Abstract
Background:
Ligustilide, an active ingredient in a traditional Chinese medicine, has anti-inflammatory and analgesic effects. The underlying mechanisms of the anti-inflammatory pain effects of ligustilide are not completely understood.
Objective:
The aim of this study to investigate whether ligustilide conducts its analgesic effects on the complete Freund's adjuvant (CFA)-induced inflammatory pain through regulating the c-Jun N-terminal kinase (JNK)/c-Jun pathway in the spinal cord.
Materials and Methods:
Paw withdrawal thresholds (PWTs) and paw withdrawal latencies (PWLs) were tested to examine the analgesic effect of ligustilide on CFA-induced inflammatory pain in rats. The change of spinal JNK/c-Jun activation was detected by western blotting after CFA injection with or without consecutive intrathecal ligustilide administration. After SP600125 (JNK inhibitor) was intrathecally injected in CFA rats, PWTs and PWLs were tested to investigate the change of ligustilide's analgesic effect.
Results:
Repeated intravenous injection of ligustilide could attenuate the pain hypersensitivity induced by CFA. CFA caused increased activation of spinal JNK/c-Jun, which could be inhibited by ligustilide administration. Intrathecal injection of JNK inhibitor inhibited the CFA-induced mechanical hyperalgesia.
Conclusion:
Ligustilide could inhibit the upregulation of spinal p-JNK/p-c-Jun caused by CFA, and the inhibition of JNK/c-Jun activation is closely related to its anti-mechanical hyperalgesia effect in inflammatory pain.
SUMMARY
Ligustilide, an active ingredient in a popular traditional Chinese medicine, has effective anti-inflammatory and analgesic effects. Ligustilide inhibits the complete Freund's adjuvant-induced activation of spinal c-Jun N-terminal kinase-(JNK)/c-Jun pathway in rats. The inhibition of JNK/c-Jun activation is closely related to the anti-mechanical hyperalgesia effect of ligustilide.
Abbreviations used: CFA: Complete Freund's adjuvant, JNK: c-Jun N-terminal kinase, MAPK: Mitogen-activated protein kinase, PWT: Paw withdrawal threshold, PWL: Paw withdrawal latency.
Keywords: c-Jun, c-Jun N-terminal kinase, complete Freund's adjuvant, ligustilide, mechanical hyperalgesia, pain
INTRODUCTION
Ligustilide is one of the main bioactive components of Danggui, which is a popular traditional Chinese medicine and has been widely used for treating cardio- and cerebrovascular diseases through exerting myocardial protection.[1,2,3,4] Ligustilide has other positive pharmacological effects, such as protecting neurons from neurotoxicity and reducing memory deficits in Alzheimer's disease.[5] Recently, researchers have found that ligustilide also has anti-inflammatory and analgesic effects and can relieve inflammatory pain induced by acetic acid, formalin, or complete Freund's adjuvant (CFA).[6,7] It has been reported that ligustilide may conduct its anti-inflammatory effects through inhibiting glial cell-mediated proinflammatory cytokines production in the spinal cord.[8] The underlying mechanisms of the anti-inflammatory pain effects of ligustilide are still not completely understood.
The active participation of glial cells, especially astrocytes and microglial cells, in the induction and maintenance of chronic pain have been well established in numerous studies on pain research.[9] Glial cells act as immunoresponsive cells in the central nervous system and modulate the pathological processes of chronic pain partially through accelerating the production of proinflammatory cytokines.[10,11] Activation of some cellular events such as the mitogen-activated protein kinases (MAPKs) in the glial cells can enhance the release of proinflammatory mediators.[12] The c-Jun N-terminal kinase (JNK), a major member of the MAPK family, has been demonstrated to have a critical role in intracellular signal transduction and contribute to peripheral and central sensitization in chronic pain.[13] Peripheral inflammation or nerve injury causes JNK activation in the spinal astrocytes, and intrathecal administration of JNK inhibitor can block the activation of astrocytes.[14,15] Activation of JNK pathway can lead to increased production and release of some proinflammatory cytokines, which act as important biological mediators in chronic pain.[16,17] c-Jun, an immediate early gene, is a well-known downstream transcription factor of MAPK pathway and a certified substrate of JNK.[18] Nerve injury or peripheral inflammation causes increased phosphorylation of JNK in the spinal astrocytes. P-JNK (phosphorylated JNK, the activated form of JNK) could activate its downstream transcription factor c Jun and enhance the transcription of some pain related genes, contributing to the development of chronic pain.[19,20,21] It has been confirmed that the spinal JNK/c-Jun activation contributes to spinal astrocytes activation and is required for the development of hyperalgesia and allodynia under persistent inflammatory pain condition.[9] Given the critical role of JNK/c-Jun pathway in regulating spinal astrocytes activation and proinflammatory cytokines production, which have been proved to be involved in the mechanism of ligustilide's anti-inflammatory effects, the current study aims at investigating whether ligustilide conducts its analgesic effects on CFA-induced inflammatory pain through regulating JNK/c-Jun pathway in the spinal cord.
