Abstract
Background:
The development of alternatives for insulin secretion control in vivo or in vitro represents an important aspect to be investigated. In this direction, natural products have been progressively explored with this aim. In particular, flavonoids are potential candidates to act as insulin secretagogue.
Objective:
To study the influence of flavonoid on overall modulation mechanisms of insulin secretion.
Methods:
The research was conducted in the following databases and platforms: PubMed, Scopus, ISI Web of Knowledge, SciELO, LILACS, and ScienceDirect, and the MeSH terms used for the search were flavonoids, flavones, islets of Langerhans, and insulin-secreting cells.
Results:
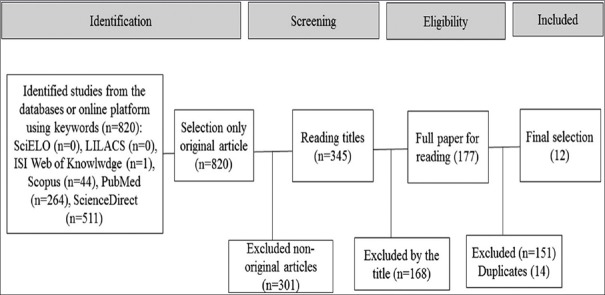
Twelve articles were included and represent the basis of discussion on mechanisms of insulin secretion of flavonoids. Papers in ISI Web of Knowledge were in number of 1, Scopus 44, PubMed 264, ScienceDirect 511, and no papers from LILACS and SciELO databases.
Conclusion:
According to the literature, the majority of flavonoid subclasses can modulate insulin secretion through several pathways, in an indication that corresponding molecule is a potential candidate for active materials to be applied in the treatment of diabetes.
SUMMARY
The action of natural products on insulin secretion represents an important investigation topic due to their importance in the diabetes control
In addition to their typical antioxidant properties, flavonoids contribute to the insulin secretion
The modulation of insulin secretion is induced by flavonoids according to different mechanisms.
Abbreviations used: KATP channels: ATP-sensitive K+ channels, GLUT4: Glucose transporter 4, ERK1/2: Extracellular signal-regulated protein kinases 1 and 2, L-VDCCs: L-type voltage-dependent Ca+2 channels, GLUT1: Glucose transporter 1, AMPK: Adenosine monophosphate-activated protein kinase, PTP1B: Protein tyrosine phosphatase 1B, GLUT2: Glucose transporter 2, cAMP: Cyclic adenosine monophosphate, PKA: Protein kinase A, PTK: Protein tyrosine kinase, CaMK II: Ca2+/calmodulin-dependent protein kinase II, GSIS: Glucose-stimulated insulin secretion, Insig-1: Insulin-induced gene 1, IRS-2: Insulin receptor substrate 2, PDX-1: Pancreatic and duodenal homeobox 1, SREBP-1c: Sterol regulatory element binding protein-1c, DMC: Dihydroxy-6’-methoxy-3’,5’-dimethylchalcone, GLP-1: Glucagon-like peptide-1, GLP-1R: Glucagon-like peptide 1 receptor.
Keywords: Diabetes, flavones, flavonoids, insulin, insulin-secreting cells, islets of Langerhans
INTRODUCTION
Insulin is produced in β-cells being considered as a key metabolic hormone[1] and essential for maintaining glucose homeostasis. Insulin circulates in the blood and acts at skeletal myocytes and adipocytes to facilitate glucose uptake through membrane insertion of the insulin-sensitive glucose transporter 4 (GLUT4), stimulating fuel storage in liver, fat, and skeletal muscle.[2]
Insulin secretion is extremely sensitive to changes in blood glucose.[3] The stimulus for insulin secretion by glucose takes place with increase in the intracellular ATP levels. In the intracellular medium, the increase of ATP levels leads to closure of ATP-sensitive K+ channels (KATP) resulting in the membrane depolarization capable of opening the voltage-dependent Ca2+ channels, which promote influx of extracellular Ca2+, inducing insulin secretion.[4,5,6,7]
The glucose is the most potent stimulator of insulin secretion and can achieve acute and long-term regulation of insulin secretion.[8] However, other nutrients also are capable of triggering insulin release or amplify glucose-stimulated insulin secretions (GSISs)[9] such as hormones,[10] proteins,[11,12] and pharmacological agents.[13]
In recent years, different therapeutic strategies to increasing or improving insulin secretion in vivo or in vitro by the use of natural products have been explored. Scheen reported that new insulin secretagogues should offer advantages over sulfonylureas in the near feature.[14] Most of the plants contain substances that have antidiabetic effect and insulin secretagogue as plant extracts, functional foods,[15,16,17,18] and some isolated as triterpenes and derivatives,[19,20] phytosterols,[21,22] stilbene,[23] and iridoid glycoside[24] have shown activity which stimulates or potentiates insulin secretion in pancreatic islets.
Flavonoids represent a class of phenolic compounds (benzopyran heterocycle linked to a benzene ring) divided into subclasses based on the degree of oxidation of the C4 position, hydroxylation pattern, and substitution of the C3 position.[25] The basic flavonoid skeleton offers numerous substituents that can subdivide into major subclasses as flavonols, flavones, flavan-3-ols, anthocyanidins, flavanones, and isoflavones and into smaller subclasses as dihydroflavonols, flavan-3,4-diols, coumarins, chalcones, dihydrochalcones, and aurones.[26]
Studies indicate several benefits of flavonoids due to their antioxidant properties.[27,28,29] Furthermore, the plant extracts and/or its fractions containing flavonoids can act on insulin secretagogue.[30] Thus, in this review, we focus on findings related to the insulin secretion due to the flavonoids action, highlighting the mechanism of modulation of insulin secretion.
METHODS
Search strategy and databases
The internet bibliographic search on flavonoids and their insulin secretion mechanisms was conducted in 2016. The following databases and platforms were consulted: PubMed, Scopus, ISI Web of Knowledge, SciELO, LILACS, and ScienceDirect on April 8, 2016. The MeSH terms used for the search were flavonoids, flavones, islets of Langerhans, and insulin-secreting cells. Different combinations of these keywords were used. This systematic review was conducted in accordance with the guidelines of Transparent Reporting of Systematic Reviews and Meta-analyses (PRISMA statement).[31]
Selection of papers
Complete articles were included in the review by observing the following inclusion criteria: studies that show the mechanism of insulin secretion of isolated flavonoids. Studies were excluded according to the following exclusion criteria: studies which reported the insulin secretion mechanism of the extracts or fractions containing flavonoids or their metabolites and/or derivatives, studies that no explain the mechanism of insulin secretion, and those articles related to review articles, meta-analysis, abstracts, conference proceedings, editorials/letters, and others.
Discrepancies on the study inclusion/exclusion were decided with the reach of a consensus. All electronic search titles, selected abstracts, and full-text articles were reviewed by a minimum of two reviewers.
Data extraction
Data were extracted employing standardized forms. Extracted information included data regarding type of flavonoid, doses, concentration or quantity utilized, animal used, parameters assessed, and results and mechanisms of action of insulin secretion.
RESULTS
Corresponding literature was in the order of 820 productions/scientific papers, including original articles, reviews, and book chapters. Papers in ISI Web of Knowledge were in number of 1, Scopus 44, PubMed 264, ScienceDirect 511, and no papers from LILACS and SciELO databases.
