Abstract
Background
Specimen labeling errors have long plagued the laboratory industry putting patients at risk of transfusion-related death, medication errors, misdiagnosis, and patient mismanagement. Many interventions have been implemented and deemed to be effective in reducing sample error rates. The objective of this review was to identify and evaluate the effectiveness of laboratory practices/ interventions to develop evidence based recommendations for the best laboratory practices to reduce labeling errors.
Content
The standardized LMBP™ A-6 methods were used to conduct this systematic review. Total evidence included 12 studies published during the time periods of 1980 to September 2015. Combined data from seven studies found that the interventions developed as a result of improved communication and collaboration between the laboratory and clinical staff resulted in substantial decrease in specimen labeling errors (Median relative percent change in labeling errors: −75.86; IQI: −84.77, −58.00). Further data from subset of four studies showed a significant decrease in specimen labeling errors after the institution of the standardized specimen labeling protocols (Median relative percent decrease in specimen labeling errors: −72.45; IQI: −83.25, −46.50).
Summary
Based on the evidence included in this review, the interventions that enhance the communication and collaboration between laboratory and healthcare professionals can decrease the specimen identification errors in healthcare settings. However, more research is needed to make the conclusion on the effectiveness of other evaluated practices in this review including training and education of the specimen collection staff, audit and feedback of labeling errors, and implementation of new technology (other than barcoding).
INTRODUCTION
Sample labeling errors have long plagued the laboratory industry putting patients at risk of transfusion-related death, medication errors, misdiagnosis, and patient mismanagement. It has been estimated over 160,000 adverse patient events occur each year in the U.S. because of patient or specimen identification errors involving the laboratory.1 Eleven percent of all transfusion deaths occur as a result of the phlebotomist not properly identifying the patient or mislabeling the tube of blood.2 Inadequately labeled samples account for 5.6–6.7% of all rejected samples.3, 4 A 2009 Q-Probes study found the rate of tube mislabeling of blood bank samples to be 1.12 percent. 5
The use of barcoding systems for specimen labeling and point-of-care test barcoding was established by the Centers for Disease Control and Prevention (CDC) as a Best Practice in 2010 to reduce identification errors and improve the accuracy of patient specimen and laboratory testing identification in hospital settings.6, 7 However, between 2007 and 2015 the incidence of wrong-blood-in-tube errors (WBIT) remained unchanged even though barcode scanner usage increased from eight percent to 38 percent during the same period.8
A thorough literature review and establishment of Best Practices for the industry are necessary to protect patients from the threats of specimen labeling errors. The objective of this review is to identify and evaluate the effectiveness of existing interventions /practices to develop evidence based recommendations for the best laboratory practices to reduce sample labeling errors.
DESCRIPTION OF EVALUATED PRACTICES
In this review we evaluated the effectiveness of four laboratory practices to reduce the specimen labeling errors at the time of specimen collection,
Improved Communication and Collaboration between Laboratory and Healthcare Professionals: Formation of Multidisciplinary Teams
Education and Training of healthcare staff responsible for specimen collection
Audit and Feedback of Labeling Errors: Real time event reporting
Implementation of new Technology
Improved Communication and Collaboration between Laboratory and Healthcare Professionals: Formation of Multidisciplinary Teams (MDT)
MDT approach help to improve the communication and collaboration between the key stakeholders including pathologists, radiologists, diagnosticians, management, and treating health care professionals (clinicians and nurses) to reduce diagnostic errors related to patient misidentification due to labeling errors.9 Generally the improved collaboration in the form of MDT result into emphasize that health-care providers have primary responsibility for checking/verifying a patient’s identity, development of standardized organization policies and protocols to emphasize the importance of positive patient identification that are compatible with the values and needs of the medical facilities, e.g., requirement of unique patient identifiers on specimen labels, implementation of zero tolerance policy, staff performance assessment, availability of adequate number of qualified personnel to perform specimen collection, reinforcement of specimen labeling at the bed side, delta checks etc.
Education and Training of Healthcare Staff Responsible for Specimen Collection
These interventions include education and training of laboratory staff (e.g., technicians/scientists, phlebotomists) and clinical staff (e.g., nurses) who are responsible for collection and labeling of patient specimen in clinical settings. Education and training sessions serve to maintain and increase the knowledge and skills of the staff that involve patient preparation, filling of test requisition form (TRF), collection and labeling of patient specimen.
Education and training can be conducted through different outreach methods, e.g., educational training modules, dissemination of information through seminars, bulletins, newsletter, courses, infographics, and technical briefs, training in phlebotomy practices, training in technology and practical demonstrations during training sessions.
Audit and Feedback of Labeling Errors: Real time event reporting
Collection of information/ error data about mislabeled specimens on regular basis and feedback to the management and the involved staff with the aim to eliminate these errors or minimize the relative risk of errors. Literature has shown that sharing trending data on mislabeled samples on regular basis to patient care areas can change phlebotomy practices and reduce specimen mislabeling.
METHODS
The standardized LMBP A-6 methods were used to conduct LMBP systematic reviews have been described elsewhere.6 For this review, a systematic review team was formed including review coordinator, data abstractors, CDC liaison, and the advisory group called Expert Panel Team comprised of experts with varied professional experience. (Supplemental Appendix A lists the members of Expert Panel team for this review). The systematic review team worked under the oversight of the independent, unpaid, nonfederal LMBP Workgroup team. (Supplemental Appendix B lists the LMBP Workgroup members).
Ask (A-1): Review Question and Analytic Framework
Review Question (s)
What practices are effective at reducing patient identification (ID) errors due to specimen mislabeling at the time of sample collection in all types of healthcare settings?
