Abstract
Background:
Polyphenolic phytochemicals are natural compounds, easily found in fruits and vegetables. Importantly, polyphenols have been intensively studied as excellent antioxidant activity which contributes to anticancer function of the natural compounds. Lung cancer has been reported to mainly account for cancer-related deaths in the world. Moreover, epidermal growth factor receptor tyrosine kinase inhibitor (TKI) resistance is one of the biggest issues in cancer treatment, especially in nonsmall cell lung cancer (NSCLC). Even though several studies both in preclinical and clinical trials have showed promising therapeutic effects of polyphenolic compounds in anticancer therapy, the function of the natural compounds in TKI-resistant (TKIR) lung cancer remains poorly studied.
Objective:
The aim of this study is to screen polyphenolic compounds as potential anticancer adjuvants which suppress TKIR lung cancer.
Materials and Methods:
Colony formation and thiazolyl blue tetrazolium blue assay were performed in the pair-matched TKI-sensitive (TKIS) versus TKIR tumor cell lines to investigate the therapeutic effect of polyphenolic compounds in TKIR NSCLC.
Results:
Our data show that equol, kaempferol, resveratrol, and ellagic acid exhibit strong anticancer effect in HCC827 panel. Moreover, the inhibitory effect of most of tested polyphenolic compounds was highly selective for TKIR lung cancer cell line H1993 while sparing the TKIS one H2073.
Conclusion:
This study provides an important screening of potential polyphenolic compounds for drug development to overcome TKI resistance in advanced lung cancer.
SUMMARY
The study provides an important screening of potential polyphenolic compounds for drug development to overcome tyrosine kinase inhibitor (TKI) resistance in advance lung cancer
Equol, kaempferol, resveratrol, and ellagic acid show strong anticancer effect in HCC827 panel, including TKI-sensitive (TKIS) and TKI-resistant clones
The inhibitory effect of polyphenolic compounds such as equol, kaempferol, resveratrol, ellagic acid, gallic acid, p-Coumaric, and hesperidin is highly selective for TKI-resistant lung cancer cell line H1993 while sparing the TKIS one H2073.
Abbreviations used: EGFR: Epidermal growth factor receptor, EMT: Epithelial-to-mesenchymal transition, GTP: Green tea polyphenols, IGF1R: Insulin-like growth factor 1 receptor, MET: Met proto-oncogene, MTT: Thiazolyl blue tetrazolium blue, NSCLC: Non-small cell lung cancer, ROS: Reactive oxygen species, RTK: Receptor tyrosine kinase, STAT3: Signal transducer and activator of transcription 3, TKIR: TKI-resistant, TKIs: Tyrosine kinase inhibitors, TKIS: TKI-sensitive.
Keywords: Epidermal growth factor receptor tyrosine kinase inhibitor resistance, lung cancer, polyphenolic compounds
INTRODUCTION
Lung cancer is the highest cause of cancer deaths in the world.[1] The initiation of lung cancer is induced by diverse oncogenic activation and tumor suppressor inhibition. Receptor tyrosine kinase epidermal growth factor receptor (EGFR) is usually strongly active in non-small cell lung cancer (NSCLC) and regulates various important tumorigenic processes.[2] Targeting EGFR signaling, using tyrosine kinase inhibitors (TKIs) such as gefitinib or erlotinib, provides dramatic survival benefit for EGFR-mutant lung cancer patients.[3] However, majority of patients, initially responsive to TKI treatment, eventually develop TKI resistance and tumor relapse.[4] Molecular mechanisms of acquired TKI resistance in EGFR-mutant NSCLC are vary from patients to patients, due to the biological heterogeneity among tumor cells existing before treatment and continually increasing through selection pressure of the treatment. EGFR secondary mutation T790M is among the most popular TKI-acquired resistant mechanisms. The mutation prevents TKIs, such as gefitinib and erlotinib, to bind the kinase cleft of EGFR and reduces drug effectiveness.[4] Other tracks bypassing EGFR signaling account for 20% of TKI-resistant (TKIR) mechanism, such as amplification of the met proto-oncogene (MET), activation of insulin-like growth factor 1 receptor, feedback activation of signal transducer and activator of transcription 3 (Stat3), Kras mutations, or epithelial-to-mesenchymal transition.[5] Different therapeutic approaches to overcome TKI resistance in lung cancer have been established, such as development of next generations of TKIs (CO-1686 and AZD-9291), combination of surgery and radiation therapy for the region surgically removed, or combination of radiation therapy and chemotherapy.[4] However, despite advances in early prognosis and standard therapy, prevention and treatment strategies for advanced lung cancer are unmet needs, especially to overcome TKI resistance.
