Abstract
Multicenter, longitudinal studies on nontuberculous mycobacteria (NTM) pulmonary infection (PI) are lacking. This study provides a 5-year epidemiological overview of NTM-PI in Taiwan and investigated its predictors. The clinical relevance of each respiratory NTM isolate in six hospitals between 2008 and 2014 was determined according to current guidelines. Recurrent episodes were judged by serial bacteriological results. New episodes of NTM-PI and pulmonary colonization (PC) occurring since 2010 were analyzed. Logistic regression analysis was performed to identify the predictors of NTM-PI. Between 2010 and 2014, the incidence rate of NTM-PI was 46.0 episodes per 100,000 hospital-based patient-years. Mycobacterium avium intracellulare complex (MAC) was predominant in Northern Taiwan, whereas MAC and M. abscessus were copredominant in Southern Taiwan. Multiple episodes occurred in 9.5% of NTM-PI patients. No female predominance was observed, except for MAC-PI. Previous pulmonary tuberculosis and chronic obstructive pulmonary disease (COPD) were the most common pulmonary comorbidities and independent risk factors for NTM-PI. Other risk factors included M. kansasii, M. abscessus, and southern Taiwan. Geographical variation of NTM-PI exists in Taiwan. Clinicians should keep a high suspicion on NTM-PI in the risk population. In endemic area of tuberculosis and COPD, there may be no female predominance in NTM-PI.
Introduction
Pulmonary involvement accounts for 75–94% of all nontuberculous mycobacteria (NTM)-induced human diseases1,2. In the past 20 years, a global increase has been reported in both number of clinical isolates and prevalence of NTM3–5. The most common species causing NTM pulmonary infection (PI) are Mycobacterium avium-intracellulare complex (MAC), M. kansasii, and M. abscessus. However, their relevance ranking varied in different epidemiological reports3, which indicates geographical diversity and suggests that single-center data cannot represent the general picture of NTM-PI.
Determining the incidence rate of NTM-PI is challenging because case selection and identification are difficult. Currently, NTM-PI is diagnosed based on composite and complex criteria that were established by the American Thoracic Society and Infectious Disease Society of America (ATS/IDSA) in 20072. Unlike tuberculosis (TB), a public health notification is not mandatory for NTM-PI. Although prospective cohort studies may confirm the exact incidence rate of NTM disease, the long-term follow-up is extremely time-consuming and labor-intensive in large populations. Therefore, most studies have had a single-center, retrospective, and cross-sectional design that determined prevalence rather than incidence rate1,6–9. Another limitation of the available reports on NTM-PI is the lack of data on recurrent episodes10.
Therefore, this 5-year, longitudinal study investigated the incidence rate of NTM-PI by reviewing the medical records, laboratory data, and chest radiographs of patients with respiratory NTM in six hospitals. We identified the recurrent episodes of NTM-PI and determined the predictors of NTM-PI.
Results
Identification of new episodes of NTM-PI and NTM-PC
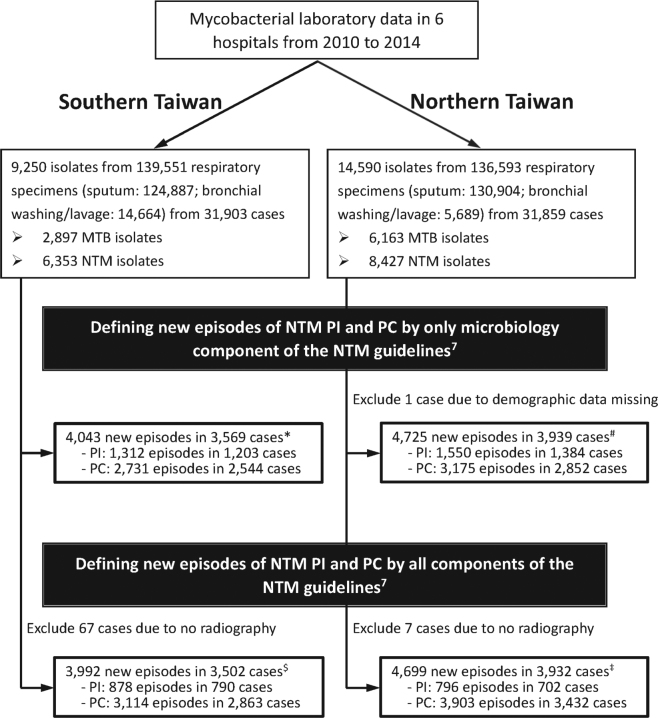
Between 2010 and 2014, a total of 1,674 new episodes of NTM-PI in 1,492 cases and 7,017 new episodes of NTM-pulmonary colonization (PC) in 6,295 cases were identified (Fig. 1). Among the 1,492 cases, 141 (9.5%) experienced multiple episodes of NTM-PI; recurrent infection occurred in 36 (25.5%) of them because of same NTM species.
