Abstract
Most pancreas and islet grafts are recovered from brain-dead (BD) donors. In this study we characterized the early inflammatory response induced by brain death in pancreata and islets from non-human primate donors and evaluated the effect of targeted anti-inflammatory intervention in the protection of pancreatic islets prior to transplantation. Brain-dead donors were monitored for 6 hours and assigned to three experimental groups. Group 1: BD-untreated donors (BD-UT) (n=7), Group 2: BD + donor pre-treatment with IL-1ra (n=6) and Group 3: Non-BD animals serving as controls (n=7). We observed an IL-1ra dependent reduction in the mobilization and activation of neutrophils from bone marrow and a significantly reduced accumulation of CD68+ leukocytes in the pancreas and islets after brain death induction. Donor treatment with IL-1ra significantly decreased chemokine mRNA expression (MCP-1, IL-8 and MIP-1a) and attenuated the activation of circulating neutrophils and intra-islet macrophages as demonstrated by a reduction in intracellular IL-1β, IL-6, MCP-1 and MIP-1α expression. As a result, IL-1ra dramatically improved viability, mitochondrial membrane polarity and islet engraftment in mice transplanted using a minimal islet mass. These results suggest that early immunomodulation targeting inflammation in the brain-dead donor may represent an effective therapeutic strategy to improve islet quality and function prior to transplantation.
Keywords: Islet Transplantation, Brain-death, IL-1 receptor antagonist, Donor management, Innate Immunity, Inflammation
INTRODUCTION
It is currently accepted that severe brain injury resulting in brain-death (BD) triggers a generalized inflammatory response that is detrimental to organ quality and function in the post-transplant period. Studies analyzing different donation modalities have consistently shown superior long term function and graft survival in organs obtained from living donors (LD) when compared to those recovered from BD donors (2,12,33,35). However, the pathophysiological mechanisms responsible for the discrepancies observed in graft survival between living and deceased donors remain poorly understood. In the last decade, several groups have reported that generalized inflammation triggered by BD plays a key role in enhancing donor tissue immunogenicity and results in a rapid and aggressive allo-response by the host associated to increased risk of delayed graft function and rejection after transplantation (23,24,35).
There is substantial evidence demonstrating that early expression of pro-inflammatory mediators is partly responsible for inducing cell migration and secondary tissue injury during the inflammatory cascade triggered by ischemia-reperfusion in various animal models (34). Interleukin-1 beta (IL-1β) is of particular interest due to its central role in the orchestration and amplification of the acute inflammatory response to tissue injury (9). Recent work by our group and others suggests that the IL-1β pathway is highly up-regulated during the acute phase response and the inflammatory process triggered by BD (7). IL-1β regulates the production and release of pro-inflammatory cytokines (PIC), chemokines and cellular elements that further potentiate the immune response and prompt tissue infiltration by inflammatory cells of the innate and adaptive immune system. This in turn leads to production of reactive oxygen species (ROS), edema, necrosis and apoptosis which are considered to be the main driving forces behind acute ischemic and inflammatory damage to visceral and thoracic organs destined for transplantation (25).
In pancreatic islets, the role of IL-1β in the pathogenesis of diabetes mellitus (type I and II) as well as islet dysfunction after pancreatic islet transplantation (PIT) has been extensively studied (1,10,13). In the pancreas, IL-1β is produced and secreted mainly by activated macrophages, neutrophils, endothelial cells and β-cells within the islet (15). Numerous reports indicate that secreted IL-1β mediates the activation of NF-kB dependent pro-inflammatory pathways within the islet cells and has been linked to impaired insulin secretion, mitochondrial damage and increased oxidative injury that ultimately result in increased levels of apoptosis and necrosis of β-cells (4,13,29).
In this study, we tested the protective effects of organ donor pre-treatment with IL-1 receptor antagonist (IL-1ra) on pancreas and islet quality and function. For this purpose, we used a non-human primate model of BD to simulate the initial cascade of inflammatory events that follow acute explosive neurological injury. Our results provide evidence indicating that blockade of the IL-1β pathway at the donor level leads to improved quality and functional potency of isolated pancreatic islets by preventing innate immune activation and attenuating the inflammatory response to BD-induced ischemia/reperfusion injury of the pancreas.
MATERIALS AND METHODS
Animals and animal care
Male rhesus macaques (n=20), age range 4–16 years and weight (3.0–14.0 kg) were used in this study. All animals were pre-screened negative for TB, Herpes B, SRV, SIV, and STLV-1. Animals were housed in accordance to NIH and USDA guidelines. All donor/recipient transplant protocols were approved by Institutional Animal Care and Use Committee.
Experimental design
Donor animals were allocated to one of three groups. Group 1: Brain-death induction and standard donor management for 6 hours prior to organ recovery (BD-UT, n=7). Group 2: Brain-death induction, standard donor management and continuous administration of IL-1ra for 6 hours prior to organ recovery (BD + IL-1ra, n=6). Group 3: Non-brain dead primates served as the control group (NBD, n=7). Control animals were sedated, anesthetized and ventilated for 1 hour prior to organ recovery. A subset of control animals (n=3) were anesthetized, intubated and monitored for 6 hours in order to document changes in hemodynamic parameters. All animals in this study were euthanized by exsanguination under anesthesia during in-situ flush with cold UW preservation solution (SPS-1 Static Preservation Solution, Organ recovery Systems, Chicago, IL).
Brain death Induction and donor management
A detailed description of the brain-death protocol used in this study can be found elsewhere (7). In brief, animals were anesthetized, mechanically ventilated and monitored. A 16F Foley catheter (Bard Medical, Covington, Georgia) was placed in the extradural space of the cranial fossa and gradually inflated until hemodynamic and neurologic signs of brain-stem herniation were documented (36). BD animals were monitored for six hours and received IV fluid resuscitation to achieve a stable mean arterial pressure and urinary output. Arginine vasopressin (AVP, American Regent, Shirley, NY) (0.01 – 0.04 unit/min) was given intravenously when mean arterial blood pressure dropped below 45 and dopamine (0.5–5.0 U/kg/min, IV) (American Regent, Shirley, NY) was started when hypotension refractory to AVP treatment was documented.
Study drug
Interleukin-1 Receptor Antagonist (Anakinra, Amgen, Inc, Thousand Oaks, CA) was administered to brain-dead animals as an intravenous bolus (10 mg/kg) at BD induction and then maintained by continuous infusion (3.3 mg/kg/hr) throughout the 6 hour experimental period.
Islet isolation, purification and culture
Pancreatic islet isolation was performed using a modification of the semi-automated method previously described by Ricordi (27). The pancreas was digested using Liberase CI (Roche, Indianapolis, IN), mechanically disrupted and islets were purified by biocoll gradient centrifugation in a COBE 2991 cell processor (COBE, Lakewood, CO) (30). Purified islets were then cultured in CMRL 1066 (Mediatech, Herndon, VA) supplemented with 1% penicillin/streptomycin (Thermo Scientific, Waltham, MA), 10% FBS (Thermo Scientific) at 25°C in an incubator with 5% CO2 for 24 hours prior to assessment.
