Abstract
Mitochondrial function has been suggested to underlie constraints on whole-organism aerobic performance and associated hypoxia and thermal tolerance limits, but most studies have focused on measures of maximum mitochondrial capacity. Here we investigated whether variation in mitochondrial oxygen kinetics could contribute to local adaptation and plasticity in response to temperature using two subspecies of the Atlantic killifish (Fundulus heteroclitus) acclimated to a range of temperatures (5, 15, and 33 °C). The southern subspecies of F. heteroclitus, which has superior thermal and hypoxia tolerances compared to the northern subspecies, exhibited lower mitochondrial O2 P50 (higher O2 affinity). Acclimation to thermal extremes (5 or 33 °C) altered mitochondrial O2 P50 in both subspecies consistent with the effects of thermal acclimation on whole-organism thermal tolerance limits. We also examined differences between subspecies and thermal acclimation effects on whole-blood Hb O2-P50 to assess whether variation in oxygen delivery is involved in these responses. In contrast to the clear differences between subspecies in mitochondrial O2-P50 there were no differences in whole-blood Hb-O2 P50 between subspecies. Taken together these findings support a general role for mitochondrial oxygen kinetics in differentiating whole-organism aerobic performance and thus in influencing species responses to environmental change.
Introduction
Both ambient temperature and O2 availability vary widely across the biosphere and this has profound implications for the geographic distributions of aquatic organisms1,2. The physiological constraints imposed by hypoxia and temperature are thought to be a consequence of their effects on aerobic metabolism and, by extension, mitochondrial function2–6. Attempts to link whole organism thermal and hypoxia performance and mitochondrial function have typically examined maximum mitochondrial capacity. Studies of this nature have had mixed success in identifying mitochondrial processes that putatively constrain whole-organism performance or that are altered following environmental stress7–17. In addition, in vitro studies of maximum mitochondrial capacity can be difficult to link back to in vivo function because mitochondria are unlikely to operate at maximum flux in vivo under most circumstances10.
One factor that can constrain mitochondrial flux is low O2 supply. The effects of O2 supply on mitochondrial function can be described using mitochondrial O2 P50 (Mito-P50), the O2 partial pressure (PO2) at which mitochondrial O2 consumption rate is 50% of maximum flux18. Because Mito-P50 is measured during the aerobic to anoxic transition, it provides information on mitochondrial function over a PO2 range that is relevant to in vivo performance. In theory, a low Mito-P50 results in a greater capacity to extract O2 from the cytosol. Consequently, there has been interest in this parameter as a predictor of whole-organism performance. Indeed, intraspecific variation in Mito-P50 is a predictor of basal metabolic rate in humans19. Moreover, Lau et al.19 provide evidence for putative adaptation of Mito-P50 as a mechanism underlying variation in hypoxia tolerance among intertidal sculpin species (family: Cottidae). Links between Mito-P50 and hypoxia tolerance are not universal, however, as Du et al.7 did not observe altered Mito-P50 following hypoxic acclimation in Fundulus heteroclitus. By comparison, nothing is known about variation in Mito-P50 in the context of putative local thermal adaptation or thermal acclimation. It has been suggested that there may be a link between whole-organism thermal performance and systemic hypoxemia, although there is some debate about the role of systemic O2 limitation as a general mechanism underlying thermal tolerance limits in fishes20,21. However, given the relationship between ambient temperature and aerobic metabolism, prolonged thermal stress likely alters mitochondrial function and thus an investigation of temperature effects on Mito-P50 is necessary3,4,6.
To address this question, here we utilize F. heteroclitus, a eurythermal teleost found in estuarine salt marshes along a large latitudinal range that spans a steep thermal gradient [(Northern Florida, USA (mean monthly southern temperature range Sapelo Island, GA, USA: 11–30 °C22) to Nova Scotia, Canada (mean monthly northern temperature range 3–11 °C Wells Inlet, ME, USA22)]. This species is highly tolerant of both thermal and hypoxic stress, experiencing considerable variation in these abiotic factors over diel as well as seasonal cycles23–25. Furthermore, northern and southern F. heteroclitus subspecies exhibit variation in thermal and hypoxia tolerance that is consistent with apparent adaptation to their local environments24,25. This species recruits a wide array of physiological responses to thermal stress, including altered mitochondrial function12,15,25–27. Thus, this species is an ideal model in which to investigate the potential roles of mitochondrial kinetic properties in thermal acclimation and adaptation.
The objectives of this study were three-fold, (1) determine if intraspecific variation in Mito-P50 exists between putatively thermally adapted northern (Fundulus heteroclitus heteroclitus) and southern (Fundulus heteroclitus macrolepidotus) Atlantic killifish subspecies, (2) investigate the effects of thermal acclimation (5, 15, and 33 °C) on Mito-P50 and, (3) characterize the acute thermal response of Mito-P50 between F. heteroclitus subspecies acclimated to different temperatures. We also assessed intraspecific variation and thermal acclimation effects on hemoglobin (Hb) O2-P50, as there is some evidence of intraspecific variation in this parameter in F. heteroclitus 28 and any variation in this parameter could result in changes in PO2 gradients between the circulatory system and the mitochondrion.
Results
Whole-organism hypoxia tolerance (LOEhyp)
We measured time to loss of equilibrium in hypoxia (LOEhyp) at 15 °C in fish acclimated to 15 °C to confirm subspecies differences in whole organism hypoxia tolerance25. The northern and southern subspecies of killifish acclimated to 15 °C differed in LOEhyp at Tassay = 15 °C across a range of PO2 (Fig. 1). Southern killifish exhibited greater hypoxia tolerance, as indicated by time to LOEhyp, when compared to northern killifish (psubspecies < 0.001). PO2 also affected LOEhyp, which decreased with decreasing PO2 (ppartial pressure < 0.001), as did the difference between the subspecies (ppartial pressure*subspecies < 0.05).
Figure 1.
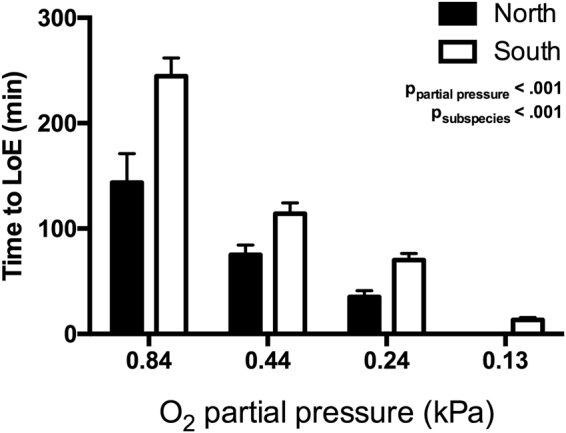
Intraspecific differences in whole-organism hypoxia tolerance. Northern and southern F. heteroclitus were acclimated to and assayed at 15 °C. Hypoxia tolerance was estimated using a time to loss of equilibrium assay across a range of low O2 partial pressures. Data are mean ± SEM, n = 9–10.
