Abstract
Background
Breast cancer incidence and mortality are influenced by early-detection methods, including mammographic screening. Demographic changes in US statistics serve as a model for changes that can be anticipated in countries where mammographic screening has not been implemented.
Methods
SEER statistics (1973–2013) for breast cancer mortality, incidence, stage at diagnosis, and age at diagnosis were examined. Temporal associations between screening changes and breast cancer demographics in the US were documented.
Findings
Before 1982 (pre-screening), breast cancer incidence in the US remained stable, with similar incidence of localized and regional cancers, and with in-situ disease comprising <2% of diagnosed disease1. During the transitional phase of mammographic screening, breast cancer incidence increased. In 1991, breast cancer age-adjusted mortality rates began decreasing and have continued to decrease. In the post-screening phase, stage distribution stabilized, but now with localized and in-situ disease representing the majority of diagnosed cases. The median age at diagnosis has increased to 61 years.
Discussion
Mammographic screening increases breast cancer incidence, shifts the stage distribution toward earlier stage disease, and, in high-income countries, is associated with improved survival. Whether similar improvement in breast cancer survival can be achieved in the absence of mammographic screening has yet to be conclusively demonstrated.
INTRODUCTION
Between 1990 and 2013, breast cancer mortality in the United States (US) declined by 34 percent2, but this trend has not yet been mirrored in low and middle income countries (LMICs). Over the past four decades, mammographic screening has been one of the most significant changes in breast cancer control strategies in the US and in other high income countries that have similarly seen dropping breast cancer mortality. While much focus has been directed at the mortality impact of mammographic screening3, less attention has been paid to how population-based mammographic screening impacts on overall breast cancer demographics, which for countries preparing to implement mammographic screening is important to anticipate. The purpose of this overview is to examine how breast cancer incidence, stage at diagnosis and age at diagnosis changes as mammographic screening is introduced into a previously unscreened population, using historical evidence from SEER in the US as a model. Implications for LMICs where mammographic screening has not been fully implemented will be examined.
METHODS
All women diagnosed with a first invasive or in situ breast cancer in the US from 1973 to 2013 were identified through nine population-based cancer registries that participate in the National Cancer Institute’s Surveillance, Epidemiology, and End Results Program (SEER). Registries included Atlanta, Connecticut, Detroit, Hawaii, Iowa, New Mexico, San Francisco-Oakland, Seattle-Puget Sound, and Utah. SEER 9 (n= 86,355,485) covers approximately 9.4% of the U.S. population based on the 2010 census, and it is estimated that greater than 95% of all incident cases in the populations under surveillance are ascertained. The population covered by SEER is comparable to the general US population according to measures of socioeconomic status and education, although the SEER population tends to have a higher proportion of foreign-born persons than the general U.S. population4.
Using SEER*Stat software version 8.2.3 (National Cancer Institute, Bethesda, MD; released April 11, 2016)5, these estimates were used to calculate incidence rates and mortality rates of female breast carcinoma that were age-adjusted to the 2000 U.S. population (19 age groups – Census P25-1130). Rates of both invasive and in situ breast cancer were evaluated. Incidence rates were evaluated for all ages, and were also stratified by age at diagnosis (30–39 years, 40–49 years, 50–59 years, 60-60 years, 70–79 years, and ≥80 years). For invasive cases, SEER historic stage A was chosen to evaluate extent of disease at diagnosis due to the relatively higher percentage of missing data in the National Cancer Institute’s adjusted American Joint Committee on Cancer Classification (AJCC) stage, 6th edition variable. The historic stage A variable defines breast cancer as localized (confined to the breast), regional (contiguous and adjacent organ spread such as lymph nodes or chest wall) and distant disease (remote metastases).