Figure 1.
Flowchart of case selection and identification of new episodes of nontuberculous mycobacteria (NTM) pulmonary infection (PI) and pulmonary colonization (PC) in six hospitals (178*, 197#, 151$, 202‡ cases had both NTM-PI and NTM-PC, and were counted in both groups).
The number of respiratory specimens was the lowest in 2010 (n = 40,657) and highest in 2012 (n = 71,771) (Table 1). The number of new infection episodes was stable in Northern Taiwan. However, in Southern Taiwan, the number of new infection episodes peaked in 2014 and that of new M. kansasii infection gradually increased (Table 1). Between 2010 and 2014, the incidence rate of pulmonary TB gradually declined in the entire cohort (i.e., both Northern and Southern Taiwan; Fig. 2). However, the incidence rate of NTM-PI remained stable and that of NTM-PC gradually increased and reached a plateau after 2012.
Table 1.
Number of respiratory samples, nontuberculous mycobacteria (NTM) isolates and new infection episodes in six hospitals (three each in northern and southern Taiwan) from 2010 to 2014
| No. of respiratory samples or NTM isolates | No. of NTM-PI episodes | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 2010 | 2011 | 2012 | 2013 | 2014 | 2010 | 2011 | 2012 | 2013 | 2014 | |
| Northern Taiwan | ||||||||||
| Respiratory samples | 21,966 | 23,158 | 37,254 | 28,165 | 26,050 | — | — | — | — | — |
| NTM isolates (Total) | 1,193 | 1,348 | 2,093 | 2,054 | 1,739 | 158 | 157 | 204 | 169 | 119 |
| M. abscessus | 189 | 257 | 472 | 364 | 395 | 25 | 42 | 34 | 31 | 33 |
| MAC | 381 | 488 | 771 | 1051 | 741 | 60 | 64 | 71 | 84 | 58 |
| M. kansasii | 68 | 102 | 155 | 106 | 161 | 18 | 12 | 19 | 12 | 11 |
| Others | 555 | 501 | 695 | 533 | 442 | 55 | 39 | 80 | 42 | 17 |
| Sothern Taiwan | ||||||||||
| Respiratory samples | 18,691 | 20,472 | 34,517 | 31,033 | 34,838 | — | — | — | — | — |
| NTM isolates (Total) | 606 | 968 | 1,821 | 1,230 | 1,728 | 87 | 114 | 230 | 172 | 253 |
| M. abscessus | 147 | 274 | 411 | 318 | 347 | 28 | 46 | 60 | 51 | 58 |
| MAC | 157 | 207 | 399 | 241 | 368 | 31 | 43 | 59 | 46 | 61 |
| M. kansasii | 15 | 55 | 147 | 108 | 232 | 3 | 10 | 25 | 17 | 57 |
| Others | 287 | 432 | 864 | 563 | 781 | 25 | 45 | 86 | 58 | 77 |
Abbreviation: MAC, Mycobacterium avium-intracellulare complex.
Figure 2.
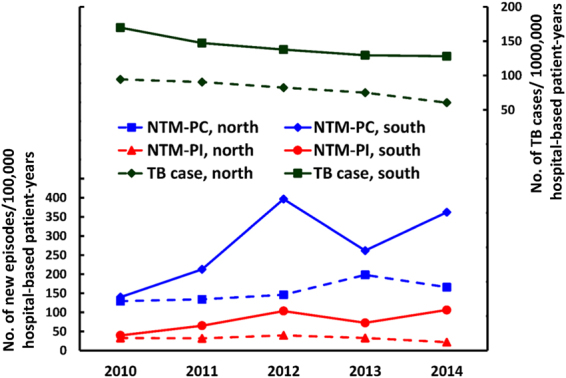
Incidence rate of new episodes of nontuberculous mycobacteria (NTM) pulmonary infection (PI) and pulmonary colonization (PC), as well as incidence of tuberculosis (TB) in northern and southern Taiwan.
Characteristics of new episodes of NTM-PI and NTM-PC
Table 2 shows the clinical characteristics and underlying comorbidities of patients with new episodes of NTM-PI and NTM-PC. Most patients were aged ≥65 years. The male: female ratio was 1.26 and 1.35 in the NTM-PI and NTM-PC groups, respectively. The most common pulmonary comorbidities were previous pulmonary TB (22.1%) and chronic obstructive pulmonary disease (COPD) (19.8%), whereas the most common systemic illnesses were malignancy (8.9%) and diabetes mellitus (DM) (8.3%). In the NTM-PI group, the most common symptom was cough (75.5%) and sputum (74.5%).