It has been reported that dietary optimization is among the most effective approaches for cancer prevention.[6] Importantly, about 20% of all cases of cancer are preventable by modified diet containing high amount and variety of vegetables and fruits.[7] Polyphenolic phytochemicals are natural compounds highly contained in fruits, vegetables, and cereal and involved in protecting plants from microbial infections, deterrence of herbivores, preventing reactive oxygen species (ROS) stress, regulation of growth processes, and ripening.[6] Polyphenol structure is characterized by the presence of multiphenol units, which might contribute to oxidative stability of plant tissue and other organoleptic characteristics, such as color, flavor, and odor.[6] Due to favorable safety profile and profound antioxidant activity, polyphenolic phytochemicals have been intensively studied as anticancer adjuvants.[8,9,10] Particularly, in mice-harboring mammary carcinoma 4T1, green tea polyphenols dramatically suppressed cancer development and inhibited tumor metastasis.[11] Furthermore, a clinical study on patients with intestinal premalignant lesions showed that 6-month treatment of quercetin and curcumin inhibited the development of polyps both in number and size.[12] Moreover, in various epidemiological studies, consumption of fruits and vegetables with high content of lycopene reduces risks of cancer development in prostate, alimentary tract, and cervix.[13,14,15] Even though polyphenolic phytochemicals showed promising effect in anticancer therapy both in preclinical and clinical studies, therapeutic effect of the natural compounds in EGFR-TKI-resistant lung cancer remains poorly studied. Here, our study investigated the anticancer effect of a set of polyphenolic phytochemicals on TKIR lung cancer cell lines. Interestingly, we found that most tested phytochemicals showed profound effect to suppress TKIR NSCLC. This study, therefore, provides a fundamental screening of potential natural compounds as adjuvants which might be included in the treatment combo to overcome TKIR NSCLC.
MATERIALS AND METHODS
Cell lines and reagents
HCC827, HCC827C1, HCC827C2, H2073, and H1993 cells were a kind gift of Dr. John D. Minna (University of Texas Southwestern Medical Center, Dallas, Texas, US). All cells were grown in 5% fetal bovine serum-supplemented RPMI 1640, at 37°C and 5% CO2 atmosphere.
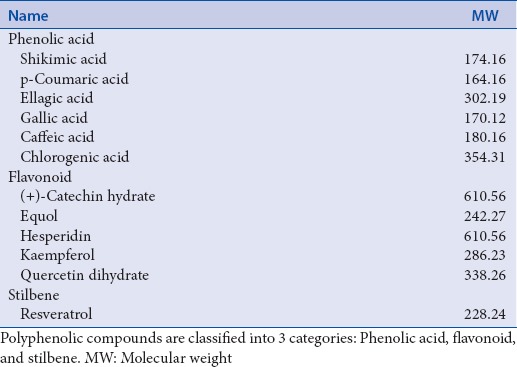
Polyphenolic compounds, including (+)-catechin hydrate, caffeic acid, chlorongeic acid, ellagic acid, equol, gallic acid, hesperidin, kaempferol, p-Coumaric acid, quercetin dihydrate, resveratrol, and shikimic acid were from Sigma.
Colony formation assay
About 3000 cells/well were seeded into 6-well plates containing RPMI medium in final volume of 2 mL/well. After 24 h, cells were incubated with indicated polyphenolic compounds for 7 days. To visualize the colonies, cells were stained with methylene blue solution (0.4% in methanol 50%).
Thiazolyl blue tetrazolium blue assay
About 1000 cells/well were splitted into 96-well microplates containing RPMI medium in final volume of 0.1 mL/well. After 24 h, cells were incubated with indicated polyphenolic compounds. Five days after treatment, thiazolyl blue tetrazolium blue (MTT) from Sigma was used to measure cell viability.