Hb and Mito-P50
In this study, we examined mitochondrial O2 binding affinity using isolated mitochondria from the liver because high-quality mitochondria can be isolated from this tissue in this species15,27. These assays were conducted using a mixture of substrates at saturating levels to be representative of working or stressed states. For Mito-P50, there were significant effects of subspecies (psubspecies < 0.05), assay temperature (passay < 0.0001), thermal acclimation (pacclimation < 0.0005), and the interaction between thermal acclimation and assay temperature (pacclimation*assay < 0.0001) on Mito-P50. No additional significant interaction effects were detected (psubspecies*acclimation = 0.181, psubspecies*assay = 0.144, ppopulation*acclimation*assay = 0.273) (Supplementary Fig. S1).
In contrast, there was no significant effect of subspecies (psubspecies = 0.230) on Hb-P50, but we detected significant effects of thermal acclimation (pacclimation < 0.05), assay temperature (passay < 0.0001), the interaction between subspecies and assay temperature (psubspecies*assay < 0.001), and the interaction between subspecies, acclimation and assay temperature (psubspecies*acclimation*assay < 0.05) but no other significant interaction effects (psubspecies*acclimation = 0.146, pacclimation*assay = 0.071). (Supplementary Fig. S2). Hill coefficients derived from Hb-O2 equilibrium curves did not differ between subspecies (Supplementary Fig. S3; psubspecies = 0.196) or with acclimation (pacclimation < 0.181), but were significantly affected by assay temperature (passay < 0.05), and there were no significant interactions (psubspecies*acclimation = 0.070, psubspecies*assay = 0.634, pacclimation*assay = 0.637, psubspecies*acclimation*assay = 0.714). Hematocrit did not differ following thermal acclimation (Supplementary Fig. S4; pacclimation = 0.080), or between subspecies (psubspecies = 0.992), and there were no significant interactions (pacclimation*subspecies = 0.350).
Because of the complex interactive effects of subspecies, acclimation temperature, and assay temperature on both Mito-P50 and Hb-P50, we tested a series of specific hypotheses regarding the effects of individual factors on both of these parameters.
Subspecies effects on mitochondrial and Hb-O2 affinity
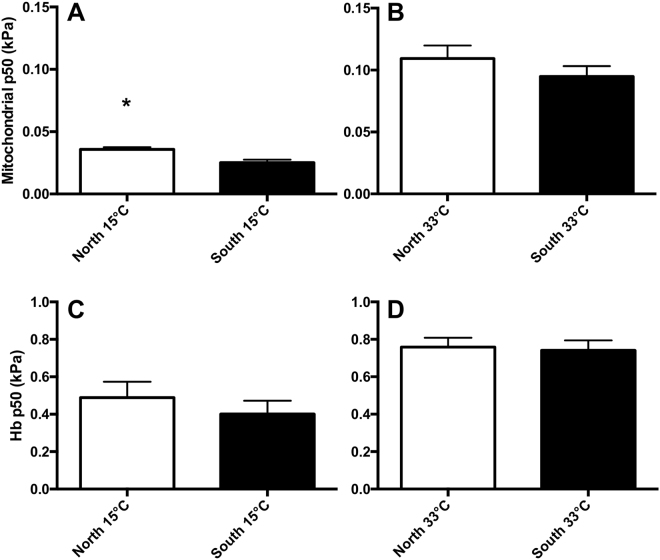
We compared mitochondrial and Hb-P50 between subspecies when assayed at their acclimation temperature to test our prediction that southern killifish maintain lower O2 P50 when compared to northern killifish (Fig. 2). Mito-P50 was lower in southern killifish compared to northern killifish when acclimated to 15 °C and assayed at 15 °C (Fig. 2A; psubspecies < 0.005), but not in 33 °C acclimated killifish assayed at 33 °C (Fig. 2B; psubspecies = 0.301). Hb-O2 binding affinity did not differ between subspecies at either acclimation temperature (Fig. 2C,D; 15 °C: psubspecies = 0.446, 33 °C: psubspecies = 0.817).
Figure 2.
Intraspecific differences in mitochondrial. (A,B; n = 7–8) and hemoglobin (C,D; n = 7–20) O2 binding affinity. Northern and southern Fundulus heteroclitus were acclimated to 15 (A,C) or 33 °C (B,D). The assay temperature for subspecies comparisons was the same as acclimation temperature (e.g., 33 °C acclimated fish were compared at Tassay = 33 °C). Asterisks indicate a significant subspecies effect within an acclimation treatment (T-test, p < 0.05). Data are mean ± SEM.
Thermal acclimation effects on mitochondrial and Hb-O2 affinity
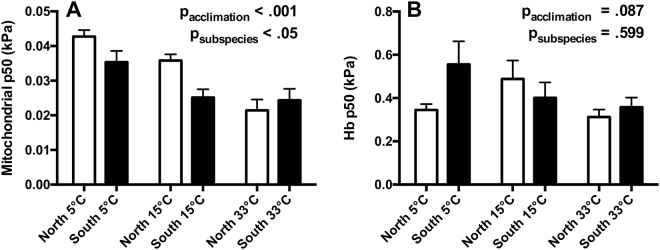
We compared thermal acclimation effects (5, 15, and 33 °C) on mitochondrial (Fig. 3A) and Hb-P50 (Fig. 3B) between subspecies at a common assay temperature of 15 °C to test our prediction that acclimation to higher temperatures would result in lower O2 P50 for both parameters. We used 5 and 33 °C as our experimental acclimation temperatures as these are temperatures at which effects on whole-organism aerobic metabolism are first observed29. In addition, we acclimated F. heteroclitus to 33 °C to avoid the induction of substantial breeding physiology at slightly lower temperatures (i.e., 25 to 30 °C). These acclimation treatments represent the majority of temperatures experienced by natural F. heteroclitus populations22.
Figure 3.
Thermal acclimation effects on mitochondrial. (A, n = 7–8) and hemoglobin (B, n = 7–20) O2 binding affinity. Northern and southern Fundulus heteroclitus were acclimated to 5, 15 or 33 °C (Tassay = 15 °C). Data are mean ± SEM.
