Abstract
Background:
Previous studies of Citrus spp. peel have shown hypoglycemic and antioxidant activities. Citrus limetta has been studied for its therapeutic properties. Diabetes mellitus (DM) is a health problem in Mexico and worldwide, that takes a vital importance due to its high incidence. Recently, scientists have searched natural sources to control the disease.
Materials and Methods:
In this study, we evaluated the in vitro hypoglycemic activity and in vivo postprandial glycemic effect of C. limetta peel flour by glucose adsorption and retardation assays as well as postprandial serum glucose levels using a group of female Balb-c mice, respectively.
Results:
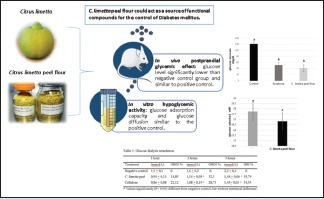
C. limetta peel flour showed a glucose adsorption capacity of 16.58 mM, having a similar effect regarding the positive control. The glucose diffusion in the dialysate was elevated, with a glucose dialysis retardation index of 33.79% in a period of 3 h, showing similar results to positive control. Postprandial serum glucose levels in the animal group treated with C. limetta peel flour showed a glucose level of 41.4 mg/dL, being this value significantly lower than negative control group and similar to positive control. Toxicity tests showed good tolerance to the dose of 2000 mg/kg.
Conclusion:
C. limetta peel flour could act as a source of functional compounds for the control of DM.
SUMMARY
Citrus limetta peel flour showed a glucose adsorption capacity similar to the positive control
The glucose diffusion in the dialysate was elevated, showing similar results to positive control
Postprandial serum glucose levels in the animal group treated with C. limetta peel flour showed a glucose level significantly lower than negative control group and similar to positive control
Toxicity tests showed good tolerance
C. limetta peel flour could act as a source of functional compounds for the control of diabetes mellitus.
Abbreviations used: CIATEJ: Center for Research and Assistance in Technology and Design of Jalisco; DM: Diabetes mellitus; FGC: Final glucose concentration; GDRI: Glucose dialysis retardation index; IGC: Initial glucose concentration; OECD: Organization for Economic Cooperation and Development.
Keywords: Citrus limetta, diabetes mellitus, hypoglycemic, peel flour
INTRODUCTION
Diabetes is a metabolic disorder caused by pancreas incapacity for insulin production in sufficient quantities resulting in hyperglycemia in the organisms, which may result in other serious effects.[1] Diverse pharmacological approaches are used to diabetes treatment through different action modes, such as the carbohydrate enzyme inhibitors.[2] However, they are responsible for a large number of side effects, making them less attractive as therapeutic agents and placing to the natural remedies as viable alternative. High glucose levels can be effectively controlled through the use of natural products, presenting side effects less frequently and implies a low-cost alternative.[3,4,5,6]
Among the products that have shown hypoglycemic activity are citrus peels from orange, grapefruit, lemon, and others. They have been used as traditional medicine in rural communities for prevention and treatment of diabetes.[7] Citrus limetta, an edible fruit from Central America known as sweet lime used for human consumption, is comprised 8%–10% peel, in case of juice industries; the peel is a byproduct without any use, becoming an environmental problem.[8] However, the C. limetta peel contains physiological beneficial metabolites such as glucosides, flavonoids, and abscisic acid derivatives.[9,10] We previously reported the ability of the C. limetta peel to inhibit α-glucosidase and α-amylase enzymes responsible for carbohydrate digestion, which could be exploited for use as an alternative for the control of hyperglycemia in people with type 2 diabetes mellitus (DM).[11] In the present study, in vitro hypoglycemic activity and in vivo postprandial glycemic effect and acute toxicity study of C. limetta peel flour were determined.
MATERIALS AND METHODS
Plant material
C. limetta fruits were obtained from a market in Jalisco, Mexico. The pulp was discarded and the peel was dried at 37°C, and then, it was ground and sieved in 50 mesh. The C. limetta peel flour was packaged until its use.
Experimental animals
Eight-week-old female Balb-c mice (~22 g body weight) were purchased from the University of Guadalajara, Mexico. They were housed under environmental conditions according to national normativity for the care of laboratory animals. This study was conducted in accordance with the National Institute of Health's “Guide for the Care and Use of Laboratory Animals,” and the animals were handled following the animal care guidelines in accordance with regulations enacted by the Federal Government of Mexico (NOM-062-ZOO-1999). An internal committee of the Center for Research and Assistance in Technology and Design of Jalisco reviewed the protocol for the care of laboratory animals.