Histology and pathology
Pancreas biopsies were fixed in 10% buffered formalin, embedded in paraffin, and mounted onto slides for staining with anti-CD68 (clone KP1, DAKO North America, Carpinteria, CA), anti-insulin (Abcam) and hematoxylin-eosin for evaluation by microscopy. Slides were analyzed blindly and quantification was performed using ImageJ software (NIH, Bethesda, MD). Color-separation, background-subtraction and automatic thresholding and particle-analysis algorithms were used to quantify positive-staining area and cell counts for each image.
RNA extraction and reverse-transcription
A 1mm3 sample of pancreatic tissue was homogenized 1 mL Trizol (Invitrogen, Carlsbad, CA) and purified using the Qiagen RNeasy Kit (Qiagen, Germantown, MD) following instructions for RNA clean-up and including the on-column DNase digestion step. RNA (0.5 μg) was reverse transcribed using Omniscript Reverse Transcription kit (Qiagen) following manufacturer’s instructions.
Quantitative PCR and data analysis
Quantitative PCR was performed using Taqman Universal PCR Master Mix and a Taqman gene expression assays (Applied Biosystems, Inc, Foster City, CA) with human and primate gene specific primers (Applied Biosystems) on a GeneAmp 5700 or 7500 Sequence Detection System (Applied Biosystems, Inc). We used commercially available Rhesus macaque validated primers (Life Technologies, Grand Island, NY) to determine changes in gene expression for CCL2 (Rh02621753_m1), IL-8 (Rh02789781_m1), CXCL10 (Rh02788358_m1) and used Beta-Actin (Rh03043379_gH) as the endogenous control. Fold change was calculated using the ΔΔCt method relative to untreated with HPRT as the endogenous control. Error was calculated as fold change ranges within one standard deviation of the mean.
Quantification of serum cytokine levels
Serum levels of IL-1ra were determined by ELISA (Cellsciences, Canton, MA) following manufacturer’s instructions. Circulating levels of IL-2, IL-4, IL-5, IL-6, TNF-α and IFN-γ were determined using a non-human primate Th1/Th2 cytokine cytometric bead array (BD Biosciences, San Diego, CA) analyzed using a FACS-calibur flow cytometer (BD Biosciences, San Diego, CA).
In-vitro and in-vivo assessment of islet function
For the glucose stimulated insulin secretion (GSIS) assay islets were handpicked into oxygen saturated basal Krebs-Ringer Bicarbonate Buffer (KRB, 137 mM NaCl, 4.7 mM KCl, 1.2 mM KH2PO4, 1.2 mM MgSO4-7H2O, 2.5 mM CaCl2-2H2O, 25 mM NaHCO3, Sigma), 0.25% BSA (Sigma-Aldrich), 3.3 mM glucose (Acros Organics, Thermo Scientific) followed by incubation at 37°C for 30 min. in a 5%CO2/95% air incubator. In each group 10 islets from the equilibration cultures were then transferred to fresh oxygen saturated KRB containing either 3.3 mM or 16.7 mM glucose and incubated an additional 60 min. in a 37°C water bath with gentle shaking. Secreted insulin in the media was measured by ELISA (Millipore, Billerica, MA) and values normalized to extracted islet DNA (Quant-iT Picogreen, Invitrogen). Islet viability was evaluated by fluorescence microscopy as previously described in other publications (18).
NOD.scid male mice (>8 weeks of age), purchased from The Jackson Laboratory (Bar Harbor, MA), were used as islet recipients. Mice were rendered diabetic by a single intraperitoneal injection of Streptozotocin (150 mg/kg) (Sigma-Aldrich Corp.) and only animals with sustained blood glucose level > 300 mg/dl for 3 consecutive days were used in this assay. Islets (1000 IEQ) were transplanted under the kidney capsule of diabetic mice and blood glucose levels followed daily for a period of 30 days using an Ascensia Elite glucometer (Bayer, Burr Ridge, IL).
Multi-parametric flow cytometry
For assessment of islet cell populations, purified islets were dispersed into single cell suspensions by incubation at 37°C in 0.05% Trypsin / 0.53mM EDTA (Mediatech, Manassas, VA) for 5 minutes, followed by gentle trituration through a narrow gauge pipet tip. Dispersed cells were then re-suspended in staining buffer. For intra-islet assessments, cells were suspended in 1x PBS (Mediatech) with 1% BSA (Sigma-Aldrich).
For viability assessment of islet cell populations, dispersed islet cells were suspended in modified Kreb’s Ringer Bicarbonate containing 3.3 mM glucose and 0.25% bovine serum albumin (BSA) and stained with probes for viability (FDA, Sigma- Aldrich), apoptosis (R-PE Annexin V, Invitrogen, Carlsbad, CA; VADFMK-FITC, Promega, Madison, WI), and mitochondrial membrane polarity (JC-1, Invitrogen). TO-PRO3-Iodide (Invitrogen) was added immediately prior to acquisition in order to identify necrotic cells. Samples were analyzed on a BD FACS Calibur with Cytek upgrade.
For assessment of leukocyte populations, PBMC were generated from blood was processed on lymphocyte separation medium (Mediatech) and re-suspended in 1x PBS with 1% BSA. They were then incubated with 10uL FC-Block (Biolegend, San Diego, CA) and stained for surface: HLA-DR, CD11b, CD3, CD4, CD8, CD20 (BD Biosciences, San Jose, CA), CD14, CD11c (Biolegend). Cells were then fixed and permeabilized using the BD Cytofix/CytoPerm kit, and stained for internal antigens: IL-6, IL-8, TNF-α, MCP-1, MIP-1a (BD Biosciences, San Jose, CA), and IL-1β (AbCam, Cambridge, MA). Samples were analyzed on a BD LSRII and analyzed using FlowJo (Treestar Inc., San Carlos, CA).
Statistical analysis
Statistical analysis was performed using the GraphPad Prism V4.03. All data are shown as mean ± standard error of the mean. Comparison of more than two groups was performed by One Way ANOVA or Kruskal-Wallis test with either Bonferroni’s or Dunn’s post-test correction where appropriate. Differences between treatment groups were considered significant with P < 0.05. Survival analysis was performed with Kaplan–Meier survival analysis using the log-rank test.