Reviews, book chapters, and posters were excluded, and it was performed after a screening for relevant titles and full papers and also removed all repeated. Twelve articles met the inclusion and exclusion criteria established. All of the checked steps and article description are described in the following flow chart [Figure 1].
Figure 1.
Flowchart of the selection of the articles included
From these documents, it was observed the increasing interest in the application of flavonoids to treatment of diabetes. The development of new medications from natural compounds offers potential for the management of diabetes through therapies with products of natural sources solely or by association with synthetic drugs, which can avoid the side effects of antidiabetic drugs, such as insulin and oral hypoglycemic agents.[32,33]
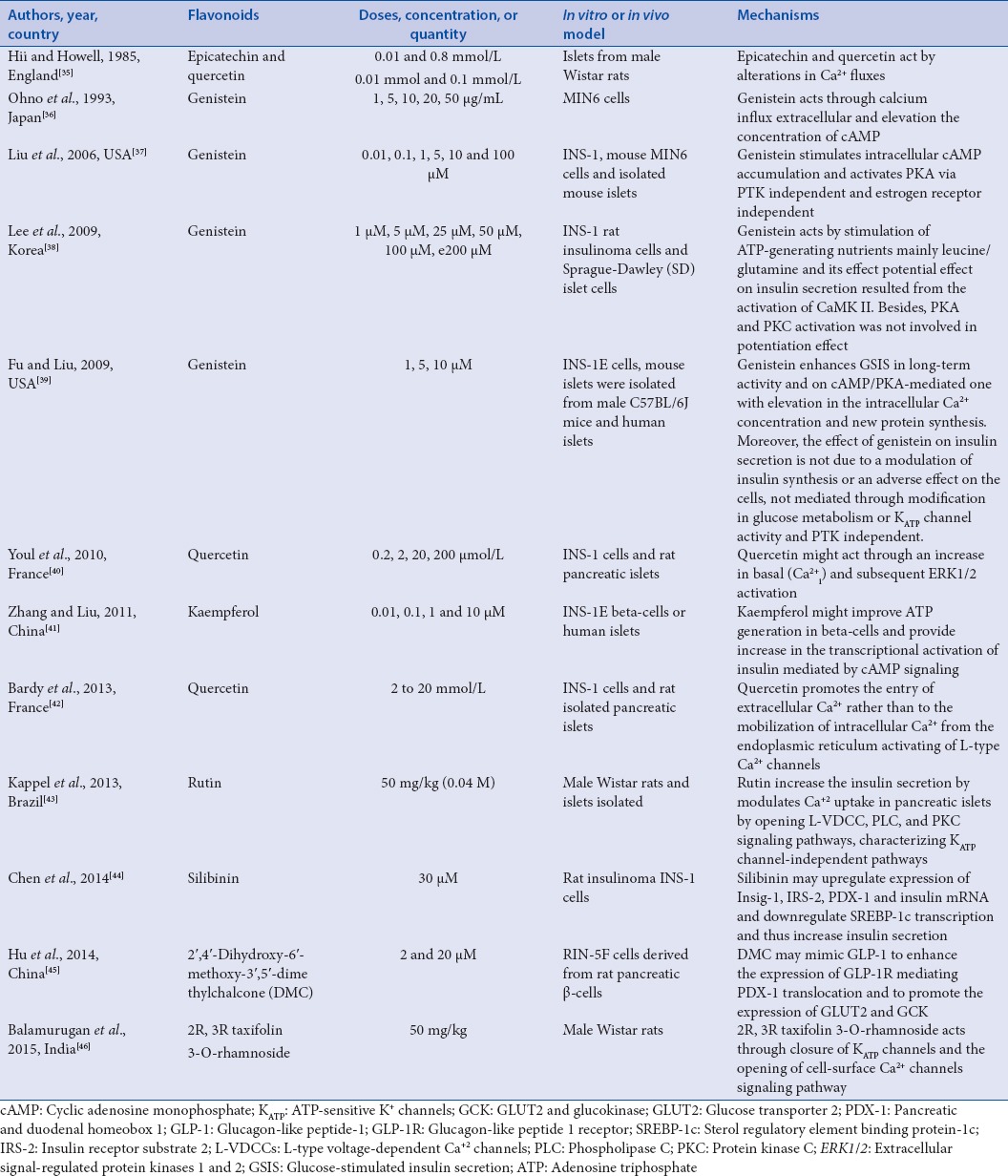
In general, flavonoids or its fractions have shown that their hypoglycemic activities are due to the inhibition of glucose transport, upregulatory activities of glucose uptake, improved GSIS, and/or restoration of insulin secretion capacity;[34] besides that, it plays a protective role on osmotic fragility of cells, resembling the insulin. Table 1 summarizes the insulin secretion mechanism described by the papers included in this study.
Table 1.
Insulin secretion mechanism of flavonoids
DISCUSSION
All of the types of flavonoids have an important antioxidant activity, which explain some of its beneficial effects. They can exhibit antiviral,[47,48,49] anti-inflammatory,[50,51,52,53] anticancer,[54,55,56] and hypoglycemic activity,[57,58,59,60,61,62] among others.[63,64] Thus, to better comment on the major classes of flavonoids and corresponding characteristics, we discuss the insulin secretion mechanism by subgroups of flavonoids.
Flavonol
The flavonols comprise one of the major groups of flavonoids. Usually, flavonols are found in woody angiosperms.[65] The main flavonols are most commonly found as O-glycosides.[26] Different disposition of phenolic OH groups on 3 hydroxyflavone molecule can be explored for production of new compounds.
Quercetin is the most abundant of the flavonol. It is found in a variety of food and medicinal botanicals.[66,67] Studies reported that quercetin can improve glucose uptake in peripheral insulin,[68] providing reduction in the blood glucose level of diabetic rats to normal values, which is attributed to its ability to regenerate pancreatic β-cells and to increase insulin release.[69] Furthermore, quercetin may be considered as a partially useful supplement for the treatment of diabetic depression.[70] However, the oral bioavailability of quercetin is poor which hinders wide applications,[71] which are directed to the determination of improved bioavailability from the incorporation in nanomaterials.[72,73]
Kaempferol is a flavonol present in a variety of plants and fruits showing several antioxidants effects. Kaempferol may act as α-glucosidase inhibitor,[74] improving the insulin resistance[75] and glucose homeostasis.[76] Concerning rutin, it is chemically a flavonol glycoside compound of quercetin and disaccharide rutinose that present multiple pharmacological activities.[77] Rutin showed antidiabetic activity.[78,79,80,81]
In terms of insulin secretion mechanisms (described in the literature), until now, there are five studies describing such modulation mechanisms of insulin secretion by flavonol. Reports that used the flavonol quercetin involve the modulation of Ca+2, either through influx or increase basal or by intracellular mobilization from the endoplasmic reticulum activating of L-type Ca2+ channels.[35,40,42] The rutin has a similar activity in comparison with calcium uptake in pancreatic islets through L-type voltage-dependent Ca+2 channels (L-VDCCs).[43] Kaempferol might improve ATP generation in β-cells and provide an increase in the transcriptional activation of insulin mediated by cyclic adenosine monophosphate (cAMP) signaling.[41] These processes are integrated by Ca2+ signals in the insulin granule exocytosis.[82]
The increased level of Ca2+ stimulates processes to trigger many different cellular pathways[83] and into β-cells since calcium is crucial to insulin secretion. Thus, the flavonols may directly or indirectly act on the calcium modulation and thus can be an alternative to available hypoglycemic drugs. However, more studies are necessary to validate the bioavailability and adverse effects of these flavonols.