MATERIALS AND METHODS
Animals
Male Sprague–Dawley rats (200–250 g) were used in the present study. The animals had free access to food and water and were maintained on a 12 h/12 h light–dark cycle at the room temperature (RT) of 23°C ± 1°C. All the animals were housed under controlled condition to adjust to the new surroundings for 7 days before any experimental procedure. The experiments were performed under the guidelines of the International Association for the Study of Pain.
Model of inflammatory pain
For inducing peripheral inflammation, the rats received a subcutaneous injection of CFA (100 μl, Sigma) into the plantar surface of the left hind paw. The injection was performed under brief anesthesia with sevoflurane. The same dose of normal saline (NS, 100 μl) was injected as control.
Intravenous drug administration
The ligustilide, which was used for intravenous injection in the present study, was extracted from Danggui. Danggui was purchased from Gansu Province, China. The procedure of extracting ligustilide from Danggui was performed according to the method described by Kuang et al.[22] In brief, the essential oil of Danggui was extracted by petroleum ether. From the oil, ligustilide was isolated by silica gel column chromatography and was identified using electron impact ionization MS, 1H NMR, and 13C NMR spectrometric technique. In the present study, the purity of ligustilide was found to be >98% based on the percentage of total peak area by the high-performance liquid chromatography analysis. The ligustilide was mixed with 2% Lutrol F68 poloxamer 188 (BASF Corporation) for intravenous injection. The same dose of 2% Lutrol F68 poloxamer 188 was injected as vehicle control. The ligustilide and vehicle were delivered into the tail vein of the rat by a 5-gauge needle.
Intrathecal drug administration
The inhibitor of JNK, SP600125 (Abcam, ab120065), was dissolved in 10% dimethyl sulfoxide (DMSO) and was used for intrathecal injection in this study. The procedure of intrathecal injection was performed under inhalational anesthesia: 1.375% isoflurane mixed in the flow of oxygen was continuously delivered into a Plexiglas observation chamber in which the rat was placed. When the rat lost its righting reflex, it was taken out and put on an operation panel with its nose placed in a nose cone, into which isoflurane was continually administrated. The lower back of the rat was shaved and sterilized. At the rat's intervertebral space of L4-5, lumbar puncture was operated. When the rat showed a sudden slight flick of the tail, SP600125 (50 μg in 20 μl DMSO) was slowly injected into the subarachnoid space. The same dose of DMSO (20 μl) was injected as vehicle control.
Measurement of mechanical hyperalgesia
The mechanical allodynia of rats was measured by paw withdrawal threshold (PWT). The rats were placed separately in transparent plastic cages with wire mesh bottom and were allowed 30 min for habituation. The PWT value of rat was tested using von Frey hairs with logarithmically incrementing stiffness (0.6, 1, 1.4, 2, 4, 6, 8, 10, 15, and 26 g). We used the von Frey filament (began with 2 g) to press the plantar surface of the rat's left hind paw for 5–6 s. If the rat showed paw withdrawal or paw-licking response, which was taken as positive response, we switched to a lower filament (1.4 g). If the rat showed no positive response, a higher filament (4 g) was used. The 50% withdrawal threshold was assayed according to the Dixon's up–down method described by Chaplan et al.[23] We performed three measurements on each rat and recorded the average value of three measurements as the final PWT.
