Ramachandran T, Nair BG, Satheesh Babu TG. Tantalum Oxide Honeycomb Architectures for the Development of a Non-enzymatic Glucose Sensor with Wide Detection Range. Biosens. Bioelectron. 2013;50:472–477. doi: 10.1016/j.bios.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 18.Gholivand MA, Simultaneous MB. Detection of Dopamine and Acetaminophen by Modified Gold Electrode with Polypyrrole/Aszophloxine. J. Electroanal Chem. 2012;676:53–59. doi: 10.1016/j.jelechem.2012.05.001. [DOI] [Google Scholar]
- 19.Teles FRR, Fonseca LP. Applications of Polymers for Biomolecule Immobilization in Electrochemical Biosensors. Mater. Sci. Eng. C. 2008;28:1530–1543. doi: 10.1016/j.msec.2008.04.010. [DOI] [Google Scholar]
- 20.Rong Q, Han H, Feng F, Ma Z. Network Nanostructured Polypyrrole Hydrogel/Au Composites as Enhanced Electrochemical Biosensing Platform. Sci. Rep. 2015;5:11440. doi: 10.1038/srep11440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wang J, Musameh M. Carbon-nanotubes Doped Polypyrrole Glucose Biosensor. Anal. chim. Acta. 2005;539:209–213. doi: 10.1016/j.aca.2005.02.059. [DOI] [Google Scholar]
- 22.Hui N, Wang J. Electrodeposited Honeycomb-like Cobalt Nanostructures on Graphene Oxide Doped Polypyrrole Nanocomposite for High Performance Enzymeless Glucose Sensing. J. Electroanal. Chem. 2017;798:10–16. doi: 10.1016/j.jelechem.2017.05.021. [DOI] [Google Scholar]
- 23.Wang J. Carbon-nanotube Based Electrochemical Biosensors: A Review. Electroanalysis. 2005;17:7–14. doi: 10.1002/elan.200403113. [DOI] [Google Scholar]
- 24.Haddad R, Cosnier S, Maaref A, Holzinger M. Non-covalent Biofunctionalization of Single-walled Carbon Nanotubesviabiotin Attachment by π-stacking Interactions and Pyrrole Polymerization. Analyst. 2009;134:2412–2418. doi: 10.1039/b916774a. [DOI] [PubMed] [Google Scholar]
- 25.Xu L, Zhu Y, Yang X, Li C. Amperometric Biosensor Based on Carbon Nanotubes Coated with Polyaniline/Dendrimer-encapsulated Pt Nanoparticles for Glucose Detection. Mater. Sci. Eng. C. 2009;29:1306–1310. doi: 10.1016/j.msec.2008.10.031. [DOI] [Google Scholar]
- 26.Barsan MM, Ghica ME, Brett CMA. Electrochemical Sensors and Biosensors Based on Redox Polymer/Carbon Nanotube Modified Electrodes: A Review. Anal. Chim. Acta. 2015;881:1–23. doi: 10.1016/j.aca.2015.02.059. [DOI] [PubMed] [Google Scholar]
- 27.Choudhury A, Kar P. Doping Effect of Carboxylic Acid Group Functionalized Multi-Walled Carbon Nanotube on Polyaniline. Composites Part B: Eng. 2011;42:1641–1647. doi: 10.1016/j.compositesb.2011.04.005. [DOI] [Google Scholar]
- 28.Wang J, Musameh M, Lin Y. Solubilization of Carbon Nanotubes by Nafion toward the Preparation of Amperometric Biosensors. J. Am. Chem. Soc. 2003;125:2408–2409. doi: 10.1021/ja028951v. [DOI] [PubMed] [Google Scholar]
- 29.Zhang J, et al. Dispersion of Single-Walled Carbon Nanotubes by Nafion in Water/Ethanol for Preparing Transparent Conducting Films. J. Phys. Chem. C. 2008;112:16370–16376. doi: 10.1021/jp8053839. [DOI] [Google Scholar]
- 30.Gao C, Gao Z, Liu J-H, Huang X-J. The New Age of Carbon Nanotubes; An Updated Review of Functionalized Carbon Nanotubes in Electrochemical Sensor. Nanoscale. 2012;4:1948–19603. doi: 10.1039/c2nr11757f. [DOI] [PubMed] [Google Scholar]
- 31.Pham X-H, et al. Electrochemical Characterization of a Single-Walled Carbon Nanotube Electrode for Detection of Glucose. Anal. Chimi. Acta. 2010;671:36–40. doi: 10.1016/j.aca.2010.05.010. [DOI] [PubMed] [Google Scholar]
- 32.Tsampas MN, Pikos A, Brosda S, Katsaounis A, Vayenas CG. The Effect of Membrane Thickness on the Conductivity of Nafion. Electrochim. Acta. 2006;51:2743–2755. doi: 10.1016/j.electacta.2005.08.021. [DOI] [Google Scholar]
