Abstract
Background:
Limonium tetragonum, a naturally salt-tolerant halophyte, has been studied recently and is of much interest to researchers due to its potent antioxidant and hepatoprotective activities.
Objective:
In the present study, we attempted to elucidate bioactive compounds from ethyl acetate (EtOAc) soluble fraction of L. tetragonum extract. Furthermore, the simultaneous analysis method of bioactive EtOAc fraction of L. tetragonum has been developed using high-performance liquid chromatography (HPLC).
Materials and Methods:
Thirteen compounds have been successfully isolated from EtOAc fraction of L. tetragonum, and the structures of 1–13 were elucidated by extensive one-dimensional and two-dimensional spectroscopic methods including 1H-NMR, 13C-NMR, 1H-1H COSY, heteronuclear single quantum coherence, heteronuclear multiple bond correlation, and nuclear Overhauser effect spectroscopy. Hepatoprotection of the isolated compounds against liver fibrosis was evaluated by measuring inhibition on hepatic stellate cells (HSCs) undergoing proliferation.
Results:
Compounds 1–13 were identified as gallincin (1), apigenin-3-O-β-D-galactopyranoside (2), quercetin (3), quercetin-3-O-β-D-galactopyranoside (4), (−)-epigallocatechin (5), (−)-epigallocatechin-3-gallate (6), (−)-epigallocatechin-3-(3″-O-methyl) gallate (7), myricetin-3-O-β-D-galactopyranoside (8), myricetin-3-O-(6″-O-galloyl)-β-D-galactopyranoside (9), myricetin-3-O-α-L-rhamnopyranoside (10), myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside (11), myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside (12), and myricetin-3-O-α-L-arabinopyranoside (13), respectively. All compounds except for 4, 8, and 10 are reported for the first time from this plant.
Conclusion:
Myricetin glycosides which possess galloyl substituent (9, 11, and 12) showed most potent inhibitory effects on the proliferation of HSCs.
SUMMARY
In the present study, we have successfully isolated 13 compounds from bioactive fraction of Limonium tetragonum. The structures of compounds isolated have been fully elucidated, and hepatoprotective activities of compounds against liver fibrosis were evaluated by measuring inhibition on hepatic stellate cells undergoing proliferation. Furthermore, the simultaneous analysis method of bioactive ethyl acetate fraction of L. tetragonum has been developed using HPLC. Ten compounds identified herein are reported for the first time from this plant.
Abbreviations used: HSQC: Heteronuclear single quantum coherence; HMBC: Heteronuclear multiple bond correlation; NOESY: Nuclear Overhauser effect spectroscopy; EGCG: Epigallocatechin-3-gallate; EGC: Epigallocatechin; HSC: Hepatic stellate cell; MTT: 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide.
Keywords: Constituents, halophyte, hepatoprotective, Limonium tetragonum, simultaneous analysis
INTRODUCTION
Salty soils such as marshes and muddy seashores contain a variety halophyte species. The edible halophytes have a long history of usage for their high nutritional content, for medicinal value, or as an excellent source of salt. The potential of halophytes as nutraceuticals and/or medicinal sources is mainly due to the enriched natural antioxidants contained in halophytes. To protect itself, halophyte is known to involve powerful antioxidant defense system that can tolerate unfavorable environmental condition. Enzymatic or nonenzymatic components in halophytes make possible to block the harmful action of internally generated reactive oxygen species and sometimes by directly scavenging free radicals.[1,2] With this respect, various medicinal halophytes are shown to be potential sources of high levels of bioactive compounds including terpenoids (carotenoids, diterpenes, sesquiterpenes and essential oil), predominantly polyphenols.[3,4,5,6,7]
Limonium tetragonum (Thunb.) Bullock is a biennial halophyte in the family of Plumbaginaceae. L. tetragonum is a naturally salt-tolerant plant growing in muddy seashores and salt marches. The edible young leaves and shoots of this plant (known as “Jin-Chi-Ye-Cao” or “Bu-Xue-Cao”) have been locally used by people living near foreshores for their salty taste and their high nutritional content. Recently, L. tetragonum has been received much attention for its potent hepatoprotective effects. The methanolic extract of L. tetragonum effectively suppressed the proliferation of hepatic stellate cells (HSCs) stimulated by culturing environment or platelet-derived growth factor (PDGF-BB) with little affect on hepatocytes. The production of inflammatory cytokines, tumor necrosis factor-alpha, was also attenuated by L. tetragonum in RAW264.7 macrophages.