RESULTS
Hemodynamic alterations induced by brain death and drug delivery to brain-dead non- human primates
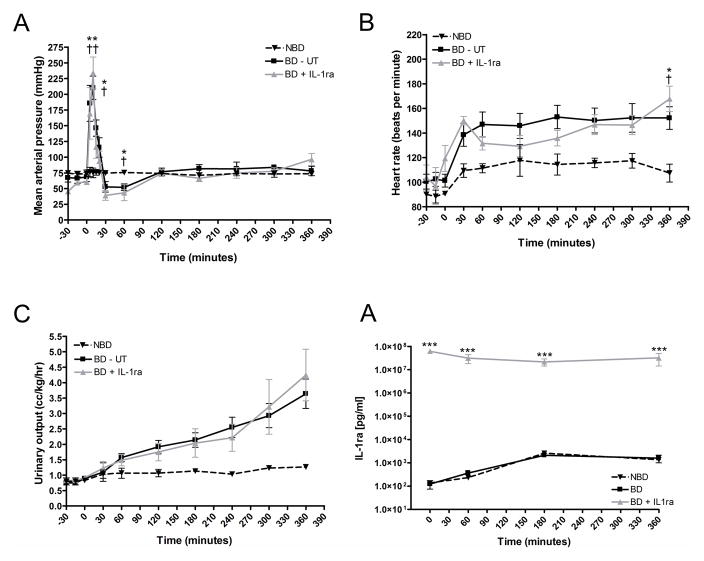
Following the inflation of the intracranial catheter, we observed a sharp increase in systolic and diastolic blood pressure (Figure 1A) along with sustained tachycardia (Figure 1B) and a progressive increase in urinary output suggestive of diabetes insipidus (Figure 1C). BD untreated (UT) and BD + IL-1ra treated animals evidenced a decrease in blood pressure at 30 minutes post-BD induction followed by a return to hemodynamic stability comparable to that of NBD donors for the remaining experimental period.
Figure 1.
Hemodynamic alterations induced by brain death induction in non-human primates. Mean arterial (A) blood pressure, (B) heart-rate and (C) urinary output were monitored for a six hour period following BD induction. (D) Serum IL-1ra levels measured at 0, 60, 180 and 360 min after BD induction. NBD controls (dashed line, n=3), BD-UT group (black line, n=7) and BD + IL1ra (gray line, n=6). Data are expressed as mean values ± SEM (*p<0.05, **p<0.01 and ***p<0.001 between NBD controls and BD-UT group and †p<0.05, ††p<0.01 and †††p<0.001 between NBD and BD+IL-1ra group).
We measured serum levels of circulating IL-1ra in the serum of donors at 0, 60, 180 and 360 min post BD induction and documented a significant increase to desired therapeutic levels in IL-1ra treated animals (Figure 1D).
IL-1ra mitigates the brain death induced recruitment of innate immune cells into peripheral circulation
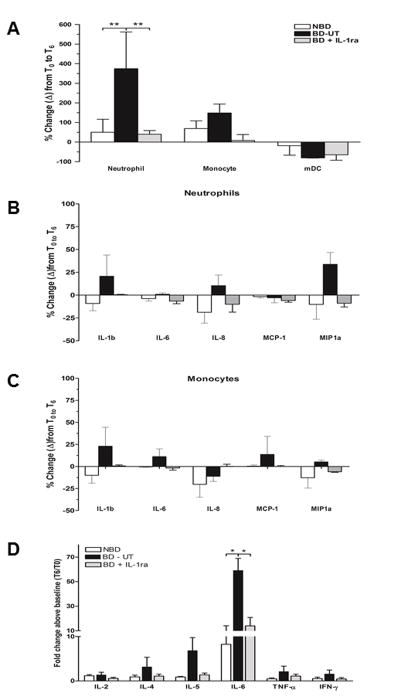
We examined the phenotype and activation state of circulating innate immune cell populations by flow cytometry. To assess the time dependent changes in circulating leukocyte populations we measured the relative change from baseline to organ recovery. At six hours post BD induction, the BD-UT group showed a significant increase in PMN levels (374.33% ± 187.70) when compared to baseline PMN counts (p <0.01). Conversely, levels of PMN decreased in NBD and IL-1ra treated animals (50.5% ± 38.2% and 39.8% ± 11.2%, respectively). Monocyte levels showed a similar trend, with a negligible increase observed in the BD-UT group at six hours and relatively no change observed in the NBD and IL-1ra treated groups; however, these results were not statistically different (Figure 2A). To determine the level of activation, we analyzed intracellular cytokine staining and documented an increase in IL-1β, IL-8 and MIP-1a expression in circulating neutrophils (Figure 2B) and IL-1β, IL-6 and MCP-1 expression in circulating monocytes obtained from the BD untreated group (Figure 2C). However, these changes did not achieve statistical significance. The frequency of positive staining cells from T0 to T6 in animals in the BD + IL1ra group demonstrated a decrease that paralleled changes observed in the NBD group. In addition, we observed a significant increase in serum IL-6 levels from BD untreated donors, which was suppressed to control levels by IL-1 blockade. Circulating levels of IL-2, IL-4, IL5, TNF-α and IFNγ were not statistically different among all three groups (Figure 2D).
Figure 2.
Peripheral blood mononuclear cells were analyzed by flow cytometry prior to BD induction (T0) and 6 hours after (T6), at organ recovery. (A) Changes in neutrophil, monocyte and myeloid dendritic cells in blood from brain death induction (T0) to 360 minutes (T6) post-induction. (B) Changes in neutrophil expression of IL-1β, IL-8 and MIP1a between T0 and T6 and (C) changes in monocyte expression of IL-1β, IL-6 and CCL3 (MIP-1a) between T0 and T6 in all groups. (D) Quantification of pro-inflammatory cytokine levels in serum between T0 and T6 was performed by ELISA. Data expressed as mean percent change from baseline ± SEM. NBD (n =7), BD-UT (n =7) and BD + IL-1ra (n=6) (*p<0.05 and ** p<0.01).
Brain death induces pancreatic injury that is attenuated by donor pre-treatment with IL-1ra
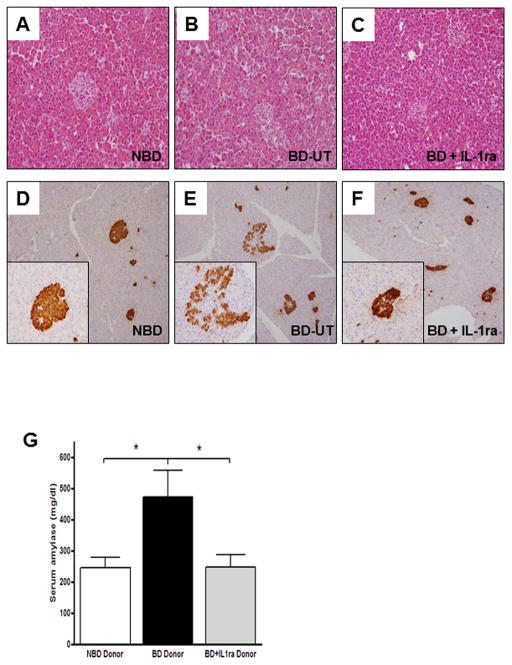
To determine the overall impact of brain-death on pancreatic injury, we analyzed tissue sections of pancreata recovered from BD donors and compared them to those obtained from NBD controls and IL-1ra treated animals. Although pancreatic H&E sections obtained from BD donors did not exhibit gross changes in morphology and cyto-architecture (Figure 3A–C), immunohistochemical analysis of several sections stained with anti-insulin antibodies revealed decreased insulin staining intensity and disruption of the cytoarchitecture and morphological integrity of the islets from BD donors when compared to those of NBD controls or IL-1β treated animals (Figure 3D–F). In addition, levels of serum amylase were markedly increased at 6 hours post brain death induction in the untreated BD donor group but remained unchanged in NBD and IL-1ra treated donors (BD-UT: 473.1 ± 86.2 U/L; n=5, vs. NBD: 270.5 ± 43.2 U/L; n=4, vs. BD + IL1ra: 268.0 ± 33.4 U/L; n=4, p=0.028) (Figure 3G).