FINDINGS
Breast cancer mortality
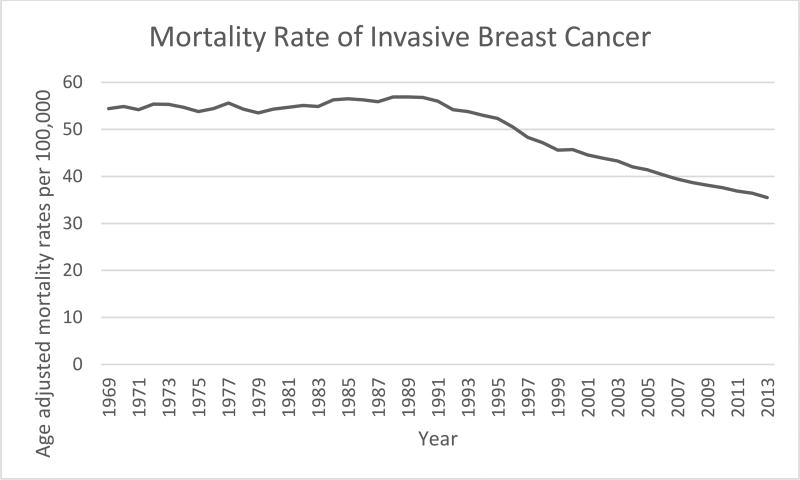
Breast cancer age-adjusted mortality rates were unchanged from 1973 when SEER statistics were first recorded until 1990 at approximately 55 cases per 100,000 (Figure 1). In 1991, breast cancer mortality has been dropping by between 1 and 2% per year.
Figure 1.
Trends in Age-Standardized Breast Cancer Mortality, United States, 1973–2013
Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) SEER*Stat Database: Mortality - All COD, Aggregated With State, Total U.S. (1969–2013 <Katrina/Rita Population Adjustment>, National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016. Underlying mortality data provided by NCHS (www.cdc.gov/nchs).
Breast cancer incidence
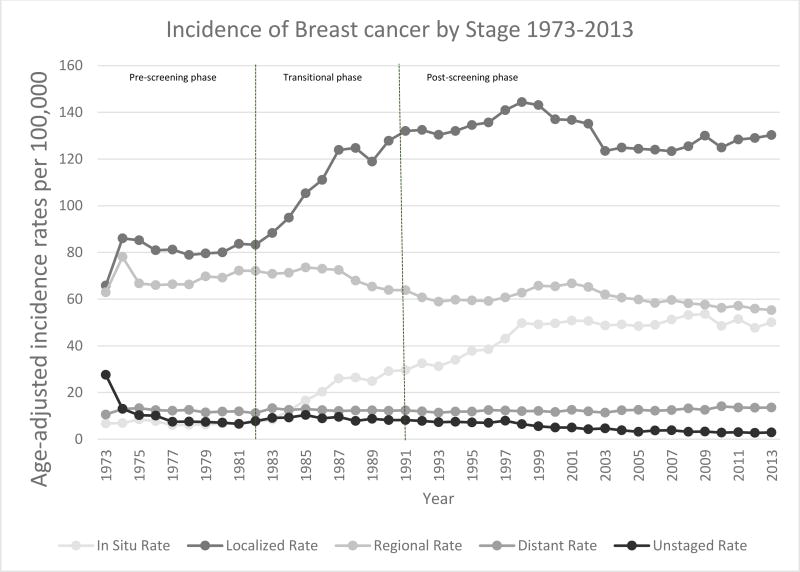
Mammographic screening was instituted in the early 1980s as a result of the Health Insurance Plan (HIP) trial in New York6. Before 1982 in the pre-screening phase, overall breast cancer incidence had been relatively stable, with a similar incidence of both localized (node negative) and regional (node positive) cancers at time of diagnosis, and with in situ disease comprising less than 2% of diagnosed disease1. With the introduction of mammographic screening (Figure 2), breast cancer incidence rose by about 4% per year between 1982 and 1986, continued to increase through 1991when breast cancer age-adjusted mortality rates first began to decrease (Figure 1). In the post-screening phase after 1991, breast cancer incidence continued to rise to an age-adjusted rate of 141.4 per 100,000 women by 1999, then declined between 1999–2003, and now have stabilized at approx. 127 per 100,000 women since 20037.