[8] Ethyl acetate (EtOAc) soluble fraction of L. tetragonum was further verified for its hepatoprotection in experimental animal models. The administration of the EtOAc fraction of L. tetragonum rapidly decreased blood ethanol concentration through activating alcohol metabolic enzymes, alcohol dehydrogenase, and aldehyde dehydrogenase following acute ethanol intoxication in Sprague-Dawley rats. The increased MDA level was decreased, and the reduced activities of superoxide dismutase (SOD), glutathione peroxidase, and catalase were markedly preserved by L. tetragonum.[9] In diethylnitrosamine (DEN)-induced hepatotoxic rat model, the EtOAc soluble fraction of L. tetragonum showed protection against DEN-induced hepatotoxicity that attenuated the increase in serum levels of alanine transaminase, aspartate aminotransferase, alkaline phosphatase, gamma-glutamyl transferase, and triglycerides. The increased oxidative stress, mononuclear cell infiltration, and fibrotic process in the liver were markedly attenuated by L. tetragonum.[10] Nevertheless of hepatoprotective functionality of L. tetragonum, scientific evidence on molecular actions and the involved bioactive constituents of this plant are still insufficient. In regard to constituents, four flavonoids with peroxide scavenging activities have been only reported from this plant so far.[11]
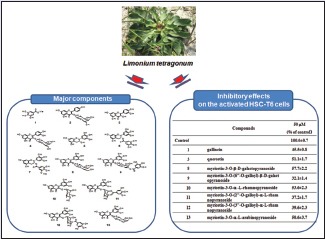
In the present study, we have attempted to isolate bioactive constituents from the EtOAc fraction of L. tetragonum extract and tested the hepatoprotective activities of the isolated compounds in the activated HSC-T6 cells.
MATERIALS AND METHODS
General methods
Optical rotations were measured on a JASCO P-1010 digital polarimeter. All NMR spectra were measured on a Varian VNMRS 500 spectrometer (500 and 125 MHz for 1H and 13C, respectively) using CD3OD as solvent, with proton and carbon chemical shifts recorded at δH 3.30 and δC 49.0. The ESI mass spectra were acquired using an ABSCIEX QTRAP 3200 instrument. High-performance liquid chromatography (HPLC) was performed using a Varian Prostar system with a 355 refractive index (RI) detector or Agilent 1200 Chemstation with diode array detector. The separation was performed using the Phenomenex or YMC C18 column (10 mm × 250 mm, 5 μm) with solvents that were distilled before use.
Plant material
The whole plants of L. tetragonum were collected from the foreshore in Sinan-gun, Korea, in July 2013. A voucher specimen (GNP-70) has been deposited in the Laboratory of Pharmacognosy, College of Life Sciences, Gyeongnam National University of Science and Technology.
Extraction and isolation
The leaves of L. tetragonum were dried using freezing dryer and then extracted three times with methanol for 3 h each in an ultrasonic apparatus. Removal of the solvent in vacuo yielded a methanolic extract (569.9 g). The methanolic extract was then suspended in distilled water and partitioned successively with n-hexane (96.0 g), CHCl3 (1.2 g), EtOAc (42.6 g), and n-BuOH (56.3 g). Each fraction was evaluated for its inhibitory activity on the proliferation of the activated HSC-T6 cells. Among the four fractions, the EtOAc fraction which showed the significant antiproliferative activity was subjected to column chromatography on a silica gel column using mixtures of CHCl3−MeOH of increasing polarity as eluents to give 19 fractions (E1~19). E5 was further subjected to ODS gel column chromatography with a gradient elution of MeOH−H2O (25→62% MeOH) to give five fractions (E5-1~5). Compounds 1 and 3 were obtained from E5-1 and E5-3, respectively. E9 subjected to ODS gel column chromatography with a gradient elution of MeOH−H2O (5→35% MeOH) to give seven fractions (E9-1~7). E9-5 was further applied to C18 RP preparative HPLC (MeOH−H2O 55:45, 5.0 mL/min, 220 nm) to give 11 fractions (E9-5-1~11). Compounds 2 and 5 were obtained from E9-5-1 and E9-5-2, respectively. E10 was subjected to column chromatography on Sephadex LH-20 (MeOH) to give 14 fractions (E10-1~14). Compounds 6 and 7 were obtained from E10-11 and E10-13, respectively. E14 and E15 were combined and subjected to ODS gel column chromatography with a gradient elution of MeOH − H2O (5→80% MeOH) to give 11 fractions (E1415-1~11). Compounds 8~10 and 12 were isolated from E1415-10 through C18 RP HPLC using as eluent a mixture of AcCN and H2O (20:80) at a flow rate of 2.0 mL/min with a RI detector. E1415-11 was applied to C18 RP HPLC using as eluent a mixture of AcCN and H2O (26:74) at a flow rate of 2.0 mL/min with a RI detector to yield compounds 11 and 13.