Flavan-3-ol
Catechin is a phytonutrient member of flavan family such as epicatechin (EC), epigallocatechin (EGC), epicatechin-3-gallate, and epigallocatechin-3-gallate.[84] Studies indicate that flavan-3-ol can exert both inhibitory activities[85,86] as stimulus for insulin secretion.[87] EC is found in large amounts in grape pomace[88] and has been considered a strong antioxidant. Typical mechanism of insulin secretion was described in 1985 by Hii and Howell, who showed that EC and quercetin (as above) may act by alterations in Ca2+ fluxes to enhance insulin secretion.[35] Indeed, the increase in intracellular calcium concentration promotes the insulin secretion.
Isoflavones
Isoflavone aglycones are two aromatic rings linked by a heterocyclic pyrane ring. They are present mainly in plants of Leguminosae family and generally found in soybeans, red clover, and kudzu root in highest concentrations.[89] The main isoflavones in soybean are genistein and daidzein.
Genistein is a phytoestrogen characterized by a wide variety of health benefits,[90] considered a pluripotent and promising therapeutic agent to diabetes, with activity in inhibition of glucose uptake in mature adipocyte, enhancement in the GSIS, improvement in insulin resistance state, and reduction in the reactive oxygen species-induced β cell damage.[91]
El-Kordya EA, Alshahrani AM recently reported that genistein has a protective effect on pancreatic β-cells damage, and in high dosage, it possesses the ability to regenerate β-cells and consequently improves serum levels and decreases high serum glucose in diabetic rats.[92] Furthermore, the study of Lee et al. showed that genistein derivatives stimulates glucose uptake and the mechanism can be established by adenosine monophosphate-activated protein kinase activation, GLUT4 and GLUT1 expressions, and protein tyrosine phosphatase 1B inhibition.[93]
Until now, a few studies described the corresponding mechanism, in which genistein is able to improve or increase insulin secretion. In 1993, Ohno et al. demonstrated that genistein increases insulin secretion in a dose-dependent manner up to 20 μg/mL and this should be through calcium influx extracellular and elevation the concentration of cAMP.[36] Liu et al. showed that genistein stimulates insulin secretion in a dose of 0.01–5 μM stimulating intracellular cAMP accumulation, which subsequently activates protein kinase A (PKA) via protein tyrosine kinase (PTK) independent and estrogen receptor independent.[37]
In 2009, Lee et al. reported that the concentration of 50 μM of genistein exhibit improved effect on insulin secretion stimulated by ATP-generating nutrients mainly leucine/glutamine activating of Ca2+/calmodulin-dependent protein kinase II (CaMK II) and PKA and PKC independent.[38] Fu and Liu showed that genistein at 5 μM has the maximal increase in the insulin secretion in long-term acting in the cAMP/PKA and the elevation of intracellular Ca2+ concentration is independent on KATP channel activity and PTK.[39]
Based on this information, genistein has the potential to improve and increase insulin secretion by increasing the calcium concentration into intracellular medium which characterizes the primary signal in the regulation of insulin secretion and cAMP, an important molecule that acts as a type of cellular secondary messenger. When activated, the cAMP exerts a regulatory effect in multiple peripheral tissues, enhances insulin sensitivity, stimulates glucose uptake, and promotes gene expression.[94,95] It might also generate ATP into cells and activate CaMK II. CaMKII participates in GSIS in several steps of this process, such as the modulation of cytoplasmic content of ATP and the synthesis of insulin granules.[96] Furthermore, all possible mechanisms are independent of PKC.
Flavonolignans
Flavonolignan has in its composition a portion of flavonoid and other of lignan. Silibinin is the major pharmacologically active compound of Silymarin, an isomeric mixture from the Silybum marianum that containing at least six flavonolignans.[97,98,99,100] Extracts and flavonoids from S. marianum have been reported in the literature as hypoglycemiant and applied for pancreas recovery for diabetics.[101,102] This activity can be associated to Silibinin. According to Chen et al., Silibinin protects β-cells from glucotoxicity and can improve GSIS and upregulate expression of insulin-induced gene 1 (Insig-1), insulin receptor substrate 2 (IRS-2), pancreatic and duodenal homeobox 1 (PDX-1), and insulin mRNA and downregulate sterol regulatory element binding protein-1c (SREBP-1c) transcription.[44]
PDX-1 is involved in the regulation factor in the cascade regulating insulin secretion, regulating the transcription of insulin and other insulin secretion-related genes,[103] IRS-2 mediates effects of insulin and insulin-like growth factor 1, and Insig-1 negatively regulates SREBPs. Therefore, this transcription factors and genes contribute to insulin secretion, as well as some studies have showed.[104,105,106]
Chalcone
Chalcones are aromatic ketone with two phenyl rings that are precursors of flavonoids and isoflavonoids and exert several of biological and pharmacological activities.[107,108] Generally, natural chalcones occur as petal pigments and have been found in the heartwood, bark, leaf, fruit, and root.[109] Different studies have showed that chalcones present antihyperglycemic properties, while chalcone with iodine substitution showed great potential in reducing glucose medium concentration,[110] increasing insulin secretion,[111] and increasing glucose uptake.[112]
In a study published Hu et al., it was reported that 2’,4’-dihydroxy-6’-methoxy-3’,5’-dimethyl chalcone may increase insulin secretion under the condition of elevated glucose by mimicking glucagon-like peptide-1 (GLP-1) to enhance the expression of glucagon-like peptide 1 receptor (GLP-1R)-mediated PDX-1 translocation and to promote the expression of GLUT2 and glucokinase (GCK).[45] GLP-1 promotes insulin secretion, maintains a blood sugar balance, and improves pancreatic islet cell function.[113,114] Besides, it has been used as alternative to conventional hypoglycemic agents and insulin injectable therapies since it has a lower risk of causing hypoglycemia[115] because the GLP-1 has alternative mechanisms may act as negative feedback pathway for insulin secretion, activating the KATP channels through phosphatidylinositol-4,5-bisphosphate 3-kinase (PI3K)-dependent pathway.[10]
The different mechanisms of activity of GLP-1 are regulated by the GLP-1R. The GLP-1R induces in the cytosol PKA activity that regulates the insulin gene transcription factor of pancreatic duodenal homeobox-1 (PDX-1) by increasing its expression and its nuclear translocation[116,117] and this activation leads to insulin secretion. On the other hand, the 2’,6’-dihydroxy-4’-methoxy-3’,5’-dimethyl chalcone might stimulate the secretion of insulin by increasing the GLUT2 and GCK. The GLUT 2 facilitates transport of glucose and thus initiates the GSIS by the uptake of glucose.[118] After, glucose is then phosphorylated by glucokinase and further metabolized through the glycolytic route. Thereby, this process increases the production of ATP into the cell that increases calcium influx and leads to insulin secretion.