To address the applicability of the implementation of evaluated interventions to reduce specimen labeling errors, we also investigated whether the effectiveness of these practices vary according to the,
Type of setting/ population (e.g., emergency, in-patient, out-patient)
Organization type (e.g., academic institution, private clinic)
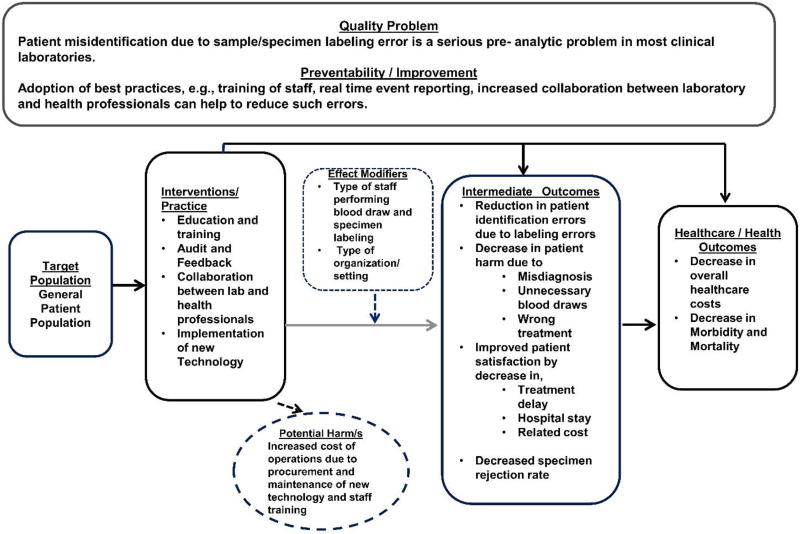
The conceptual approach in Figure 1 illustrates the causal relationship of the laboratory interventions to the relevant intermediate outcomes, e.g., reduction in patient labeling errors and associated harm to patient health due to missed/ delayed diagnosis, unnecessary blood draws, wrong treatment and improved patient satisfaction by a decrease in treatment delay, hospital stay, and related costs. Ultimately, these interventions may lead to decrease in overall morbidity and mortality and decrease in healthcare costs at organizational level.
Figure 1.
Analytic Framework
Following PICO elements were considered for this review:
Population
General patients attending all types of healthcare settings who require specimen collection for diagnostic laboratory testing.
Intervention
Following practices to reduce patient identification due to labeling errors were evaluated
Improved Communication and Collaboration between Laboratory and Healthcare Professionals: Formation of Multidisciplinary Teams
Education and Training of healthcare staff responsible for specimen collection
Audit and Feedback of Labeling Errors: Real time event reporting
Implementation of new Technology (other than barcoding): e.g., automatic identification and data capture (AIDC) systems include radio frequency identification (RFID), biometrics (e.g., optical character recognition), magnetic stripes, smart cards, point-of-care label printers and scanners, voice recognition.
Comparison group
Group with no exposure to the intervention/practice of interest
Outcomes of interest
Primary outcome(s) of interest
Decrease in specimen labeling errors at the time of specimen collection
- Decrease in patient harm due to,
- Misdiagnosis
- Unnecessary blood draws
- Wrong treatment
- Increase in patient satisfaction by decrease in,
- Treatment delay
- Hospital stay
- Related cost
Decreased specimen rejection rate
Long term Healthcare outcomes
Decrease in related,
Morbidity and Mortality
Overall Healthcare costs
There is no generally accepted taxonomy of identification errors. Varied definitions for ‘specimen mislabeling’ have been used interchangeably in the existing literature. For example, when a specimen from one patient is labeled with another patient’s name some studies described this error as ‘mislabeled specimens’ when as others categories this error as ‘wrong blood in tube’. For the analyses purposes, we lumped subsequent categories as ‘specimen labeling errors’ in this review and each category was defined as follows,
Mislabeled/ misidentified specimen
Specimen label with patent identifiers from wrong patient, mostly referred as wrong blood in tube (WBIT) in the literature.
Mismatched labels
Patient information on the label does not meet with the accompanying requisition form or patient information on specimen label does not match with the patient’s wrist band
Incomplete, illegible or unlabeled specimen label
A specimen with a label that lists only partial information of required unique patient identifier; a specimen without a label or without any patient identifiers on the label; and finally specimen label that had illegible patient identifiers that could be read electronically or manually respectively.
Inclusion /Exclusion Criteria for Evidence to be Included in this Review
Exclusion Criteria
To be included in this review the study had to
evaluate the effectiveness of at least one of the interventions/ practices of interest to reduce specimen labeling error;
report at least one of the outcomes of interest (listed above) after the intervention implementation;
be primary research published in an English-language journal, or available as a dissertation or a technical or government report;
employ a study design that compared outcomes of interest with and without the new practice implementation to reduce specimen mislabeling e.g., pre- and post- intervention data, concurrent comparison data such as RCTs. In addition, this review included labeling errors associated with all types of patient specimens collected from the patients for laboratory diagnostic testing (e.g., blood, urine, CSF, sputum).