Chromatographic conditions
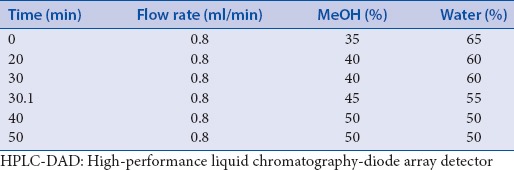
The HPLC system consisted of an Agilent 1100 series HPLC system with a quaternary pump, an autosampler, a diode array detector (DAD). Chromatographic separation was achieved on a Phenomenex hydro-RP18 (5 μm, 4.6 × 250 mm) using a gradient methanol–water with 0.1% acetic acid. The flow rate was 0.8 ml/min and the solvent gradient conditions are shown in Table 1. The eluent was detected with DAD (ultraviolet [UV] 220, 254, 280, and 365 nm).
Table 1.
Solvent gradient conditions for HPLC-DAD
Sample preparation for high-performance liquid chromatography analysis
The EtOAc fraction of L. tetragonum extract was freeze-dried and weighed accurately. The dried fraction was dissolved with 50% ethanol and was filtered through 0.45 um membrane filter (Millipore, Nylon, 170 mm) and analyzed with HPLC.
Culture of hepatic stellate cell-T6
An immortalized rat HSC line, HSC-T6, was provided by Prof. SL Friedman (Columbia University, New York, USA). HSC-T6 cells were maintained in DMEM supplemented with 10% heat-inactivated fetal bovine serum, 100 IU/ml penicillin, and 100 μg/ml streptomycin at 37°C in a humidified atmosphere of 95% air–5% CO2.
Assessment of cell viability
Compounds to be tested were dissolved in dimethyl sulfoxide (DMSO) (final culture concentration, 0.1%). Our preliminary study showed that DMSO at a final concentration of 0.1% in media did not affect the cell viability. For the assay, HSC-T6 cells were seeded in 48-well plates at a density of 5 × 104 cells/mL and incubated for 24 h. The cells were treated with vehicle or compounds to be tested at the concentration as indicated for 48 h. The cell viability was assessed by the 3-(4,5-dimethylthiazol-2-yl)-2.5-diphenyltetrazolium bromide (MTT) assay. The cells were incubated with 2 mg/mL of MTT for 2 h. The reduction of MTT to formazan was assessed in an ELISA plate reader at 450 nm. Data were expressed as the mean of three independent experiments.