Figure 2.
Trends in Age-Standardized Breast Cancer Incidence by Stage at Diagnosis, United States, 1963–2013
Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2013), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission
The mortality and incidence patterns are similar among racial groups in the US, but differences in time course, especially comparing white and black women. While white women have historically had higher incidence rates than black women, the rates converged in 20128. The decrease in breast cancer mortality rates among black women was delayed and did not begin its decrease until after 1995. Unfortunately, the mortality disparity between black and white women nationwide has persisted. In 2012, death rates were still 42% higher among black women than in white women. Whether this difference represents differences in early detection, access to and utilization of treatment or biological differences remains an area of ongoing investigation.
Stage at diagnosis
The increased breast cancer incidence seen in the US in association with mammographic screening was accompanied by a shift in breast cancer stage distribution toward earlier staged disease. During the transitional phase between the introduction of mammographic screening in 1982 and the subsequent decrease in overall age-adjusted breast cancer mortality in 1991, the rate of localized (node-negative) breast cancers rose from 80 to over 130 cases per 100,000 while DCIS increased from fewer than 5 to more than 30 cases per 100,000. Today in the post-screening phase, stage distribution again appears to have stabilized, but now with localized and in situ breast cancer representing the significant majority of diagnosed cases (Figure 2). Today, 20.6% of breast cancer cases diagnosed in the US are in situ disease8.
Notably, any decrease in incidence rates of advanced stage disease since the introduction of mammographic screening has been modest and quantitatively has not mirrored the corresponding increase in early stage disease during the same time period. The rate of regional (node-positive) disease declined from greater than 70 to less than 60 cases per 100,000 between 1982 and 2012 (Figure 2), suggesting that some less favorable breast cancer biological subtypes may continue to present as more advanced disease despite the utilization of mammographic screening. Screening mammography increases the proportion of estrogen receptor positive, HER2 negative (Luminal A and Luminal B) breast cancer subtypes9, which when appropriately treated have a more favorable prognosis.10
Age at diagnosis
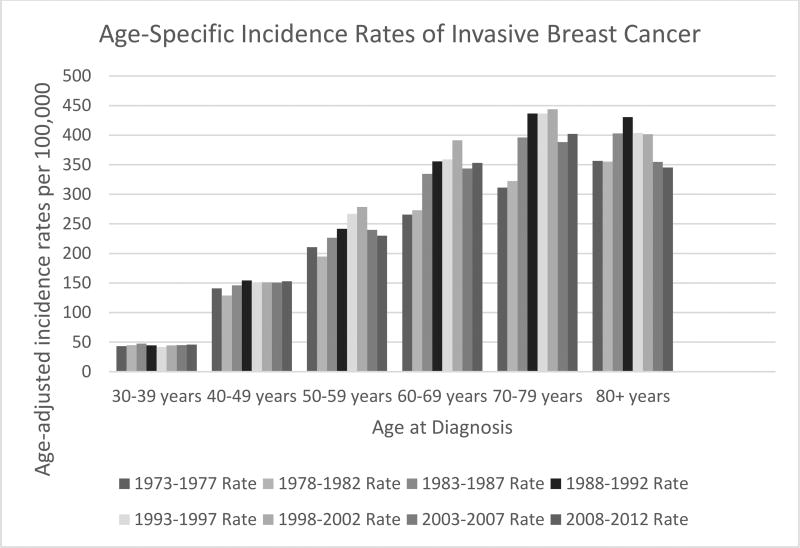
The increase in breast cancer incidence corresponds to an increase in cancer diagnosis in women aged 50 to 80 years; stratifying incidence rates over time by different age groups demonstrates increasing incidence rates during the transitional phase, but only in women aged 50 years or older and peaking during the 1998 – 2002 epoch (Figure 3). The median age of breast cancer diagnosis has now risen to 61 years in the US11.