Figure 3.
Hematoxylin-eosin stained sections of pancreas tissue in (A) NBD (n=7), (B) BD-UT (n=7) and (C) BD + IL-1ra (n=6) treated donors at six hours post BD induction. Insulin staining of pancreas sections in (D) NBD, (E) BD-UT and (F) BD + IL-1ra treated donors. (G) Quantification of serum amylase levels at 6 hours post BD induction. Data are expressed as mean values ± SEM (*p<0.05).
Donor management with IL-1ra prevents macrophage migration, accumulation, activation and differentiation within islets recovered from brain-dead donors
Next, we investigated the migration of PMN and monocytes into pancreatic tissue. Immunohistochemical staining of the pancreas showed that a higher number of CD68 positive staining cells accumulated in pancreas from BD-UT donors and in some cases exclusively surrounding pancreatic islets when compared to NBD controls and IL-1ra treated animals (11.47 ± 0.03% vs. 2.32 ± 0.01 vs. 4.70 ± 3.63%, respectively, n=5 for each group, p<0.001) (Figure 4A–D). Flow cytometric analysis also revealed an increase in activated macrophages/dendritic cells (CD11C+ cells) in dispersed isolated islets from BD untreated donors when compared to those receiving IL-1ra treatment and more importantly, compared to NBD donors (Figure 5A–D). These CD11C+ cells had significantly elevated expression of IL-1β (p < 0.01) and IL-8 (p <0.01) when compared to those isolated from NBD donors and those obtained from IL-1ra treated BD donors (Figure 5E). Expression of MCP-1 and MIP-1a was also elevated in DC from BD-UT islets, but this comparison did not achieve statistical significance.
Figure 4.
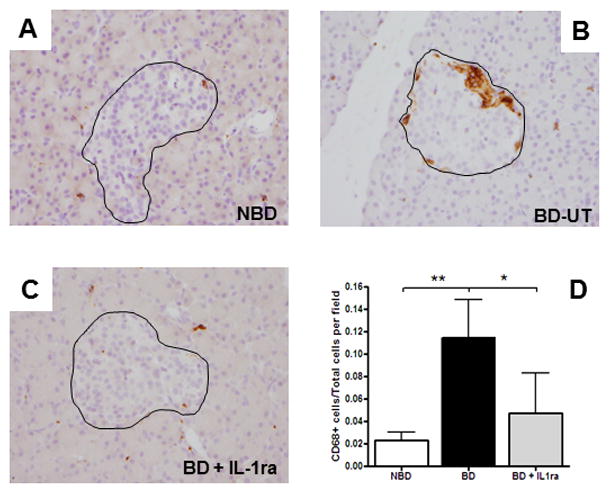
Migration and infiltration of inflammatory leukocytes into pancreatic tissue 6 hours after brain-death induction. Representative images of CD68+ cells in pancreas biopsies and islets (outlined) of (A) NBD (n=7), (B) BD-UT (n=7) and(C) BD+IL-1ra (n=6) treated animals. (D) Images were analyzed and quantification performed using image analysis software. Data are expressed as mean values ± SEM, (*p<0.05 and **p<0.01).
Figure 5.
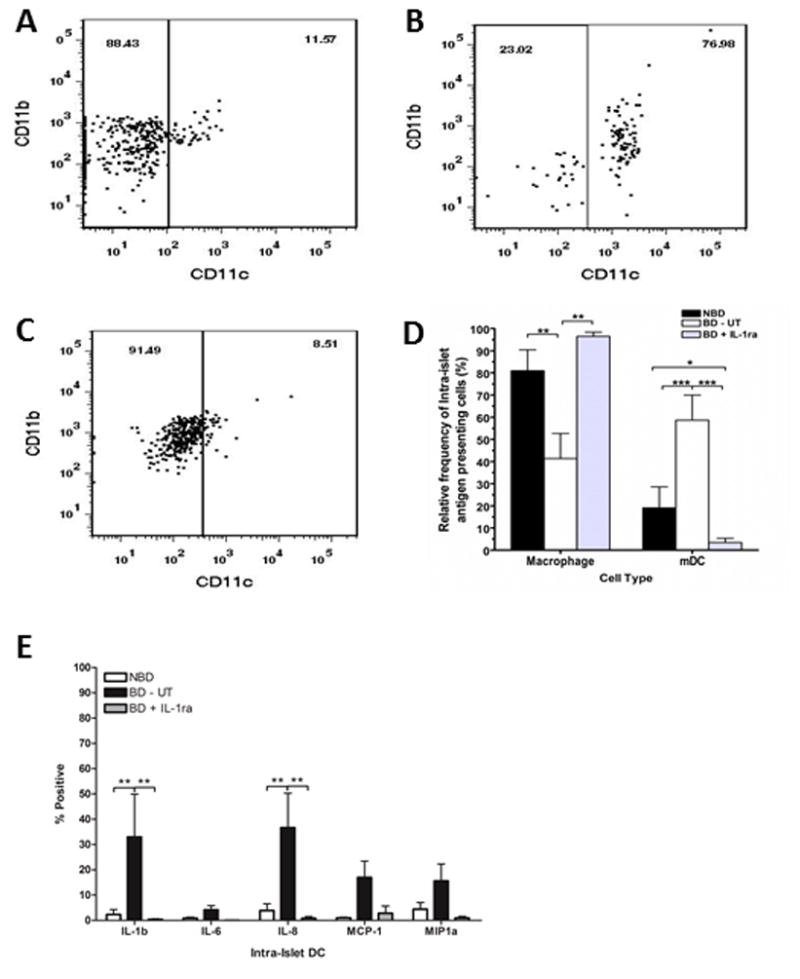
Dispersed islets were stained using mAb specific to CD11b and CD11c and analyzed by flow cytometry. The relative frequencies of immature monocytes (CD11b+, CD11c−) and activated macrophages/myeloid dendritic cells (mDC, CD11b+, CD11c+) in islets isolated from (A) NBD (n=7), (B) BD-UT (n=7) and (C) BD + IL-1ra (n=6) were measured and compared between groups (D). Intra-islet activation of mDC was then analyzed in dispersed islets stained for activation markers IL-1b, IL-6, IL-8, MCP-1 and MIP-1a. The scatter plots are representative of one experiment. Data are expressed as mean values ± SEM, (*p<0.05, **p<0.01 and ***p<0.001).