Glucose adsorption capacity
One percent of C. limetta peel flour was added to 25 mL of glucose 100 mM/L in triplicate, according to Ahmed et al.[11] The mixture was incubated in an orbital rotatory shaker at 37°C for 6 h. Then, it was centrifuged at 4000 ×g for 20 min. Finally, the amount of glucose adsorbed by C. limetta flour was measured in the supernatant using a commercial enzymatic kit (GodPap, Randox). Cellulose was utilized as positive control. Glucose bound was calculated as following:
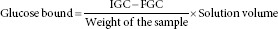
Where IGC is the initial glucose concentration of the original solution and FGC is the final glucose concentration after 6 h of incubation.
Glucose dialysis retardation index
The glucose dialysis retardation index (GDRI) was carried out according to Ou et al.[12] with some modifications. Briefly, 0.5 g of C. limetta peel flour was mixed in a 100 mM glucose solution (25 mL), and then, it was dialyzed in dialysis bags (12,000 MW cutoff) against 200 mL of distilled water at 37°C during 3 h. The glucose content in the dialysate was measured each hour with a commercial enzymatic kit (GodPap, Randox). Cellulose was used as positive control. The GRDI was determined as follows:
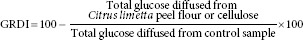
Postprandial hypoglycemic activity
Female Balb-c mice were randomly assigned to three groups of five mice in each. Blood samples were collected from tail veins for glucose levels measurement at baseline on each group after 8 h fasting. The first group (control) was orally administrated purified water (10 ml/kg), the second group was orally administrated 300 mg/kg of C. limetta peel flour diluted in purified water, and the third group was administrated the reference drug, acarbose (30 mg/kg). Then, the three groups were induced with hyperglycemia by oral administration of maltose (3 g/kg body weight). Test sample and controls were administered by oral gavage. Blood samples were collected from the mice tail vein before and 30 min after maltose administration, and glucose levels were measured based on the glucose oxidase method using a glucometer (ACCU-CHEK Meter®, Roche Diagnostics Corp., USA).[13]
Acute toxicity study
Acute toxicity was realized according to up-and-down method Organization for Economic Cooperation and Development[14] in five female mice. They were orally administrated with a single dose of 2000 mg/kg (limit test) of C. limetta peel flour. All physical and behavioral changes and death were recorded during 14 days.
Statistical analysis
All data were expressed as mean ± standard deviation on triplicate. The data were analyzed by one-way ANOVA followed by Tukey's post hoc test for multiple comparisons using Statgraphics 5.1 software (Statpoint Technologies, Inc. Warrenton, Virginia, USA). P < 0.05 was considered significant.
RESULTS AND DISCUSSION
The In vitro hypoglycemic activity was measured by the methods of glucose adsorption capacity and glucose dialysis retardation index. C. limetta peel flour had a glucose adsorption capacity of 16.58 mM showing a similar effect with the positive control (cellulose) which adsorbed 17.43 mM of glucose, without statistical difference when they are compared, indicating that they have the same effect [Figure 1]. This capacity may be attributed to its fiber content such as fiber from different sources able to adsorb glucose.[15]
Figure 1.
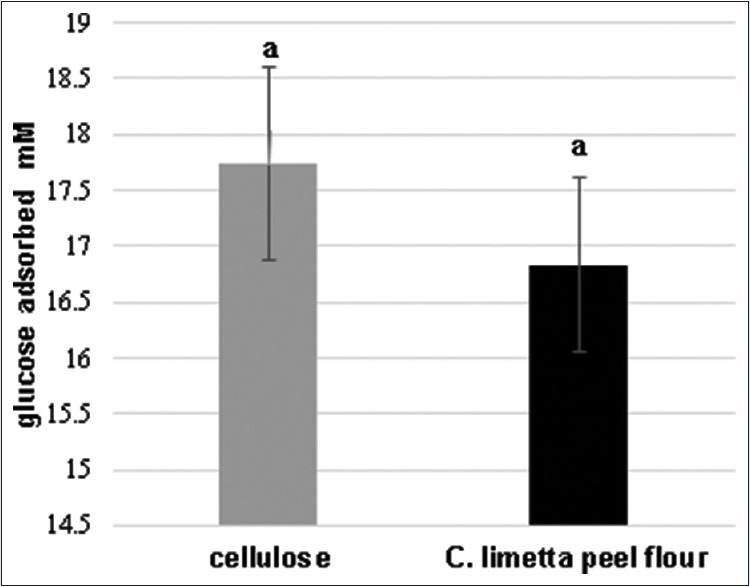
Adsorption of glucose. Cellulose had a capacity of 17.43 ± 0.86 mM of glucose and Citrus limetta peel flour a capacity of 16.58 ± 0.77 mM of glucose. Assays were performed in triplicate. Bars with same letters do not differ statistically from each other, P < 0.05
The glucose dialysis retardation test is useful to predict the effect of the samples on the delay in glucose absorption in the gastrointestinal tract.[16] In this study, glucose diffusion rates of C. limetta peel flour were time dependent. The GDRI increased considerably from 1 to 3 h, C. limetta peel flour and cellulose (positive control) showed significant inhibitory effects on glucose movement after the second hour, and both samples kept similar values at the three measurement times, without statistical difference among them. The higher GRDI was found at 3 h with 33.79% for C. limetta peel flour and 34.55% for cellulose [Table 1]. This result revealed that C. limetta peel flour might be efficient in retarding the glucose absorption. Similar effects have been observed for other citrus peels.[17,18,19,20]
Table 1.