RESULTS AND DISCUSSION
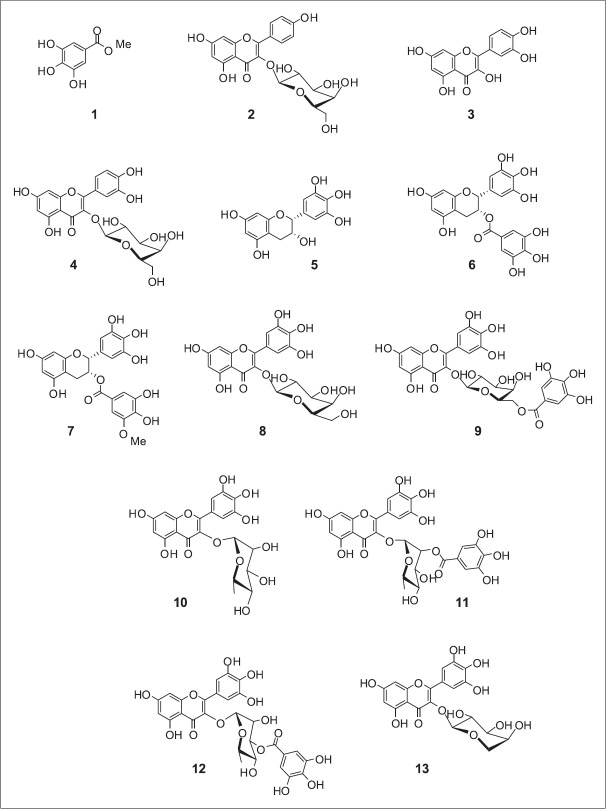
The methanolic extract of L. tetragonum leaves was suspended in water and successively fractionated with n-hexane, CHCl3, EtOAc, and n-BuOH. Each fraction was evaluated for its inhibitory activity on proliferation in the activated HSC-T6. The EtOAc fraction that previously reported for its hepatoprotective effect in vitro and in vivo[8,9,10] was subjected to repeated column chromatography to yield 13 compounds (1 − 13) [Figure 1]. The isolated compounds were identified as gallincin (1), apigenin-3-O-β-D-galactopyranoside (2), quercetin (3), quercetin-3-O-β-D-galactopyranoside (4), (−)-epigallocatechin (5), (−)-epigallocatechin-3-gallate (6), (−)-epigallocatechin-3-(3″-O-methyl) gallate (7), myricetin-3-O-β-D-galactopyranoside (8), myricetin-3-O-(6″-O-galloyl)-β-D-galactopyranoside (9), myricetin-3-O-α-L-rhamnopyranoside (10), myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside (11), myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside (12), and myricetin-3-O-α-L-arabinopyranoside (13), respectively, by comparison of spectroscopic data with those previously reported [Table 2]. All compounds except for 4, 8, and 10 are reported for the first time from this plant.
Figure 1.
Chemical structures of compounds 1–13 identified in the ethyl acetate fraction of Limonium tetragonum extract. 1: gallincin, 2: apigenin-3-O-β-D-galactopyranoside, 3: quercetin, 4: quercetin-3-O-β-D-galactopyranoside, 5: (−)-epigallocatechin, 6: (−)-epigallocatechin-3-gallate, 7: (−)-epigallocatechin-3-(3″-O-methyl) gallate, 8: myricetin-3-O-β-D-galactopyranoside, 9: myricetin-3-O-(6″-O-galloyl)-β-D-galactopyranoside, 10: myricetin-3-O-α-L-rhamnopyranoside, 11: myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside, 12: myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside, 13: myricetin-3-O-α-L-arabinopyranoside
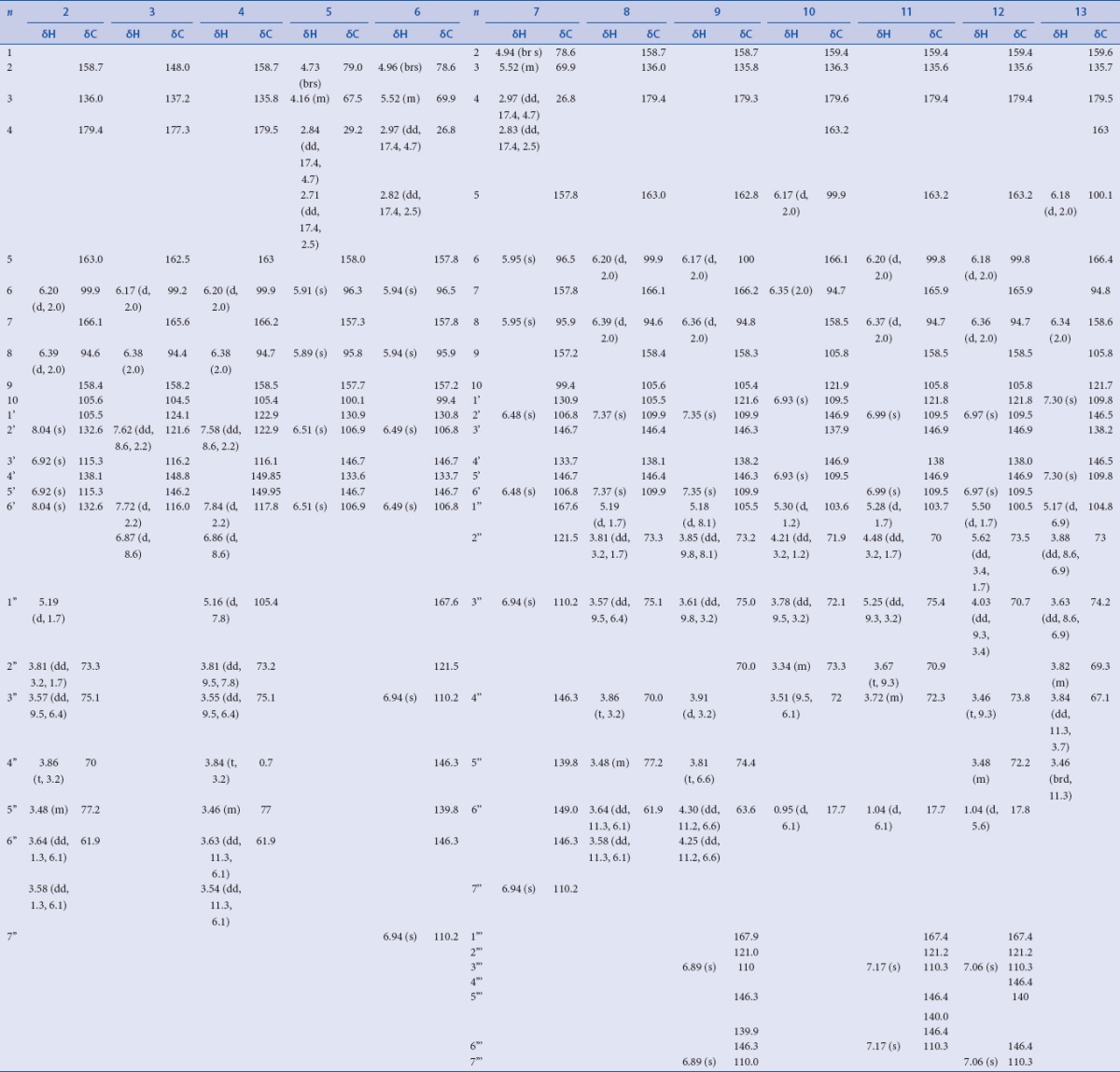
Table 2.
1H-, 13C-NMR data of compounds 2-13 isolated from Limonium tetragonum