Table 2.
Clinical characteristics, radiographic findings and laboratory data of new episodes of nontuberculous mycobacterial (NTM) pulmonary infection and colonization in six hospitals.
| Characteristics | New Infection (1,674 episodes) | New Colonization (7,016 episodes) | p-value |
|---|---|---|---|
| Age group | |||
| Age < 25 | 11 (0.7%) | 129 (1.8%) | 0.001 |
| Age 25~44 | 110 (6.6%) | 684 (9.7%) | <0.001 |
| Age 45~64 | 560 (33.5%) | 2,202 (31.4%) | 0.103 |
| Age >=65 | 993 (59.3%) | 4,001 (57.0%) | 0.088 |
| Gender | |||
| Male | 932 (55.7%) | 4027 (57.4%) | 0.200 |
| Comorbidity | |||
| History of pulmonary TB | 425 (25.4%) | 1495 (21.3%) | <0.001 |
| COPD | 392 (23.4%) | 1328 (18.9%) | <0.001 |
| Bronchiectasis | 261 (15.6%) | 535 (7.6%) | <0.001 |
| Interstitial lung disease | 101 (6.0%) | 261 (3.7%) | <0.001 |
| Asthma | 87 (5.2%) | 312 (4.4%) | 0.304 |
| Pneumoconiosis | 13 (0.8%) | 36 (0.5%) | 0.199 |
| Cancer | 154 (9.2%) | 623 (8.9%) | 0.680 |
| Diabetes mellitus | 121 (7.2%) | 602 (8.6%) | 0.072 |
| Congestive heart failure | 100 (6.0%) | 323 (4.6%) | 0.020 |
| Autoimmune disease | 63 (3.7%) | 140 (2.0%) | <0.001 |
| HIV infection | 48 (2.9%) | 134 (1.9%) | 0.015 |
| Liver cirrhosis | 29 (1.7%) | 121 (1.7%) | 0.983 |
| Transplant | 15 (0.9%) | 46 (0.7%) | 0.292 |
| Chronic kidney disease | 13 (0.8%) | 50 (0.7%) | 0.782 |
| Steroid user | 108 (6.5%) | 331 (4.7%) | 0.004 |
| NTM species | |||
| MAC | 577 (34.4%) | 1818 (26.0%) | <0.001 |
| M. abscessus | 408 (24.3%) | 1059 (15.1%) | <0.001 |
| M. fortuitum | 164 (9.8%) | 1266 (18.0%) | <0.001 |
| M. kansasii | 184 (11.0%) | 403 (5.7%) | <0.001 |
| M. gordonae | 78 (4.7%) | 826 (11.7%) | <0.001 |
| Other NTM species | 263 (15.7%) | 1644 (23.4%) | <0.001 |
| CXR pattern | |||
| Fibocavitary | 517 (30.9%) | ||
| Nodular bronchiectatic | 1157 (69.1%) | ||
| CXR extent | |||
| Focal | 475 (28.4%) | ||
| Multifocal | 1199 (71.6%) | ||
| Blood tests* | |||
| Hemoglobin <12 g/dL | 432 (39.7%) | 1492 (39.2%) | 0.782 |
| Platelet count <140 K/uL | 140 (12.9%) | 574 (15.1%) | 0.067 |
| Leukocyte <4000 or >10500/uL | 299 (27.5%) | 986(25.9%) | 0.304 |
| Segment >70% | 456 (54.9%) | 1440 (48.5%) | 0.004 |
| C-reactive protein >5 mg/L | 522 (70.6%) | 1640 (67.7%) | 0.135 |
| AST >40 U/L | 184 (18.3%) | 616 (17.7%) | 0.678 |
| ALT >40 U/L | 138 (13.1%) | 522 (14.0%) | 0.454 |
| Total bilirubin >1.0 mg/dL | 146 (18.2%) | 565 (20.9%) | 0.096 |
| Creatinine >1.4 mg/dL | 125 (11.4%) | 511 (13.2%) | 0.099 |
Data are number (%).
Abbreviation: ALT, alanine transaminase; AST, aspartate transaminase; COPD, chronic obstructive pulmonary disease; MAC, Mycobacterium avium-intracellulare complex; TB, tuberculosis.
*Data are the percentage of episodes with the characteristics among all tested episodes.
Compared with patients with NTM-PC, those with NTM-PI were older and were more likely to have structural lung diseases (including a history of pulmonary TB, COPD, bronchiectasis, or interstitial lung disease [ILD]), congestive heart failure, an autoimmune disease, HIV infection, and steroid usage (Table 2).