Measurement of thermal hyperalgesia
The thermal hyperalgesia was measured by paw withdrawal latency (PWL), which was assayed according to the method described by Hargreaves et al.[24] The rats were placed in transparent plastic boxes separately and were allowed 30 min to adapt the environment. The planter surface of the each rat's left paw was exposed to a beam of radiant heat through the glass plate. If the rat showed paw withdrawal or paw-licking response, which was taken as positive response, the radiant heat source was cutoff and the total irradiating time was recorded. If the rat showed no paw withdrawal or paw-licking response till 30 s, radiant heat source was automatically cutoff to prevent potential skin injury, and the “30 s” was recorded. We performed three measurements on each rat and recorded the average value of three measurements as the final PWL.
Western blotting
The rats were rapidly killed after deep anesthesia. The L4-5 segments of the spinal cord were quickly extracted and were immediately homogenized in lysis buffer which contains phenylmethylsulfonyl fluoride (100:1) for 30 min. The samples were then centrifuged at 10,000 rpm for 15 min at 4°C. The supernatants of the samples were collected and the protein concentration was estimated according to the Bradford method.[25] The samples were heated at 100°C for 5 min mixed with 1× loading buffer. Protein samples and the marker (26616, Thermo Scientific) were separated on a 10% sodium dodecyl sulfate–polyacrylamide gel electrophoresis gel and were transferred onto polyvinylidene difluoride membranes. The membranes were blocked with 5% nonfat dry milk for 1 h at RT. The membranes were then incubated at 4°C overnight with rabbit anti-JNK antibody (ab59227, 1:1000, Abcam), rabbit anti-p-JNK (ab124956, 1:5000, Abcam), rabbit anti-p-c-Jun (Ser73, 1:1000, cell signaling), and mouse anti-glyceraldehyde-3-phosphate dehydrogenase (GAPDH) (60004-1-lg, 1:1000, Proteintech) primary antibodies. After primary incubation, the membranes were washed for 3 × 5 min with Tris-buffered saline Tween-20 (TBST). Membranes containing JNK, p-JNK, and p-c-Jun were incubated with secondary antibody-goat anti-rabbit peroxidase (horseradish peroxidase [HRP], 1:2500; A0208, Beyotime) at RT for 2 h. Membranes containing GAPDH were incubated with goat anti-mouse peroxidase (HRP, 1:2500, RS0001, Ruiying Biological) at RT for 2 h. Then, the membranes were extensively washed again for 3 × 5 min with TBST. The specific bands of protein signals were detected by enhanced chemiluminescence solution. The western blot densitometry analysis was performed by Quantity One 4.6.2 (Molecular Imager ChemiDocXRS+ Imaging System, Bio Rad, USA).
Statistical analysis
All data were expressed as mean ± standard deviation (SD). Statistical analyses were performed using GraphPad Prism 5 (GraphPad Software, Inc., San Diego, CA, USA). Student's t-test was used to compare differences between two groups of samples. One-way repeated analysis of variance (ANOVA) and two-way repeated ANOVA, followed by Bonferroni test, were adopted to compare differences among more than two groups. The criterion for statistical significance was P < 0.05.
RESULTS
Ligustilide prevented the mechanical and thermal hyperalgesia caused by complete Freund's adjuvant peripheral injection
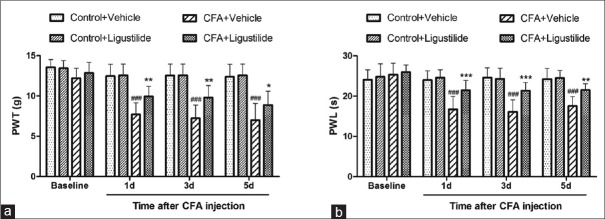
Peripheral subcutaneous injection of CFA is a well-established model of chronic inflammatory pain.[26] In the present study, we examined the effect of ligustilide on CFA-induced thermal and mechanical hyperalgesia. At 1 h after CFA or NS injection, ligustilide (60 mg/kg) or vehicle was intravenously injected in rats. The ligustilide or vehicle was given for 3 consecutive days. PWT and PWL were measured on day 1, 3, and 5 after CFA injection. As shown in Figure 1a and b, CFA caused both mechanical and thermal hyperalgesia from day 1 after injection. However, compared to the vehicle group, consecutive ligustilide administration for 3 days inhibited the CFA-induced hyperalgesia. Both the inhibition of mechanical and thermal hyperalgesia lasted for more than 2 days after the last injection of ligustilide [Figure 1a and b].