Figure 3.
Trends in Age-Standardized Breast Cancer Incidence by Age at Diagnosis in Five Year Intervals, United States, 1973–2012
Rates are per 100,000 and age-adjusted to the 2000 US Std Population (19 age groups - Census P25-1130) standard.
Surveillance, Epidemiology, and End Results (SEER) Program (www.seer.cancer.gov) Research Data (1973–2013), National Cancer Institute, DCCPS, Surveillance Research Program, Surveillance Systems Branch, released April 2016, based on the November 2015 submission
DISCUSSION
International breast cancer demographics
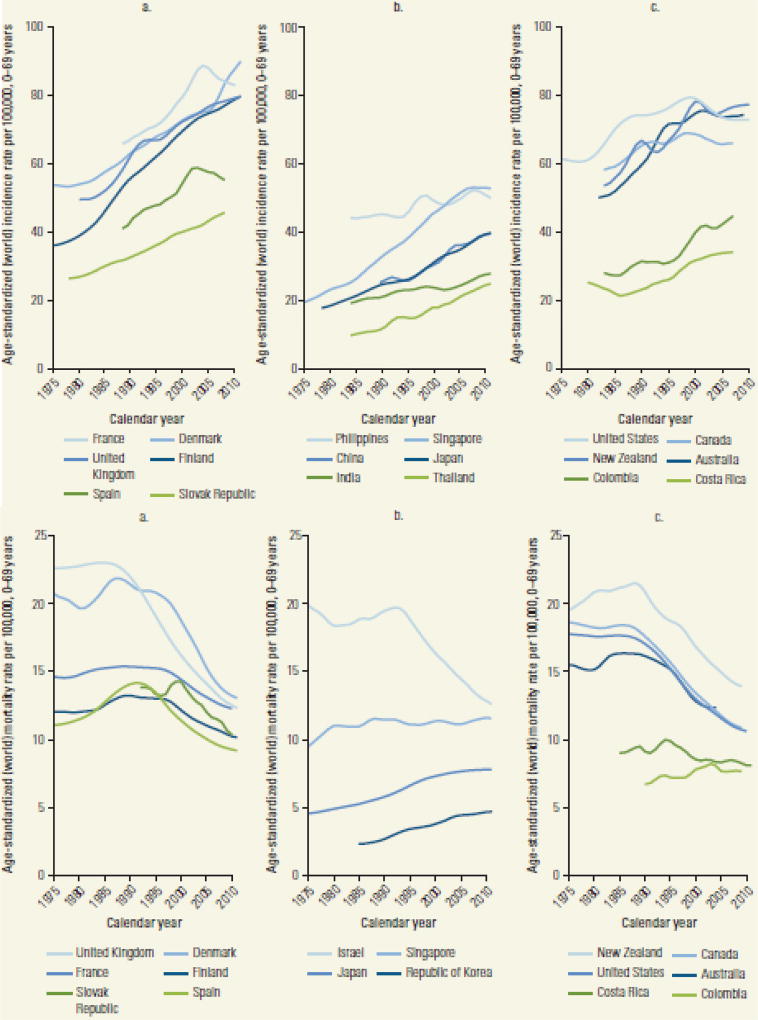
The shifting patterns of breast cancer incidence in the US have been replicated in other countries with successful national screening programs. For example, the United Kingdom (U.K.) NHS breast screening programme (BSP), which began in 1988, had screening rates of over 90% of the target population (women aged 50 years and older) by 199512. Like the US, incidence rates increased, and the majority of breast cancer cases were diagnosed at an earlier stage13. Similar patterns in incidence and mortality can be observed in many countries with widespread use of mammography (Figure 4); a review by Anderson et al14 summarized increases in breast cancer incidence in 18 countries, with accompanying mortality declines in sixteen. Only two countries had stable mortality rates; both had more a modest increase in incidence over time compared to the other countries.