IL-1 receptor blockade decreased the expression of pro-inflammatory chemokines in the pancreas of brain-dead donors
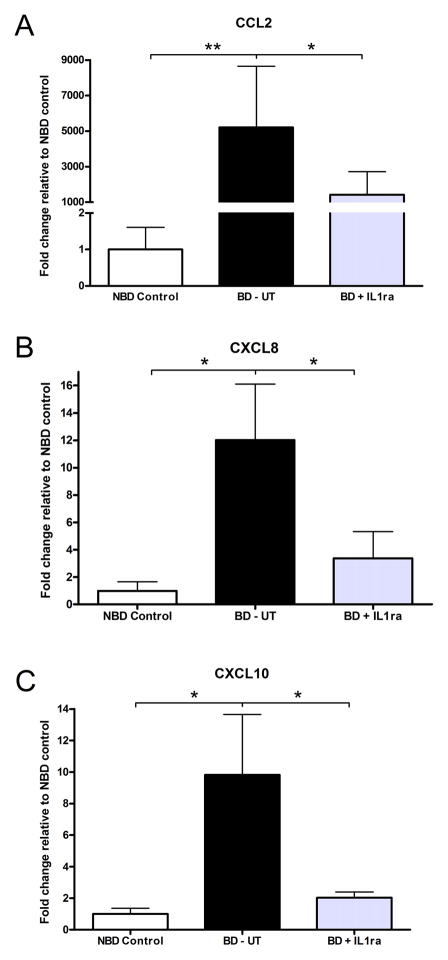
We measured chemokine expression to determine if systemic delivery of IL-1ra was decreasing leukocyte recruitment to tissue by interfering with these signals. We observed a significant increase in the level of CCL2 (MCP-1), CXCL (IL-8) and CXCL10 (IP-10) transcripts in pancreas tissue from BD-UT donors. Animals receiving IL-1ra treatment had a marked down-regulation of chemokine mRNA and the expression levels were similar to those observed in the NBD control group (Figure 6A–C).
Figure 6.
Analysis of chemokine mRNA expression in pancreas tissue 6 hours after induction of brain-death. Expression levels of (A) CCL2 (MCP1), (B) CXCL8 (IL-8) and (C) CXCL10 (IP-10) were analyzed and compared in all groups. Data are shown as relative fold induction in BD-UT (n=7) and IL-1ra (n=6) treated donors, compared to control untreated pancreatic tissue from NBD control animals (n=7). Data are shown as mean values ± SEM (*p<0.05 and **p<0.01).
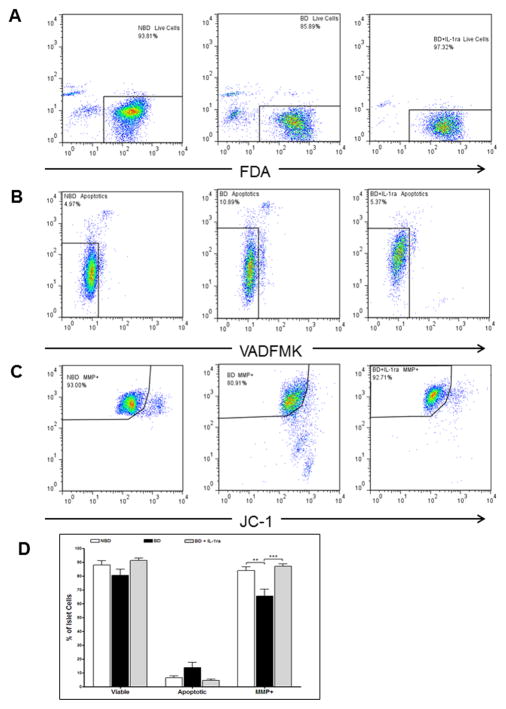
Donor pre-treatment with IL-1 receptor blockade increased islet functional potency in-vitro and in-vivo
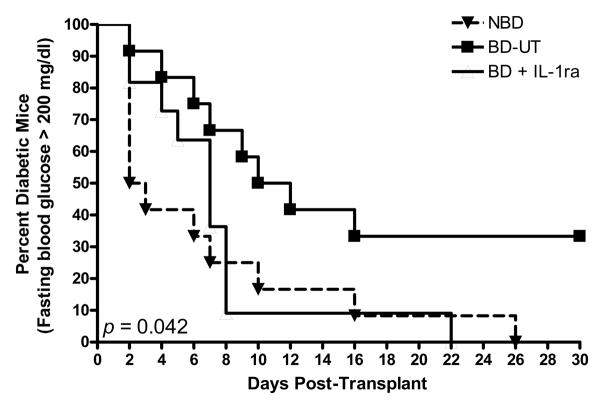
Isolated islets were dispersed and analyzed by flow cytometry using probes designed to test cell viability (FDA and Topro3), apoptosis (Annexin V and VAD-FMK) and mitochondrial membrane polarity (JC-1). Islets recovered from BD-UT donors showed lower viability (%), higher apoptosis (%) and significantly decreased MMP (%) when compared to their NBD counterparts. More importantly, islets from donors pretreated with IL-1ra did not exhibit reduction in viability or MMP and maintained an apoptosis level comparable to that of islets from NBD donors (Figure 7A–D). In addition, isolated islets from donors in all groups were transplanted into the renal subcapsular space of diabetic immunodeficient NOD.scid mice (1000 IEQ/mouse) to evaluate functional potency. Recipients transplanted with islets from NBD (n=13) and IL-1ra treated donors (n=11) showed a markedly reduced median time to cure (7 and 3 days, respectively) and significantly higher cure rate of 72.7 for NBD donors and 84.6% for recipients of IL-1ra treated islets (p <0.05) when compared to animals transplanted with islets from BD-UT donors (n=10) in which median time to cure was 11 days and overall cure efficiency rate was 33.3% at 14 days post implantation, (p <0.05) (Figure 8). No differences between time to cure, cure percentage and blood glucose were observed between recipients of islets from brain death donors treated with IL-1ra and recipients from the NBD control group. We did not observe significant differences in post-isolation yield and glucose stimulated insulin release or viability by fluorescein diacetate/propidium iodide assay 24 hours after isolation (Table 1).
Figure 7.
Donor pretreatment with IL-1ra prevents mitochondrial dysfunction induced by brain-death and the pro-inflammatory response. Islets cultured for 24h after isolation were evaluated for changes in viability by (A) FDA staining, (B) apoptosis by VADFMK staining, and (C) mitochondrial integrity and function by JC-1 staining. (D) Data shown are the percentage of islet cells with that are viable, apoptotic and have polarized ΔΨm indicated by high JC-1 red/JC-1 green fluorescence. Data are expressed as mean ± SEM (NBD (n=7), BD-UT (n=7) and BD + IL-1ra (n=6), **p<0.01 and ***p<0.001).
Figure 8.
Percent diabetes-free NOD.scid mice after xenogeneic islet transplantion. Immunodeficient mice received 1000 IEQ implanted in the subcapsular space of the left kidney. Mice in the NBD control group (n=11), BD-UT group (n=10) and BD+IL-1ra (n=13) were followed for 30 days after implantation and monitored daily for return to normoglycemia (defined as random blood glucose < 200 mg/dl). The differences between groups were significant (p = 0.0428 established by the log-rank test).
Table 1.