Figure 1.
Ligustilide inhibited the mechanical and thermal hyperalgesia caused by CFA. (a and b) Consecutive ligustilide administration inhibited the mechanical and thermal hyperalgesia caused by CFA. Both the inhibition lasted for more than 2 days after the last injection of ligustilide. Data were all expressed as mean ± standard deviation ###P < 0.001, compared with the “Control + Vehicle” group. *P < 0.05, **P < 0.01, ***P < 0.001, compared with the “CFA + Vehicle” group. n = 8 in each group. CFA: Complete Freund's adjuvant
Ligustilide inhibited complete Freund's adjuvant-induced increased activation of c-Jun N-terminal kinase and c-Jun in the spinal cord
In the current study, we detected the change of JNK activation in the spinal cord on day 3 after CFA injection and explored whether ligustilide administration could affect the change of spinal JNK/c-Jun activation. As shown in Figure 2a and c, western blotting showed that compared to the “control + vehicle” group, CFA injection caused an upregulation of the expression of spinal p-JNK (the activated state of JNK through phosphorylation) and p-c-Jun (phosphorylation of c-Jun) in the spinal cord. The upregulation of spinal p-JNK/p-c-Jun was inhibited by ligustilide treatment for 3 consecutive days after CFA injection [Figure 2a-c].
Figure 2.
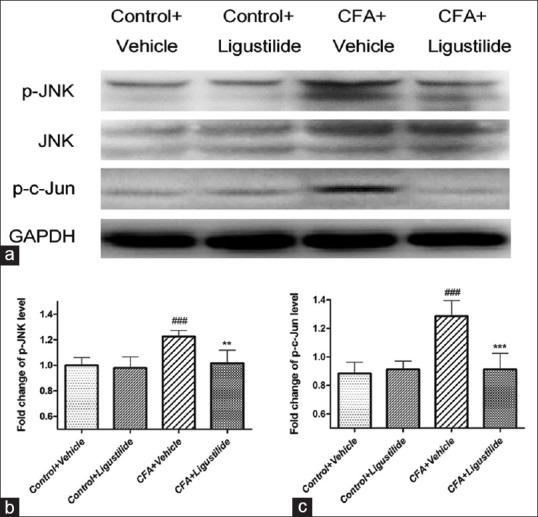
Ligustilide inhibited the increased activation of spinal c-Jun N-terminal kinase caused by CFA. (a) Western blotting showed that CFA injection caused an upregulation of p-c-Jun N-terminal kinase/p-c-Jun in the spinal cord and administration of ligustilide blocked the increased expression of spinal p-c-Jun N-terminal kinase/p-c-Jun induced by CFA. (b and c) The fold change for the density of p-c-Jun N-terminal kinase level normalized to c-Jun N-terminal kinase; and the density of p-c-Jun normalized to glyceraldehyde-3 phosphate dehydrogenase, as shown in Figure 2a. Data were all expressed as mean ± standard deviation ###P < 0.001, compared with “Control + Vehicle” group. **P < 0.01, ***P < 0.001, compared with “CFA + Vehicle” group. CFA: Complete Freund's adjuvant
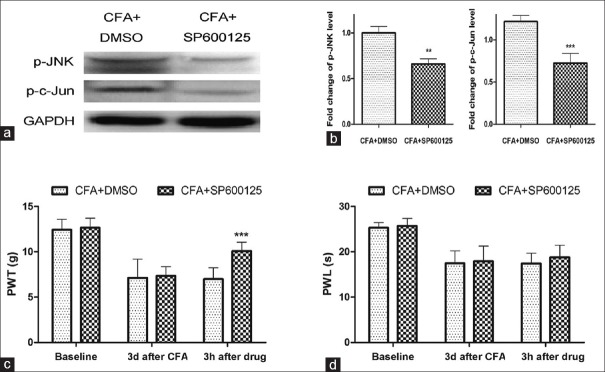
Intrathecal injection of SP600125 blocked the expression of spinal p-c-Jun N-terminal kinase and p-c-Jun and inhibited the complete Freund's adjuvant-induced mechanical hyperalgesia
To confirm the specific role of JNK/c-Jun activation in the maintenance of CFA-induced inflammatory pain, we applied intrathecal injection of SP600125 (a pharmacological inhibitor of JNK) on day 3 after subcutaneous CFA injection in rats. Compared to the “CFA + DMSO” group, western blotting showed a reduction of spinal p-JNK and p-c-Jun level at 3 h after SP600125 administration [Figure 3a and b]. The PWTs and PWLs of rats were also measured at 3 h after SP600125 injection. As shown in Figure 3c, compared to the “CFA + DMSO” group, the mechanical hyperalgesia caused by CFA injection was partially inhibited by SP600125 administration in the “CFA + SP600125” group. However, SP600125 administration had no effect on the CFA-induced thermal hyperalgesia [Figure 3d], suggesting that the spinal JNK/c-Jun pathway played an important role in CFA-induced mechanical allodynia.