Hepatic fibrosis is scarring resulting from several chronic liver diseases that is well characterized by the increased proliferation and excessive production and deposition of extracellular matrix.[12] Activation of HSCs plays an important role in the development of liver fibrosis. HSC-T6, an immortalized rat HSC line, retains major features of activated stellate cells, including expression of desmin, α-smooth muscle actin, and glial fibrillary acidic protein, and it can esterify retinol into retinyl esters.[13] The addition of serum, cytokines, and other factors such as specific culturing conditions in vitro is well known to promote the activation of HSCs.[14,15] Culturing HSCs on uncoated plastic plates causes spontaneous activation leading to myoblastic phenotype, mimicking the process seen in liver fibrosis in vivo. The accelerated proliferation of HSCs by culturing on plastic plate was found to be attenuated by the treatment of EtOAc fraction of L. tetragonum in MTT and BrdU incorporation assays.[8] Hepatoprotective activities of the isolated compounds from the EtOAc fraction against live fibrosis have been measured in HSC-T6 cells. The HSC-T6 cells were treated with 25 and 50 μM of each compound for 48 h. As a result, the significant cellular loss was observed by the treatment of compounds 1–13 [Table 3]. (−)-epigallocatechin-3-gallate (6) and (−)-epigallocatechin-3-(3″-O-methyl) gallate (7) inhibited the cellular proliferation while (−)-epigallocatechin (5) did not show significant effects in HSC-T6 cells consistent with the previous report demonstrating that EGCG inhibited collagen production and collagenase activity while EGC was inactive in TWNT-4 HSCs.[16] Myricetin glycosides (8–13) effectively suppressed the HSCs proliferation at both concentrations of 25 and 50 μM regardless of glucose type. To exclude the nonselective cytotoxicity of candidates, the effect of compounds on the viability of primary cultured rat hepatocytes was examined using MTT assay. Hepatocytes were intact up to 48 h in response to all compounds tested (data not shown). No cytotoxicity induced by the treatment of compound itself was observed in the concentrations tested.