Figure 4.
Trends in Age-Standardized Breast Cancer Incidence and Mortality, Selected Countries, 1975–2010
Source: Ferlay and others 2013; WHO Mortality Database (http://www.who.int/healthinfo/statistics/mortality_rawdata/en/index.html). Previously published in reference 7.
In a review of 32 countries beginning in 1995, De Santis and colleagues15 found that mortality declines did not occur in countries without accompanying increases in incidence. Some lower-income countries (e.g. Guatemala, Moldova, Philippines and South Africa) showed stable or even increasing mortality over time, but incidence trends for these countries were not provided. These findings suggest that early detection efforts, which necessarily increase breast cancer incidence rates but also increase the relative fraction of more favorable biology breast cancers, may be a necessarily prerequisite to improving breast cancer mortality at the population level.
Early detection programs without mammographic screening
In November 2014, experts from 16 countries met at the International Agency for Research on Cancer (IARC) to assess the cancer-preventive and adverse effects of different methods of breast cancer screening. This diverse expert group concluded that existing evidence is sufficient to conclude that mammographic screening reduces breast-cancer mortality in women 50 to 69 years of age3. Although mammography screening has contributed to improved breast cancer survival in many countries, such screening programs are often unavailable in LMICs. These countries continue to struggle with increasing morbidity and mortality from advanced breast cancers, which in many situations comprise the majority of cases diagnosed.16 Mammographic screening is generally neither affordable nor appropriate for detecting tumors in the advanced stages usually seen in LMICs, where women often present with tumors that are easily palpable, visible or ulcerated through the skin17, necessitating an exploration of alternatives to mammographic screening.
A core question is whether practical alternatives exist to mammographic screening that will achieve a similar early stage cancer distribution where improved population-based breast cancer mortality is possible. Clinical detection through patient awareness education and clinical evaluation including clinical breast examination (CBE) is the mainstay of breast cancer detection in the absence of mammographic screening, but evidence that CBE-based detection programs can improve breast cancer mortality is lacking.
Though designed to evaluate the effect of mammography screening, data from the Canadian National Breast Screening Study18 provides some evidence that breast cancer early detection can be achieved without the routine implementation of mammographic screening. This Canadian randomized control trial comparing mammography (intervention arm) to clinical breast examination (CBE; control) demonstrated that early detection may be feasible in a mature healthcare system. This trial found no difference in breast cancer survival comparing mammographic screening with the control arm. However, the mean tumor size of cancers diagnosed in women randomized to the control arm was approximately 2cm, which compares very favorably to a mean tumor size of ~3cm in the U.S. from 1979–1983 prior to the introduction of mammography screening, and 2cm from 1989 to 199319.
Further investigations of clinical screening based on CBE are underway. A cluster randomized controlled trial in India in 2006 evaluated whether three rounds of triennial CBE can reduce the incidence rate of advanced disease and breast cancer mortality. Results reported after the first round of screening demonstrate an increased proportion of small, early-stage, node-negative breast cancer in the intervention group. Furthermore, more women in this group received more conservative surgery, and fewer deaths were reported20. Another RCT in Mumbai, India, is investigating breast cancer screening with CBE performed by trained primary health workers at 24-month intervals. While down-staging at diagnosis was not evident in the first and second rounds of screening, the third round of screening demonstrated improved stage distribution with the majority of breast cancers being stage I or II in the intervention group but not the control group.13
It should be noted that, in all these settings, available and improving systemic therapies have also contributed to the mortality declines. In light of the projected increase in breast cancer incidence and mortality in LMICs, exploring alternative methods for achieving down-staging of disease and concomitant reductions in disease via access to appropriate and timely treatment should be a priority. Therefore it should be noted that in any setting, breast early detection efforts, regardless of technology or clinical approach used to achieve this outcome, should be offered alongside timely and effective treatment.