Figure 3.
Intrathecal injection of SP600125 blocked the activation of spinal c-Jun N-terminal kinase/c-Jun and inhibited the mechanical hyperalgesia caused by CFA. (a) Western blotting analysis of the expression of spinal p-c-Jun N-terminal kinase/p-c-Jun at 3 h after SP600125 or DMSO intrathecal injection in rats. (b) The fold change for the density of p-c-Jun N-terminal kinase and p-c-Jun level normalized to glyceraldehyde-3-phosphate dehydrogenase, as shown in Figure 3a. (c and d) SP600125 administration inhibited the mechanical hyperalgesia but did not change the value of paw withdrawal latency in CFA rats. Data were all expressed as mean ± standard deviation **P < 0.01, ***P < 0.001, compared with “CFA + DMSO” group. CFA: Complete Freund's adjuvant, DMSO: Dimethyl sulfoxide
DISCUSSION
Chronic pain is one of the most intractable diseases worldwide.[27,28] By now, pharmacotherapy, such as opioids and nonsteroid anti-inflammatory drugs, still remains the main treatment for chronic pain. However, these drugs were limited by the high incidence of side effects.[29] Therefore, it is necessary to exploit new therapeutic drugs for chronic pain and study their underlying analgesia mechanisms comprehensively.
Ligustilide, extracted from Danggui, has been proved to have anti-inflammatory effects and is effective in ameliorating inflammatory pain.[8,30] Previous studies have shown that ligustilide had neuroprotective and anti-inflammatory effects by inhibiting spinal glial cell-mediated proinflammatory cytokines production.[8,22] Many researches supported the role of ligustilide in inhibiting neuroinflammation, which has been found to contribute to the development and maintenance of inflammatory pain. Inhibition of glial cell-mediated neuroinflammation was involved in the underlying mechanism of the analgesic effects of ligustilide.[31,32] A recent study conducted by Qian et al. showed that ligustilide ameliorated CFA-induced inflammatory pain through inhibiting Toll-like receptor (TLR4) in the spinal cord.[33] Another study reported that spinal TLRs promoted the release of proinflammatory cytokines in astrocytes, the process of which could be mediated by JNK activation.[34] Furthermore, it has been reported that in a lipopolysaccharide-induced inflammatory pain model, ligustilide conducted its anti-inflammatory effects by suppressing MAPK and nuclear factor-kappa B (NF-κB) pathway mice.[30] JNK, also called stress-activated protein kinase, is found to play important roles in neurodegeneration apoptosis and inflammatory responses.[21] JNK consists of three isoforms: JNK1, JNK2, and JNK3, among which JNK1 and JNK2 are largely expressed in the spinal cord.[35] A lot of evidence have shown that JNK pathway is a crucial signaling, which contributes to the initiation and maintenance of chronic pain through distinct mechanisms in the spinal cord. The JNK/c-Jun cascade plays an important role in regulating the activation of spinal astrocytes and was required for the development of hyperalgesia and allodynia under persistent inflammatory pain condition.[14,36]
Based on the above researches, we hypothesized that ligustilide relieves CFA-induced inflammatory pain through regulating activation of spinal JNK and its downstream target c-Jun, and we have confirmed this hypothesis in the present study. First, we examined the analgesic effect of ligustilide on inflammatory pain caused by CFA injection in rats. Second, we detected the change of spinal JNK and c-Jun activation after CFA injection and found that the CFA-induced increased activation of JNK/c-Jun could be inhibited by consecutive ligustilide administration. To further investigate the role of spinal JNK/c-Jun pathway in regulating mechanical and thermal hyperalgesia, we injected SP600125 (JNK inhibitor) intrathecally in CFA rats and found that blocking spinal JNK/c-Jun could inhibit mechanical hyperalgesia, rather than thermal hyperalgesia. This result was in accordance with a previous study conducted by Gao et al., which also revealed that spinal JNK pathway was critical for maintaining mechanical allodynia under chronic inflammatory pain station.[9]
In conclusion, this study is the first to demonstrate that ligustilide inhibits the activation of spinal JNK/c-Jun pathway caused by CFA in rats, and this inhibition of JNK/c-Jun activation is closely related to the anti-mechanical hyperalgesia effect of ligustilide. Our findings help to provide new insights into the molecular mechanism underlying ligustilide's analgesic effects. Ligustilide may be of vital therapeutic value in treating chronic inflammatory pain in the future.