Statistical analysis
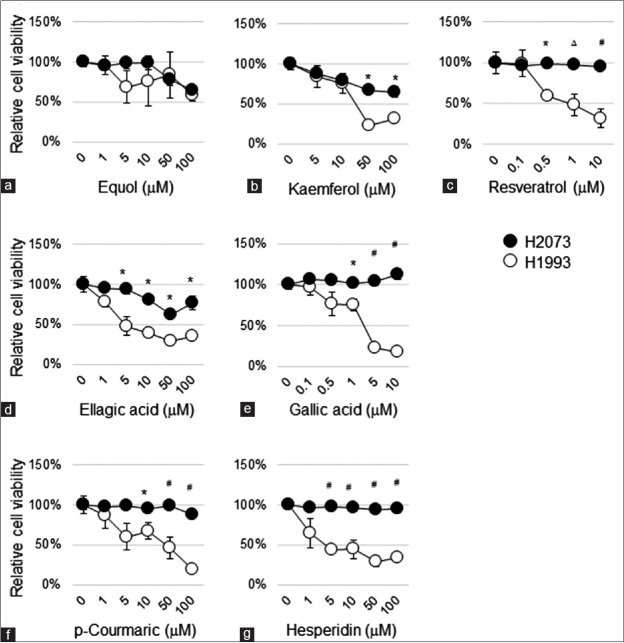
The data are showed as mean ± standard error of the mean two-way ANOVA was used to assess the significant difference in MTT assays in Figure 1. The difference between viability of H1993 and H2073 treated with a single dose in Figure 1 was examined by two-tailed unpaired t-test. *, P < 0.05; Δ, P < 0.01; #, P < 0.001.
Figure 1.
Selective therapeutic effect of polyphenolic phytochemicals on tyrosine kinase inhibitor resistant lung cancer cells H1993 thiazolyl blue tetrazolium blue assay was used to evaluate therapeutic effects of polyphenols in the panel of tyrosine kinase inhibitor-sensitive cells, H2073 and tyrosine kinase inhibitor-resistant cells, H1993. Lung cancer cells were treated with equol (a), kaempferol (b), resveratrol (c), ellagic acid (d), gallic acid (e), p-Coumaric acid (f), and hesperidin (g) at indicated concentration for 5 days. In every thiazolyl blue tetrazolium blue assay, values are mean ± standard error of the mean of five replicate assays. Polyphenolic compounds suppressed cancer growth of tyrosine kinase inhibitor-resistant cells H1993 while sparing tyrosine kinase inhibitor-sensitive cells H2073
RESULTS
Therapeutic effect of polyphenolic phytochemicals on HCC827 panel
The chemical structures and molecular weight of tested polyphenolic phytochemicals are represented [Figure 2 and Table 1]. Structurally, the polyphenolic compounds are classified into three groups of phenolic acids, flavonoids, and stilbenes. Shikimic acid, p-coumaric acid, ellagic acid, gallic acid, caffeic acid, and chlorogenic acid are included in phenolic acid class; flavonoids consist of (+)-catechin hydrate, equol, kaempferol, queretin dihydrate, and hesperidin; and resveratrol is a stilbene compound. Most of the polyphenolic compounds arise from a common precursor, shikimic acid.[6] Principally, they appear in conjugated structures, with one or more sugar molecules linked to hydroxyl groups. The hydroxyl groups in polyphenolic compounds can uptake free electrons to form a stable phenoxyl radicals that subsequently interrupts cellular oxidative reaction and reduces ROS in the cells.[6]
Figure 2.