The female sex was not associated with NTM-PI in the entire cohort, and in different NTM species except for MAC (Table 2, Table S2 and Figure S2). Moreover, compared with men, women had a higher prevalence of bronchiectasis (19.7% vs. 12.3%, p < 0.001) and ILD (7.8% vs. 4.6%, p = 0.007), and a lower prevalence of previous pulmonary TB (21.6% vs. 28.4%, p = 0.001), COPD (18.9% vs. 27.0%, p < 0.001), and pneumoconiosis (0% vs. 1.4%, p < 0.001; Table S3).
MAC, M. abscessus, and M. kansasii were the most common species of NTM-PI in either the entire cohort (Table 2) or in all age groups (Figure S3). Among the 878 new episodes of NTM-PI in Southern Taiwan, the most common species were M. abscessus (n = 243, 27.7%) and MAC (n = 240, 27.3%). However, in Northern Taiwan, only MAC was predominant (n = 337, 42.3%).
The FC and NB patterns were the major radiographic findings in 517 (30.9%) and 1157 (69.1%) episodes of NTM-PI, respectively (Table 2). The proportion of men was higher in the FC group than in the NB group (65.4% vs. 51.3%, p < 0.001). A history of TB was more common in the FC group than in the NB group (33.5% vs. 21.8%, p < 0.001). Lesions with an FC pattern were more common in M. kansasii (42.4%) infection than MAC (28.6%, p < 0.001) or M. abscessus (26.2%, p < 0.001) infections (Table S4). Multifocal involvement was most common in M. kansasii infection (78.8%), followed by MAC (75.4%) and M. abscessus (69.6%) infections.
Blood examination (Table 2) mostly revealed similarities between the NTM-PI and NTM-PC groups, although neutrocytosis was more common in the NTM-PI group (54.9% vs. 48.5%, p = 0.004). Elevation in the serum C-reactive protein (CRP) level, anemia (hemoglobin < 12 g/dL) and thrombocytopenia (platelet < 140 k/µL) were observed in 70.6%, 39.7% and 12.9% of patients, respectively, in the NTM-PI group.
Predictors of NTM-PI
Multivariate logistic regression analysis on the new episodes of NTM-PI and NTM-PC revealed that NTM isolates in Southern Taiwan were more likely to cause pulmonary infection than those in Northern Taiwan (Table 3). An age of 25–65 years was a protector of NTM-PI; other independent predictors for NTM-PI included the underlying pulmonary diseases (history of pulmonary TB, COPD, and bronchiectasis) and systemic comorbidities (autoimmune disease and HIV infection). NTM-PI was more commonly caused by MAC (OR: 2.34 [2.03–2.70]), M. abcessus (OR: 2.92 [2.50–3.42]), or M. kansasii (OR: 3.41 [2.78–4.19]).
Table 3.
Independent risk factors of new episode of pulmonary infection by nontuberculous mycobacteria in multivariate logistic regression analysis.
| Characteristics | Odds Ratio (95% CI) | p-value |
|---|---|---|
| Location (southern vs. northern Taiwan) | 1.73 (1.54–1.94) | <0.001 |
| Age between 25~45 (yes vs. no) | 0.36 (0.19–0.67) | 0.001 |
| Age between 45~65 (yes vs. no) | 0.66 (0.54–0.82) | <0.001 |
| Previous history of tuberculosis (yes vs. no) | 1.31 (1.15–1.49) | <0.001 |
| Chronic obstructive pulmonary disease (yes vs. no) | 1.17 (1.01–1.34) | 0.032 |
| Bronchiectasis (yes vs. no) | 2.14 (1.80–2.54) | <0.001 |
| Autoimmune (yes vs. no) | 1.79 (1.30–2.46) | 0.001 |
| Acquired immunodeficiency syndrome (yes vs. no) | 1.51 (1.06–2.16) | 0.022 |
| M. avium-intracellulare complex (yes vs. no) | 2.34 (2.03–2.70) | <0.001 |
| M. abscessus (yes vs. no) | 2.92 (2.50–3.42) | <0.001 |
| M. kansasii (yes vs. no) | 3.41 (2.78–4.19) | <0.001 |
All variables in Table 2 except radiographic pattern and extent were included in multivariate logistic regression analysis.
Predictors of NTM-PI were further investigated by ‘patients’, instead of ‘episodes’. Among the 7434 patients, 809 had multiple episodes at different time points. For them only the first episode was included. Another 178 had NTM isolates of different species at the same time and was excluded from this analysis. For the 7256 patients analyzed, the independent predictors of NTM-PI were very similar as those identified in the analysis done by ‘episodes’ (Table S5).