CONCLUSION
In the present study, we demonstrated the following findings: (1) repeated intravenous injection of ligustilide attenuated the pain hypersensitivity induced by CFA in rats, (2) CFA caused increased activation of spinal JNK/c-Jun, which could be inhibited by ligustilide administration, and (3) intrathecal injection of JNK inhibitor inhibited the CFA-induced mechanical hyperalgesia.
Financial support and sponsorship
This work was supported by grants from the National Nature Science Foundation of China (81101371).
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by the National Nature Science Foundation of China (81101371). Authors sincerely thank the staff from the Basic Research Laboratory of Wenzhou Medical University for their technical guidance.
REFERENCES
- 1.Yim TK, Wu WK, Pak WF, Mak DH, Liang SM, Ko KM. Myocardial protection against ischaemia-reperfusion injury by a Polygonum multiflorum extract supplemented ‘Dang-Gui decoction for enriching blood’, a compound formulation, ex vivo. Phytother Res. 2000;14:195–9. doi: 10.1002/(sici)1099-1573(200005)14:3<195::aid-ptr629>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 2.Zhao KJ, Dong TT, Tu PF, Song ZH, Lo CK, Tsim KW. Molecular genetic and chemical assessment of radix Angelica (Danggui) in China. J Agric Food Chem. 2003;51:2576–83. doi: 10.1021/jf026178h. [DOI] [PubMed] [Google Scholar]
- 3.Wang Y, Yan J, Li S, Wang W, Cai X, Huang D, et al. Composition of the essential oil from Danggui-zhiqiao herb-pair and its analgesic activity and effect on hemorheology in rats with blood stasis syndrome. Pharmacogn Mag. 2016;12:271–5. doi: 10.4103/0973-1296.192195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ying L, Si-Wang W, Hong-Hai T, Wei C. Simultaneous quantification of six main active constituents in Chinese Angelica by high-performance liquid chromatography with photodiode array detector. Pharmacogn Mag. 2013;9:114–9. doi: 10.4103/0973-1296.111255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Kuang X, Du JR, Chen YS, Wang J, Wang YN. Protective effect of Z-ligustilide against amyloid beta-induced neurotoxicity is associated with decreased pro-inflammatory markers in rat brains. Pharmacol Biochem Behav. 2009;92:635–41. doi: 10.1016/j.pbb.2009.03.007. [DOI] [PubMed] [Google Scholar]
- 6.Du J, Yu Y, Ke Y, Wang C, Zhu L, Qian ZM. Ligustilide attenuates pain behavior induced by acetic acid or formalin. J Ethnopharmacol. 2007;112:211–4. doi: 10.1016/j.jep.2007.02.007. [DOI] [PubMed] [Google Scholar]
- 7.Zhao LX, Jiang BC, Wu XB, Cao DL, Gao YJ. Ligustilide attenuates inflammatory pain via inhibition of NFκB-mediated chemokines production in spinal astrocytes. Eur J Neurosci. 2014;39:1391–402. doi: 10.1111/ejn.12502. [DOI] [PubMed] [Google Scholar]
- 8.Zhu MD, Zhao LX, Wang XT, Gao YJ, Zhang ZJ. Ligustilide inhibits microglia-mediated proinflammatory cytokines production and inflammatory pain. Brain Res Bull. 2014;109:54–60. doi: 10.1016/j.brainresbull.2014.10.002. [DOI] [PubMed] [Google Scholar]
- 9.Gao YJ, Xu ZZ, Liu YC, Wen YR, Decosterd I, Ji RR. The c-Jun N-terminal kinase 1 (JNK1) in spinal astrocytes is required for the maintenance of bilateral mechanical allodynia under a persistent inflammatory pain condition. Pain. 2010;148:309–19. doi: 10.1016/j.pain.2009.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.DeLeo JA, Yezierski RP. The role of neuroinflammation and neuroimmune activation in persistent pain. Pain. 2001;90:1–6. doi: 10.1016/s0304-3959(00)00490-5. [DOI] [PubMed] [Google Scholar]