Exclusion Criteria
The studies on the effectiveness of barcoding practices for reducing patient specimen and laboratory testing identification errors were excluded from the ‘Implementation of New Technology’ category as these practices are already evaluated in one of the previous LMBP reviews.7
Acquire (A-2): Search for Evidence
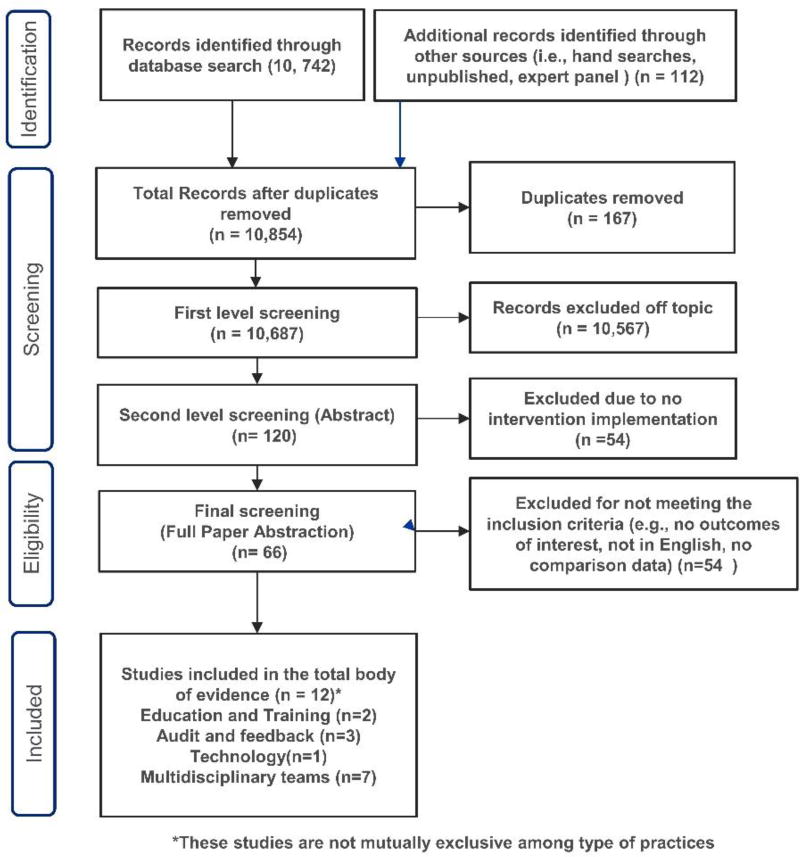
Published evidence was searched between the time periods of 1990 to September 2015 using the following databases: Medline OVID (R), Embase OVID, CINAHL EbscoHost, Cochrane Library Database, Dissertation Abstracts, and PubMed to identify studies relevant to evaluations of interventions to reduce specimen mislabeling. Search details are available at Supplemental Appendix C. In addition, the systematic review team retrieved evidence from other informal sources such as hand searches including relevant references from all retrieved articles and additional studies identified by subject matter experts on the e-SBI systematic review team were incorporated into the review. We also received relevant unpublished data from the researchers, laboratories, and institutions in the field through personal requests and LMBP™ website but none of the unpublished data qualified to be included in this review. A total of 10,854 relevant records (both published and unpublished) were retrieved as a result of formal and informal literature searches. (Figure 2)
Figure 2.
Patient Misidentification due to Specimen Labeling Errors Review Search Flow Diagram
Appraise (A-3): Screening, Data abstraction and Quality scoring of Individual Studies
Retrieved evidence was screened at different levels, e.g., title screening and abstract screening to identify studies meeting the inclusion criteria. The data was abstracted from each study that met the inclusion criteria by 2 reviewers using the Standardized LMBP™ abstraction methods and abstraction form. Any discrepancies among the reviewers were reconciled by consensus. Based on the abstracted data, each study was rated using a 10 point scale for study quality as good (8–10 score), fair (5–7 score), or poor (≤4 score). Details for the LMBP quality scoring process can be found elsewhere.10 Studies with good and fair quality of execution were included in this review analysis. Supplemental Appendix D describes the Evidence Summary Tables containing detail information and quality ratings for each study.
Analyze (A-4): Summarization of results and strength of the Effect Magnitude
Effect estimates for each practice effectiveness were calculated as relative percentage point change (pct pt) where the studies reported the percent change in specimen labeling errors due to the intervention implementation. For each study, the effect estimates were calculated separately using the last available data point.
Following formula was used to calculate the relative percentage change in labeling errors:
Where the Intervention Pre and Intervention Post represent the percent of labeling errors before and after the intervention implementation respectively.
The effect size strength rating for each individual study was based on the range of percentage change in labeling errors due to the intervention, the reduction in labeling errors between 0% to ≤ 40% was considered ‘minimal’ magnitude of effect; any decrease in labeling errors between >40% to ≤ 75% was considered ‘moderate’ magnitude and finally any decrease in labeling errors > 75% was considered ‘substantial’ magnitude of effect.
LMBP criteria were used to make conclusions on the overall strength of evidence on effectiveness which is based on the total body of evidence by taking into account the number of studies included in the evidence, quality of available evidence, consistency of results, magnitude of effect estimates, and applicability considerations. More details about these criteria can be found elsewhere.6
RESULTS
From the broader search for evidence, a total of 10,854 references were retrieved. After removing 167 duplicates and 10,567 off the topic, 66 were considered for full abstraction. After the full abstraction and quality scoring, eleven studies were qualified to be included in the systematic review. (Figure 2) Majority of the studies were conducted in USA11–20, one in India21 and one in Spain.22
Practice 1: Improved Communication and Collaboration between Laboratory and Healthcare Professionals: Formation of Multidisciplinary Teams
Seven studies11, 15–20 were identified to be included in the analyses to investigate the effectiveness of interventions that were developed and implemented due to the improved communication and collaboration between clinical staff and laboratory personnel. Three18–20 studies were of ‘good’ quality and four studies11, 15–17 were of ‘fair’ quality. All identified interventions for this category comprised of formation of multidisciplinary teams including the representatives from diverse disciplines (e.g., testing laboratory personnel, clinicians, nurses or other healthcare professionals). The teams met on regular basis to develop collaborative approaches according to the organizational needs that were acceptable and sustainable to staff in order to reduce specimen identification errors. The interventions were targeted to the general population in five studies15–18, 20, children and adults in one study,19 and in one study the target population was newborn children.11 The total evidence was derived from varied types of healthcare settings, i.e., three studies15–17 were conducted in entire facilities including different settings such as inpatient, ambulatory, surgical services areas, emergency department, ICU, general care unit, one study19 in inpatient setting, one20 in surgical unit, one18 in dermatology unit, and one11 in pediatric department.