Discussion
This first multicenter NTM study conducted in Taiwan yielded several important findings. First, NTM-PI is common, with an average incidence rate of 46.0 episodes per 100,000 hospital-based patient-years between 2010 and 2014. In total, 9.5% of patients with NTM-PI experienced multiple episodes, and recurrent infections in 24.8% of them were caused by the same NTM species. In Southern Taiwan, the incidence rate of NTM-PI increased slightly over 5 years, particularly that caused by M. kansasii. Second, NTM-PI occurs equally in men and women in Taiwan, and is more likely to occur in the elderly, patients with structural lung diseases, and those living in southern Taiwan. Third, M. abscessus and MAC are copredominant in Southern Taiwan, whereas only MAC is dominant in Northern Taiwan.
The diagnosis of NTM-PI based on the ATS/IDSA guidelines requires a detailed review of extensive medical records, which is extremely time-consuming and labor-intensive2. Therefore, in many studies, clinical symptoms and radiographic findings were omitted and only the microbiological criteria were used as the diagnostic standard of NTM-PI3,11–13, sometimes with modifications14,15. According to present study, among those who fulfilled the microbiological component of the ATS diagnostic criteria for NTM-PI, 41.5% had no typical radiographic findings or no compatible clinical symptoms. Therefore, they were not initially deemed to be NTM-PI by primary care physicians. Furthermore, most studies have determined the prevalence rate, rather than the incidence rate1,6–9.
Because a large proportion of patients with NTM isolates have underlying structural lung diseases, clinical symptoms and radiographic abnormalities may indeed result from structural lung diseases rather than from NTM-PI. Diagnosing NTM-PI is difficult unless patients were longitudinally followed.
Previous studies revealed female predominance in NTM-PI2,16. Though several hypotheses have been proposed1,17–20, the underlying mechanism remains controversial. One possible explanation for the discrepancies in female predominance in this study is that TB is endemic in Taiwan, with a male: female ratio of 2.2–2.321. COPD is also more prevalent in men (male: female ratio: 1.9)22. Because both old pulmonary TB and COPD were the most common underlying pulmonary comorbidities in this study, the male predominance in the two diseases could offset the effects of female sex on the risk of NTM-PI.
In concordance with previous reports, the current study revealed that the incidence rate of NTM-PI increased with age13,23. This may reflect the accumulation of structural changes in lung parenchyma during aging. Thus, with the global trend of population aging24, we can expect a progressive increase in the prevalence of NTM diseases and their consequent medical expenses in the future.
The species distribution of NTM-PI varies worldwide; however, the exact mechanism remains unclear. MAC and M. kansasii are the most common species in most parts of the United States, Australia (Queensland), South Africa, and Japan2,11,14. In Singapore, southeastern United States (from Florida to Texas), Okinawa, and South Australia, rapidly growing mycobacteria (RGM), particularly M. abscessus, are more predominant25–27. In a recent meta-analysis of 105 publications from China, the ratio of MAC in NTM isolates was reported to increase with the latitude, whereas that of RGM isolates showed a contrasting trend5. Taiwan lies on the Tropic of Cancer; thus, the climate of Northern and Central Taiwan is subtropical, whereas that of Southern Taiwan is tropical. This might partially explain the MAC predominance in Northern Taiwan and the increased proportion of M. abscessus in Southern Taiwan13,15.
The increase of the incidence rate of M. kansasii-PI in southern Taiwan is a serious concern for its high clinical relevance28. The industrial activities, such as gold mining and iron manufacturing, may be associated with M. kansasii infection, particularly in cases of previous TB scarring or silicosis14,29,30. Southern Taiwan is famous for its iron and steel industries, as well as shipbuilding. Industrial water pollution and chlorination for disinfection may further favor the emergence of M. kansasii because of its natural resistance to disinfectants, acid, and heat, and its affinity for pipe surfaces31,32. The intense air pollution caused by heavy doses of particulates may also aid mycobacterial invasion and infection33, and the relatively wetter and hotter climate in Southern Taiwan might facilitate the survival of M. kansasii 27,34. These environmental factors might also explain the finding that southern Taiwan was an independent risk factor of NTM-PI.
The study has some limitations. First, it is a retrospective study without a standard protocol. Data on some critical factors, such as lifestyle, living environment, smoking history, occupation, and body mass index, are lacking. Second, the NTM isolates were not identified to the subspecies level, which have been revealed associated with different clinical manifestations33. Third, because data from only two medical centers and their branch hospitals were analyzed in this study, the findings may not entirely represent the incidence of NTM-PI in Taiwan. Forth, even we try to identify active lung lesions by reviewing clinical symptoms and serial chest images for the 141 cases suffering from ≥2 episodes of NTM-PI, the later episodes may be misjudged due to the radiographic sequelae of previous NTM-PI episodes. Lastly, due to lacks of results of antibiotic susceptibility test, it was not possible to predict the clinical effectiveness of specific antimicrobials. A prospective study with comprehensive case information and a standardized follow-up protocol in more hospitals in different areas of Taiwan is recommended to confirm the present findings.