Table 3.
Inhibitory effects of compounds 1-13 on the activated HSC-T6 cells

Antioxidant and hepatoprotective properties of myricetin and its glycosides have only relatively recently been reported in hepatotoxin-induced liver injury animal models. Myricetin, a major component of Cotinus coggygria Scop., promoted restoration of hepatic function against pyrogallol-induced toxicity and reduced DNA damage through pro-surviving Akt activity and STAT3 protein expression.[17] In carbon tetrachloride (CCl4)-induced intoxicated animal, the chloroform extract of Launaea procumbens which contains myricetin as a main component protected liver with concomitant increase in the activities of antioxidant enzymes, catalase, SOD, glutathione-S-transfers, glutathione reductase, and glutathione peroxidase in Rats.[18] Furthermore, Domitrovic et al.[19] demonstrated that myricetin-3-O-α-rhamnoside (myricitrin) significantly ameliorated CCl4-induced profibrotic responses evidenced by the reduced expression of transforming growth factor-beta1 (TGF-β1) and alpha-smooth muscle actin (α-SMA) in BALB/cN mice. Myricitrin improved the regeneration of hepatic tissue by inducing proliferating cell nuclear antigen expression. In this animal model, myricitrin was demonstrated to provide a better hepatoprotection when compared to silymarin, which is consistent with its higher in vitro antioxidant potential. EtOAc fraction of L. tetragonum which is enriched with various myricetin glycosides might therefore be useful for the prevention and/or treatment of liver diseases including fibrosis.
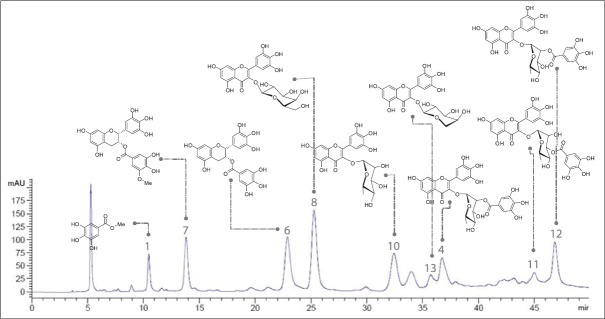
For the extensive usage of medicinal plants as new drug leads or functional food resources, the quality control of plant extract should be preceded. Standardization of plant extract guarantees the reproducible bioactivity and phytoequivalence as well as minimizing undesirable effects. For this, the identification of bioactive constituents in the extract and the selection of one or more constituents as marker compounds is necessary. Furthermore, the development of efficient analytical methods is required. In the present study, we could isolate active compounds classified into catechins and flavonoid glycosides from the EtOAc fraction of L. tetragonum extract. Using these isolated compounds, we tried to develop a simultaneous analytical method using HPLC. For the simultaneous determination of major constituents of L. tetragonum, the chromatographic condition was first investigated. Various mixtures of acetonitrile, water, and methanol in combination with acetic acid or formic acid in concentration range from 0.01% to 1% were tested as a mobile phase. Acid is known to be effective to improve the separation for phenolic compounds by reducing the tailing of the peaks. Under our chromatographic condition, the addition of 0.1% acetic acid in water was found to improve the resolution of the peaks detected, whereas the distortion of peaks was observed when <0.05% of acetic acid was added. The wavelength for detection was set at 254 nm, where the peaks showed high absorption with the improved S/N (signal-to-noise ratio) measured by DAD. As a result, the optimal mobile phase consisting of acetonitrile–water with 0.1% acetic acid was subsequently employed for the analysis of the EtOAc fraction of L. tetragonum extract, which led to a good resolution and satisfactory peak shape at 254 nm [Table 1]. The presence of eight compounds including gallincin (1), quercetin-3-O-β-D-galactopyranoside (4), (−)-epigallocatechin-3-gallate (6), myricetin-3-O-β-D-galactopyranoside (8), myricetin-3-O-α-L-rhamnopyranoside (10), myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside (11), myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside (12), myricetin-3-O-α-L-arabinopyranoside (13) in the extract was verified by comparing each retention time and UV spectrum with those of each standard compound and spiking with authentic standards [Figure 2].