In conclusion, population-based mammographic screening promotes well-defined and specific changes in breast cancer demographics. Screening increases breast cancer incidence, particularly of favorable biology, early staged cancers. The median age of diagnosis rises in conjunction with mammographic screening. These features may be necessary for improving breast cancer mortality at the population level, since it has been a near ubiquitous finding among countries where improved mortality has been achieved. It remains unknown if early detection efforts yielding these outcomes can be achieved without mammographic screening using clinical methods based on CBE. Regardless of the early detection method used, cancer detection must be followed by effective and timely cancer treatment if improved mortality rates are to be realized.
Synopsis.
Breast cancer incidence and mortality are influenced by early-detection methods, including mammographic screening. Mammographic screening increases breast cancer incidence, shifts the stage distribution toward earlier stage disease, and, in high-income countries, is associated with improved survival. Demographic changes in US statistics serve as a model for changes that can be anticipated in countries where mammographic screening has not been implemented.
Acknowledgments
Funding:
Dr. Verdial was supported by a training grant from the National Institute of Diabetes and Digestive and Kidney Diseases of the National Institutes of Health under Award Number T32DK070555. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
References
- 1.Ernster VL, Barclay J, Kerlikowske K, Grady D, Henderson C. Incidence of and treatment for ductal carcinoma in situ of the breast. Jama. 1996;275(12):913–918. http://www.ncbi.nlm.nih.gov/pubmed/8598618. [PubMed] [Google Scholar]
- 2.Desantis C, Ma J, Bryan L, Jemal A. Breast Cancer Statistics, 2013. CA Cancer J Clin. 2014;64:52–62. doi: 10.3322/caac.21203. [DOI] [PubMed] [Google Scholar]
- 3.Lauby-Secretan B, Scoccianti C, Loomis D, et al. Breast-Cancer Screening — Viewpoint of the IARC Working Group. N Engl J Med. 2015;372(24):2353–2358. doi: 10.1056/NEJMsr1504363. [DOI] [PubMed] [Google Scholar]
- 4.SEER. SEER*Stat Database: Incidence - SEER 9 Regs Research Data, Nov 2015 Sub (1973–2013) <Katrina/Rita Population Adjustment> - Linked To County Attributes - Total U.S., 1969–2014 Counties. 2015 www.Seer.Cancer.Gov.
- 5.Surveillance Research Program National Cancer Institute. SEER*Stat software. seer.cancer.gov/seerstat.
- 6.Chu KC, Smart CR, Tarone RE. Analysis of Breast Cancer Mortality and Stage Distribution by Age for the Health Insurance Plan Clinical Trial. JNCI J Natl Cancer Inst. 1988;80(14):1125–1132. doi: 10.1093/jnci/80.14.1125. [DOI] [PubMed] [Google Scholar]
- 7.Gangnon RE, Sprague BL, Stout NK, et al. The contribution of mammography screening to breast cancer incidence trends in the United States: an updated age-period-cohort model. Cancer Epidemiol Biomarkers Prev. 2015;24(6):905–912. doi: 10.1158/1055-9965.EPI-14-1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Desantis CE, Fedewa SA, Sauer AG, Kramer JL, Smith RA, Jemal A. Breast Cancer Statistics, 2015 : Convergence of Incidence Rates Between Black and White Women. 2016;66(1):31–42. doi: 10.3322/caac.21320. [DOI] [PubMed] [Google Scholar]
- 9.Dawson S-J, Duffy SW, Blows FM, et al. Molecular characteristics of screen-detected vs symptomatic breast cancers and their impact on survival. Br J Cancer. 2009;101(8):1338–1344. doi: 10.1038/sj.bjc.6605317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Valla M, Vatten LJ, Engstrøm MJ, et al. Molecular Subtypes of Breast Cancer: Long-term Incidence Trends and Prognostic Differences. Cancer Epidemiol Prev Biomarkers. 2016;25(12):1625–1635. doi: 10.1158/1055-9965.EPI-16-0427. [DOI] [PubMed] [Google Scholar]
- 11.Howlader N, Noone AM, Krapcho M, Miller D, Bishop K, Altekruse SF, Kosary CL, Yu M, Ruhl J, Tatalovich Z, Mariotto A, Lewis DR, Chen HS, Feuer EJCK, editors. SEER Cancer Statistics Review, 1975–2013. Bethesda, MA: 2016. http://seer.cancer.gov/csr/1975_2013/ [Google Scholar]
- 12. [Accessed January 8, 2017];Breast Cancer Screening Programs in 26 ICSN Countries, 2012: Organization, Policies, and Program Reach. https://healthcaredelivery.cancer.gov/icsn/breast/screening.html. Published 2016.