In conclusion, this is the first multicenter, longitudinal NTM epidemiological report to show geographical diversity in NTM distribution and describe patient characteristics and the recurrent nature of NTM-PI. The findings remind clinicians that NTM-PI is common in Taiwan (46.0 episodes per 100,000 hospital-based patient-years), especially in elderly patients with structural lung disease in southern Taiwan. There may be no female predominance in endemic area of TB and COPD. NTM-PI should not be diagnosed solely based on the microbiological criteria of the ATS/IDSA guidelines because the overestimation of disease incidence is likely.
Methods
Study population
This retrospective study was conducted in six hospitals, namely a 2600-bed medical center (Taipei) and its two branch hospitals (Taipei and Hsinchu) in Northern Taiwan, and a 2665-bed medical center (Kaohsiung) and its two branch hospitals (both in Kaohsiung) in Southern Taiwan. This study was approved by the institutional ethic committees of both hospitals (NTUH REC 201508017RIND and KMUHIRB-SV[I]-2015200266).
Between 2008 and 2014, respiratory specimens were retrieved from the mycobacteriological databases of these six hospitals. NTM were classified into the following groups: M. kansasii, MAC, M. abscessus, M. fortuitum, M. gordonae, and other NTM species (see the supplementary file for mycobacteriology methodology). The clinical relevance of each NTM isolate was determined according to the ATS/IDSA guidelines2. First, patients with NTM isolates who fulfilled the microbiological criteria were identified, and those with typical radiographic findings suggestive of NTM-PI (fibrocavitary [FC] or nodular bronchiectatic [NB]) were further selected (detailed methodology in the supplementary file and Table S1). Subsequently, clinical symptoms, medical history, and other laboratory data were reviewed to exclude the diagnoses of diseases other than NTM-PI (Fig. 1). Clinical symptoms included cough, sputum, hemoptysis, chest tightness, dyspnea, fever, weight loss, night fever, and poor appetite. NTM-pulmonary colonization (PC) was considered if not fulfilling all of the aforementioned factors.
To determine the hospital-based incidence rate and to record the recurrent nature, new episodes of NTM-PI or NTM-PC occurring between 2010 and 2014 were used as the numerator, and the denominator was hospital-based patient-years, for which one patient with any outpatient or inpatient visit within one specific year was counted as 1 patient-year. A new episode of NTM-PI or NTM-PC was considered if either of the following conditions was established: (1) no culture was performed or no same NTM species were isolated in the previous 2 years, or (2) at least two respiratory samples were cultured and no isolates of the same NTM species were obtained in the previous year.
Data collection
Patient demographics and underlying comorbidities before obtaining each NTM isolate were retrieved. The comorbidities were categorized as pulmonary (e.g., COPD, asthma, previous pulmonary TB, bronchiectasis, pneumoconiosis, and ILD), and extrapulmonary (e.g., DM, autoimmune disease, chronic kidney disease [CKD], post-transplantation, HIV infection, congestive heart failure [CHF], and steroid usage). Furthermore, the TB registry databases of the Taiwan Centers for Disease Control were used to retrieve the history of pulmonary TB. Laboratory data included hemogram (leukocyte count and percentage of segment, hemoglobin level, platelet count) and serum biochemistry (creatinine, aspartate transaminase [AST], alanine transaminase [ALT], total bilirubin, and CRP) within 3 months of each sample yielding NTM isolate.
Statistical analysis
All data were expressed as numbers (percentage) or means ± standard deviations. Intergroup differences were analyzed using independent sample t tests for continuous variables and chi-square tests for categorical variables. Multivariate logistic regression analysis was performed to identify the independent risk factors for NTM-PI among all episodes of NTM-PI and NTM-PC. Statistical significance was set at p < 0.05. All statistical analyses were performed using SAS (Version 9.3; SAS Institute Inc., Cary, NC, USA).
Electronic supplementary material
Acknowledgements
The authors thank the Office of Medical Affairs and Information Technology, National Taiwan University Hospital and Statistical Analysis Laboratory, Department of Medical Research, Kaohsiung Medical University Hospital (KMUH104-4M04), and Kaohsiung Medical University for their assistance with data retrieval and analysis. We also thank Dr. Meng-Rui Lee, Lih-Yu Chang, Chia-Hao Chang, Chun-Ta Huang, Ying-Chun Chien, Chun-Kai Huang, and Yen-Lin Chen for their interpretations of the chest radiographs.