Four studies15, 18–20 measured the effect of standardized specimen labeling policies developed as a result of multidisciplinary approach that required inclusion of specific patient identifiers in specimen labeling (e.g., patient full legal name, DOB, Date and time of collection, initials of person collecting the specimen) and also educated staff about those standardized policies. Three studies18–20 implemented policies that required to print specimen labels at the time of specimen labeling process and use of one sheet for blood and other bodily fluid specimens from the same patient.
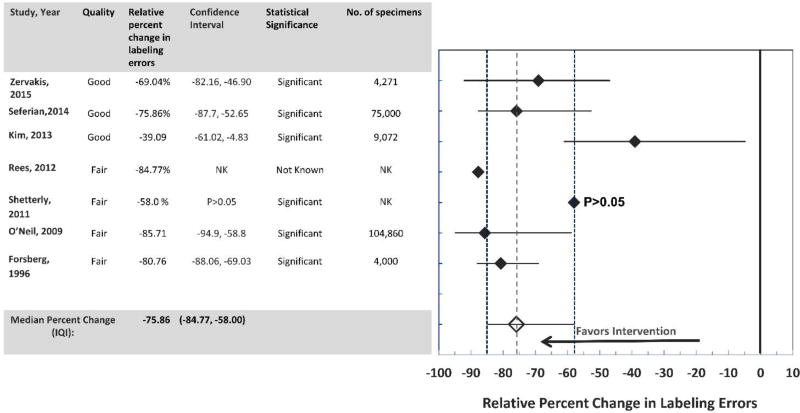
The overall findings from the combined data from seven studies11, 15–20 found that the interventions developed as a result of improved communication and collaboration between the laboratory and clinical staff resulted in substantial decrease in specimen labeling errors (Median relative percent change in labeling errors: −75.86; IQI: −84.77, −58.00). (Figure 3). Results from all studies were statistically significant but for one study17 due to the limited information available to calculate the significance of results. The strength of the effect size was considered ‘substantial’ from four studies11, 15, 17, 19 ‘moderate’ from two studies, 16, 20 and ‘minimal’ for one study18. Overall data showed that improved communication and collaborative efforts by the clinical and laboratory staff (e.g., development of standardized policies and practices for specimen labeling and organizational workflow chart to determine strategies to check for any scope of errors, establishment of processes to check specimen slips for missing collection dates) resulted in significant decrease in the rate of specimen identification errors.
Figure 3.
Improved communication and collaboration between laboratory and healthcare professionals: Formation of Multidisciplinary Teams
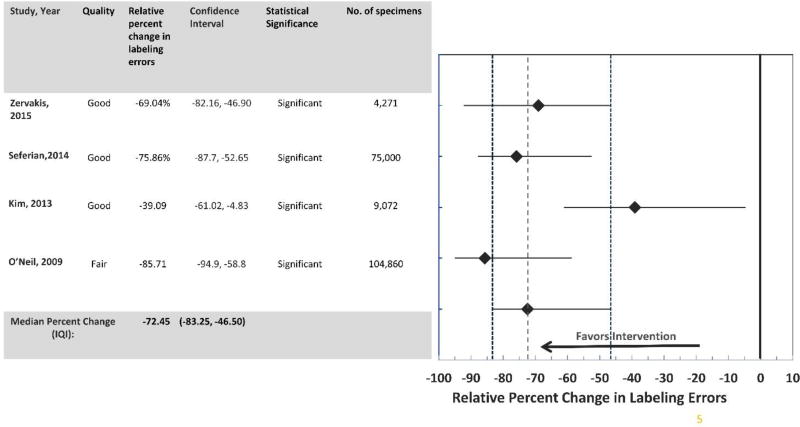
In addition, the results from the sub-analyses performed for four studies15, 18–20 showed a ‘moderate’ decline in specimen labeling errors after the institution of the standardized specimen labeling policy, i.e., inclusion of unique patient identifiers on the specimen labeling. (Median relative percent decrease in specimen labeling errors: −72.45; IQI: −83.25, −46.50). The results from all these studies were statistically significant and were consistently in a favorable direction. (Figure 4).
Figure 4.
Improved communication and collaboration between laboratory and healthcare professionals: Development of Standardized Policies and Practices
Conclusions
Applying the LMBP™ criteria, the overall strength of evidence from seven studies11, 15–20 is considered ‘sufficient’ to recommend that the improved communication and collaboration between laboratory and healthcare professionals by forming multidisciplinary teams is effective to decrease the specimen identification errors. (Table 1) Based on sub- group analysis, there was sufficient evidence of ‘moderate’ strength’ (based on four studies)15, 18–20 to recommend that the implementation standardized policies that require specific patient identifiers on specimen label developed by collaborative efforts between laboratory and healthcare staff are effective in decreasing specimen labeling in healthcare settings. (Table 2)
Table 1.
Improved Communication and Collaboration between Laboratory and Healthcare Professionals by Formation of Multidisciplinary Teams Interventions: Overall Practices
| Studies (Published) | Study Quality Rating | Effect Size Rating |
|---|---|---|
|
| ||
| Zervakis, 2015 | Good | Moderate |
|
| ||
| Seferian, 2014 | Good | Substantial |
|
| ||
| Kim, 2013 | Good | Minimal |
|
| ||
| Rees, 2012 | Fair | Substantial |
|
| ||
| Pa Patient/ Shetterly, 2011 | Fair | Moderate |
|
| ||
| O’Neil, 2009 | Fair | Substantial |
|
| ||
| Foresberg, 1996 | Fair | Substantial |
|
| ||
| BODY OF EVIDENCE RATINGS | 1 Good/ Substantial | |
| 1 Good/ Moderate | ||
| 1 Good/ Minimal | ||
| 1 Fair/ Moderate | ||
| 3 Fair/ Substantial | ||
|
| ||
| CONSISTENCY | Consistent | |
|
| ||
| OVERALL STRENGTH | Moderate | |
Table 2.