- 11.Gao YJ, Ji RR. Chemokines, neuronal-glial interactions, and central processing of neuropathic pain. Pharmacol Ther. 2010;126:56–68. doi: 10.1016/j.pharmthera.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Raghavendra V, Tanga F, DeLeo JA. Inhibition of microglial activation attenuates the development but not existing hypersensitivity in a rat model of neuropathy. J Pharmacol Exp Ther. 2003;306:624–30. doi: 10.1124/jpet.103.052407. [DOI] [PubMed] [Google Scholar]
- 13.Ji RR, Woolf CJ. Neuronal plasticity and signal transduction in nociceptive neurons: Implications for the initiation and maintenance of pathological pain. Neurobiol Dis. 2001;8:1–10. doi: 10.1006/nbdi.2000.0360. [DOI] [PubMed] [Google Scholar]
- 14.Zhuang ZY, Wen YR, Zhang DR, Borsello T, Bonny C, Strichartz GR, et al. A peptide c-Jun N-terminal kinase (JNK) inhibitor blocks mechanical allodynia after spinal nerve ligation: Respective roles of JNK activation in primary sensory neurons and spinal astrocytes for neuropathic pain development and maintenance. J Neurosci. 2006;26:3551–60. doi: 10.1523/JNEUROSCI.5290-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Obata K, Yamanaka H, Kobayashi K, Dai Y, Mizushima T, Katsura H, et al. Role of mitogen-activated protein kinase activation in injured and intact primary afferent neurons for mechanical and heat hypersensitivity after spinal nerve ligation. J Neurosci. 2004;24:10211–22. doi: 10.1523/JNEUROSCI.3388-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gao YJ, Zhang L, Samad OA, Suter MR, Yasuhiko K, Xu ZZ, et al. JNK-induced MCP-1 production in spinal cord astrocytes contributes to central sensitization and neuropathic pain. J Neurosci. 2009;29:4096–108. doi: 10.1523/JNEUROSCI.3623-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Park C, Lee S, Cho IH, Lee HK, Kim D, Choi SY, et al. TLR3-mediated signal induces proinflammatory cytokine and chemokine gene expression in astrocytes: Differential signaling mechanisms of TLR3-induced IP-10 and IL-8 gene expression. Glia. 2006;53:248–56. doi: 10.1002/glia.20278. [DOI] [PubMed] [Google Scholar]
- 18.Li JK, Nie L, Zhao YP, Zhang YQ, Wang X, Wang SS, et al. IL-17 mediates inflammatory reactions via p38/c-Fos and JNK/c-Jun activation in an AP-1-dependent manner in human nucleus pulposus cells. J Transl Med. 2016;14:77. doi: 10.1186/s12967-016-0833-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sanna MD, Ghelardini C, Galeotti N. Blockade of the spinal BDNF-activated JNK pathway prevents the development of antiretroviral-induced neuropathic pain. Neuropharmacology. 2016;105:543–52. doi: 10.1016/j.neuropharm.2016.02.016. [DOI] [PubMed] [Google Scholar]
- 20.Lindwall C, Kanje M. Retrograde axonal transport of JNK signaling molecules influence injury induced nuclear changes in p-c-Jun and ATF3 in adult rat sensory neurons. Mol Cell Neurosci. 2005;29:269–82. doi: 10.1016/j.mcn.2005.03.002. [DOI] [PubMed] [Google Scholar]
- 21.Gao YJ, Ji RR. Activation of JNK pathway in persistent pain. Neurosci Lett. 2008;437:180–3. doi: 10.1016/j.neulet.2008.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kuang X, Yao Y, Du JR, Liu YX, Wang CY, Qian ZM. Neuroprotective role of Z-ligustilide against forebrain ischemic injury in ICR mice. Brain Res. 2006;1102:145–53. doi: 10.1016/j.brainres.2006.04.110. [DOI] [PubMed] [Google Scholar]