Flavanone
Flavanones are flavonoids that have generally a disaccharide glycosylated at position seven. This derivative is characterized by a wide variety of biological activities[119] with antihyperglycemic activity.[120,121,122] However, there are few reports on flavanone with antidiabetic activity. In 2015, Balamurugan et al. isolated an flavanone: 2R, 3R taxifolin 3-O-rhamnosid, and tested its action as insulin secretagogue, which showed action through closure of KATP channels and the opening of cell surface Ca2+ channels signaling pathway.[46] This mechanism is similar to the mechanism of sulfonylureas.[123] Thus, further studies are needed to confirm effective and low side effects that sulfonylureas to be a more effective alternative.
CONCLUSIONS
Emerging evidence indicates that flavonoids can be an alternative in the treatment diabetes. Insulin secretagogues’ effects may be due to different mechanisms. Flavonoids can modulate insulin secretion through alterations in Ca2+ fluxes by L-type Ca2+ channels or L-VDCCs or by others mechanisms, intracellular cAMP accumulation, sometimes PKA-mediated, activation of CaMK II, or by the transcription factors or its products (genes), as PDX-1, GLP-1, IRS-2, Insig-1, and so on. The subclasses flavonol and isoflavones show the better activities of modulation and evidence that can be promising to treatment of diabetes. Despite of these findings, more studies are needed to prove the long-term effects of flavonoids in insulin secretion and to ensure its applicability in diabetic patients.
Financial support and sponsorship
This work was supported by Brazilian Funding Agency FACEPE, CAPES, FINEP, CNPq, and FAPESB.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Fu Z, Gilbert ER, Liu D. Regulation of insulin synthesis and secretion and pancreatic Beta-cell dysfunction in diabetes. Curr Diabetes Rev. 2013;9:25–53. [PMC free article] [PubMed] [Google Scholar]
- 2.Amisten S, Salehi A, Rorsman P, Jones PM, Persaud SJ. An atlas and functional analysis of G-protein coupled receptors in human islets of Langerhans. Pharmacol Ther. 2013;139:359–91. doi: 10.1016/j.pharmthera.2013.05.004. [DOI] [PubMed] [Google Scholar]
- 3.Cantley J, Ashcroft FM. Q and A: Insulin secretion and type 2 diabetes: Why do β-cells fail? BMC Biol. 2015;13:33. doi: 10.1186/s12915-015-0140-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Henquin JC. Regulation of insulin secretion: A matter of phase control and amplitude modulation. Diabetologia. 2009;52:739–51. doi: 10.1007/s00125-009-1314-y. [DOI] [PubMed] [Google Scholar]
- 5.Henquin JC, Ravier MA, Nenquin M, Jonas JC, Gilon P. Hierarchy of the beta-cell signals controlling insulin secretion. Eur J Clin Invest. 2003;33:742–50. doi: 10.1046/j.1365-2362.2003.01207.x. [DOI] [PubMed] [Google Scholar]
- 6.Bryan J, Crane A, Vila-Carriles WH, Babenko AP, Aguilar-Bryan L. Insulin secretagogues, sulfonylurea receptors and K (ATP) channels. Curr Pharm Des. 2005;11:2699–716. doi: 10.2174/1381612054546879. [DOI] [PubMed] [Google Scholar]
- 7.Ashcroft FM, Harrison DE, Ashcroft SJ. Glucose induces closure of single potassium channels in isolated rat pancreatic beta-cells. Nature. 1984;312:446–8. doi: 10.1038/312446a0. [DOI] [PubMed] [Google Scholar]
- 8.Szollosi A, Nenquin M, Aguilar-Bryan L, Bryan J, Henquin JC. Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old β-cells lacking ATP-sensitive K+ channels. J Biol Chem. 2007;282:1747–56. doi: 10.1074/jbc.M609875200. [DOI] [PubMed] [Google Scholar]
- 9.Baumgard LH, Hausman GJ, Sanz Fernandez MV. Insulin: Pancreatic secretion and adipocyte regulation. Domest Anim Endocrinol. 2016;54:76–84. doi: 10.1016/j.domaniend.2015.07.001. [DOI] [PubMed] [Google Scholar]
- 10.Kwon HJ, Park HS, Park SH, Park JH, Shin SK, Song SE, et al. Evidence for glucagon-like peptide-1 receptor signaling to activate ATP-sensitive potassium channels in pancreatic beta cells. Biochem Biophys Res Commun. 2016;469:216–21. doi: 10.1016/j.bbrc.2015.11.127. [DOI] [PubMed] [Google Scholar]
- 11.Gao J, Zhong X, Ding Y, Bai T, Wang H, Wu H, et al. Inhibition of voltage-gated potassium channels mediates uncarboxylated osteocalcin-regulated insulin secretion in rat pancreatic β cells. Eur J Pharmacol. 2016;777:41–8. doi: 10.1016/j.ejphar.2016.02.060. [DOI] [PubMed] [Google Scholar]
- 12.Chen Y, Carlessi R, Walz N, Cruzat VF, Keane K, John AN, et al. Pigment epithelium-derived factor (PEDF) regulates metabolism and insulin secretion from a clonal rat pancreatic beta cell line BRIN-BD11 and mouse islets. Mol Cell Endocrinol. 2016;426:50–60. doi: 10.1016/j.mce.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 13.Anderson RL, Randall MD, Chan SL. The complex effects of cannabinoids on insulin secretion from rat isolated islets of Langerhans. Eur J Pharmacol. 2013;706:56–62. doi: 10.1016/j.ejphar.2013.02.034. [DOI] [PubMed] [Google Scholar]
- 14.Scheen AJ. Investigational insulin secretagogues for type 2 diabetes. Expert Opin Investig Drugs. 2016;25:405–22. doi: 10.1517/13543784.2016.1152260. [DOI] [PubMed] [Google Scholar]
- 15.Schmidt S, Jakab M, Jav S, Streif D, Pitschmann A, Zehl M, et al. Extracts from Leonurus sibiricus L. increase insulin secretion and proliferation of rat INS-1E insulinoma cells. J Ethnopharmacol. 2013;150:85–94. doi: 10.1016/j.jep.2013.08.013. [DOI] [PubMed] [Google Scholar]
- 16.Aggarwal S, Shailendra G, Ribnicky DM, Burk D, Karki N, Qingxia Wang MS. An extract of Artemisia dracunculus L. stimulates insulin secretion from β cells, activates AMPK and suppresses inflammation. J Ethnopharmacol. 2015;170:98–105. doi: 10.1016/j.jep.2015.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Meenatchi P, Purushothaman A, Maneemegalai S. Antioxidant, antiglycation and insulinotrophic properties of Coccinia grandis (L.) in vitro: Possible role in prevention of diabetic complications. J Tradit Complement Med. 2016;7:54–64. doi: 10.1016/j.jtcme.2016.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Kazeem MI, Davies TC. Anti-diabetic functional foods as sources of insulin secreting, insulin sensitizing and insulin mimetic agents. J Funct Foods. 2016;20:122–38. [Google Scholar]