- 13.National Cancer Registration and Analysis Service (NCRAS) 2016 [Google Scholar]
- 14.Anderson BO, Lipscomb J, Murillo R, Thomas DB. Disease Control Priorities. 3. Vol. 3. World Bank Publications; 2015. Breast cancer (3) pp. 473–480. http://www.ncbi.nlm.nih.gov/entrez/query.fcgi?cmd=Retrieve&db=PubMed&dopt=Citation&list_uids=1625737\ nhttp://www.nejm.org/doi/pdf/10.1056/NEJM199208133270706. [Google Scholar]
- 15.DeSantis CE, Bray F, Ferlay J, Lortet-Tieulent J, Anderson BO, Jemal A. International Variation in Female Breast Cancer Incidence and Mortality Rates. Cancer\tEpidemiology\tBiomarkers\t&\tPrevention. 2015;24(10):OF1–OF12. doi: 10.1158/1055-9965.EPI-15-0535. [DOI] [PubMed] [Google Scholar]
- 16.Goss PE, Lee BL, Badovinac-Crnjevic T, et al. Planning cancer control in Latin America and the Caribbean. Lancet Oncol. 2013;14(5):391–436. doi: 10.1016/S1470-2045(13)70048-2. [DOI] [PubMed] [Google Scholar]
- 17.Tsu VD, Jeronimo J, Anderson BO. Why the time is right to tackle breast and cervical cancer in low-resource settings. Bull World Health Organ. 2013;91(9):683–690. doi: 10.2471/BLT.12.116020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Miller AB, Wall C, Baines CJ, Sun P, To T, Narod Sa. Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. Bmj. 2014;348(feb11 9):g366–g366. doi: 10.1136/bmj.g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Cady B, MD S, JG S, Thakur R, MA W, PT L. The new era in breast cancer: Invasion, size, and nodal involvement dramatically decreasing as a result of mammographic screening. Arch Surg. 1996;131(3):301–308. doi: 10.1001/archsurg.1996.01430150079015. http://dx.doi.org/10.1001/archsurg.1996.01430150079015. [DOI] [PubMed] [Google Scholar]
- 20.Sankaranarayanan R, Ramadas K, Thara S, et al. Clinical breast examination: Preliminary results from a cluster randomized controlled trial in India. J Natl Cancer Inst. 2011;103(19):1476–1480. doi: 10.1093/jnci/djr304. [DOI] [PubMed] [Google Scholar]
- 21.Mittra I, Mishra GA, Singh S, et al. A cluster randomized, controlled trial of breast and cervix cancer screening in Mumbai, India: Methodology and interim results after three rounds of screening. Int J Cancer. 2010;126(4):976–984. doi: 10.1002/ijc.24840. [DOI] [PubMed] [Google Scholar]
- 22.Mishra G, Dhivar H, Gupta S, Kulkarni S, Shastri S. A population-based screening program for early detection of common cancers among women in India – methodology and interim results. Indian J Cancer. 2015;52(1):139. doi: 10.4103/0019-509X.175581. [DOI] [PubMed] [Google Scholar]