We detected a significant effect of acclimation on Mito-P50 (Fig. 3A; pacclimation < 0.001). This effect was driven by higher Mito-P50 in 5 °C acclimated killifish and lower Mito-P50 in 33 °C acclimated northern killifish. We also detected a significant effect of subspecies that was driven by greater Mito-P50 in northern killifish (psubspecies < 0.05). Significant interactions between subspecies and thermal acclimation effects were a result of subspecies differences being removed as Tacclimation increased to 33 °C (psubspecies*acclimation < 0.05).
We did not detect a significant effect of acclimation on Hb-P50 (Fig. 3B; pacclimation = 0.087) or an effect of subspecies (psubspecies = 0.599) or an interaction between subspecies and acclimation temperature (psubspecies*acclimation = 0.140).
Acute thermal effects on mitochondrial and Hb-O2 affinity
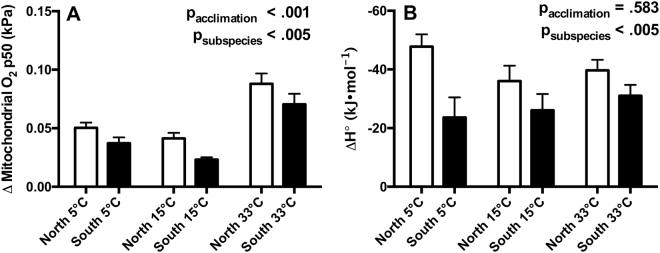
We assessed the effects of thermal acclimation and local adaptation on Δ Mito-P50 (i.e., the difference in Mito-P50 between Tassay = 15 and 33 °C) and the heat of oxygenation of Hb, ΔH (Fig. 4). We predicted that thermal acclimation and putative local adaptation would alter the acute thermal response for both parameters. Acute changes in temperature alter buffer pH, and this has the potential to affect both Mito-P50 and Hb-P50. But this variation in pH does not affect the interpretation of our specific predictions as most comparisons were made at the same assay temperature. The only exception is our assessment of acute thermal effects on O2 binding affinity (Fig. 4), which might be subject to an interaction between intraspecific variation or thermal acclimation effects and pH variation induced by the acute temperature shift between 15 and 33 °C.
Figure 4.
Acute thermal sensitivity (Tassay = 15 to 33 °C) of mitochondrial (A) Δ mitochondrial O2 P50; n = 7–8) and hemoglobin (B) ΔH°; n = 7–20) O2 P50. Northern and southern Fundulus heteroclitus were acclimated to 5, 15, or 33 °C. A more negative ΔH° is indicative of favorable Hb-O2 binding at lower temperatures. Data are mean ± SEM.
Acclimation temperature affected Δ Mito-P50 (Fig. 4A; pacclimation < 0.001). Northern F. heteroclitus exhibited a greater Δ Mito-P50 when compared to the southern subspecies (psubspecies < 0.005). No significant interaction effects were detected (pacclimation*subspecies = 0.907).
Thermal acclimation did not significantly change ΔH (Fig. 4B; pacclimation = 0.583). Northern F. heteroclitus maintained more negative ΔH when compared to southern F. heteroclitus (psubspecies < 0.005). No significant interaction effects were detected (pacclimation*subspecies = 0.371).
Discussion
Here we demonstrate that Mito-P50 differs between putatively thermally adapted subspecies of killifish and is sensitive to thermal acclimation. These effects on Mito-P50 are consistent with intraspecific variation and the effects of thermal acclimation on whole-organism thermal and hypoxia tolerance25. These data provide evidence for altered mitochondrial oxygen affinity as a potential mechanism for maintaining whole-organism performance under environmental stress, which may ultimately contribute to subspecies-specific differences in thermal tolerance.
There were clear intraspecific differences in Mito-P50 between northern and southern F. heteroclitus subspecies, with southern fish maintaining lower O2-P50 (Fig. 2A, S1). We propose that variation in Mito-P50 partially underlies intraspecific variation in hypoxia tolerance (Fig. 1 25). Intraspecific variation in Mito-P50 may be a consequence of variation in genes encoding cytochrome c oxidase (CCO) subunits. CCO is the terminal acceptor of the electron transport chain and the primary site of O2 consumption in the mitochondrion. In addition, CCO subunits are encoded by both nuclear and mitochondrial genomes, the latter being subject to high mutation rates. Consequently, CCO function has been demonstrated to be a target of selection30–32. Genome sequencing efforts in F. heteroclitus 9 have revealed mixed evidence for the presence of functionally significant variation in CCO among F. heteroclitus populations, but at present there have been no comprehensive examinations of sequence variation between populations from the extremes of the species distribution. In addition, differences in CCO function could be a consequence of variation in mitochondrial membrane composition or post-translational modifications of the enzyme33.
In contrast to the variation between subspecies in Mito-P50, we did not observe significant differences in Hb-P50 between F. heteroclitus subspecies (Fig. 2C,D), consistent with previous observations in a hybrid F. heteroclitus population28. However, in this population individuals with differing LDH-B genotypes differed in Hb-P50 following exhaustive swimming, implicating a genotype-specific Bohr shift (i.e., low pH decreasing O2 affinity) due to allosteric modification of Hb by ATP28. These differences are thought to result from differences in glycolytic metabolism due to variation in LDH-B34. Both subspecies maintain equivalent hematocrit (Supplementary Fig. S4 28), indicating that if Hb characteristics differentiate aerobic performance between subspecies it likely occurs through allosteric mechanisms.
Hb-P50 in killifish (approximately 0.4 kPa) is low relative to other fish species (e.g., Hb-P50 = 3.6 kPa in Oncorhynchus mykiss 35; 2.6 kPa in Kryptolebias marmoratus 36; and ranges between 3–8 kPa among intertidal sculpins37) suggesting that selection may have acted on F. heteroclitus to maximize O2 extraction from the environment. However, for Hb there is a trade-off between the ability to load O2 at the gills and unload at the tissues. The fact that we do not observe much variation between subspecies in Hb-P50 may reflect this trade-off. Thus, variation in Mito-P50 could represent an adaptation to maximize tissue O2 diffusion in the hypoxia tolerant southern subspecies. The hypothesis of a tissue O2 diffusion limitation in this species is supported by electron microscope observations of the location of the mitochondrion in this species, which at least in muscle are localized immediately below the plasma membrane26. However, both Hb-P50 and Mito-P50 are subject to considerable regulation in vivo, and this is an effect that we are unable to account for in our assays. Nevertheless, the difference in O2 binding affinity between Hb and the mitochondrion likely contributes to the shape of O2 diffusion gradients at the tissue38.