Figure 2.
High-performance liquid chromatography of the ethyl acetate fraction of Limonium tetragonum extract at the wavelength of 254 nm. 1: gallincin, 4: quercetin-3-O-β-D-galactopyranoside, 6: (−)-epigallocatechin-3-gallate, 7: (−)-epigallocatechin-3-(3″-O-methyl) gallate, 8: myricetin-3-O-β-D-galactopyranoside, 10: myricetin-3-O-α-L-rhamnopyranoside, 11: myricetin-3-O-(2″-O-galloyl)-α-L-rhamnopyranoside, 12: myricetin-3-O-(3″-O-galloyl)-α-L-rhamnopyranoside, 13: myricetin-3-O-α-L-arabinopyranoside
CONCLUSION
Our data demonstrated that flavonoids and flavonoid glycosides (apigenin, quercetin, and myricetin) and catechins derived from L. tetragonum attenuated the proliferation of the activated HSC-T6. Furthermore, a rapid and reliable HPLC method for the simultaneous determination of eight active constituents in EtOAc fraction of L. tetragonum has been developed. The EtOAc fraction and the isolated compounds may have promise as candidates for the treatment of liver diseases.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This work was supported by Korea Institute of Planning and Evaluation for Technology in Food, Agriculture, Forestry and Fisheries(IPET) through High Value-added Food Technology Development Program(116016-3), funded by Ministry of Agriculture, Food and Rural Affairs(MAFRA), and partially supported by Gyeongnam National University of Science and Technology Grant (2016).
REFERENCES
- 1.Ksouri R, Megdiche W, Debez A, Falleh H, Grignon C, Abdelly C. Salinity effects on polyphenol content and antioxidant activities in leaves of the halophyte Cakile maritima. Plant Physiol Biochem. 2007;45:244–9. doi: 10.1016/j.plaphy.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 2.Ksouri R, Megdiche W, Falleh H, Trabelsi N, Boulaaba M, Smaoui A, et al. Influence of biological, environmental and technical factors on phenolic content and antioxidant activities of Tunisian halophytes. C R Biol. 2008;331:865–73. doi: 10.1016/j.crvi.2008.07.024. [DOI] [PubMed] [Google Scholar]
- 3.Sehrawat A, Sultana S. Evaluation of possible mechanisms of protective role of Tamarix gallica against DEN initiated and 2-AAF promoted hepatocarcinogenesis in male Wistar rats. Life Sci. 2006;79:1456–65. doi: 10.1016/j.lfs.2006.04.009. [DOI] [PubMed] [Google Scholar]
- 4.Hanen F, Riadh K, Samia O, Sylvain G, Christian M, Chedly A. Interspecific variability of antioxidant activities and phenolic composition in Mesembryanthemum genus. Food Chem Toxicol. 2009;47:2308–13. doi: 10.1016/j.fct.2009.06.025. [DOI] [PubMed] [Google Scholar]
- 5.Ksouri R, Falleh H, Megdiche W, Trabelsi N, Mhamdi B, Chaieb K, et al. Antioxidant and antimicrobial activities of the edible medicinal halophyte Tamarix gallica L. and related polyphenolic constituents. Food Chem Toxicol. 2009;47:2083–91. doi: 10.1016/j.fct.2009.05.040. [DOI] [PubMed] [Google Scholar]
- 6.Oueslati S, Ksouri R, Falleh H, Pichette A, Abdelly C, Legault J. Phenolic content, antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Suaeda fruticosa Forssk. Food Chem. 2012;132:943–47. [Google Scholar]