- 33.Huang Y, et al. Extremely Stable Polypyrrole Achieved via Molecular Ordering for Highly Flexible Supercapacitors. ACS Appl. Mater. Interfaces. 2016;8:2435–2440. doi: 10.1021/acsami.5b11815. [DOI] [PubMed] [Google Scholar]
- 34.Li X-G, Li A, Huang M-R, Liao Y, Lu Y-G. Efficient and Scalable Synthesis of Pure Polypyrrole Nanoparticles Applicable for Advanced Nanocomposites and Carbon Nanoparticles. J. Phys. Chem. C. 2010;114:19244–19255. doi: 10.1021/jp107435b. [DOI] [Google Scholar]
- 35.Wepasnick, et al. Surface and Structural Characterization of Multi-walled. Carbon Nanotubes Following Different Oxidative Treatments. Carbon. 2011;49:24–36. [Google Scholar]
- 36.Inoue F, Ando RA, Izumi CMS, Corio P. Spectroscopic Characterization of Carbon Nanotube-Polypyrrole Composites. J. Phys. Chem. C. 2014;118:18240–18248. doi: 10.1021/jp505525k. [DOI] [Google Scholar]
- 37.Woo JY, Kim D, Kim J, Park J, Han C-S. Fast and Efficient Purification for Highly Conductive Transparent Carbon Nanotube Films. J. Phys. Chem. C. 2010;114:19169–19174. doi: 10.1021/jp107691q. [DOI] [Google Scholar]
- 38.Chang C-M, liu Y-L. Electrical Conductivity Enhancement of Polymer/Multiwalled Carbon Nanotube (MWCNT) Composite by Thermally-Induced Difunctionalization of MWCNTs. ACS Appl. Mater. Interfaces. 2011;7:2204–2208. doi: 10.1021/am200558f. [DOI] [PubMed] [Google Scholar]
- 39.Biswas S, Drzal LT. Multilayered Nanoarchitecture of Graphene Nanosheets and Polypyrrole Nanowires for High Performance Supercapacitor Electrodes. Chem. Mater. 2010;22:5667–5671. doi: 10.1021/cm101132g. [DOI] [Google Scholar]
- 40.Ijeri VK, et al. Nafion and Carbon Nanotube Nanocomposites for Mixed Proton and Electron Conduction. J. Membr. Sci. 2010;363:265–270. doi: 10.1016/j.memsci.2010.07.037. [DOI] [Google Scholar]
- 41.Kanga X, et al. Glucose Oxidase-Graphene-Chitosan Modified Electrode for Direct Electrochemistry and Glucose Sensing. Biosens. Bioelectron. 2009;25:901–905. doi: 10.1016/j.bios.2009.09.004. [DOI] [PubMed] [Google Scholar]
- 42.Chen Y, et al. Fabrication of Gold Nanoparticles on Bilayer Graphene for Glucose Electrochemical Biosensing. J. Mater. Chem. 2011;21:7604–7611. doi: 10.1039/c1jm10293a. [DOI] [Google Scholar]
- 43.Terse-Thakoor T, Komori K, Ramnani P, Lee I, Mulchandani A. Electrochemically Functionalized Seamless Three-Dimensional Graphene-Carbon Nanotube Hybrid for Direct Electron Transfer of Glucose Oxidase and Bioelectrocatalysis. Langmuir. 2015;31:13054–13061. doi: 10.1021/acs.langmuir.5b03273. [DOI] [PubMed] [Google Scholar]
- 44.Yu Y, et al. Direct Electron Transfer of Glucose Oxidase and Biosensing for Glucose Based on PDDA-Capped Gold Nanoparticle Modified Graphene/Multi-Walled Carbon Nanotubes Electrode. Biosens. Bioelectron. 2014;52:147–152. doi: 10.1016/j.bios.2013.08.043. [DOI] [PubMed] [Google Scholar]
- 45.Zhao Y, et al. Zno-Nanorods/Graphene Heterostructure: A Direct Electron Transfer Glucose Biosensor. Sci. Rep. 2016;6:32327. doi: 10.1038/srep32327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Luo Z, et al. Reduced Graphene oxide/PAMAM–Silver Nanoparticles Nanocomposite Modified Electrode for Direct Electrochemistry of Glucose Oxidase and Glucose Sensing. Biosens. Bioelectron. 2012;36:179–185. doi: 10.1016/j.bios.2012.04.009. [DOI] [PubMed] [Google Scholar]
- 47.Zhang X, et al. Direct Electrochemistry of Glucose Oxidase on Novel Free-Standing Nitrogen-Doped Carbon Nanospheres@Carbon Nanofibers Composite Film. Sci. Rep. 2015;5:9885. doi: 10.1038/srep09885. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Jose MV, Marx S, Murata H, Koepsel RR, Russell AJ. Direct Electron Transfer in a Mediator-Free Glucose Oxidase Based. Carbon Nanotube-Coated Biosensor. Carbon. 2012;50:4010–4020. [Google Scholar]