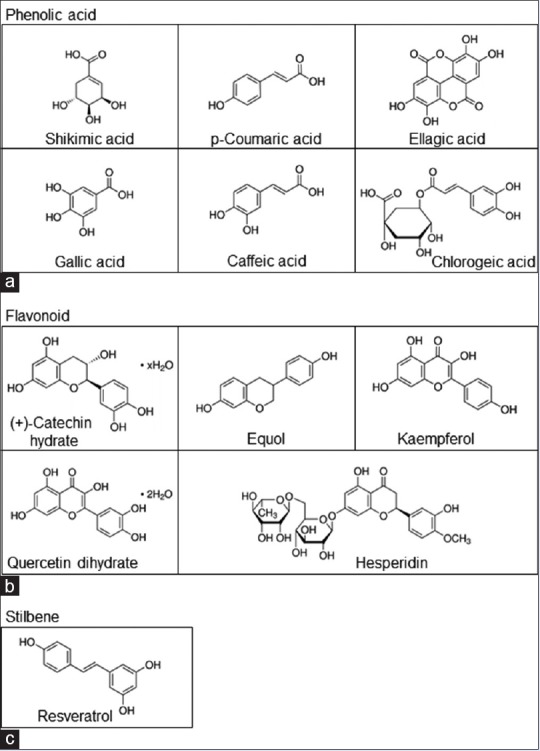
Chemical structure of polyphenolic compounds (a-c). Polyphenolic compounds were structurally classified into three categories as follows: phenolic acid (a), shikimic acid, p-Coumaric acid, ellagic acid, gallic acid, caffeic acid, and chlorogenic acid; flavonoid (b), (+)-catechin hydrate, equol, kaempferol, quercetin dihydrate, and hesperidin; and stilbene (c), resveratrol
Table 1.
List of polyphenolic phytochemicals
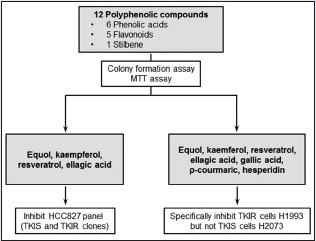
To explore therapeutic function of polyphenolic phytochemicals on TKIR lung cancer, we first used a panel of NSCLC cells including parental TKI-sensitive (TKIS) cells HCC827 and TKIR clones HCC827C1 and HCC827C2. As previous described, parental HCC827 cells, harboring active mutation EGFRE746-A750del, are very sensitive to TKIs, while HCC827C1 and HCC827C2 are resistant to TKI treatment.[16] To investigate inhibitory effect of polyphenolic compounds on HCC827 panel, we carried out colony formation assay in a dose-dependent manner [Figure 3]. Interestingly, we found that equol, kaempferol, resveratrol, and ellagic acid dramatically suppressed cell growth of HCC827C2 [Figure 3a]. On the other hand, chlorogenic acid, hesperidin, caffeic acid, and queretin dihydrate showed strong inhibitory effect on HCC827C2 growth only at 200 μM [Figure 3a]. (+)-Catechin hydrate, shikimic acid, and p-Coumaric acid did not show any growth inhibitory effect on HCC827C2 [Figure 3a]. We next tested growth response of parental HCC827 and HCC827C1 to equol, kaempferol, resveratrol, and ellagic acid treatment. Similar to HCC827C2, liquid colony forming assay showed that the compounds strongly suppressed cell growth of HCC827 and HCC827C1 [Figure 3b and c]. These data provide a systemic screening of polyphenolic compounds that strongly sensitize TKIR lung cancer cells.
Figure 3.
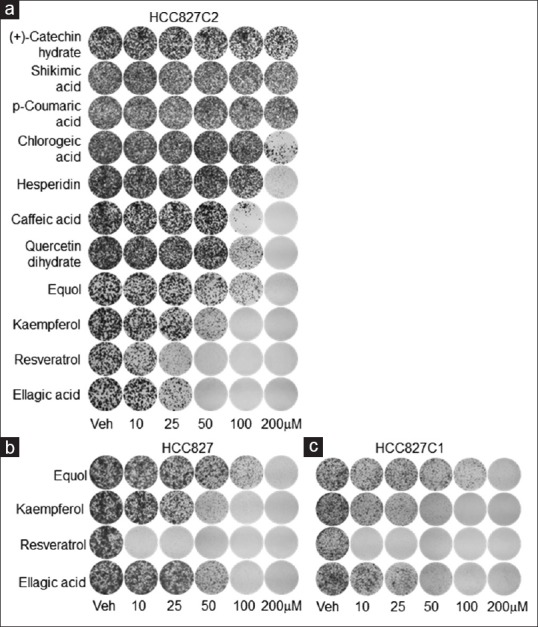
Therapeutic effect of polyphenols in the panel of HCC827 cells (a-c). Therapeutic effects of polyphenols were evaluated using colony formation assay in HCC827 clones, including tyrosine kinase inhibitor-resistant cells, HCC827C2 (a) and HCC827C1 (c); and tyrosine kinase inhibitor-sensitive cells, parental HCC827 (b). Lung cancer cells were treated with polyphenolic compounds at indicated concentration for 7 days
Therapeutic effect of polyphenolic phytochemicals on H2073 and H1993
We next tested therapeutic effect of some screened polyphenols in a panel of cancer cells including H2073 and H1993. Note that H2073 and H1993, respectively, are primary and metastatic tumor cells established from the same patient as previous described.[17] Mechanically, H2073, with overexpression of EGFR, is highly sensitive to TKI treatment, whereas H1993-harboring MET amplification is strongly resistant to TKIs.[17] We then tested growth inhibition of H2073 and H1993 cells by the polyphenolic phytochemicals which can suppress growth of HCC827 parental and the TKIR clones as described above. Surprisingly, we found that most phytochemicals sensitized the TKIR lung cancer cells H1993 which showed minor suppressive effect on H2073 growth. Particularly, equol, kaemferol, resveratrol, and ellagic acid strongly inhibited H1993 in a dose-dependent manner, while H2073 did not significantly respond to the compounds’ treatment [Figure 1a-d]. A recent study has reported that gallic acid showed strong inhibitory effect, both in vitro and in vivo, on cell growth of TKIR lung cancer, while sparing other TKIS cells.[16] We then tested the viability response of H2073 and H1993 to gallic acid treatment. Consistently, we found that H1993 is extremely sensitive to gallic acid at 5 μM, while H2073 was not responsive to gallic acid treatment [Figure 1e]. Similarly, hesperidin and p-Coumaric acid strongly inhibited H1993 and showed only modest effect on H2073 [Figure 1f and g]. Interestingly, even though p-Coumaric acid and hesperidin showed modest effect on HCC827C2 [Figure 3a], the compounds strongly suppressed cell viability of H1993 at the same treatment condition. In conclusion, our data suggest promising anticancer effects of screened polyphenolic compounds, especially to overcome TKI resistance in NSCLC.