- 23.Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Methods. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- 24.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 25.Bradford MM. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–54. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- 26.Zöllner C, Mousa SA, Fischer O, Rittner HL, Shaqura M, Brack A, et al. Chronic morphine use does not induce peripheral tolerance in a rat model of inflammatory pain. J Clin Invest. 2008;118:1065–73. doi: 10.1172/JCI25911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Liou JT, Lee CM, Day YJ. The immune aspect in neuropathic pain: Role of chemokines. Acta Anaesthesiol Taiwan. 2013;51:127–32. doi: 10.1016/j.aat.2013.08.006. [DOI] [PubMed] [Google Scholar]
- 28.Zhang R, Lao L, Ren K, Berman BM. Mechanisms of acupuncture-electroacupuncture on persistent pain. Anesthesiology. 2014;120:482–503. doi: 10.1097/ALN.0000000000000101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Carter GT, Duong V, Ho S, Ngo KC, Greer CL, Weeks DL. Side effects of commonly prescribed analgesic medications. Phys Med Rehabil Clin N Am. 2014;25:457–70. doi: 10.1016/j.pmr.2014.01.007. [DOI] [PubMed] [Google Scholar]
- 30.Chung JW, Choi RJ, Seo EK, Nam JW, Dong MS, Shin EM, et al. Anti-inflammatory effects of (Z)-ligustilide through suppression of mitogen-activated protein kinases and nuclear factor-κB activation pathways. Arch Pharm Res. 2012;35:723–32. doi: 10.1007/s12272-012-0417-z. [DOI] [PubMed] [Google Scholar]
- 31.Mika J, Zychowska M, Popiolek-Barczyk K, Rojewska E, Przewlocka B. Importance of glial activation in neuropathic pain. Eur J Pharmacol. 2013;716:106–19. doi: 10.1016/j.ejphar.2013.01.072. [DOI] [PubMed] [Google Scholar]
- 32.Su YW, Chiou WF, Chao SH, Lee MH, Chen CC, Tsai YC. Ligustilide prevents LPS-induced iNOS expression in RAW 264.7 macrophages by preventing ROS production and down-regulating the MAPK, NF-κB and AP-1 signaling pathways. Int Immunopharmacol. 2011;11:1166–72. doi: 10.1016/j.intimp.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 33.Qian B, Li F, Zhao LX, Dong YL, Gao YJ, Zhang ZJ. Ligustilide ameliorates inflammatory pain and inhibits TLR4 upregulation in spinal astrocytes following complete freund's adjuvant peripheral injection. Cell Mol Neurobiol. 2016;36:143–9. doi: 10.1007/s10571-015-0228-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mei XP, Zhang H, Wang W, Wei YY, Zhai MZ, Wang W, et al. Inhibition of spinal astrocytic c-Jun N-terminal kinase (JNK) activation correlates with the analgesic effects of ketamine in neuropathic pain. J Neuroinflammation. 2011;8:6. doi: 10.1186/1742-2094-8-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ji RR, Kawasaki Y, Zhuang ZY, Wen YR, Decosterd I. Possible role of spinal astrocytes in maintaining chronic pain sensitization: Review of current evidence with focus on bFGF/JNK pathway. Neuron Glia Biol. 2006;2:259–69. doi: 10.1017/S1740925X07000403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Manassero G, Repetto IE, Cobianchi S, Valsecchi V, Bonny C, Rossi F, et al. Role of JNK isoforms in the development of neuropathic pain following sciatic nerve transection in the mouse. Mol Pain. 2012;8:39. doi: 10.1186/1744-8069-8-39. [DOI] [PMC free article] [PubMed] [Google Scholar]