Islet isolation and quality control assesment of isolated islets
| Non-BD (n=7) | BD-Untreated (n=7) | BD + IL-1ra (n=6) | P value | |
|---|---|---|---|---|
| Post-isolation Yield (IEQ) | 64311 ± 39186 | 53403± 24212 | 77415 ± 30672 | ns |
| Viability by FDA/PI assay | 90.8 ± 3.1 | 89.1 ± 7.2 | 91.9 ± 3.8 | ns |
| GSIS S.I. (16.7 mM/3.3 mM) | 3.4 ± 1.2 | 2.5 ± 1.8 | 3.1 ± 1.5 | ns |
| Mouse bioassay | ||||
| Cure rate | 11/13 (84.6 %) | 3/10 (30.0 %) | 8/11 (72.7 %) | <0.05 |
| Blood glucose (mg/dl) at day 7 | 217.9 ± 31.6 | 335.0 ± 49.9 | 183.9 ± 30.4 | <0.05 |
DISCUSSION
In this study we investigated the effect of early anti-inflammatory intervention in the brain-dead donor and its impact on pancreas quality and islet function following islet isolation and transplantation. Brain-death is characterized by hemodynamic instability and the release of vasoactive hormones that result in tissue hypo-perfusion and rapid activation of innate immunity (2,22). This systemic and organ specific inflammatory response has been associated with increased tissue immunogenicity and host allo-reactivity, known to increase the risk of delayed graft function and acute rejection (23,24).
The role of pro-inflammatory cytokines has been documented extensively in the pathogenesis of type I and type II diabetes mellitus as well as in the inflammatory process associated with islet isolation and subsequent intra-portal transplantation (3,5). We chose to target the IL-1 receptor based on previous work showing IL-1ra protected islets from IL-1β induced necrosis and apoptosis triggered by cytokines in culture from and data from our own laboratory showing that during BD the IL-1 pathway is strongly up-regulated in NHP following BD induction (7,29). In the context of islet transplantation, Contreras et al. showed a detrimental effect of BD in post-isolation islet yields and islet functional potency both in-vitro and after intra-portal transplantation into diabetic immunodeficient mice (6). Although we did not observe significant differences in islet yields, our results did indicate that BD results in a marked reduction in islet cell and exocrine tissue viability as shown by increased serum amylase levels, a substantial loss in insulin staining and alterations in morphological integrity of the islets within the pancreas.
We documented a rapid mobilization of neutrophils into peripheral circulation six hours after BD induction. Interestingly, donor animals receiving treatment with IL-1ra did not show significant changes in the numbers of circulating immunocytes compared to NBD controls. In addition, intracellular staining of these cell populations showed that IL-1ra treated animals had a reduced expression of pro-inflammatory cytokines and chemokines suggesting an IL-1 dependent anti-inflammatory effect that impacted systemic cell activation and mobilization. These findings are in agreement with data from previous studies in which administration of IL-1ra was found to decrease neutrophil activation when tested in mouse models of cerebral focal ischemia and in patients after acute ischemic injury and stroke (11,37). Ischemia/reperfusion is also known to increase expression and secretion of pro-inflammatory mediators that modulate inflammatory responses and play a role in inflammatory injury to end organs (31,32). We observed that expression for all measured cytokines and chemokines was significantly higher in cells obtained from untreated donors at six hours post induction. Interestingly, animals that received IL-1ra throughout the BD period revealed no changes in the level of cytokine and chemokine expression compared to baseline levels and these results paralleled those observed in NBD donors. Elevated levels of IL-6 in deceased donors and transplant recipients have been previously associated with increased graft immunogenicity, early allograft dysfunction and lower recipient survival (8,20). We observed a systemic release of IL-6 that was suppressed to control levels by IL-1 blockade in our model. Our data support a generalized anti-inflammatory effect of IL-1ra during BD through the suppression of pro-inflammatory cytokine and chemokine production and release. Flow cytometric data in our model suggest that IL-1ra prevented activation of monocytes and mDC in systemic circulation and also within the pancreatic islet. The inactivation of pro-inflammatory leukocytes at the tissue level may likely prevent the formation of reactive oxygen species and cytokines that induce apoptosis and cell necrosis, and suggests a potential mechanism by which IL-1ra confers protection when administered after neurological injury has occurred. These data are supported by findings from studies in which steroid treatment in the donor aimed at preventing inflammatory injury led to improved organ quality and function after transplantation (14,21).
The most important finding in our study was the observed increase in tissue engraftment and islet function after islet transplantation into diabetic immune-deficient recipients when using islets isolated from IL-1ra treated donors. We chose the mouse bioassay as a simple method to monitor islet functional potency and avoid the confounding effects of allo-reactivty in the primate model. The improved cure efficiency and sustained normoglycemia in mice receiving islets from IL-1ra treated donors was comparable to that of islets transplanted from NBD donors. Since transplant recipients did not receive treatment, the protective effect of IL-1ra was likely explained by attenuation of the inflammatory response at the donor level and more importantly, protection was sustained during the isolation process and through tissue culture. To our knowledge, this is the first report describing the use of anti-inflammatory therapy targeting IL-1 in the context of donor management. However, there is already some evidence of the beneficial effect of IL-1ra treatment in islet transplantation. We previously described the protective effect IL-1ra on isolated islets and demonstrated that blocking endogenously produced IL-1β during islet isolation and culture led to an important reduction in cell necrosis, apoptosis and mitochondrial dysfunction (29). Moreover, a recent study by Montolio et al. reported a pivotal role for IL-1β driven nitric oxide (NO) islet injury responsible for extensive beta cell necrosis after islet transplantation (19). In addition, recent reports have shown that blockade of IL-1β in culture through the use of IL-1 receptor antagonists prior to transplantation prevents islet injury, improves islet quality and facilitates engraftment (16,17,28). The combined use of IL-1ra and TNF-α blockade is being used in pre-clinical models of rodent and human islet transplantation and has been found to improve tissue engraftment. These reports expanded on preliminary results by Matsumoto et al. showing that combined IL-1 and TNF-α blockade facilitates tissue engraftment and improved islet transplantation efficacy (16,17).
For the purpose of understanding the immunobiology of BD donors, we created a unique non-human primate model that recreates clinical brain-death and constitutes a powerful tool to study the role of intervention at the organ donor level. However, the implementation of this model involves some important limitations that need to be addressed. First, we focused our efforts on understanding the effect of therapy in the organ donor and did not address the impact of treatment in an intra-portal non-human primate allo-transplantation model. We used the mouse bioassay as a simple method to evaluate islet functional potency in the absence of confounding variables commonly associated with the use of a large animal islet transplantation models. However, further investigation is needed to determine the impact of donor IL-1 blockade using a large animal allo-transplant model to validate our preliminary results. Furthermore, the objective of this study was to provide a proof of concept and the data presented here are only representative of the early events of BD induced acute inflammatory injury. We acknowledge that six hours are a relatively narrow time frame and further studies evaluating the effect of therapy in clinically relevant time-frames are warranted. Lastly, we did not test the additive benefit of anti-inflammatory therapy at any other stage of the transplant process. It is possible that treatment with IL-1ra alone or in combination with other potent anti-inflammatory therapies (donor management, during cold preservation, tissue culture and/or at the level of the recipient) may hold greater potential in the protection of islets from inflammatory injury. We did not consider the use of steroids since they are known to negatively impact islet and beta cell function in the peri-transplant period and have failed to consistently show protective effects when used in donor management prior to transplantation(26).