DISCUSSION
Even though advances in standard treatment and diagnosis techniques have improved patient overall survival recently, emergence of TKI resistance become a serious problem in NSCLC treatment. Moreover, developing new drugs or new targets for cancer treatment remains to be a remarkable clinical challenge both effectively and economically, especially in TKIR NSCLC. Polyphenolic phytochemicals are natural-derived compounds which play critical functions in protecting plants from microbial infections, preventing ROS stress, or deterrence of herbivores.[18,19,20] Notably, growing evidence shows that dietary polyphenols such as curcumin, resveratrol, and EGCG exert their chemopreventive effects against different types of cancers in preclinical studies.[21,22,23,24] Intensive studies on antioxidant as well as anticancer activity of polyphenolic phytochemicals suggested supplement of polyphenols in daily diet as a prevention strategy for cancer development.[7,22] However, therapeutic function of polyphenolic compounds in TKIR NSCLC remains poorly studied.
In the present study, for systemic screening of polyphenolic phytochemicals as anticancer adjuvants in TKIR lung cancer, we firstly selected various polyphenolic compounds from three major classes of polyphenols, including phenolic acid, flavonoid, and stilbene. These compounds were then investigated for therapeutic effect in TKIR lung cancer cells.
Next, we used a panel of HCC827 cell lines including parental cells and two independent TKIR clones HCC827C1 and HCC827C2 to address cancer-suppressing effects of the polyphenolic compounds. Interestingly, we found that equol, kaempferol, resveratrol, and ellagic acid are potent anticancer agents in both HCC827 and TKIR cells. The data, therefore, provide a fundamental screening of polyphenolic compounds to sensitize TKIR NSCLC. We next confirmed growth-inhibitory function of tested natural compounds in another set of lung cancer cells including H2073 and H1993 which are primary and pair-matched metastasis tumor cells, respectively, isolated from the same patient. Note that H2073, harboring overexpressed EGFR, are sensitive to TKI treatment, while H1993, containing amplification of MET, are highly resistant to TKIs.[17] To our surprise, most of test polyphenolic compounds, including equol, kaempferol, resveratrol, ellagic acid, p-Coumaric acid, hesperidin, and gallic acid dramatically suppressed cancer growth of TKIR cells H1993 while sparing TKIS cells H2073. Importantly, gallic acid has been reported in a previous study to specifically inhibit tumor development of TKIR cells but not of TKIS cells, both in vitro and in xenograft model.[16] Consistently, our data showed the selective cancer-suppressive effect of gallic acid in TKIR cells H1993 but not in TKIS H2073 cells. Gallic acid has been suggested to target Src-mediated Stat3 signaling, subsequently suppress TKIR cells specifically.[16] However, the molecular targets of other polyphenolic compounds such as equol, kaempferol, resveratrol, ellagic acid, p-coumaric acid, and hesperidin remain elusive, especially in TKIR cells. Therefore, efforts are required to identify molecular mechanism by which these phytochemicals specifically suppress TKI-resistant NSCLC.