In humans, there is an inverse relationship between basal metabolic rate and Mito-P5032. We therefore predicted that northern F. heteroclitus would have lower Mito-P50 than southern fish, because northern populations maintain higher routine metabolic rates than do their southern counterparts29. In contrast, we found that the southern subspecies has a lower Mito-P50, suggesting that the relationship between metabolic rate and Mito-P50 does not represent a functional constraint in F. heteroclitus. Alternatively, the inconsistent relationship between Mito-P50 and estimates of basal metabolism in humans and F. heteroclitus may be a consequence of the energetic demands imposed by endothermy when compared with ectothermy. The combination of low routine metabolic rate and Mito-P50 exhibited by the southern subspecies may represent a beneficial strategy in high temperature hypoxic environments, allowing for greater tissue O2 extraction while also decreasing overall demand in the face of environmental hypoxia.
Organisms’ thermal tolerance limits are thought to be shaped by temperature effects on aerobic metabolism that may, at least in part, be due to effects at the level of the mitochondrion3,6,13. However, these effects have mostly been examined in the context of mitochondrial respiratory capacity. Here we show that northern and southern F. heteroclitus subspecies that differ in thermal tolerance24 also exhibit differences in Mito-P50. The lower Mito-P50 in the southern subspecies, which reflects a greater mitochondrial oxygen affinity, could aid in the maintenance of mitochondrial O2 diffusion gradients, particularly at high temperatures, which could help to sustain aerobic metabolism at high temperatures. Similarly, low Mito-P50 has the potential to aid O2 delivery during environmental hypoxia. Thus, in southern habitats where temperatures are higher and hypoxic events are more likely, low Mito-P50 could be favored. Alternatively, the relatively low temperatures and higher oxygen levels in northern habitats might result in relaxed selection on these traits, reducing the constraints on Mito-P50 in this subspecies. Taken together, our demonstration of intraspecific variation in Mito-P50 thus provides a candidate trait accounting for potential covariation of both whole-organism hypoxia and thermal tolerance.
Acclimation to both 5 and 33 °C resulted in clear changes in Mito-P50 that were subspecies-dependent (Fig. 3, S1). In contrast, Hb-P50 did not exhibit a clear response to thermal acclimation (Fig. 4, S2). This previously undescribed phenomenon identifies potential targets and mechanisms of thermal acclimation and provides support for a role for mitochondrial function in maintaining performance following prolonged thermal stress.
We predicted that acclimation to 33 °C would decrease Mito-P50 thereby compensating for the aerobic challenges associated with high temperature3,8,15,39. When compared at a common assay temperature of 15 °C, acclimation to 33 °C decreased Mito-P50 in northern but not southern F. heteroclitus (Fig. 3A). Decreases in Mito-P50 under these conditions may alleviate limitations on total O2 flux suggested to occur with elevated temperatures3 and is consistent with the maintenance of a PO2 gradient to the mitochondrion. In contrast, southern F. heteroclitus exhibit low Mito-P50 (high oxygen affinity) at both intermediate and high temperatures, indicating a difference in strategy between the subspecies. However, when compared at a higher assay temperature of 33 °C, acclimation to 33 °C increased Mito-P50 in both subspecies (Supplementary Fig. S1). This paradoxical response could represent maladaptive acclimation as this would presumably decrease O2 diffusion gradients to the tissues. Thus, it is possible that this response may instead be a consequence of sub-lethal effects associated with 33 °C acclimation. 33 °C is a non-lethal temperature to which F. heteroclitus can acclimate for prolonged periods24. However, acclimation to 33 °C is associated with decreases in whole body mass and routine O2 consumption, perhaps indicative of trade-offs necessary to mitigate the effects of extremely high acclimation temperatures on energetic balance8,29. Thus, our observed change in Mito-P50 may be a consequence of other mitochondrial responses associated with high temperature acclimation (e.g., altered mitochondrial morphology and membrane composition40,41). This raises interesting questions as to the mitochondrial responses of organisms under conditions of sub-lethal stress, which are likely to have a profound influence on species' fitness42–44.
In both subspecies, we observed higher Mito-P50 in fish acclimated to low temperature when assayed at 15 °C (Fig. 3A). Indeed, cold acclimation is associated with increases in mitochondrial respiratory capacity, mitochondrial volume density and lipid content in aquatic ectotherms14,15,26,41. Increases in lipid content increase O2 solubility45 which may alleviate potential mitochondrial O2 limitations in systemic tissues at low temperatures that are brought on by decreased O2 diffusion rates46. This 5 °C acclimation response may reveal a mechanism for life at low temperatures as it is consistent with the greater Mito-P50 exhibited by northern F. heteroclitus (Fig. 2).
Similar to our demonstration of thermal acclimation effects on Mito-P50 (Fig. 3A), prolonged exposure to hypoxia might be predicted to decrease Mito-P507,17, if reductions in Mito-P50 are important for maintaining O2 diffusion gradients. However, hypoxia acclimated northern F. heteroclitus exhibit no modification of Mito-P507. This suggests that declines in Mito-P50 at high temperatures are not directly mediated by the associated environmental hypoxia or resulting hypoxemia. Thus, the effects of thermal acclimation that we observe may play a role other than maintaining oxygen diffusion gradients. Alternatively, during hypoxic acclimation this species could recruit other mechanisms for maintaining O2 supply and demand balance, such reductions in demand23 would reduce the necessity for decreased Mito–P50 following acclimation.
Although we detected intraspecific variation and thermal acclimation effects on liver Mito-P50, this effect might not be present in mitochondria from other tissues47. Indeed, thermal acclimation and intraspecific variation effects on F. heteroclitus mitochondrial respiratory capacity vary among the heart, liver and brain8,15,27, suggesting that mitochondria from different tissues may respond differently. The role of liver mitochondria in constraining whole-organism hypoxia and thermal tolerance is not clear, whereas other tissues such as the heart and brain may be more important3,5,21. Consistent with this idea, variation in Mito-P50 from brain mitochondria has been shown to be associated with evolutionary variation in hypoxia tolerance19 whereas Mito-P50 from liver mitochondria does not change in response to hypoxic acclimation in F. heteroclitus despite changes in whole-organism hypoxia tolerance7. Thus, the role of the differences in liver Mito-P50 that we observe in setting whole organism thermal or hypoxia tolerance requires further investigation.
As assay temperature increased (assay temperature: 15–33 °C), both Hb and Mito-P50 increased (Figs S1, S2). The loss of Hb O2 affinity associated with increasing assay temperature is often observed and is primarily a consequence of the exothermic nature of Hb oxygenation48. Decreased Hb O2 binding affinity with acute increases in temperature might aid in unloading O2 to the tissues but might also compromise O2 loading at the gills. However, these effects are likely subject to considerable regulation in vivo. In contrast, decreased mitochondrial O2 affinity with increased assay temperature has not been previously characterized and is presumably a result of temperature effects on enzyme stability. This decrease in mitochondrial O2 binding affinity at high assay temperatures might cause a decline in mitochondrial ATP synthesis, perhaps revealing an aspect of declining mitochondrial function associated with acute increases in temperature.