- 19.Castro AJ, Cazarolli LH, de Carvalho FK, da Luz G, Altenhofen D, dos Santos AR, et al. Acute effect of 3β-hidroxihop-22 (29) ene on insulin secretion is mediated by GLP-1, potassium and calcium channels for the glucose homeostasis. J Steroid Biochem Mol Biol. 2015;150:112–22. doi: 10.1016/j.jsbmb.2015.03.011. [DOI] [PubMed] [Google Scholar]
- 20.da Luz G, Frederico MJ, Castro AJ, Moraes AL, de Carvalho FK, Espíndola L, et al. Triterpene derivative: A potential signaling pathway for the fern-9(11)-ene-2α,3β-diol on insulin secretion in pancreatic islet. Life Sci. 2016;154:58–65. doi: 10.1016/j.lfs.2016.04.027. [DOI] [PubMed] [Google Scholar]
- 21.Balamurugan R, Duraipandiyan V, Ignacimuthu S. Antidiabetic activity of γ-sitosterol isolated from Lippia nodiflora L. in streptozotocin induced diabetic rats. Eur J Pharmacol. 2011;667:410–8. doi: 10.1016/j.ejphar.2011.05.025. [DOI] [PubMed] [Google Scholar]
- 22.Subash-Babu P, Ignacimuthu S, Alshatwi AA. Nymphayol increases glucose-stimulated insulin secretion by RIN-5F cells and GLUT4-mediated insulin sensitization in type 2 diabetic rat liver. Chem Biol Interact. 2015;226:72–81. doi: 10.1016/j.cbi.2014.12.011. [DOI] [PubMed] [Google Scholar]
- 23.Chen WP, Chi TC, Chuang LM, Su MJ. Resveratrol enhances insulin secretion by blocking K(ATP) and K(V) channels of beta cells. Eur J Pharmacol. 2007;568:269–77. doi: 10.1016/j.ejphar.2007.04.062. [DOI] [PubMed] [Google Scholar]
- 24.Zhang Y, Ding Y, Zhong X, Guo Q, Wang H, Gao J, et al. Geniposide acutely stimulates insulin secretion in pancreatic β-cells by regulating GLP-1 receptor/cAMP signaling and ion channels. Mol Cell Endocrinol. 2016;430:89–96. doi: 10.1016/j.mce.2016.04.020. [DOI] [PubMed] [Google Scholar]
- 25.Testai L. Flavonoids and mitochondrial pharmacology: A new paradigm for cardioprotection. Life Sci. 2015;135:68–76. doi: 10.1016/j.lfs.2015.04.017. [DOI] [PubMed] [Google Scholar]
- 26.Crozier A, Jaganath IB, Clifford MN. Dietary phenolics: Chemistry, bioavailability and effects on health. Nat Prod Rep. 2009;26:1001–43. doi: 10.1039/b802662a. [DOI] [PubMed] [Google Scholar]
- 27.Hu Q, Yu J, Yang W, Kimatu BM, Fang Y, Ma N, et al. Identification of flavonoids from Flammulina velutipes and its neuroprotective effect on pheochromocytoma-12 cells. Food Chem. 2016;204:274–82. doi: 10.1016/j.foodchem.2016.02.138. [DOI] [PubMed] [Google Scholar]
- 28.Ben Sghaier M, Skandrani I, Nasr N, Franca MG, Chekir-Ghedira L, Ghedira K. Flavonoids and sesquiterpenes from Tecurium ramosissimum promote antiproliferation of human cancer cells and enhance antioxidant activity: A structure-activity relationship study. Environ Toxicol Pharmacol. 2011;32:336–48. doi: 10.1016/j.etap.2011.07.003. [DOI] [PubMed] [Google Scholar]
- 29.Maruyama H, Sumitou Y, Sakamoto T, Araki Y, Hara H. Antihypertensive effects of flavonoids isolated from brazilian green propolis in spontaneously hypertensive rats. Biol Pharm Bull. 2009;32:1244–50. doi: 10.1248/bpb.32.1244. [DOI] [PubMed] [Google Scholar]
- 30.Bharucha B, Dwivedi M, Laddha NC, Begum R, Hardikar AA, et al. Antioxidant rich flavonoids from Oreocnide integrifolia enhance glucose uptake and insulin secretion and protects pancreatic β-cells from streptozotocin insult. BMC Complement Altern Med. 2011;11:126. doi: 10.1186/1472-6882-11-126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moher D, Liberati A, Tetzlaff J, Altman DG PRISMA Group. Preferred reporting items for systematic reviews and meta-analyses: The PRISMA Statement. PLoS Med. 2009;6:e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Mentreddy S, Mohamed AI, Rimando AM. Medicinal plants with hypoglycemic/anti hyperglycemic properties: A review. Proc Assoc Adv Ind Crop Conf. 2005;20:341–53. [Google Scholar]
- 33.Mentreddy SR. Medicinal plant species with potential antidiabetic properties. J Sci Food Agric. 2007;87:743–50. [Google Scholar]
- 34.Hanhineva K, Törrönen R, Bondia-Pons I, Pekkinen J, Kolehmainen M, Mykkänen H, et al. Impact of dietary polyphenols on carbohydrate metabolism. Int J Mol Sci. 2010;11:1365–402. doi: 10.3390/ijms11041365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hii CS, Howell SL. Effects of flavonoids on insulin secretion and 45Ca2+handling in rat islets of Langerhans. J Endocrinol. 1985;107:1–8. doi: 10.1677/joe.0.1070001. [DOI] [PubMed] [Google Scholar]
- 36.Ohno T, Kato N, Ishii C, Shimizu M, Ito Y, Tomono S, et al. Genistein augments cyclic adenosine 3’5’-monophosphate(cAMP) accumulation and insulin release in MIN6 cells. Endocr Res. 1993;19:273–85. doi: 10.1080/07435809309026682. [DOI] [PubMed] [Google Scholar]
- 37.Liu D, Zhen W, Yang Z, Carter JD, Si H, Reynolds KA. Genistein acutely stimulates insulin secretion in pancreatic beta-cells through a cAMP-dependent protein kinase pathway. Diabetes. 2006;55:1043–50. doi: 10.2337/diabetes.55.04.06.db05-1089. [DOI] [PubMed] [Google Scholar]
- 38.Lee SJ, Kim HE, Choi SE, Shin HC, Kwag WJ, Lee BK, et al. Involvement of Ca2+/calmodulin kinase II (CaMK II) in genistein-induced potentiation of leucine/glutamine-stimulated insulin secretion. Mol Cells. 2009;28:167–74. doi: 10.1007/s10059-009-0119-7. [DOI] [PubMed] [Google Scholar]