Taken together, our study provides a fundamental yet important screening of potential natural compounds for anticancer drug development, especially to overcome TKI resistance in advanced lung cancer. Therefore, the administration of polyphenolic compounds might serve as a potent adjuvant therapeutic strategy, supporting long-term TKI treatment in lung cancer patients.
Financial support and sponsorship
This study was financially supported by a research grant from Yonsei University Wonju College of Medicine (YUWCM-2008-19), Yonsei University Future-leading Research Initiative of 2015 (RMS2-2015-22-0094), JJ-2017-1, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology (NRF-2017R1D1A3B03033851) and (NRF-2016R1D1A3B03930581).
Conflicts of interest
There are no conflicts of interest.
Acknowledgement
This study was financially supported by a research grant from Yonsei University Wonju College of Medicine (YUWCM-2008-19), Yonsei University Future-leading Research Initiative of 2015 (RMS2-2015-22-0094), JJ-2017-1, and the Basic Science Research Program through the National Research Foundation of Korea (NRF) funded by the Ministry of Education, Science and Technology.
REFERENCES
- 1.Gridelli C, Rossi A, Carbone DP, Guarize J, Karachaliou N, Mok T, et al. Non-small-cell lung cancer. Nat Rev Dis Primers. 2015;1:15009. doi: 10.1038/nrdp.2015.9. [DOI] [PubMed] [Google Scholar]
- 2.Herbst RS, Heymach JV, Lippman SM. Lung cancer. N Engl J Med. 2008;359:1367–80. doi: 10.1056/NEJMra0802714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Farmer G. Targeted lung cancer therapies. Nat Rev Drug Discov. 2004;3:547–8. doi: 10.1038/nrd1444. [DOI] [PubMed] [Google Scholar]
- 4.Camidge DR, Pao W, Sequist LV. Acquired resistance to TKIs in solid tumours: Learning from lung cancer. Nat Rev Clin Oncol. 2014;11:473–81. doi: 10.1038/nrclinonc.2014.104. [DOI] [PubMed] [Google Scholar]
- 5.Chong CR, Jänne PA. The quest to overcome resistance to EGFR-targeted therapies in cancer. Nat Med. 2013;19:1389–400. doi: 10.1038/nm.3388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Pandey KB, Rizvi SI. Plant polyphenols as dietary antioxidants in human health and disease. Oxid Med Cell Longev. 2009;2:270–8. doi: 10.4161/oxim.2.5.9498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Glade MJ. Food, nutrition, and the prevention of cancer: A global perspective. American Institute for Cancer Research/World Cancer Research Fund, American Institute for Cancer Research, 1997. Nutrition. 1999;15:523–6. doi: 10.1016/s0899-9007(99)00021-0. [DOI] [PubMed] [Google Scholar]
- 8.Lai HY, Lim YY, Kim KH. Isolation and characterisation of a proanthocyanidin with antioxidative, antibacterial and anti-cancer properties from fern Blechnum orientale. Pharmacogn Mag. 2017;13:31–7. doi: 10.4103/0973-1296.197659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Akowuah G, Mariam A, Chin J. The effect of extraction temperature on total phenols and antioxidant activity of Gynura procumbens leaf. Pharmacogn Mag. 2009;5:81–5. [Google Scholar]