Improved Communication and Collaboration between Laboratory and Healthcare Professionals by Formation of Multidisciplinary Teams Interventions: Specimen labeling Policies and Processes
| Studies (Published) | Study Quality Rating | Effect Size Rating |
|---|---|---|
|
| ||
| Zervakis, 2015 | Good | Moderate |
|
| ||
| Seferian, 2014 | Good | Substantial |
|
| ||
| Kim, 2013 | Good | Minimal |
|
| ||
| O’Neil, 2009 | Fair | Substantial |
|
| ||
| BODY OF EVIDENCE RATINGS | 1 Good/ Substantial | |
| 1 Good/ Moderate | ||
| 1 Good/ Minimal | ||
| 1 Fair/ Substantial | ||
|
| ||
| CONSISTENCY | Consistent | |
|
| ||
| OVERALL STRENGTH | Moderate | |
Practice 2: Education and Training
Two studies13, 21 were identified that evaluated the effectiveness of education and training interventions to decrease the specimen labeling errors. One study21 was good quality and one13 was of fair quality. Results from one study21 showed substantial reduction in patients with wrong identification due to the labeling errors. (Relative percent change: −90.89; IQI: −97.86, −61.14) after introducing training and education sessions for medical, nursing, and laboratory staff as part of the continuous medical education. Another study13 showed minimal decrease in the specimen labeling after the nursing in-service education over the period of six months along with the provision of 24-hour phlebotomy services at the facility. (Relative percent change: −35.77; IQI: −51.58, −14.80). (Figure 5)
Figure 5.
Relative Percent Point Change in Specimen Labeling Erros Due To Educational and Training Interventions
Conclusions
Both included studies showed a consistent decrease in sampling errors, however due to the small number of studies, according to the LMBP rules,6 evidence is considered ‘insufficient’ to determine if education and training interventions are effective in reducing patient misidentification due to the labeling errors. (Table 3)
Table 3.
Body of Evidence LMBPTM Ratings for Educational and Training Interventions
| Studies (Published) | Study Quality Rating |
Effect Size Rating |
|---|---|---|
|
| ||
| Agarwal, 2012 | Good | Substantial |
|
| ||
| Wagar, 2006 | Fair | Minimal |
|
| ||
| BODY OF EVIDENCE RATINGS | 1 Fair/ Moderate | |
| 1 Good/ | ||
|
| ||
| CONSISTENCY OF RESULTS | Consistent | |
|
| ||
| OVERALL STRENGTH | Insufficient | |
Practice 3: Audit and Feedback
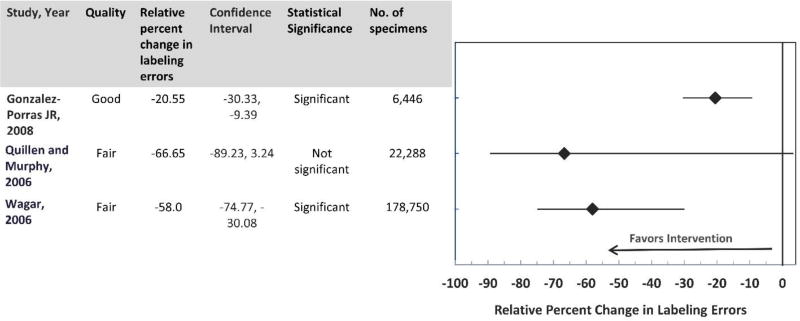
Three studies12, 13, 22 were included in the analyses to investigate the effectiveness of audit and feedback interventions. One study22 was of ‘good’ quality and two12, 13 studies were of ‘fair’ quality of execution. Two studies12, 13 were conducted in the USA and one study in Spain. 22 The interventions in the included studies involved reporting or feedback of specimen mislabeling data from the laboratories to the management and the staff responsible for specimen collection and labeling on a regular basis. The combined results from three studies showed that after the intervention implementation there was a significant decrease in labeling errors (Overall decrease in median relative percent change in labeling errors: −58.0; IQI: −74.77, −30.08). Results from the two studies13, 22 were statistically significant and from one study12 were not statistically significant. (Figure 6) The effect size from two studies12, 13 was of ‘moderate’ strength and from one study22 was of ‘minimal’ strength. (Table 4)
Figure 6.
Relative Percent Point Change in Specimen Lebeling Errors Due Audit and Feedback Interventions
Table 4.
Body of Evidence LMBPTM Ratings for Educational and Training Interventions
| Studies (Published) | Study Quality Rating | Effect Size Rating |
|---|---|---|
|
| ||
| Gonzalez-Porras JR, 2008 | Good | Minimal |
|
| ||
| Quillen and Murphy, 2006 | Fair | Moderate |
|
| ||
| Wagar, 2006 | Fair | Moderate |
|
| ||
| BODY OF EVIDENCE RATINGS | 2 Fair/ Moderate | |
| 1 Good/ Minimal | ||
|
| ||
| CONSISTENCY | Consistent | |
|
| ||
| OVERALL STRENGTH | Insufficient | |
Conclusions
Applying the LMBP™ criteria, the overall strength of evidence is considered ‘insufficient’ due to small number of included studies and weak effect size to draw any conclusions on the effectiveness of ‘audit and feedback’ interventions at reducing errors related to specimen labeling in all types of clinical settings. (Table 4)
Practice 4: Implementation of new Technology
No study qualified to be included in the analyses to evaluate the effectiveness this intervention category.