Glucose dialysis retardation
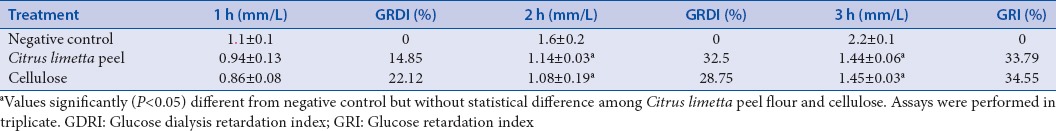
It is known that citrus peel has high-fiber content and some studies have revealed that fiber can help lowering postprandial serum glucose levels. Three mechanisms have been proposed: the viscosity increase in the intestine to hinder the glucose diffusion, glucose uptake, and the retardation of α-amylase activity.[13,14,21] Taking into account our previous study which revealed that C. limetta peel flour has high ability to inhibit α-glucosidase and α-amylase and now the in vitro results showed capacity to glucose adsorption and its retardation, we suspected that C. limetta peel flour sample plays a role in lowering postprandial serum glucose level in the intestinal lumen as other fibers.[18]
Therefore, it was carried out a test to show if C. limetta peel flour played a role in lowering postprandial serum glucose levels. The results of this test showed that the animal group treated with C. limetta peel flour showed blood glucose levels of 41.4 mg/dL and that value was significantly lower than the negative control group which had a value of 120 mg/dL; meanwhile, mice treated with positive control (acarbose) showed a glucose level of 51.7 mg/dL. There was no statistical difference between C. limetta peel flour and acarbose values [Figure 2]. Our results coincide with the reported by Kundusen et al.,[22] whereby a methanolic extract obtained from C. limetta showed antihyperglycemic effectiveness in streptozotocin-induced diabetic rats.
Figure 2.
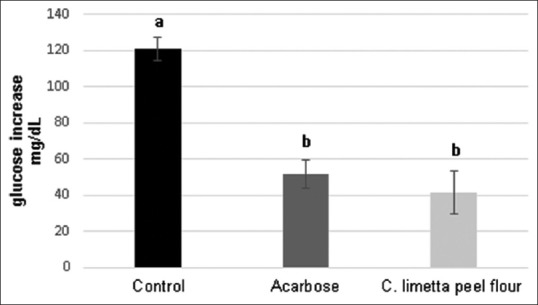
Postprandial hypoglycemic activity. Glucose levels in mice serum 30 min after hyperglycemia induced by maltose. Each of the three groups was treated with the vehicle, acarbose (30 mg/kg), and Citrus limetta peel flour (300 mg/kg). Assays were performed in triplicate. Bars with different letters denote statistically significant differences from each other, P < 0.05
A great variety of natural products inhibits the enzymes responsible for carbohydrate digestion due to the presence of compounds such as terpenes, flavonoids, alkaloids, phenylpropanoid, among others. Recently, Barreca et al.[23] identified eight glycosylated flavonoids compounds in C. limetta juice, and it has been suggested that these types of compounds are related to antihyperglycemic and antioxidant activity. It is known that the presence of flavonoids in therapeutic plants, confers antioxidant, antibacterial, anti-inflammatory, among other properties.[2,24,25]
In addition to these beneficial properties, several studies indicate that content of phenolic compounds is higher in the peel in relation to other sections of the citrus fruit. The peel acts as a protective barrier; in the fact that, it is outside, favors the synthesis of phenolic compounds involved in the antihyperglycemic activity, and is also a source of antioxidants.[2,5,25,26]
The safety of plants is of vital important when they are used clinically; therefore, we tested the acute toxicity in mice, finding that all animals showed good tolerance at the dose of 2000 mg/kg. The treated mice did not show noticeable signs of toxic effects on behavior or appearance, and all mice survived during the whole experimental period, and also, the body weight and food consumption were normal during the study period, which indicate that the use of C. limetta peel flour would be safe because the study did not show toxic effects.