- 10.Taparia S, Khanna A. Effect of procyanidin-rich extract from natural cocoa powder on cellular viability, cell cycle progression, and chemoresistance in human epithelial ovarian carcinoma cell lines. Pharmacogn Mag. 2016;12(Suppl 2):S109–15. doi: 10.4103/0973-1296.182164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Baliga MS, Meleth S, Katiyar SK. Growth inhibitory and antimetastatic effect of green tea polyphenols on metastasis-specific mouse mammary carcinoma 4T1 cells in vitro and in vivo systems. Clin Cancer Res. 2005;11:1918–27. doi: 10.1158/1078-0432.CCR-04-1976. [DOI] [PubMed] [Google Scholar]
- 12.Cheng AL, Hsu CH, Lin JK, Hsu MM, Ho YF, Shen TS, et al. Phase I clinical trial of curcumin, a chemopreventive agent, in patients with high-risk or pre-malignant lesions. Anticancer Res. 2001;21:2895–900. [PubMed] [Google Scholar]
- 13.Zackheim HS. Re: Tomatoes, tomato-based products, lycopene, and prostate cancer: Review of the epidemiologic literature. J Natl Cancer Inst. 1999;91:1331. doi: 10.1093/jnci/91.15.1331. [DOI] [PubMed] [Google Scholar]
- 14.Giovannucci E, Rimm EB, Liu Y, Stampfer MJ, Willett WC. A prospective study of tomato products, lycopene, and prostate cancer risk. J Natl Cancer Inst. 2002;94:391–8. doi: 10.1093/jnci/94.5.391. [DOI] [PubMed] [Google Scholar]
- 15.García-Closas R, Castellsagué X, Bosch X, González CA. The role of diet and nutrition in cervical carcinogenesis: A review of recent evidence. Int J Cancer. 2005;117:629–37. doi: 10.1002/ijc.21193. [DOI] [PubMed] [Google Scholar]
- 16.Phan AN, Hua TN, Kim MK, Vo VT, Choi JW, Kim HW, et al. Gallic acid inhibition of Src-Stat3 signaling overcomes acquired resistance to EGF receptor tyrosine kinase inhibitors in advanced non-small cell lung cancer. Oncotarget. 2016;7:54702–13. doi: 10.18632/oncotarget.10581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Wairagu PM, Park KH, Kim J, Choi JW, Kim HW, Yeh BI, et al. Combined therapeutic potential of nuclear receptors with receptor tyrosine kinase inhibitors in lung cancer. Biochem Biophys Res Commun. 2014;447:490–5. doi: 10.1016/j.bbrc.2014.04.018. [DOI] [PubMed] [Google Scholar]
- 18.Kiliçgün H, Altiner D. Correlation between antioxidant effect mechanisms and polyphenol content of Rosa canina. Pharmacogn Mag. 2010;6:238–41. doi: 10.4103/0973-1296.66943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Wan C, Yu Y, Zhou S, Liu W, Tian S, Cao S. Antioxidant activity and free radical-scavenging capacity of Gynura divaricata leaf extracts at different temperatures. Pharmacogn Mag. 2011;7:40–5. doi: 10.4103/0973-1296.75900. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Dimitrova-Dyulgerova I, Zhelev I, Mihaylova D. Phenolic profile and in vitro antioxidant activity of endemic bulgarian carduus species. Pharmacogn Mag. 2015;11(Suppl 4):S575–9. doi: 10.4103/0973-1296.172964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Vallianou NG, Evangelopoulos A, Schizas N, Kazazis C. Potential anticancer properties and mechanisms of action of curcumin. Anticancer Res. 2015;35:645–51. [PubMed] [Google Scholar]
- 22.Amin AR, Kucuk O, Khuri FR, Shin DM. Perspectives for cancer prevention with natural compounds. J Clin Oncol. 2009;27:2712–25. doi: 10.1200/JCO.2008.20.6235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stoner GD, Mukhtar H. Polyphenols as cancer chemopreventive agents. J Cell Biochem Suppl. 1995;22:169–80. doi: 10.1002/jcb.240590822. [DOI] [PubMed] [Google Scholar]
- 24.Kampa M, Nifli AP, Notas G, Castanas E. Polyphenols and cancer cell growth. Rev Physiol Biochem Pharmacol. 2007;159:79–113. doi: 10.1007/112_2006_0702. [DOI] [PubMed] [Google Scholar]