Author Contributions
Study concept and design: H.L.H., J.Y.W., I.W.C. Acquisition of data: H.L.H., M.H.C., P.L.L., C.C.S., J.T.W. Analysis and interpretation of data: H.L.H., M.H.C., P.L.L., J.Y.W., J.T.W., L.N.L. Drafting of the manuscript: H.L.L. Critical revision of the manuscript for important intellectual content: M.H.C., J.Y.W., I.W.C. Statistical analysis: H.L.H., J.Y.W., J.T.W., L.N.L. Study supervision: I.W.C., L.N.L. Guarantor of the paper: J.Y.W.
Competing Interests
The authors declare that they have no competing interests.
Footnotes
Electronic supplementary material
Supplementary information accompanies this paper at 10.1038/s41598-017-16559-z.
Publisher's note: Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
References
- 1.Cassidy PM, Hedberg K, Saulson A, McNelly E, Winthrop KL. Nontuberculous mycobacterial disease prevalence and risk factors: a changing epidemiology. Clin Infect Dis. 2009;49:e124–129. doi: 10.1086/648443. [DOI] [PubMed] [Google Scholar]
- 2.Griffith DE, et al. An official ATS/IDSA statement: diagnosis, treatment, and prevention of nontuberculous mycobacterial diseases. Am J Respir Crit Care Med. 2007;175:367–416. doi: 10.1164/rccm.200604-571ST. [DOI] [PubMed] [Google Scholar]
- 3.Johnson MM, Odell JA. Nontuberculous mycobacterial pulmonary infections. J Thorac Dis. 2014;6:210–220. doi: 10.3978/j.issn.2072-1439.2013.12.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Kendall BA, Winthrop KL. Update on the epidemiology of pulmonary nontuberculous mycobacterial infections. Semin Respir Crit Care Med. 2013;34:87–94. doi: 10.1055/s-0033-1333567. [DOI] [PubMed] [Google Scholar]
- 5.Yu X, et al. The prevalence of non-tuberculous mycobacterial infections in mainland China: Systematic review and meta-analysis. J Infect. 2016;73:558–567. doi: 10.1016/j.jinf.2016.08.020. [DOI] [PubMed] [Google Scholar]
- 6.Wu J, et al. Increase in nontuberculous mycobacteria isolated in Shanghai, China: results from a population-based study. PLoS One. 2014;9:e109736. doi: 10.1371/journal.pone.0109736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Marras TK, Mendelson D, Marchand-Austin A, May K, Jamieson FB. Pulmonary nontuberculous mycobacterial disease, Ontario, Canada, 1998–2010. Emerg Infect Dis. 2013;19:1889–1891. doi: 10.3201/eid1911.130737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gallivan M, Shah N, Flood J. Epidemiology of human Mycobacterium bovis disease, California, USA, 2003–2011. Emerg Infect Dis. 2015;21:435–443. doi: 10.3201/eid2103.141539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Prevots DR, et al. Nontuberculous mycobacterial lung disease prevalence at four integrated health care delivery systems. Am J Respir Crit Care Med. 2010;182:970–976. doi: 10.1164/rccm.201002-0310OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Diagnosis and treatment of disease caused by nontuberculous mycobacteria. This official statement of the American Thoracic Society was approved by the Board of Directors, March 1997. Medical Section of the American Lung Association. Am J Respir Crit Care Med156, S1–25 (1997). [DOI] [PubMed]
- 11.Hoefsloot W, et al. The geographic diversity of nontuberculous mycobacteria isolated from pulmonary samples: an NTM-NET collaborative study. Eur Respir J. 2013;42:1604–1613. doi: 10.1183/09031936.00149212. [DOI] [PubMed] [Google Scholar]
- 12.Simons S, et al. Nontuberculous mycobacteria in respiratory tract infections, eastern Asia. Emerg Infect Dis. 2011;17:343–349. doi: 10.3201/eid170310060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chien JY, Lai CC, Sheng WH, Yu CJ, Hsueh PR. Pulmonary infection and colonization with nontuberculous mycobacteria, Taiwan, 2000–2012. Emerg Infect Dis. 2014;20:1382–1385. doi: 10.3201/eid2008.131673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Chou MP, Clements AC, Thomson RM. A spatial epidemiological analysis of nontuberculous mycobacterial infections in Queensland, Australia. BMC Infect Dis. 2014;14:279. doi: 10.1186/1471-2334-14-279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lai CC, et al. Increasing incidence of nontuberculous mycobacteria, Taiwan, 2000–2008. Emerg Infect Dis. 2010;16:294–296. doi: 10.3201/eid1602.090675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bodle EE, Cunningham JA, Della-Latta P, Schluger NW, Saiman L. Epidemiology of nontuberculous mycobacteria in patients without HIV infection, New York City. Emerg Infect Dis. 2008;14:390–396. doi: 10.3201/eid1403.061143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chan ED, Bai X, Kartalija M, Orme IM, Ordway DJ. Host immune response to rapidly growing mycobacteria, an emerging cause of chronic lung disease. Am J Respir Cell Mol Biol. 2010;43:387–393. doi: 10.1165/rcmb.2009-0276TR. [DOI] [PubMed] [Google Scholar]
- 18.Kim RD, et al. Pulmonary nontuberculous mycobacterial disease: prospective study of a distinct preexisting syndrome. Am J Respir Crit Care Med. 2008;178:1066–1074. doi: 10.1164/rccm.200805-686OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Straub RH. The complex role of estrogens in inflammation. Endocr Rev. 2007;28:521–574. doi: 10.1210/er.2007-0001. [DOI] [PubMed] [Google Scholar]
- 20.Salgame P. Host innate and Th1 responses and the bacterial factors that control Mycobacterium tuberculosis infection. Curr Opin Immunol. 2005;17:374–380. doi: 10.1016/j.coi.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 21.Centers for Disease Control, T. Statistics of Communicable Diseases and Surveillance Report 2015, (Centers for Disease Control, Taiwan, Taipei, Taiwan, 2016).