DISCUSSION
Best practices recommendations
Based on the findings from the included evidence, the interventions involving improved communication and collaboration between laboratory and healthcare professionals by forming multidisciplinary teams are recommended to decrease the specimen identification errors.11, 15–20
Particularly, the development and implementation of standardized policies and strategies (e.g., use at least two identifiers to verify a specimen/patient identity on the specimen label) by the MD teams at organizational level.15, 18–20 The findings from this review showed that other evaluated practices, i.e., training and education of the specimen collection staff, audit and feedback of labeling errors also led to decrease in specimen labeling errors. However, due to insufficient available evidence no recommendations could be made, ‘in favor or against’, the effectiveness of those practices.
In laboratory medicine, correct linking of the specimen to the patient from whom it was collected is identified as an essential and fundamental objective for improving patient health and safety as it impacts on all aspects of patient care including correct diagnosis and right treatment. In 2013, Joint Commission identified accurate specimen/ patient identification as first of the National Patient Safety Goals, and this continues to be an accredited requirement .23 The World Health Organization (WHO) considers identification a priority area for improving patient safety, recommends for staff education and training to ensure correct specimen/patient identification among all healthcare organizations. 24 Various approaches have been proved to be effective to improve specimen labeling errors when implemented among different facilities within a health-care system. Evidence showed that education and training programs for the staff responsible for specimen collection and labeling 22, 25, 26 and audit/continuous monitoring and reporting of specimen labeling 27 have been proved to reduce specimen/patient identification error. 27–29
The findings from our review appear to be similar to a recent systematic review that addressed the errors related to the wrong blood in tube (WBIT) and investigated what interventions (single or multiple) were successful in reducing WBIT. This review found that the interventions including standardized labeling policies, staff education, weekly feedback, and electronic transfusion systems were likely to be more effective when implemented in combination than when implemented individually.30 However, most of the evidence came from the studies that implemented multiple practices at the same time and did not make an attempt to investigate the weighing of effectiveness of individual practice in relation to the other.
In this review we were able to measure the effectiveness of four individual practices to reduce the specimen labeling errors at the time of specimen collection.
CONSIDERATIONS FOR IMPLEMENTATION
Lack of knowledge and training of non-laboratory staff (e.g., doctors and nurses) regarding specimen collection procedures like phlebotomy may contribute to pre analytical errors including specimen mislabeling. Educational and training interventions targeting non-laboratory staff can be more effective to improve specimen mislabeling.13, 19, 31 Other factors contributing to labeling errors are identified as lack of compliance by the staff to the specimen labeling SOPs majority of the times due to the short cuts and workarounds 32, furthermore staff turnover is a major issue in lab and nursing. 33 To mitigate these barriers incorporating staff training sessions into their orientation as well as routine continuing ongoing professional development sessions may prove to be more effective vs. one time training. Annual competency checks for new as well as existing employee(s) is required. Finally it is also suggested that patient involvement can play an important role to improve their own identification. Therefore, interventions that encourage patient and family involvement to verify and confirm patient information should not be undervalued in order to reduce patient identification errors. 24
ECONOMIC EVALUATION
No eligible economic evaluations were identified for analysis of cost-effectiveness.
POTENTIAL HARMS
Some of the interventions to reduce specimen labeling errors may have unintended disadvantages. Interventions may result in increased cost of operations due to implementation and maintenance of staff education and training. There can be additional cost associated with acquiring technical solutions and the training of the staff about the use of new technology. In addition, regular educational/trainings sessions may result in increase in staff workload and time spent away from the patients. Health care providers perceive that by repeated verification of patient identity may compromise their relationship with the patients.
STUDY LIMITATIONS AND FUTURE RESEARCH NEEDS
Due to the limited available evidence, no recommendations- ‘for or against’, the effectiveness the effectiveness of three evaluated practices could be made, i.e. interventions including education and training,13, 21 audit and feedback12, 13, 22 and implementation of new technology. More research is needed to evaluate these interventions to reduce specimen labeling errors.
Study design limitations regarding the entire evidence driven from before-and-after study design studies. Because of the uncontrolled nature of this design, there may have been unmeasured factors that changed between study periods that account for or influence the study results. Future studies of stronger research designs (e.g., randomized control trials) would be valuable to clarify effectiveness of interventions to reduce patient misidentification due to the labeling errors.
In this review, implementation of policies and strategies for specimen labeling developed as a result of improved communication and collaboration between the laboratory, management, and clinical staff remained effective for longer period (up to 3 years) of time. However, for other evaluated practices, i.e., staff education, audit and feedback interventions the follow-up period to report the results varied from 6 months to 1 year,12, 13, 21, 22 future research studies need to be conducted over a prolonged period required to examine the sustainability of the effects of these interventions for longer periods of time.
Large discrepancies in errors definitions, terminology, and error categorization strategies used in existing literature made it difficult to compare the studies. For example, the terms ‘laboratory identification error’, ‘specimen identification error’, ‘patient identification error’, ‘identification error’, ‘mislabeled specimen’, ‘unlabeled specimen’ have been interchangeably used for specimen labeling. Furthermore, use of variety of metrics/ measures for result reporting, such as percent change, change in error rate, error counts etc. made it challenging and difficult for synthesizing and summarizing findings from the total evidence. For future research it is warranted to use standardize term(s), definition(s) and error detection methods and measures for result reporting in establishing future quality control studies to allow better analysis and better result interpretation.