We detected clear subspecies effects on the acute thermal sensitivity of O2 binding affinity at all acclimation temperatures (Fig. 3). Northern F. heteroclitus exhibited greater Δ Mito-P50 and a more exothermic Hb ΔH (i.e., greater thermal sensitivity) when compared to the southern subspecies (Tassay = 15 to 33 °C). These data indicate that northern F. heteroclitus exhibit a greater relative loss of O2 binding affinity than the southern subspecies following acute increases in temperature. This loss of O2 binding affinity at high acute temperatures may result in greater constraints on aerobic metabolism in this subspecies, which could be associated with the differences between subspecies in acute thermal tolerance limits24.
Thermal acclimation also altered the acute thermal sensitivity of Mito-P50 (Supplementary Fig. S1). This is reflected by increased Δ Mito-P50 following 33 °C acclimation in both F. heteroclitus subspecies (Fig. 4A). This increase in sensitivity may be a consequence of sub-lethal effects associated with 33 °C acclimation and changes in mitochondrial morphology as discussed previously. In contrast, we did not detect significant thermal acclimation effects on hemoglobin thermal sensitivity (ΔH°, Fig. 4B), although such effects have been detected in other fish species. (e.g., Oncorhynchus mykiss 49).
In this study, we demonstrate clear effects of intraspecific variation and thermal acclimation on Mito-P50. This variation in kinetic properties is consistent with subspecies differences and the effects of thermal acclimation on hypoxia and thermal tolerance. These changes in Mito P50 may help to maintain oxygen diffusion gradients particularly at high temperatures. We thus propose that altered Mito-P50 is involved in differentiating organism-level aerobic and thermal performance between putatively adapted F. heteroclitus subspecies and in response to thermal acclimation and could represent a novel target for thermal adaptation.
Methods
Animals
All procedures were carried out at the University of British Columbia according to the University of British Columbia Animal Care Committee approved protocol #A01-0180. Northern (Fundulus heteroclitus macrolepidotus; Ogden’s Pond Estuary, NS, 45°71′N; 61°90′W) and southern (Fundulus heteroclitus heteroclitus; Jekyll Island, GA, 31°02′N; 81°25′W) Atlantic killifish were collected in summer, 2014, and were transported to UBC's Aquatics Facility where they were kept in 190 L tanks with biological filtration. Fish were held at 15 ± 2 °C, and 20 ppt salinity, with a 12:12 L:D photoperiod. Animals were fed once daily to satiation (Nutrafin Max). In June 2015, fish were transferred to 114 L tanks with biological filtration. Temperature was held at 5, 15 or 33 ± 2 °C and all other conditions were maintained. After a minimum of four weeks of thermal acclimation, fish were fasted for 24 h and sampled as described below.
Loss of equilibrium in hypoxia assay
Brackish water (Ta = 15 °C, 20 ppt salinity) was circulated in a 50 l plexiglass arena with bubblewrap at the water's surface to prevent O2 diffusion. Fish were placed in individual containers in the arena (10 fish per trial, 1 fish per container), where the former allowed for full mixing of water while preventing access to the water's surface. PO2 was monitored continuously using a fiber-optic oxygen probe (NEO-Fox, Ocean Optics, Dunedin, FL). Following 10 min of acclimation to the container, PO2 was decreased over 30 min by bubbling N2 gas to one of four PO2 values (0.84, 0.44, 0.24, or 0.13 ± .02 kPa). When the desired PO2 was reached, the trial began and time to LOEhyp was recorded. LOEhyp was determined as the time at which fish no longer responded to gentle movement of their containers following which fish were removed and placed in a recovery tank.
Hemoglobin O2 P50 and hematocrit content
Fundulus heteroclitus were removed from the 5, 15, or 33 °C thermal acclimation tanks at 8:00 AM PST, euthanized by cervical dislocation and weighed. The caudal peduncle was severed and blood was collected from the incision using micro-hematocrit tubes. Micro-hematocrit tubes were centrifuged (10 min, 12,700 g, 25 °C) and hematocrit was measured as the volume percentage of packed RBCs within the total blood sample.
Micro-hematocrit tubes were separated at the boundary between RBCs and plasma. RBCs were re-suspended to approximately the same measured hematocrit content with buffered saline (50 mM HEPES with 100 mM NaCl, final osmolality = 390 Osm kg−1, pH = 7.8 at 25 °C) for O2 equilibria experiments50. Osmolality of the buffered saline solution was set based on plasma osmolality measurements (10 μL of whole blood measured using standard protocols with an osmometer, Vapro 5520, Wescor, Logan, UT) from five randomly selected 15 °C acclimated F. heteroclitus (396 ± 14 Osm⋅kg−1 mean ± sd).
Oxygen equilibrium curves were generated using protocols described by Lilly et al.51. Re-suspended RBCs (3 μL) were sandwiched between two sheets of low density polyethylene that were secured on an aluminum ring with two plastic O-rings. Blood samples were placed in a gas tight tonometry cell modified to fit into a SpectraMax 190 microplate reader (Molecular Devices, Sunnyvale, USA). Assay temperature was maintained at 15 or 33 °C, and blood from the three acclimation temperatures were assayed at both assay temperatures. Samples were equilibrated with pure N2 for 30 min to achieve full Hb deoxygenation (deoxyHb). O2 tension was increased by 6 to 12 increments of air (21% O2) balanced with N2 using a Wӧsthoff DIGAMIX gas mixing pump (H. Wösthoff Messtechnik, Bochum, Germany). Optical density (OD) was measured every 10 s at 390 nm, and 430 nm, which correspond to the isosbestic point (i.e., OD is independent of Hb-O2 saturation), and maxima for deoxygenated Hb, respectively. Hb was assumed to be fully saturated (oxyHb) with O2 (100% air) when no change in OD at 430 nm was detected after three equilibration steps.
Hb-O2 saturation for each equilibration step was calculated using Eqn. 1.
| 1 |
Oxygen equilibrium curves (OECs) were constructed for each sample by non-linear least squares curve fitting to fit the Hb-O2 saturation data to the Hill equation (Eqn. 2).
| 2 |
Where P50 is the PO2 at which Hb is 50% saturated and is a measure of Hb-O2 affinity, and n is the cooperativity (Hill) coefficient51. All curves used in the final analyses fit well to the data (r2 > 0.99).
We estimated the effects of acute thermal shifts (Tassay = 15 to 33 °C) on Hb-O2 affinity by calculating the apparent heat of oxygenation using the van't Hoff isochore (ΔH, Eqn. 3 52).
| 3 |
Where R is the gas constant and T1 and T2 are the absolute temperatures 306 and 288 K respectively.