- 7.Ksouri WM, Medini F, Mkadmini K, Legault J, Magné C, Abdelly C, et al. LC-ESI-TOF-MS identification of bioactive secondary metabolites involved in the antioxidant, anti-inflammatory and anticancer activities of the edible halophyte Zygophyllum album Desf. Food Chem. 2013;139:1073–80. doi: 10.1016/j.foodchem.2013.01.047. [DOI] [PubMed] [Google Scholar]
- 8.Yang MH, Kim NH, Heo JD, Sung SH, Jeong EJ. Hepatoprotective effects of Limonium tetragonum, edible medicinal halophyte growing near seashores. Pharmacogn Mag. 2014;10(Suppl 3):S563–8. doi: 10.4103/0973-1296.139783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Kim NH, Sung SH, Heo JD, Jeong EJ. The extract of Limonium tetragonum protected liver against acute alcohol toxicity by enhancing ethanol metabolism and antioxidant enzyme activities. Nat Prod Sci. 2015;21:1–5. [Google Scholar]
- 10.Kim NH, Heo JD, Kim TB, Rho JR, Yang MH, Jeong EJ. Protective effects of ethyl acetate soluble fraction of Limonium tetragonum on diethylnitrosamine-induced liver fibrosis in rats. Biol Pharm Bull. 2016;39:1022–8. doi: 10.1248/bpb.b15-01047. [DOI] [PubMed] [Google Scholar]
- 11.Lee JI, Kong CS, Jung ME, Hong JW, Noh I, Seo Y. Peroxynitrite-scavenging activity of the halophyte Limonium tetragonum. Ocean Polar Res. 2011;33:185–91. [Google Scholar]
- 12.Bataller R, Brenner DA. Liver fibrosis. J Clin Invest. 2005;115:209–18. doi: 10.1172/JCI24282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vogel S, Piantedosi R, Frank J, Lalazar A, Rockey DC, Friedman SL, et al. An immortalized rat liver stellate cell line (HSC-T6): A new cell model for the study of retinoid metabolism in vitro. J Lipid Res. 2000;41:882–93. [PubMed] [Google Scholar]
- 14.Wu J, Zern MA. Hepatic stellate cells: A target for the treatment of liver fibrosis. J Gastroenterol. 2000;35:665–72. doi: 10.1007/s005350070045. [DOI] [PubMed] [Google Scholar]
- 15.Chen YW, Li DG, Wu JX, Chen YW, Lu HM. Tetrandrine inhibits activation of rat hepatic stellate cells stimulated by transforming growth factor-beta in vitro via up-regulation of Smad 7. J Ethnopharmacol. 2005;100:299–305. doi: 10.1016/j.jep.2005.03.027. [DOI] [PubMed] [Google Scholar]
- 16.Nakamuta M, Higashi N, Kohjima M, Fukushima M, Ohta S, Kotoh K, et al. Epigallocatechin-3-gallate, a polyphenol component of green tea, suppresses both collagen production and collagenase activity in hepatic stellate cells. Int J Mol Med. 2005;16:677–81. [PubMed] [Google Scholar]
- 17.Matic S, Stanic S, Bogojevic D, Vidakovic M, Grdovic N, Dinic S, et al. Methanol extract from the stem of Cotinus coggygria Scop. and its major bioactive phytochemical constituent myricetin modulate pyrogallol-induced DNA damage and liver injury. Mutat Res. 2013;755:81–9. doi: 10.1016/j.mrgentox.2013.03.011. [DOI] [PubMed] [Google Scholar]
- 18.Khan RA, Khan MR, Ahmed M, Sahreen S, Shah NA, Shah MS, et al. Hepatoprotection with a chloroform extract of Launaea procumbens against CCl4-induced injuries in rats. BMC Complement Altern Med. 2012;12:114. doi: 10.1186/1472-6882-12-114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Domitrovic R, Rashed K, Cvijanovic O, Vladimir-Kneževic S, Škoda M, Višnic A. Myricitrin exhibits antioxidant, anti-inflammatory and antifibrotic activity in carbon tetrachloride-intoxicated mice. Chem Biol Interact. 2015;230:21–9. doi: 10.1016/j.cbi.2015.01.030. [DOI] [PubMed] [Google Scholar]