The results from this study indicate that inflammatory tissue injury begins at the donor level and support results by others suggesting a detrimental effect of ischemia/reperfusion injury that is triggered early in the transplantation process. More importantly, it provides evidence suggesting that early intervention in the BD donor using IL-1ra may be beneficial in the protection of pancreas and islets prior to transplantation. Based on our results, we consider that early donor management targeting IL-1 receptor signaling is a powerful and practical approach to improve current clinical islet transplant outcomes.
Acknowledgments
This study was supported in part by internal funds of the Department of Surgery/Division of Transplantation at the University of Wisconsin-Madison. We gratefully acknowledge Kimberly Maurer, Dan Consigny and Drew Roenneburg for expert technical assistance.
Abbreviations
- BD
brain death
- DGF
delayed graft function
- HIV
human immunodeficiency virus
- IL
interleukin
- IL-1ra
Interleukin 1 Receptor Antagonist
- MHC
major histocompatibility complex
- NHP
non-human primate
- NIH
National Institutes of Health
- PBMC
Peripheral blood mononuclear cells
- ROS
reactive oxygen species
- TB
tuberculosis
- USDA
United States Department of Agriculture
Footnotes
DISCLOSURE
The authors of this manuscript have no conflicts of interest to disclose.
References
- 1.Arnush M, Heitmeier MR, Scarim AL, Marino MH, Manning PT, Corbett JA. IL-1 produced and released endogenously within human islets inhibits beta cell function. J Clin Invest. 1998;102(3):516–526. doi: 10.1172/JCI844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Barklin A. Systemic inflammation in the brain-dead organ donor. Acta Anaesthesiol Scand. 2009;53(4):425–435. doi: 10.1111/j.1399-6576.2008.01879.x. [DOI] [PubMed] [Google Scholar]
- 3.Barshes NR, Wyllie S, Goss JA. Inflammation-mediated dysfunction and apoptosis in pancreatic islet transplantation: implications for intrahepatic grafts. J Leukoc Biol. 2005;77(5):587–597. doi: 10.1189/jlb.1104649. [DOI] [PubMed] [Google Scholar]
- 4.Bendtzen K, Mandrup-Poulsen T, Nerup J, Nielsen JH, Dinarello CA, Svenson M. Cytotoxicity of human pI 7 interleukin-1 for pancreatic islets of Langerhans. Science. 1986;232(4757):1545–1547. doi: 10.1126/science.3086977. [DOI] [PubMed] [Google Scholar]
- 5.Cnop M, Welsh N, Jonas JC, Jörns A, Lenzen S, Eizirik DL. Mechanisms of pancreatic beta-cell death in type 1 and type 2 diabetes: many differences, few similarities. Diabetes. 2005;54(Suppl 2):S97–107. doi: 10.2337/diabetes.54.suppl_2.s97. [DOI] [PubMed] [Google Scholar]
- 6.Contreras J, Eckstein C, Smyth C, Sellers M, Vilatoba M, Bilbao G, Rahemtulla F, Young C, Thompson J, Chaudry I, Eckhoff DE. Brain death significantly reduces isolated pancreatic islet yields and functionality in vitro and in vivo after transplantation in rats. Diabetes. 2003;52(12):2935–2942. doi: 10.2337/diabetes.52.12.2935. [DOI] [PubMed] [Google Scholar]
- 7.Danobeitia JS, Sperger JM, Hanson MS, Park EE, Chlebeck PJ, Roenneburg DA, Sears ML, Connor JX, Schwarznau A, Fernandez LA. Early activation of the inflammatory response in the liver of brain-dead non-human primates. J Surg Res. 2012;176(2):639–648. doi: 10.1016/j.jss.2011.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Deng MC, Erren M, Kammerling L, Gunther F, Kerber S, Fahrenkamp A, Assmann G, Breithardt G, Scheld HH. The relation of interleukin-6, tumor necrosis factor-alpha, IL-2, and IL-2 receptor levels to cellular rejection, allograft dysfunction, and clinical events early after cardiac transplantation. Transplantation. 1995;60(10):1118–1124. doi: 10.1097/00007890-199511270-00011. [DOI] [PubMed] [Google Scholar]
- 9.Dinarello CA. Immunological and inflammatory functions of the interleukin-1 family. Annu Rev Immunol. 2009;27:519–550. doi: 10.1146/annurev.immunol.021908.132612. [DOI] [PubMed] [Google Scholar]
- 10.Dinarello CA, Donath MY, Mandrup-Poulsen T. Role of IL-1beta in type 2 diabetes. Curr Opin Endocrinol Diabetes Obes. 2010;17(4):314–321. doi: 10.1097/MED.0b013e32833bf6dc. [DOI] [PubMed] [Google Scholar]
- 11.Emsley HC, Smith CJ, Georgiou RF, Vail A, Hopkins SJ, Rothwell NJ, Tyrrell PJ Investigators AS. A randomised phase II study of interleukin-1 receptor antagonist in acute stroke patients. J Neurol Neurosurg Psychiatry. 2005;76(10):1366–1372. doi: 10.1136/jnnp.2004.054882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Floerchinger B, Oberhuber R, Tullius SG. Effects of brain death on organ quality and transplant outcome. Transplant Rev (Orlando) 2012;26(2):54–59. doi: 10.1016/j.trre.2011.10.001. [DOI] [PubMed] [Google Scholar]
- 13.Hoorens A, Stangé G, Pavlovic D, Pipeleers D. Distinction between interleukin-1-induced necrosis and apoptosis of islet cells. Diabetes. 2001;50(3):551–557. doi: 10.2337/diabetes.50.3.551. [DOI] [PubMed] [Google Scholar]
- 14.Kotsch K, Ulrich F, Reutzel-Selke A, Pascher A, Faber W, Warnick P, Hoffman S, Francuski M, Kunert C, Kuecuek O, Schumacher G, Wesslau C, Lun A, Kohler S, Weiss S, Tullius SG, Neuhaus P, Pratschke J. Methylprednisolone therapy in deceased donors reduces inflammation in the donor liver and improves outcome after liver transplantation: a prospective randomized controlled trial. Ann Surg. 2008;248(6):1042–1050. doi: 10.1097/SLA.0b013e318190e70c. [DOI] [PubMed] [Google Scholar]
- 15.Mandrup-Poulsen T, Zumsteg U, Reimers J, Pociot F, Mørch L, Helqvist S, Dinarello CA, Nerup J. Involvement of interleukin 1 and interleukin 1 antagonist in pancreatic beta-cell destruction in insulin-dependent diabetes mellitus. Cytokine. 1993;5(3):185–191. doi: 10.1016/1043-4666(93)90003-n. [DOI] [PubMed] [Google Scholar]