- 39.Fu Z, Liu D. Long-term exposure to genistein improves insulin secretory function of pancreatic beta-cells. Eur J Pharmacol. 2009;616:321–7. doi: 10.1016/j.ejphar.2009.06.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Youl E, Bardy G, Magous R, Cros G, Sejalon F, Virsolvy A, et al. Quercetin potentiates insulin secretion and protects INS-1 pancreatic β-cells against oxidative damage via the ERK1/2 pathway. Br J Pharmacol. 2010;161:799–814. doi: 10.1111/j.1476-5381.2010.00910.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Zhang Y, Liu D. Flavonol kaempferol improves chronic hyperglycemia-impaired pancreatic beta-cell viability and insulin secretory function. Eur J Pharmacol. 2011;670:325–32. doi: 10.1016/j.ejphar.2011.08.011. [DOI] [PubMed] [Google Scholar]
- 42.Bardy G, Virsolvy A, Quignard JF, Ravier MA, Bertrand G, Dalle S, et al. Quercetin induces insulin secretion by direct activation of L-type calcium channels in pancreatic beta cells. Br J Pharmacol. 2013;169:1102–13. doi: 10.1111/bph.12194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Kappel VD, Frederico MJ, Postal BG, Mendes CP, Cazarolli LH, Silva FR. The role of calcium in intracellular pathways of rutin in rat pancreatic islets: Potential insulin secretagogue effect. Eur J Pharmacol. 2013;702:264–8. doi: 10.1016/j.ejphar.2013.01.055. [DOI] [PubMed] [Google Scholar]
- 44.Chen K, Zhao L, He H, Wan X, Wang F, Mo Z. Silibinin protects β cells from glucotoxicity through regulation of the Insig-1/SREBP-1c pathway. Int J Mol Med. 2014;34:1073–80. doi: 10.3892/ijmm.2014.1883. [DOI] [PubMed] [Google Scholar]
- 45.Hu YC, Hao DM, Zhou LX, Zhang Z, Huang N, Hoptroff M, et al. 2’,4’-Dihydroxy-6’-methoxy-3’,5’-dimethylchalcone protects the impaired insulin secretion induced by glucotoxicity in pancreatic β-cells. J Agric Food Chem. 2014;62:1602–8. doi: 10.1021/jf405365d. [DOI] [PubMed] [Google Scholar]
- 46.Balamurugan R, Vendan SE, Aravinthan A, Kim JH. Isolation and structural characterization of 2R, 3R taxifolin 3-O-rhamnoside from ethyl acetate extract of Hydnocarpus alpina and its hypoglycemic effect by attenuating hepatic key enzymes of glucose metabolism in streptozotocin-induced diabetic rats. Biochimie. 2015;111:70–81. doi: 10.1016/j.biochi.2015.02.003. [DOI] [PubMed] [Google Scholar]
- 47.Olivero-Verbel J, Pacheco-Londoño L. Structure-activity relationships for the anti-HIV activity of flavonoids. J Chem Inf Comput Sci. 2002;42:1241–6. doi: 10.1021/ci020363d. [DOI] [PubMed] [Google Scholar]
- 48.Cole AL, Hossain S, Cole AM, Phanstiel O. Synthesis and bioevaluation of substituted chalcones, coumaranones and other flavonoids as anti-HIV agents. Bioorg Med Chem. 2016;24:2768–76. doi: 10.1016/j.bmc.2016.04.045. [DOI] [PubMed] [Google Scholar]
- 49.Fan W, Qian S, Qian P, Li X. Antiviral activity of luteolin against Japanese encephalitis virus. Virus Res. 2016;220:112–6. doi: 10.1016/j.virusres.2016.04.021. [DOI] [PubMed] [Google Scholar]
- 50.Rauf A, Uddin G, Siddiqui BS, Khan H, Shah SU, Ben Hadda T, et al. Antinociceptive and anti-inflammatory activities of flavonoids isolated from Pistacia integerrima galls. Complement Ther Med. 2016;25:132–8. doi: 10.1016/j.ctim.2016.02.002. [DOI] [PubMed] [Google Scholar]
- 51.Freitas M, Ribeiro D, Tomé SM, Silva AM, Fernandes E. Synthesis of chlorinated flavonoids with anti-inflammatory and pro-apoptotic activities in human neutrophils. Eur J Med Chem. 2014;86:153–64. doi: 10.1016/j.ejmech.2014.08.035. [DOI] [PubMed] [Google Scholar]
- 52.Abdallah HM, Almowallad FM, Esmat A, Shehata IA, Abdel-Sattar EA. Anti-inflammatory activity of flavonoids from Chrozophora tinctoria. Phytochem Lett. 2015;13:74–80. [Google Scholar]
- 53.Honmore VS, Kandhare AD, Kadam PP, Khedkar VM, Sarkar D, Bodhankar SL, et al. Isolates of Alpinia officinarum Hance as COX-2 inhibitors: Evidence from anti-inflammatory, antioxidant and molecular docking studies. Int Immunopharmacol. 2016;33:8–17. doi: 10.1016/j.intimp.2016.01.024. [DOI] [PubMed] [Google Scholar]
- 54.Lee JC, Won SJ, Chao CL, Wu FL, Liu HS, Ling P, et al. Morusin induces apoptosis and suppresses NF-κB activity in human colorectal cancer HT-29 cells. Biochem Biophys Res Commun. 2008;372:236–42. doi: 10.1016/j.bbrc.2008.05.023. [DOI] [PubMed] [Google Scholar]
- 55.Yang DS, Peng WB, Yang YP, Liu KC, Li XL, Xiao WL. Cytotoxic prenylated flavonoids from Macaranga indica. Fitoterapia. 2015;103:187–91. doi: 10.1016/j.fitote.2015.04.002. [DOI] [PubMed] [Google Scholar]
- 56.Zheng ZP, Yan Y, Xia J, Zhang S, Wang M, Chen J, et al. A phenylacetaldehyde-flavonoid adduct, 8-C-(E-phenylethenyl)-norartocarpetin, exhibits intrinsic apoptosis and MAPK pathways-related anticancer potential on HepG2, SMMC-7721 and QGY-7703. Food Chem. 2016;197(Pt B):1085–92. doi: 10.1016/j.foodchem.2015.11.104. [DOI] [PubMed] [Google Scholar]
- 57.Keshari AK, Kumar G, Kushwaha PS, Bhardwaj M, Kumar P, Rawat A, et al. Isolated flavonoids from Ficus racemosa stem bark possess antidiabetic, hypolipidemic and protective effects in albino Wistar rats. J Ethnopharmacol. 2016;181:252–62. doi: 10.1016/j.jep.2016.02.004. [DOI] [PubMed] [Google Scholar]
- 58.Sheliya MA, Rayhana B, Ali A, Pillai KK, Aeri V, Sharma M, et al. Inhibition of α-glucosidase by new prenylated flavonoids from Euphorbia hirta L. herb. J Ethnopharmacol. 2015;176:1–8. doi: 10.1016/j.jep.2015.10.018. [DOI] [PubMed] [Google Scholar]
- 59.Li D, Peng C, Xie X, Mao Y, Li M, Cao Z, et al. Antidiabetic effect of flavonoids from Malus toringoides (Rehd.) Hughes leaves in diabetic mice and rats. J Ethnopharmacol. 2014;153:561–7. doi: 10.1016/j.jep.2014.02.026. [DOI] [PubMed] [Google Scholar]
- 60.Zhang X, Huang H, Zhao X, Lv Q, Sun C, Li X, et al. Effects of flavonoids-rich Chinese bayberry (Myrica rubra Sieb. et Zucc.) pulp extracts on glucose consumption in human HepG2 cells. J Funct Foods. 2015;14:144–53. [Google Scholar]
- 61.Yan F, Zhang J, Zhang L, Zheng X. Mulberry anthocyanin extract regulates glucose metabolism by promotion of glycogen synthesis and reduction of gluconeogenesis in human HepG2 cells. Food Funct. 2016;7:425–33. doi: 10.1039/c5fo00841g. [DOI] [PubMed] [Google Scholar]