- 49.Ahmad R, Tripathy N, Kim JH, Hahn Y-B. Highly Selective Wide Linear-Range Detecting Glucose Biosensors Based on Aspect-Ratio Controlled ZnO Nanorods Directly Grown on Electrodes. Sens. Actuators B. 2012;174:195–201. doi: 10.1016/j.snb.2012.08.011. [DOI] [Google Scholar]
- 50.Arslan F, Ustabaş S, Arslan H. An Amperometric Biosensor for Glucose Determination Prepared from Glucose Oxidase Immobilized in Polyaniline-Polyvinylsulfonate Film. Sensors. 2011;11:8152–8163. doi: 10.3390/s110808152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Vidal J-C, Garcia E, Castillo J-R. Electropolymerization of Pyrrole and Immobilization of Glucose Oxidase in a Flow System: Influence of the Operating Conditions on Analytical Performance. Biosens. Bioelectron. 1998;13:371–381. doi: 10.1016/S0956-5663(97)00116-4. [DOI] [PubMed] [Google Scholar]
- 52.Ahmad R, Vaseem M, Tripathy N, Hahn Y-B. Wide Linear-Range Detecting Nonenzymatic Glucose Biosensor Based on CuO Nanoparticles Inkjet-Printed on Electrodes. Anal. Chem. 2013;85:10448–10454. doi: 10.1021/ac402925r. [DOI] [PubMed] [Google Scholar]
- 53.Eitan A, Jiang K, Dukes D, Andrews R, Schadler LS. Surface Modification of Multiwalled Carbon Nanotubes: Toward the Tailoring of the Interface in Polymer Composites. Chem. Mater. 2003;15:3198–3201. doi: 10.1021/cm020975d. [DOI] [Google Scholar]
- 54.Sadki S, Schottland P, Brodie N, Sabouraud G. The Mechanisms of Pyrrole Electropolymerization. Chem. Soc. Rev. 2000;29:283–293. doi: 10.1039/a807124a. [DOI] [Google Scholar]
- 55.Boccaccini AR, et al. Electrophoretic Deposition of Carbon Nanotubes. Carbon. 2006;44:3149–3160. doi: 10.1016/j.carbon.2006.06.021. [DOI] [Google Scholar]
- 56.George PM, et al. Fabrication and Biocompatibility of Polypyrrole Implants Suitable for Neural Prosthetics. Biomaterials. 2005;26:3511–3519. doi: 10.1016/j.biomaterials.2004.09.037. [DOI] [PubMed] [Google Scholar]
- 57.Zhang J, Kong L-B, Wang B, Luo YC, Kang L. In-situ Electrochemical Polymerization of Multi-Walled Carbon Nanotube/Polyaniline Composite Films for Electrochemical Supercapacitors. Synth. Mat. 2009;159:260–266. doi: 10.1016/j.synthmet.2008.09.018. [DOI] [Google Scholar]
- 58.Tian F, Zhu G. Bienzymatic Amperometric Biosensor for Glucose Based on Polypyrrole/Ceramic Carbon as Electrode Material. Anal. Chim. Acta. 2002;451:251–258. doi: 10.1016/S0003-2670(01)01405-2. [DOI] [Google Scholar]
- 59.Ekanayake MEMI, Preethichandra DMG, Kaneto K. Polypyrrole Nanotube Array Sensor for Enhanced Adsorption of Glucose Oxidase in Glucose Biosensors. Biosens. Bioelectron. 2007;23:107–113. doi: 10.1016/j.bios.2007.03.022. [DOI] [PubMed] [Google Scholar]
- 60.Tang L, Zhu Y, Xu L, Yang X, Li C. Properties of Dendrimer-Encapsulated Pt Nanoparticles Doped Polypyrrole Composite Films and Their Electrocatalytic Activity for Glucose Oxidation. Electroanalysis. 2007;19:1677–1682. doi: 10.1002/elan.200703904. [DOI] [Google Scholar]
- 61.Raicopol M, Prună A, Damian C, Pilan L. Functionalized Single-Walled Carbon Nanotubes/Polypyrrole Composites for Amperometric Glucose Biosensors. Nanoscale research lett. 2013;8:316–323. doi: 10.1186/1556-276X-8-316. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Uang Y-M, Chou T-C. Fabrication of Glucose Oxidase/Polypyrrole Biosensor by Galvanostatic Method in Various pH Aqueous Solutions. Biosens. Bioelectron. 2003;19:141–147. doi: 10.1016/S0956-5663(03)00168-4. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.