Liver mitochondrial isolation
Seven F. heteroclitus were removed from the 5, 15, or 33 °C thermal acclimation tanks and euthanized for liver mitochondrial isolation as described previously15. Liver tissue was excised and pooled into one aliquot of ice-cold homogenization buffer (250 mM sucrose, 50 mM KCl, 0.5 mM EGTA, 25 mM KH2PO4, 10 mM HEPES, 1.5% BSA, pH = 7.4 at 20 °C). Liver tissue was cut into approximately 1 mm3 pieces and homogenized with 5 passes of a loose-fitting Teflon pestle followed by filtration through 1-ply cheesecloth. Crude liver homogenate was centrifuged at 4 °C for 10 min at 600 g. The fat layer was removed with aspiration and the remaining supernatant was filtered through 4-ply cheesecloth. Filtered supernatant was centrifuged at 4 °C for 10 min at 6000 g. The resulting pellet was washed twice and suspended in 800 μL of homogenization buffer and stored on ice until experimentation. Protein content was determined using a Bradford assay with BSA as a standard53.
Mitochondrial assays
Mitochondrial O2 binding affinity (Mito-P50) was measured using a high-resolution respirometry system (O2k MiPNetAnalyzer; Oroboros Instruments, Innsbruck, Austria). Air-saturated and O2-depleted (achieved with a yeast suspension) calibrations of respiration buffer (MiRO5; 110 mM sucrose, 0.5 mM EGTA, 3 mM MgCl2, 60 mM K-lactobionate, 20 mM taurine, 10 mM KH2PO4, 20 mM HEPES, 0.1% BSA, pH = 7.1 at 30 °C54) were taken at each assay temperature (15, 33 and 37 °C) using published O2 solubilities55. Mito-P50 was determined using DatLab 2 software (Oroboros Instruments). Mitochondrial respiration rate during the transition into anoxia was fit against Eqn. 4.
| 4 |
where is the mitochondrial respiration rate, J max is maximal respiration rate and P 50 is the at which respiration rate is half of J max. We accounted for the time delay of the O2 sensor, background O2 consumption and internal zero drift at each assay temperature18.
Two ml of MiRO5 was air-equilibrated at each assay temperature (15, 33 and 37 °C) followed by the addition of liver mitochondria (0.5 mg protein). A saturating mixture of substrates (glutamate 10 mM, pyruvate 10 mM, malate 2 mM, succinate 10 mM, palmitoyl-carnitine 20 mM) was added to the chamber followed by ADP (2.5 mM) to fuel mitochondrial respiration. Mitochondrial respiration was allowed to proceed until all O2 was consumed (i.e., anoxia). Anoxic conditions were maintained for 15 min, followed by the termination of the experiment.
The effects of acute temperature shifts on Mito-P50 (i.e., Δ Mito-P50) were estimated by calculating the difference in Mito-P50 between Tassay = 15 and 33 °C for each treatment.
Statistics and calculations
Statistical tests were completed using R software (v3.3.3). Data are presented as mean ± SEM and α was set at.05. We confirmed the presence of normal distributions and homogeneity of variance in our data using Shapiro-Wilk and Bartlett's tests respectively. Sample size is indicated (n) in the respective figure captions.
We compared the effects of subspecies, thermal acclimation, assay temperature using three-separate linear mixed effect models (individual as the random effect) to compare Hb and Mito-P50 and Hill coefficients.
We used separate t-tests to compare subspecies effects on Hb and mitochondrial O2 binding affinity. Comparisons were made between subspecies assayed at their acclimation temperature (e.g., 33 °C acclimated northern and southern fish assayed at 33 °C). We accounted for an increased false discovery rate by adjusting α using a Benjamini-Hochberg correction for our specific test of subspecies effects.
Thermal acclimation effects (5, 15, and 33 °C) were assessed between subspecies at Tassay = 15 °C using a two-way ANOVA. The effects of acute thermal shifts (Tassay = 15 to 33 °C) on mitochondrial (Δ Mito-P50) and Hb- P50 (ΔH) were assessed between subspecies and among thermal acclimation treatments using a two-way ANOVA.
Thermal acclimation and subspecies effects on hematocrit content were assessed using a two-way ANOVA. The effects of subspecies and decreasing PO2 on time to LOEhyp were assessed using a two-way ANOVA.
Data availability
The data generated during the current study are available from the corresponding author on reasonable request.
Electronic supplementary material
Acknowledgements
A. Cooper, K. Crowther T. Healy, and B. Marshall for assistance with animal collection. Funding was provided by the Natural Sciences and Engineering Research Council of Canada (NSERC) Discovery grants to PMJ and CJB, and an NSERC Canada Graduate Scholarship to DJC.