- 22.Lee CH, et al. Pulmonary tuberculosis and delay in anti-tuberculous treatment are important risk factors for chronic obstructive pulmonary disease. PLoS One. 2012;7:e37978. doi: 10.1371/journal.pone.0037978. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang Z, et al. Differences in risk factors and drug susceptibility between Mycobacterium avium and Mycobacterium intracellulare lung diseases in China. Int J Antimicrob Agents. 2015;45:491–495. doi: 10.1016/j.ijantimicag.2015.01.012. [DOI] [PubMed] [Google Scholar]
- 24.Organization, W.H. World report on ageing and health. Geneva: World Health Organization (2015).
- 25.Kirschner RA, Jr., Parker BC, Falkinham JO. 3rd. Epidemiology of infection by nontuberculous mycobacteria. Mycobacterium avium, Mycobacterium intracellulare, and Mycobacterium scrofulaceum in acid, brown-water swamps of the southeastern United States and their association with environmental variables. Am Rev Respir Dis. 1992;145:271–275. doi: 10.1164/ajrccm/145.2_Pt_1.271. [DOI] [PubMed] [Google Scholar]
- 26.Tang SS, Lye DC, Jureen R, Sng LH, Hsu LY. Rapidly growing mycobacteria in Singapore, 2006–2011. Clin Microbiol Infect. 2015;21:236–241. doi: 10.1016/j.cmi.2014.10.018. [DOI] [PubMed] [Google Scholar]
- 27.van Ingen J, Boeree MJ, Dekhuijzen PN, van Soolingen D. Environmental sources of rapid growing nontuberculous mycobacteria causing disease in humans. Clin Microbiol Infect. 2009;15:888–893. doi: 10.1111/j.1469-0691.2009.03013.x. [DOI] [PubMed] [Google Scholar]
- 28.van Ingen J, et al. Clinical relevance of non-tuberculous mycobacteria isolated in the Nijmegen-Arnhem region, The Netherlands. Thorax. 2009;64:502–506. doi: 10.1136/thx.2008.110957. [DOI] [PubMed] [Google Scholar]
- 29.Kubin M, Svandova E, Medek B, Chobot S, Olsovsky Z. Mycobacterium kansasii infection in an endemic area of Czechoslovakia. Tubercle. 1980;61:207–212. doi: 10.1016/0041-3879(80)90040-9. [DOI] [PubMed] [Google Scholar]
- 30.Corbett EL, et al. Mycobacterium kansasii and M. scrofulaceum isolates from HIV-negative South African gold miners: incidence, clinical significance and radiology. Int J Tuberc Lung Dis. 1999;3:501–507. [PubMed] [Google Scholar]
- 31.Falkinham JO, III, Norton CD, LeChevallier MW. Factors influencing numbers of Mycobacterium avium, Mycobacterium intracellulare, and other Mycobacteria in drinking water distribution systems. Appl Environ Microbiol. 2001;67:1225–1231. doi: 10.1128/AEM.67.3.1225-1231.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Le Dantec C, et al. Chlorine disinfection of atypical mycobacteria isolated from a water distribution system. Appl Environ Microbiol. 2002;68:1025–1032. doi: 10.1128/AEM.68.3.1025-1032.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Aliyu G, et al. Prevalence of non-tuberculous mycobacterial infections among tuberculosis suspects in Nigeria. PLoS One. 2013;8:e63170. doi: 10.1371/journal.pone.0063170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Morimoto K, et al. A Laboratory-based Analysis of Nontuberculous Mycobacterial Lung Disease in Japan from 2012 to 2013. Ann Am Thorac Soc. 2017;14:49–56. doi: 10.1513/AnnalsATS.201607-573OC. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.