- 16.Matsumoto S, Takita M, Chaussabel D, Noguchi H, Shimoda M, Sugimoto K, Itoh T, Chujo D, Sorelle J, Onaca N, Naziruddin B, Levy MF. Improving Efficacy of Clinical Islet Transplantation With Iodixanol-Based Islet Purification, Thymoglobulin Induction, and Blockage of IL-1β and TNF-α. Cell Transplant. 2011;20(10):1641–1647. doi: 10.3727/096368910X564058. [DOI] [PubMed] [Google Scholar]
- 17.McCall M, Pawlick R, Kin T, Shapiro AM. Anakinra potentiates the protective effects of etanercept in transplantation of marginal mass human islets in immunodeficient mice. Am J Transplant. 2012;12(2):322–329. doi: 10.1111/j.1600-6143.2011.03796.x. [DOI] [PubMed] [Google Scholar]
- 18.Miyamoto M, Morimoto Y, Nozawa Y, Balamurugan AN, Xu B, Inoue K. Establishment of fluorescein diacetate and ethidium bromide (FDAEB) assay for quality assessment of isolated islets. Cell Transplant. 2000;9(5):681–686. doi: 10.1177/096368970000900514. [DOI] [PubMed] [Google Scholar]
- 19.Montolio M, Biarnés M, Téllez N, Escoriza J, Soler J, Montanya E. Interleukin-1beta and inducible form of nitric oxide synthase expression in early syngeneic islet transplantation. J Endocrinol. 2007;192(1):169–177. doi: 10.1677/joe.1.06968. [DOI] [PubMed] [Google Scholar]
- 20.Murugan R, Venkataraman R, Wahed AS, Elder M, Hergenroeder G, Carter M, Madden NJ, Powner D, Kellum JA Investigators HS. Increased plasma interleukin-6 in donors is associated with lower recipient hospital-free survival after cadaveric organ transplantation. Crit Care Med. 2008;36(6):1810–1816. doi: 10.1097/CCM.0b013e318174d89f. [DOI] [PubMed] [Google Scholar]
- 21.Pratschke J, Kofla G, Wilhelm MJ, Vergopoulos A, Laskowski I, Shaw GD, Tullius SG, Volk HD, Neuhaus P, Tilney NL. Improvements in early behavior of rat kidney allografts after treatment of the brain-dead donor. Ann Surg. 2001;234(6):732–740. doi: 10.1097/00000658-200112000-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Pratschke J, Neuhaus P, Tullius S. What can be learned from brain-death models? Transpl Int. 2005;18(1):15–21. doi: 10.1111/j.1432-2277.2004.00018.x. [DOI] [PubMed] [Google Scholar]
- 23.Pratschke J, Wilhelm M, Kusaka M, Beato F, Milford E, Hancock W, Tilney N. Accelerated rejection of renal allografts from brain-dead donors. Ann Surg. 2000;232(2):263–271. doi: 10.1097/00000658-200008000-00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Pratschke J, Wilhelm MJ, Laskowski I, Kusaka M, Beato F, Tullius SG, Neuhaus P, Hancock WW, Tilney NL. Influence of donor brain death on chronic rejection of renal transplants in rats. J Am Soc Nephrol. 2001;12(11):2474–2481. doi: 10.1681/ASN.V12112474. [DOI] [PubMed] [Google Scholar]
- 25.Rao DA, Pober JS. Endothelial injury, alarmins, and allograft rejection. Crit Rev Immunol. 2008;28(3):229–248. doi: 10.1615/critrevimmunol.v28.i3.40. [DOI] [PubMed] [Google Scholar]
- 26.Rech TH, Moraes RB, Crispim D, Czepielewski MA, Leitão CB. Management of the brain-dead organ donor: a systematic review and meta-analysis. Transplantation. 2013;95(7):966–974. doi: 10.1097/TP.0b013e318283298e. [DOI] [PubMed] [Google Scholar]
- 27.Ricordi C, Lacy PE, Finke EH, Olack BJ, Scharp DW. Automated method for isolation of human pancreatic islets. Diabetes. 1988;37(4):413–420. doi: 10.2337/diab.37.4.413. [DOI] [PubMed] [Google Scholar]
- 28.Schwarznau A, Hanson M, Sperger J, Schram B, Danobeitia J, Greenwood K, Vijayan A, Fernandez L. IL-1beta receptor blockade protects islets against pro-inflammatory cytokine induced necrosis and apoptosis. J Cell Physiol. 2009;220(2):341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schwarznau A, Hanson MS, Sperger JM, Schram BR, Danobeitia JS, Greenwood KK, Vijayan A, Fernandez LA. IL-1 beta Receptor Blockade Protects Islets Against Pro-inflammatory Cytokine Induced Necrosis and Apoptosis. Journal of Cellular Physiology. 2009;220(2):341–347. doi: 10.1002/jcp.21770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Selvaggi G, Fernandez L, Bottino R, Lehmann R, Kenyon NS, Inverardi L, Ricordi C. Improved baboon pancreatic islet cell isolation. Transplant Proc. 1997;29(4):1967–1968. doi: 10.1016/s0041-1345(97)00185-1. [DOI] [PubMed] [Google Scholar]
- 31.Skrabal C, Thompson L, Potapov E, Southard R, Joyce D, Youker K, Noon G, Loebe M. Organ-specific regulation of pro-inflammatory molecules in heart, lung, and kidney following brain death. J Surg Res. 2005;123(1):118–125. doi: 10.1016/j.jss.2004.07.245. [DOI] [PubMed] [Google Scholar]
- 32.Takada M, Nadeau K, Hancock W, Mackenzie H, Shaw G, Waaga A, Chandraker A, Sayegh M, Tilney N. Effects of explosive brain death on cytokine activation of peripheral organs in the rat. Transplantation. 1998;65(12):1533–1542. doi: 10.1097/00007890-199806270-00001. [DOI] [PubMed] [Google Scholar]
- 33.Terasaki P, Cecka J, Gjertson D, Takemoto S. High survival rates of kidney transplants from spousal and living unrelated donors. N Engl J Med. 1995;333(6):333–336. doi: 10.1056/NEJM199508103330601. [DOI] [PubMed] [Google Scholar]
- 34.Toledo-Pereyra LH, Lopez-Neblina F, Toledo AH. Reactive oxygen species and molecular biology of ischemia/reperfusion. Ann Transplant. 2004;9(1):81–83. [PubMed] [Google Scholar]
- 35.Weiss S, Kotsch K, Francuski M, Reutzel-Selke A, Mantouvalou L, Klemz R, Kuecuek O, Jonas S, Wesslau C, Ulrich F, Pascher A, Volk HD, Tullius SG, Neuhaus P, Pratschke J. Brain death activates donor organs and is associated with a worse I/R injury after liver transplantation. Am J Transplant. 2007;7(6):1584–1593. doi: 10.1111/j.1600-6143.2007.01799.x. [DOI] [PubMed] [Google Scholar]
- 36.Wijdicks EF. The diagnosis of brain death. N Engl J Med. 2001;344(16):1215–1221. doi: 10.1056/NEJM200104193441606. [DOI] [PubMed] [Google Scholar]
- 37.Yang GY, Liu XH, Kadoya C, Zhao YJ, Mao Y, Davidson BL, Betz AL. Attenuation of ischemic inflammatory response in mouse brain using an adenoviral vector to induce overexpression of interleukin-1 receptor antagonist. J Cereb Blood Flow Metab. 1998;18(8):840–847. doi: 10.1097/00004647-199808000-00004. [DOI] [PubMed] [Google Scholar]