CONCLUSIONS
C. limetta peel flour is able to decrease the concentration of blood glucose after consumption as we demonstrated in this study. It is considered that the hypoglycemic activity of C. limetta peel flour is due to its absorption in the intestine, which was corroborated by in vitro and in vivo assays. Lime peel is a byproduct that could act as a source of functional compounds in the treatment of DM and enhance the activity of synthetic oral hypoglycemic drugs. Natural products are a good alternative with broad benefits, within these few or null side effects. However, it is necessary to perform assays with flour lime peel using an induced diabetic model and finding bioactive molecules responsible of hypoglycemic effect in C. limetta peel flour.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
We thank Vineet Rathod from the University of Alberta for his contribution on this manuscript.
REFERENCES
- 1.Asante DB, Effah-Yeboah E, Barnes P, Abban HA, Ameyaw EO, Boampong JN, et al. Antidiabetic effect of young and old ethanolic leaf extracts of Vernonia amygdalina: A comparative study. J Diabetes Res. 2016;2016:13. doi: 10.1155/2016/8252741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Padilla-Camberos E, Lazcano-Díaz E, Flores-Fernandez JM, Owolabi MS, Allen K, Villanueva-Rodríguez S, et al. Evaluation of the inhibition of carbohydrate hydrolyzing enzymes, the antioxidant activity, and the polyphenolic content of Citrus limetta Peel extract. Scientific World J 2014. 2014:121760. doi: 10.1155/2014/121760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Andrade-Cetto A, Heinrich M. Mexican plants with hypoglycaemic effect used in the treatment of diabetes. J Ethnopharmacol. 2005;99:325–48. doi: 10.1016/j.jep.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 4.Malviya N, Jain S, Malviya S. Antidiabetic potential of medicinal plants. Acta Pol Pharm Drug Res. 2010;67:113–18. [PubMed] [Google Scholar]
- 5.Lv X, Zhao S, Ning Z, Zeng H, Shu Y, Tao O, et al. Citrus fruits as a treasure trove of active natural metabolites that potentially provide benefits for human health. Chem Cent J. 2015;9:68. doi: 10.1186/s13065-015-0145-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Manoka S, Sungthong B, Sato H, Sugiyama E, Sato VH. Hypoglycemic and antioxidant activities of the water extract of Aquilaria crassna Leaves in streptozotocin-nicotinamide-induced type-2 diabetic mice. Nat Prod Commun. 2016;11:757–61. [PubMed] [Google Scholar]
- 7.Figueroa-Valverde L, Díaz-Cedillo F, Camacho-Luis A, Ramos ML. Induced effects by Ruta graveolens L., Rutaceae, Cnidoscolus chayamansa McVaugh, Euphorbiaceae, and Citrus aurantium L., Rutaceae, on glucose, cholesterol and triacylglycerides levels in a diabetic rat model. Rev Bras Farmacogn. 2009;19:898–907. [Google Scholar]
- 8.Fernández-López J, Fernández-Ginés JM, Aleson-Carbonell L, Sendra E, Sayas-Barberá E, Pérez-Alvarez JA. Application of functional citrus by-products to meat products. Trends Food Sci Technol. 2004;15:176–85. [Google Scholar]
- 9.Rodríguez-Rivera MP, Lugo-Cervantes E, Winterhalter P, Jerz G. Metabolite profiling of polyphenols in peels of Citrus limetta Risso by combination of preparative high-speed countercurrent chromatography and LC-ESI-MS/MS. Food Chem. 2014;158:139–52. doi: 10.1016/j.foodchem.2014.02.077. [DOI] [PubMed] [Google Scholar]
- 10.Clement YN, Morton-Gittens J, Basdeo L, Blades A, Francis MJ, Gomes N, et al. Perceived efficacy of herbal remedies by users accessing primary healthcare in Trinidad. BMC Complement Altern Med. 2007;7:4. doi: 10.1186/1472-6882-7-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Ahmed F, Sairam S, Urooj A. In vitro hypoglycemic effects of selected dietary fiber sources. J Food Sci Technol. 2011;48:285–9. doi: 10.1007/s13197-010-0153-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Ou S, Kwok K, Li Y, Fu L. In vitro study of possible role of dietary fiber in lowering postprandial serum glucose. J Agric Food Chem. 2001;49:1026–9. doi: 10.1021/jf000574n. [DOI] [PubMed] [Google Scholar]
- 13.Jdir H, Khemakham B, Chakroun M, Zouari S, Ben AY, Zouari N, et al. Diplotaxis simplex suppresses postprandial hyperglycemia in mice by inhibiting key-enzymes linked to type 2 diabetes. Rev Bras Farmacogn. 2015;25:152–7. [Google Scholar]
- 14.OECD/OCDE. Acute Oral Toxicity – Up-and-Down-Procedure (UDP). Vol. 425. OECD Guideline for Testing of Chemicals. 2008. [Last accessed on 2017 Jan 13]. pp. 1–27. Available from: https://www.ntp.niehs.nih.gov/iccvam/suppdocs/feddocs/oecd/oecdtg425.pdf .