Author Contributions
D.J.C. and P.M.S. designed the experiment; D.J.C. wrote the manuscript; D.J.C. and P.M.S. revised the manuscript; D.J.C., P.R.M., H.J.B. and E.J. conducted experiments; D.J.C. analyzed the data; C.J.B. and P.M.S. secured equipment.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16598-6.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Sunday JM, Bates AE, Dulvy NK. Global analysis of thermal tolerance and latitude in ectotherms. Proc. Roy. Soc. B. 2011;278:1823–1830. doi: 10.1098/rspb.2010.1295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Deutsch C, Ferrel A, Seibel B, Pörtner HO, Huey RB. Climate change tightens a metabolic constraint on marine habitats. Science. 2015;348:1132–1135. doi: 10.1126/science.aaa1605. [DOI] [PubMed] [Google Scholar]
- 3.Pörtner H. Climate change and temperature-dependent biogeography: oxygen limitation of thermal tolerance in animals. Naturwissenschaften. 2001;88:137–146. doi: 10.1007/s001140100216. [DOI] [PubMed] [Google Scholar]
- 4.Pörtner HO, Farrell AP. Physiology andclimate change. Science. 2008;322:690–692. doi: 10.1126/science.1163156. [DOI] [PubMed] [Google Scholar]
- 5.Iftikar FI, Hickey AJR. Do mitochondria limit hot fish hearts? Understanding the role of mitochondrial function with heat stress in Notolabrus celidotus. PLoS ONE. 2013;8:e64120. doi: 10.1371/journal.pone.0064120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fry FEJ, Hart JS. The relation of temperature to oxygen consumption in the goldfish. Biol. Bull. 1948;94:66–77. doi: 10.2307/1538211. [DOI] [PubMed] [Google Scholar]
- 7.Du SNN, Mahalingam S, Borowiec BG, Scott GR. Mitochondrial physiology and reactive oxygen species production are altered by hypoxia acclimation in killifish (Fundulus heteroclitus) J. Exp. Biol. 2016;219:1130–1138. doi: 10.1242/jeb.132860. [DOI] [PubMed] [Google Scholar]
- 8.Chung DJ, Bryant HJ, Schulte PM. Thermal acclimation and subspecies-specific effects on heart and brain mitochondrial performance in a eurythermal teleost (Fundulus heteroclitus) J. Exp. Biol. 2017;220:1459–1471. doi: 10.1242/jeb.151217. [DOI] [PubMed] [Google Scholar]
- 9.Baris TZ, et al. Evolved genetic and phenotypic differences due to mitochondrial-nuclear interactions. PLoS Genet. 2017;13:e1006517. doi: 10.1371/journal.pgen.1006517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gnaiger E. Bioenergetics at low oxygen: dependence of respiration and phosphorylation on oxygen and adenosine diphosphate supply. Resp. Physiol. 2001;128:277–297. doi: 10.1016/S0034-5687(01)00307-3. [DOI] [PubMed] [Google Scholar]
- 11.Baris TZ, Blier PU, Pichaud N, Crawford DL, Oleksiak MF. Gene by environmental interactions affecting oxidative phosphorylation and thermal sensitivity. Am. J. Physiol. Reg. I. 2016;311:R157–65. doi: 10.1152/ajpregu.00008.2016. [DOI] [PubMed] [Google Scholar]
- 12.Baris TZ, Crawford DL, Oleksiak MF. Acclimation and acute temperature effects on population differences in oxidative phosphorylation. Am. J. Physiol. Reg. I. 2016;310:R185–96. doi: 10.1152/ajpregu.00421.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Dos Santos RS, Galina A, Da-Silva WS. Cold acclimation increases mitochondrial oxidative capacity without inducing mitochondrial uncoupling in goldfish white skeletal muscle. Biol Open. 2013;2:82–87. doi: 10.1242/bio.20122295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Guderley H. Metabolic responses to low temperature in fish muscle. Biol. Rev. 2004;79:409–427. doi: 10.1017/S1464793103006328. [DOI] [PubMed] [Google Scholar]
- 15.Chung DJ, Schulte PM. Mechanisms and costs of mitochondrial thermal acclimation in a eurythermal killifish (Fundulus heteroclitus) J. Exp. Biol. 2015;218:1621–1631. doi: 10.1242/jeb.120444. [DOI] [PubMed] [Google Scholar]
- 16.Galli GLJ, Lau GY, Richards JG. Beating oxygen: chronic anoxia exposure reduces mitochondrial F1FO-ATPase activity in turtle (Trachemys scripta) heart. J. Exp. Biol. 2013;216:3283–3293. doi: 10.1242/jeb.087155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Costa LE, Mendez G, Boveris A. Oxygen dependence of mitochondrial function measured by high-resolution respirometry in long-term hypoxic rats. Am. J. Physiol. 1997;273:C852–8. doi: 10.1152/ajpcell.1997.273.3.C852. [DOI] [PubMed] [Google Scholar]
- 18.Gnaiger E, Lassnig B, Kuznetsov A, Rieger G, Margreiter R. Mitochondrial oxygen affinity, respiratory flux control and excess capacity of cytochrome c oxidase. J. Exp. Biol. 1998;201:1129–1139. doi: 10.1242/jeb.201.8.1129. [DOI] [PubMed] [Google Scholar]
- 19.Lau GY, et al. Evolution of cytochrome c oxidase in hypoxia tolerant sculpins (Cottidae, Actinopterygii) Mol. Biol. Evol. 2017;34:2153–2162. doi: 10.1093/molbev/msx179. [DOI] [PubMed] [Google Scholar]
- 20.Brijs J, et al. Experimental manipulations of tissue oxygen supply do not affect warming tolerance of European perch. J. Exp. Biol. 2015;218:2448–2454. doi: 10.1242/jeb.121889. [DOI] [PubMed] [Google Scholar]
- 21.Ern R, et al. Some like it hot: Thermal tolerance and oxygen supply capacity in two eurythermal crustaceans. Sci. Rep. 2015;5:10743. doi: 10.1038/srep10743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.NOAA National Estuarine Research Reserve System (NERRS) (2012).
- 23.Borowiec BG, Darcy KL, Gillette DM, Scott GR. Distinct physiological strategies are used to cope with constant hypoxia and intermittent hypoxia in killifish (Fundulus heteroclitus) J. Exp. Biol. 2015;218:1198–1211. doi: 10.1242/jeb.114579. [DOI] [PubMed] [Google Scholar]
- 24.Fangue NA, Hofmeister M, Schulte PM. Intraspecific variation in thermal tolerance and heat shock protein gene expression in common killifish. Fundulus heteroclitus. J. Exp. Biol. 2006;209:2859–2872. doi: 10.1242/jeb.02260. [DOI] [PubMed] [Google Scholar]
- 25.McBryan TL, Healy TM, Haakons KL, Schulte PM. Warm acclimation improves hypoxia tolerance in Fundulus heteroclitus. J. Exp. Biol. 2016;219:474–484. doi: 10.1242/jeb.133413. [DOI] [PubMed] [Google Scholar]
- 26.Dhillon RS, Schulte PM. Intraspecific variation in the thermal plasticity of mitochondria in killifish. J. Exp. Biol. 2011;214:3639–3648. doi: 10.1242/jeb.057737. [DOI] [PubMed] [Google Scholar]
- 27.Fangue NA, Richards JG, Schulte PM. Do mitochondrial properties explain intraspecific variation in thermal tolerance? J. Exp. Biol. 2009;212:514–522. doi: 10.1242/jeb.024034. [DOI] [PubMed] [Google Scholar]