In the majority of the existing literature, across the healthcare settings combination of interventions were implemented at the same time, e.g., staff training and education, labeling policies and processes to reduce the errors. It was difficult to disentangle what specific component attributed to the intervention effectiveness (e.g., do policy components or education components contribute more to intervention effectiveness; what are the central “active ingredients” in complex interventions). Providing more description information on how different best practices were implemented as an intervention to reduce errors might also help organizations replicate successes. The unpublished data that was retrieved from the laboratories was mostly trend data that did not qualify to be included in the final analyses, e.g., did not provide any comparison data to calculate the effect estimates. In routine, clinical laboratories study patient safety and quality improvement interventions, it is desired to design future quality improvement studies in such a way that the data driven from these studies can be utilized to demonstrate intervention effectiveness.
Finally, research showed that the incidence of specimen labeling errors vary according to the type of healthcare setting. For example the risk of these events is higher in the emergency departments due to rapid patient turnover, more interruptions to the medical staff in ED,32 patients arrive unexpectedly who maybe unconscious or with no identification when compared to in patient setting where the patients are admitted for days for their treatment.33 The findings from this review may not be generalizable across different type of healthcare settings due to limited data availability to investigate whether the recommended practices are equally effective in all types of settings (e.g., ED, pediatrics).
In summary, humans tend to cause errors and multiple corrective measures exist that focus on human factor improvement but the errors related to specimen labeling and patient identification continue to happen. Due to the potential adverse consequences on patient safety associated with mislabeled laboratory specimens, each and every specimen labeling error should be treated very seriously. Based on the findings from this review, multifaceted and multidisciplinary improvement approaches such as improved communication and collaboration between laboratory and healthcare professionals to develop and implement stringent and standardized specimen labeling policies and procedures can increase the patient safety by significantly reducing the incidence of specimen labeling errors at healthcare settings.
Supplementary Material
Acknowledgments
LMBP™ Specimen labeling/ patient misidentification Expert Panel, LMBP™ Workgroup members, Joanna Taliano, Reference Librarian (CDC).
FUNDING SOURCE
This work is funded by CDC under Contract No. 200-2013-F-57569, Delivery Order, “Laboratory Medicine Preparedness: Best Practices”.
Footnotes
Disclaimer: The findings and conclusions of this article are those of the authors and do not necessarily represent the views of the CDC.
References
- 1.Ford Catching ID errors where it counts---in the lab. CAP Today. 2005;19(9):34–40. [Google Scholar]
- 2.Sauer DMC, Boshkov L. Errors in transfusion medicine. Lab Med. 2001;4(32):205–7. 4(32):205-7. 2001;4(32):205-7. [Google Scholar]
- 3.Jones BA, Calam RR, Howanitz PJ. Chemistry specimen acceptability: a College of American Pathologists Q-Probes study of 453 laboratories. Arch Pathol Lab Med. 1997;121(1):19–26. [PubMed] [Google Scholar]
- 4.Dale JC, Novis DA. Outpatient phlebotomy success and reasons for specimen rejection. Arch Pathol Lab Med. 2002;126(4):416–9. doi: 10.5858/2002-126-0416-OPSARF. [DOI] [PubMed] [Google Scholar]
- 5.Ford Disabling mislabeling---solutions for blood banks. CAP Today. 29(11):62–64. 2009;23(1):5-8. [Google Scholar]
- 6.Christenson RH, Snyder SR, Shaw CS, Derzon JH, Black RS, Mass D, et al. Laboratory medicine best practices: systematic evidence review and evaluation methods for quality improvement. Clin Chem. 2011;57(6):816–25. doi: 10.1373/clinchem.2010.157131. [DOI] [PubMed] [Google Scholar]
- 7.Snyder SR, Favoretto AM, Derzon JH, Christenson RH, Kahn SE, Shaw CS, et al. Effectiveness of barcoding for reducing patient specimen and laboratory testing identification errors: a Laboratory Medicine Best Practices systematic review and meta-analysis. Clin Biochem. 2012;45(13–14):988–98. doi: 10.1016/j.clinbiochem.2012.06.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Ford Mislabeling, wrong-blood-in-tube errors rare but there. CAP Today. 2015;29(11):62–64. [Google Scholar]
- 9.National Academies of Sciences, Engineering, and Medicine. Improving Diagnosis in Health Care. Washington, DC: The National Academies Press; 2015. https://www.nap.edu/catalog/21794/improving-diagnosis-in-health-care. [DOI] [Google Scholar]
- 10.Price CPLJ, Christenson RH, editors. Applying evidence-based laboratory medicine: a step-by-step guide. Washington (DC): AACC; 2009. [Google Scholar]
- 11.Forsberg SA. Infant metabolic screening: a total quality management approach. J Obstet Gynecol Neonatal Nurs. 1997;26(3):257–61. doi: 10.1111/j.1552-6909.1997.tb02140.x. [DOI] [PubMed] [Google Scholar]
- 12.Quillen K, Murphy K. Quality improvement to decrease specimen mislabeling in transfusion medicine. Arch Pathol Lab Med. 2006;130(8):1196–8. doi: 10.5858/2006-130-1196-QITDSM. [DOI] [PubMed] [Google Scholar]