- 15.Chau CF, Huang YL. Comparison of the chemical composition and physicochemical properties of different fibers prepared from the peel of Citrus sinensis L.Cv. liucheng. J Agric Food Chem. 2003;51:2615–8. doi: 10.1021/jf025919b. [DOI] [PubMed] [Google Scholar]
- 16.Abirami A, Nagarani G, Siddhuraju P. Measurement of functional properties and health promoting aspects-glucose retardation index of peel, pulp and peel fiber from Citrus hystrix and Citrus maxima. Bioact Carbohydrates. 2014;4:16–26. [Google Scholar]
- 17.Céspedes MA, Martínez BF, Kil Chang Y. The effect of extruded orange pulp on enzymatic hydrolysis of starch and glucose retardation index. Food Bioprocess Technol. 2010;3:684–92. [Google Scholar]
- 18.Chau CF, Chen CH, Lin CY. Insoluble fiber-rich fractions derived from Averrhoa carambola: Hypoglycemic effects determined by in vitro methods. LWT Food Sci Technol. 2004;37:331–5. [Google Scholar]
- 19.Naim M, Amjad FM, Sultana S, Islam SN, Hossain MA, Begum R, et al. Comparative study of antidiabetic activity of hexane-extract of lemon peel (Limon citrus) and Glimepiride in alloxan-induced diabetic rats. Bangladesh Pharm J. 2012;15:131–4. [Google Scholar]
- 20.Uddin N, Hasan MR, Hossain MM, Sarker A, Hasan AH, Islam AF, et al. In vitro α-amylase inhibitory activity and in vivo hypoglycemic effect of methanol extract of Citrus macroptera Montr. fruit. Asian Pac J Trop Biomed. 2014;4:473–9. doi: 10.12980/APJTB.4.2014C1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figuerola F, Hurtado ML, Estévez AM, Chiffelle I, Asenjo F. Fibre concentrates from apple pomace and citrus peel as potential fibre sources for food enrichment. Food Chem. 2005;91:395–401. [Google Scholar]
- 22.Kundusen S, Haldar PK, Gupta M, Mazumder UK, Saha P, Bala A, et al. Evaluation of antihyperglycemic activity of Citrus limetta Fruit peel in streptozotocin-induced diabetic rats. ISRN Endocrinol. 2011;2011:869273. doi: 10.5402/2011/869273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Barreca D, Bellocco E, Caristi C, Leuzzi U, Gattuso G. Flavonoid profile and radical-scavenging activity of Mediterranean sweet lemon (Citrus limetta Risso) juice. Food Chem. 2011;129:417–22. doi: 10.1016/j.foodchem.2011.04.093. [DOI] [PubMed] [Google Scholar]
- 24.Yin Z, Zhang W, Feng F, Zhang Y, Kang W. α-Glucosidase inhibitors isolated from medicinal plants. Food Sci Hum Wellness. 2014;3:136–74. [Google Scholar]
- 25.Shirosaki M, Koyama T, Yazawa K. Anti-hyperglycemic activity of Kiwifruit leaf (Actinidia deliciosa) in mice. Biosci Biotechnol Biochem. 2008;72:1099–102. doi: 10.1271/bbb.70704. [DOI] [PubMed] [Google Scholar]
- 26.Barros HR, Ferreira TA, Genovese MI. Antioxidant capacity and mineral content of pulp and peel from commercial cultivars of citrus from Brazil. Food Chem. 2012;134:1892–8. doi: 10.1016/j.foodchem.2012.03.090. [DOI] [PubMed] [Google Scholar]