- 62.Yang X, Yang J, Xu C, Huang M, Zhou Q, Lv J, et al. Antidiabetic effects of flavonoids from Sophora flavescens EtOAc extract in type 2 diabetic KK-ay mice. J Ethnopharmacol. 2015;171:161–70. doi: 10.1016/j.jep.2015.05.043. [DOI] [PubMed] [Google Scholar]
- 63.Feng LJ, Yu CH, Ying KJ, Hua J, Dai XY. Hypolipidemic and antioxidant effects of total flavonoids of Perilla frutescens leaves in hyperlipidemia rats induced by high-fat diet. Food Res Int. 2011;44:404–9. [Google Scholar]
- 64.Ai G, Liu Q, Hua W, Huang Z, Wang D. Hepatoprotective evaluation of the total flavonoids extracted from flowers of Abelmoschus manihot (L.) Medic: In vitro and in vivo studies. J Ethnopharmacol. 2013;146:794–802. doi: 10.1016/j.jep.2013.02.005. [DOI] [PubMed] [Google Scholar]
- 65.Pal D, Verma P. Flavonoids: A powerful and abundant source of antioxidants. Int J Pharm Pharm Sci. 2013;5:95–8. [Google Scholar]
- 66.Kelly GS. Quercetin. Monograph. Altern Med Rev. 2011;16:172–94. [PubMed] [Google Scholar]
- 67.Anand David AV, Arulmoli R, Parasuraman S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn Rev. 2016;10:84–89. doi: 10.4103/0973-7847.194044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Fang XK, Gao J, Zhu DN. Kaempferol and quercetin isolated from Euonymus alatus improve glucose uptake of 3T3-L1 cells without adipogenesis activity. Life Sci. 2008;82:615–22. doi: 10.1016/j.lfs.2007.12.021. [DOI] [PubMed] [Google Scholar]
- 69.Vessal M, Hemmati M, Vasei M. Antidiabetic effects of quercetin in streptozocin-induced diabetic rats. Comp Biochem Physiol C Toxicol Pharmacol. 2003;135C:357–64. doi: 10.1016/s1532-0456(03)00140-6. [DOI] [PubMed] [Google Scholar]
- 70.Demir EA, Gergerlioglu HS, Oz M. Antidepressant-like effects of quercetin in diabetic rats are independent of hypothalamic-pituitary-adrenal axis. Acta Neuropsychiatr. 2016;28:23–30. doi: 10.1017/neu.2015.45. [DOI] [PubMed] [Google Scholar]
- 71.Mukhopadhyay P, Prajapati AK. Quercetin in anti-diabetic research and strategies for improved quercetin bioavailability using polymer-based carriers – A review. RSC Adv. 2015;5:97547–62. [Google Scholar]
- 72.Da Silva Uebel L, Schmatz DA, Kuntzler SG, Dora CL, Muccillo Baisch AL, Costa JA, et al. Quercetin and curcumin in nanofibers of polycaprolactone and poly (hydroxybutyrate co hydroxyvalerate): Assessment of in vitro antioxidant activity. J Appl Polym Sci. 2016;133:43712. [Google Scholar]
- 73.Massaro M, Riela S, Guernelli S, Parisi F, Lazzara G, Baschieri A, et al. A synergic nanoantioxidant based on covalently modified halloysite-trolox nanotubes with intra-lumen loaded quercetin. J Mater Chem B. 2016;4:2229–41. doi: 10.1039/c6tb00126b. [DOI] [PubMed] [Google Scholar]
- 74.Peng X, Zhang G, Liao Y, Gong D. Inhibitory kinetics and mechanism of kaempferol on α-glucosidase. Food Chem. 2016;190:207–15. doi: 10.1016/j.foodchem.2015.05.088. [DOI] [PubMed] [Google Scholar]
- 75.Zang Y, Zhang L, Igarashi K, Yu C. The anti-obesity and anti-diabetic effects of kaempferol glycosides from unripe soybean leaves in high-fat-diet mice. Food Funct. 2015;6:834–41. doi: 10.1039/c4fo00844h. [DOI] [PubMed] [Google Scholar]
- 76.Li H, Ji HS, Kang JH, Shin DH, Park HY, Choi MS, et al. Soy leaf extract containing kaempferol glycosides and pheophorbides improves glucose homeostasis by enhancing pancreatic beta-cell function and suppressing hepatic lipid accumulation in db/db mice. J Agric Food Chem. 2015;63:7198–210. doi: 10.1021/acs.jafc.5b01639. [DOI] [PubMed] [Google Scholar]
- 77.Ganeshpurkar A, Saluja AK. The pharmacological potential of rutin. Saudi Pharm J. 2017;25:149–164. doi: 10.1016/j.jsps.2016.04.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Hunyadi A, Martins A, Hsieh TJ, Seres A, Zupkó I. Chlorogenic acid and rutin play a major role in the in vivo anti-diabetic activity of Morus alba leaf extract on type II diabetic rats. PLoS One. 2012;7:e50619. doi: 10.1371/journal.pone.0050619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Fernandes AA, Novelli EL, Okoshi K, Okoshi MP, Di Muzio BP, Guimarães JF, et al. Influence of rutin treatment on biochemical alterations in experimental diabetes. Biomed Pharmacother. 2010;64:214–9. doi: 10.1016/j.biopha.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 80.Dhanya R, Arun KB, Syama HP, Nisha P, Sundaresan A, Santhosh Kumar TR, et al. Rutin and quercetin enhance glucose uptake in L6 myotubes under oxidative stress induced by tertiary butyl hydrogen peroxide. Food Chem. 2014;158:546–54. doi: 10.1016/j.foodchem.2014.02.151. [DOI] [PubMed] [Google Scholar]
- 81.Tian R, Yang W, Xue Q, Gao L, Huo J, Ren D, et al. Rutin ameliorates diabetic neuropathy by lowering plasma glucose and decreasing oxidative stress via Nrf2 signaling pathway in rats. Eur J Pharmacol. 2016;771:84–92. doi: 10.1016/j.ejphar.2015.12.021. [DOI] [PubMed] [Google Scholar]
- 82.Shibasaki T, Sunaga Y, Fujimoto K, Kashima Y, Seino S. Interaction of ATP sensor, cAMP sensor, Ca2+sensor, and voltage-dependent Ca2+channel in insulin granule exocytosis. J Biol Chem. 2004;279:7956–61. doi: 10.1074/jbc.M309068200. [DOI] [PubMed] [Google Scholar]
- 83.Berridge MJ, Lipp P, Bootman MD. The versatility and universality of calcium signalling. Nat Rev Mol Cell Biol. 2000;1:11–21. doi: 10.1038/35036035. [DOI] [PubMed] [Google Scholar]
- 84.Lambert JD, Sang S, Yang CS. Biotransformation of green tea polyphenols and the biological activities of those metabolites. Mol Pharm. 2007;4:819–25. doi: 10.1021/mp700075m. [DOI] [PubMed] [Google Scholar]
- 85.Kaneko YK, Takii M, Kojima Y, Yokosawa H, Ishikawa T. Structure-dependent inhibitory effects of green tea catechins on insulin secretion from pancreatic β-cells. Biol Pharm Bull. 2015;38:476–81. doi: 10.1248/bpb.b14-00789. [DOI] [PubMed] [Google Scholar]