- 13.Wagar EA, Tamashiro L, Yasin B, Hilborne L, Bruckner DA. Patient safety in the clinical laboratory: a longitudinal analysis of specimen identification errors. Archives of Pathology & Laboratory Medicine. 2006;130(11):1662–8. doi: 10.5858/2006-130-1662-PSITCL. [DOI] [PubMed] [Google Scholar]
- 14.Wagar EA, Stankovic AK, Raab S, Nakhleh RE, Walsh MK. Specimen labeling errors: a Q-probes analysis of 147 clinical laboratories. Archives of Pathology & Laboratory Medicine. 2008;132(10):1617–22. doi: 10.5858/2008-132-1617-SLEAQA. [DOI] [PubMed] [Google Scholar]
- 15.O'Neill E, Richardson-Weber L, McCormack G, Uhl L, Haspel RL. Strict adherence to a blood bank specimen labeling policy by all clinical laboratories significantly reduces the incidence of "wrong blood in tube". Am J Clin Pathol. 2009;132(2):164–8. doi: 10.1309/AJCPOJA2JRVX0IWC. quiz 306. [DOI] [PubMed] [Google Scholar]
- 16.Shetterly M, Charney F. Pennsylvania Patient Safety Authority blood specimen labeling collaborative. J Healthc Risk Manag. 2011;31(2):31–6. doi: 10.1002/jhrm.20085. [DOI] [PubMed] [Google Scholar]
- 17.Rees S, Stevens L, Mikelsons D, Quam E, Darcy T. Reducing specimen identification errors. J Nurs Care Qual. 2012;27(3):253–7. doi: 10.1097/NCQ.0b013e3182510303. [DOI] [PubMed] [Google Scholar]
- 18.Kim JK, Dotson B, Thomas S, Nelson KC. Standardized patient identification and specimen labeling: a retrospective analysis on improving patient safety. J Am Acad Dermatol. 2013;68(1):53–6. doi: 10.1016/j.jaad.2012.06.017. [DOI] [PubMed] [Google Scholar]
- 19.Seferian EG, Jamal S, Clark K, Cirricione M, Burnes-Bolton L, Amin M, et al. A multidisciplinary, multifaceted improvement initiative to eliminate mislabelled laboratory specimens at a large tertiary care hospital. BMJ Quality & Safety. 2014;23(8):690–7. doi: 10.1136/bmjqs-2014-003005. [DOI] [PubMed] [Google Scholar]
- 20.Zervakis OR Specimen Labeling. AORN. 2016;103(2):164–176. doi: 10.1016/j.aorn.2015.12.018. [DOI] [PubMed] [Google Scholar]
- 21.Agarwal R, Chaturvedi S, Chhillar N, Goyal R, Pant I, Tripathi CB. Role of intervention on laboratory performance: evaluation of quality indicators in a tertiary care hospital. Indian Journal of Clinical Biochemistry. 2012;27(1):61–8. doi: 10.1007/s12291-011-0182-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Gonzalez-Porras JR, Graciani IF, Alvarez M, Pinto J, Conde MP, Nieto MJ, et al. Tubes for pretransfusion testing should be collected by blood bank staff and hand labelled until the implementation of new technology for improved sample labelling. Results of a prospective study. Vox Sang. 2008;95(1):52–6. doi: 10.1111/j.1423-0410.2008.01049.x. [DOI] [PubMed] [Google Scholar]
- 23. [accessed 15 Aug 2013];Commission. 2013 http://www.jointcommission.org/assets/1/18/NPSG_Chapter_Jan2013_HAP.pdf.
- 24.World Health Organisation. Patient Safety Solutions, Volume 1 AND Solution 2. Geneva: WHO; 2007. Patient Identification. http://www.who.int/patientsafety/solutions/patientsafety/PS-Solution2.pdf. [Google Scholar]
- 25.Lumadue JA, Boyd JS, Ness PM. Adherence to a strict specimen-labeling policy decreases the incidence of erroneous blood grouping of blood bank specimens. Transfusion. 1997;37(11–12):1169–72. doi: 10.1046/j.1537-2995.1997.37111298088047.x. [DOI] [PubMed] [Google Scholar]
- 26.Tondon R, Pandey P, Mickey KB, Chaudhary R. Errors reported in cross match laboratory: a prospective data analysis. Transfus Apher Sci. 2010;43(3):309–14. doi: 10.1016/j.transci.2010.09.014. [DOI] [PubMed] [Google Scholar]
- 27.Howanitz PJ, Renner SW, Walsh MK. Continuous wristband monitoring over 2 years decreases identification errors: a College of American Pathologists Q-Tracks Study. Arch Pathol Lab Med. 2002;126(7):809–15. doi: 10.5858/2002-126-0809-CWMOYD. [DOI] [PubMed] [Google Scholar]
- 28.van den Akker T, Mwagomba B, Irlam J, van Roosmalen J. Using audits to reduce the incidence of uterine rupture in a Malawian district hospital. Int J Gynaecol Obstet. 2009;107(3):289–94. doi: 10.1016/j.ijgo.2009.09.005. [DOI] [PubMed] [Google Scholar]
- 29.Kotagal M, Lee P, Habiyakare C, Dusabe R, Kanama P, Epino HM, et al. Improving quality in resource poor settings: observational study from rural Rwanda. Bmj. 2009;339:b3488. doi: 10.1136/bmj.b3488. [DOI] [PubMed] [Google Scholar]
- 30.Cottrell S, Watson D, Eyre TA, Brunskill SJ, Doree C, Murphy MF. Interventions to reduce wrong blood in tube errors in transfusion: a systematic review. Transfus Med Rev. 2013;27(4):197–205. doi: 10.1016/j.tmrv.2013.08.003. [DOI] [PubMed] [Google Scholar]
- 31.Varey A, Tinegate H, Robertson J, Watson D, Iqbal A. Factors predisposing to wrong blood in tube incidents: a year's experience in the North East of England. Transfus Med. 2013;23(5):321–5. doi: 10.1111/tme.12050. [DOI] [PubMed] [Google Scholar]
- 32.Goswami B, Singh B, Chawla R, Mallika V. Evaluation of errors in a clinical laboratory: a one-year experience. Clin Chem Lab Med. 2010;48(1):63–6. doi: 10.1515/CCLM.2010.006. [DOI] [PubMed] [Google Scholar]
- 33.Bhat V, Tiwari M, Chavan P, Kelkar R. Analysis of laboratory sample rejections in the pre-analytical stage at an oncology center. Clin Chim Acta. 2012;413(15–16):1203–6. doi: 10.1016/j.cca.2012.03.024. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.