- 28.Dimichele L, Powers DA. Physiological basis for swimming endurance differences between LDH-B genotypes of Fundulus heteroclitus. Science. 1982;216:1014–1016. doi: 10.1126/science.7079747. [DOI] [PubMed] [Google Scholar]
- 29.Healy TM, Schulte PM. Thermal acclimation is not necessary to maintain a wide thermal breadth of aerobic scope in the common killifish (Fundulus heteroclitus) Physiol. Biochem. Zool. 2012;85:107–119. doi: 10.1086/664584. [DOI] [PubMed] [Google Scholar]
- 30.Scott GR, et al. Molecular evolution of cytochrome c oxidase underlies high-altitude adaptation in the bar-headed goose. Mol. Biol. Evol. 2011;28:351–363. doi: 10.1093/molbev/msq205. [DOI] [PubMed] [Google Scholar]
- 31.Zhang Z-Y, Chen B, Zhao D-J, Kang L. Functional modulation of mitochondrial cytochrome c oxidase underlies adaptation to high-altitude hypoxia in a Tibetan migratory locust. Proc. Roy. Soc. B. 2013;280:20122758–20122758. doi: 10.1098/rspb.2012.2758. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Larsen FJ, et al. Mitochondrial oxygen affinity predicts basal metabolic rate in humans. FASEB J. 2011;25:2843–2852. doi: 10.1096/fj.11-182139. [DOI] [PubMed] [Google Scholar]
- 33.Kraffe E, Marty Y, Guderley H. Changes in mitochondrial oxidative capacities during thermal acclimation of rainbow trout Oncorhynchus mykiss: roles of membrane proteins, phospholipids and their fatty acid compositions. J. Exp. Biol. 2007;210:149–165. doi: 10.1242/jeb.02628. [DOI] [PubMed] [Google Scholar]
- 34.Dimichele L, Paynter K, Powers DA. Evidence of lactate dehydrogenase-B allozyme effects in the teleost. Fundulus heteroclitus. Science. 1991;253:898–900. doi: 10.1126/science.1876847. [DOI] [PubMed] [Google Scholar]
- 35.Perry S, Reid S. The effects of acclimation temperature on the dynamics of catecholamine release during acute hypoxia in the rainbow trout Oncorhynchus mykiss. J. Exp. Biol. 1994;186:289–307. doi: 10.1242/jeb.186.1.289. [DOI] [PubMed] [Google Scholar]
- 36.Turko AJ, Robertson CE, Bianchini K, Freeman M, Wright PA. The amphibious fish Kryptolebias marmoratus uses different strategies to maintain oxygen delivery during aquatic hypoxia and air exposure. J. Exp. Biol. 2014;217:3988–3995. doi: 10.1242/jeb.110601. [DOI] [PubMed] [Google Scholar]
- 37.Mandic M, Todgham AE, Richards JG. Mechanisms and evolution of hypoxia tolerance in fish. Proc. Roy. Soc. B. 2009;276:735–744. doi: 10.1098/rspb.2008.1235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Jones DP. Intracellular diffusion gradients of O2 and ATP. Am. J. Physiol. 1986;250:C663–75. doi: 10.1152/ajpcell.1986.250.5.C663. [DOI] [PubMed] [Google Scholar]
- 39.Guderley H, Johnston I. Plasticity of fish muscle mitochondria with thermal acclimation. J. Exp. Biol. 1996;199:1311–1317. doi: 10.1242/jeb.199.6.1311. [DOI] [PubMed] [Google Scholar]
- 40.Wodtke E. Temperature adaptation of biological membranes. Compensation of the molar activity of cytochrome c oxidase in the mitochondrial energy-transducing membrane during thermal acclimation of the carp (Cyprinus carpio L.) BBA - Biomembranes. 1981;640:710–720. doi: 10.1016/0005-2736(81)90101-2. [DOI] [PubMed] [Google Scholar]
- 41.Grim JM, Miles DRB, Crockett EL. Temperature acclimation alters oxidative capacities and composition of membrane lipids without influencing activities of enzymatic antioxidants or susceptibility to lipid peroxidation in fish muscle. J. Exp. Biol. 2010;213:445–452. doi: 10.1242/jeb.036939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Lemoine NP, Burkepile DE. Temperature-induced mismatches between consumption and metabolism reduce consumer fitness. Ecology. 2012;93:2483–2489. doi: 10.1890/12-0375.1. [DOI] [PubMed] [Google Scholar]
- 43.Salin K, Auer SK, Anderson GJ, Selman C, Metcalfe NB. Inadequate food intake at high temperatures is related to depressed mitochondrial respiratory capacity. J. Exp. Biol. 2016;219:1356–1362. doi: 10.1242/jeb.133025. [DOI] [PubMed] [Google Scholar]
- 44.Iles AC. Towards predicting community level effects of climate: Relative temperature scaling of metabolic and ingestion rates. Ecology. 2014;95:2657–2668. doi: 10.1890/13-1342.1. [DOI] [Google Scholar]
- 45.Widomska J, Raguz M, Subczynski WK. Oxygen permeability of the lipid bilayer membrane made of calf lens lipids. Biochim. Biophys. Acta. 2007;1768:2635–2645. doi: 10.1016/j.bbamem.2007.06.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Sidell BD. Intracellular oxygen diffusion: the roles of myoglobin and lipid at cold body temperature. J. Exp. Biol. 1998;201:1119–1128. doi: 10.1242/jeb.201.8.1119. [DOI] [PubMed] [Google Scholar]
- 47.Benard G, et al. Physiological diversity of mitochondrial oxidative phosphorylation. Am. J. Physiol. Cell Phys. 2006;291:C1172–82. doi: 10.1152/ajpcell.00195.2006. [DOI] [PubMed] [Google Scholar]
- 48.Weber RE, Jensen FB. Functional adaptations in hemoglobins from ectothermic vertebrates. Annu. Rev. Physiol. 1988;50:161–179. doi: 10.1146/annurev.ph.50.030188.001113. [DOI] [PubMed] [Google Scholar]
- 49.Weber RE, Wood SC, Lomholt JP. Temperature acclimation and oxygen-binding properties of blood and multiple haemoglobins of rainbow trout. J. Exp. Biol. 1976;65:333–345. doi: 10.1242/jeb.65.2.333. [DOI] [PubMed] [Google Scholar]
- 50.Wells RMG, Baldwin J, Seymour RS, Christian K, Brittain T. Red blood cell function and haematology in two tropical freshwater fishes from Australia. Comp. Biochem. Physiol. A. 2005;141:87–93. doi: 10.1016/j.cbpb.2005.04.005. [DOI] [PubMed] [Google Scholar]
- 51.Lilly LE, et al. Parallel assay of oxygen equilibria of hemoglobin. Anal. Biochem. 2013;441:63–68. doi: 10.1016/j.ab.2013.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wyman J., Jr. Linked Functions and Reciprocal Effects in Hemoglobin: A Second Look. Adv. Protein Chem. 1964;19:223–286. doi: 10.1016/S0065-3233(08)60190-4. [DOI] [PubMed] [Google Scholar]
- 53.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 54.Gnaiger E, Kuznetsov AV. Mitochondrial respiration at low levels of oxygen and cytochrome c. Biochem. Soc. Trans. 2002;30:252–258. doi: 10.1042/bst0300252. [DOI] [PubMed] [Google Scholar]
- 55.Gnaiger, E. & Forstner, H. In Polarographic Oxygen Sensors 321–333 (Springer Berlin Heidelberg, 1983). 10.1007/978-3-642-81863-9_28.
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Data Availability Statement
The data generated during the current study are available from the corresponding author on reasonable request.