- 86.Li C, Allen A, Kwagh J, Doliba NM, Qin W, Najafi H, et al. Green tea polyphenols modulate insulin secretion by inhibiting glutamate dehydrogenase. J Biol Chem. 2006;281:10214–21. doi: 10.1074/jbc.M512792200. [DOI] [PubMed] [Google Scholar]
- 87.Huang CF, Chen YW, Yang CY, Lin HY, Way TD, Chiang W, et al. Extract of lotus leaf (Nelumbo nucifera) and its active constituent catechin with insulin secretagogue activity. J Agric Food Chem. 2011;59:1087–94. doi: 10.1021/jf103382h. [DOI] [PubMed] [Google Scholar]
- 88.Lafka TI, Sinanoglou V, Lazos ES. On the extraction and antioxidant activity of phenolic compounds from winery wastes. Food Chem. 2007;104:1206–14. [Google Scholar]
- 89.Mortensen A, Kulling SE, Schwartz H, Rowland I, Ruefer CE, Rimbach G, et al. Analytical and compositional aspects of isoflavones in food and their biological effects. Mol Nutr Food Res. 2009;53(Suppl 2):S266–309. doi: 10.1002/mnfr.200800478. [DOI] [PubMed] [Google Scholar]
- 90.Dixon RA, Ferreira D. Genistein. Phytochemistry. 2002;60:205–11. doi: 10.1016/s0031-9422(02)00116-4. [DOI] [PubMed] [Google Scholar]
- 91.Behloul N, Wu G. Genistein: A promising therapeutic agent for obesity and diabetes treatment. Eur J Pharmacol. 2013;698:31–8. doi: 10.1016/j.ejphar.2012.11.013. [DOI] [PubMed] [Google Scholar]
- 92.El-Kordya EA, Alshahrani AM. Effect of genistein, a natural soy isoflavone, on pancreatic β-cells of streptozotocin-induced diabetic rats: Histological and immunohistochemical study. JMAU. 2015;3:108–19. doi: 10.1016/j.jmau.2015.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Lee MS, Kim CH, Hoang DM, Kim BY, Sohn CB, Kim MR, et al. Genistein-derivatives from Tetracera scandens stimulate glucose-uptake in L6 myotubes. Biol Pharm Bull. 2009;32:504–8. doi: 10.1248/bpb.32.504. [DOI] [PubMed] [Google Scholar]
- 94.Towler MC, Hardie DG. AMP-activated protein kinase in metabolic control and insulin signaling. Circ Res. 2007;100:328–41. doi: 10.1161/01.RES.0000256090.42690.05. [DOI] [PubMed] [Google Scholar]
- 95.Long YC, Zierath JR. AMP-activated protein kinase signaling in metabolic regulation. J Clin Invest. 2006;116:1776–83. doi: 10.1172/JCI29044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Easom RA. CaM kinase II: A protein kinase with extraordinary talents germane to insulin exocytosis. Diabetes. 1999;48:675–84. doi: 10.2337/diabetes.48.4.675. [DOI] [PubMed] [Google Scholar]
- 97.Hackett ES, Twedt DC, Gustafson DL. Milk thistle and its derivative compounds: A review of opportunities for treatment of liver disease. J Vet Intern Med. 2013;27:10–6. doi: 10.1111/jvim.12002. [DOI] [PubMed] [Google Scholar]
- 98.Abenavoli L, Capasso R, Milic N, Capasso F. Milk thistle in liver diseases: Past, present, future. Phytother Res. 2010;24:1423–32. doi: 10.1002/ptr.3207. [DOI] [PubMed] [Google Scholar]
- 99.Kvasnicka F, Bíba B, Sevcík R, Voldrich M, Krátká J. Analysis of the active components of silymarin. J Chromatogr A. 2003;990:239–45. doi: 10.1016/s0021-9673(02)01971-4. [DOI] [PubMed] [Google Scholar]
- 100.Cheilari A, Sturm S, Intelmann D, Seger C, Stuppner H. Head-to-head comparison of ultra-high-performance liquid chromatography with diode array detection versus quantitative nuclear magnetic resonance for the quantitative analysis of the silymarin complex in silybum marianum fruit extracts. J Agric Food Chem. 2016;64:1618–26. doi: 10.1021/acs.jafc.5b05494. [DOI] [PubMed] [Google Scholar]
- 101.Maghrani M, Zeggwagh NA, Lemhadri A, El Amraoui M, Michel JB, Eddouks M. Study of the hypoglycaemic activity of Fraxinus excelsior and Silybum marianum in an animal model of type 1 diabetes mellitus. J Ethnopharmacol. 2004;91:309–16. doi: 10.1016/j.jep.2004.01.008. [DOI] [PubMed] [Google Scholar]
- 102.Soto C, Mena R, Luna J, Cerbón M, Larrieta E, Vital P, et al. Silymarin induces recovery of pancreatic function after alloxan damage in rats. Life Sci. 2004;75:2167–80. doi: 10.1016/j.lfs.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 103.Kaneto H, Miyatsuka T, Kawamori D, Matsuoka TA. Pleiotropic roles of PDX-1 in the pancreas. Rev Diabet Stud. 2007;4:209–25. doi: 10.1900/RDS.2007.4.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 104.Yang KC, Qi Z, Yanai G, Shirouza Y, Lu DH, Lee HS, et al. Cell coupling regulates Ins1, Pdx-1 and MafA to promote insulin secretion in mouse pancreatic beta cells. Process Biochem. 2011;46:1853–60. [Google Scholar]
- 105.Nakajima-Nagata N, Sugai M, Sakurai T, Miyazaki J, Tabata Y, Shimizu A. Pdx-1 enables insulin secretion by regulating synaptotagmin 1 gene expression. Biochem Biophys Res Commun. 2004;318:631–5. doi: 10.1016/j.bbrc.2004.04.071. [DOI] [PubMed] [Google Scholar]
- 106.Chen K, Jin P, He HH, Xie YH, Xie XY, Mo ZH. Overexpression of Insig-1 protects β cell against glucolipotoxicity via SREBP-1c. J Biomed Sci. 2011;18:57. doi: 10.1186/1423-0127-18-57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Mahapatra DK, Asati V, Bharti SK. Chalcones and their therapeutic targets for the management of diabetes: Structural and pharmacological perspectives. Eur J Med Chem. 2015;92:839–65. doi: 10.1016/j.ejmech.2015.01.051. [DOI] [PubMed] [Google Scholar]
- 108.Singh P, Anand A, Kumar V. Recent developments in biological activities of chalcones: A mini review. Eur J Med Chem. 2014;85:758–77. doi: 10.1016/j.ejmech.2014.08.033. [DOI] [PubMed] [Google Scholar]
- 109.Rozmer Z, Perjési P. Naturally occurring chalcones and their biological activities. Phytochem Rev. 2016;15:87–120. [Google Scholar]
- 110.Hsieh CT, Hsieh TJ, El-Shazly M, Chuang DW, Tsai YH, Yen CT, et al. Synthesis of chalcone derivatives as potential anti-diabetic agents. Bioorg Med Chem Lett. 2012;22:3912–5. doi: 10.1016/j.bmcl.2012.04.108. [DOI] [PubMed] [Google Scholar]
- 111.Damazio RG, Zanatta AP, Cazarolli LH, Mascarello A, Chiaradia LD, Nunes RJ, et al. Nitrochalcones: Potential in vivo insulin secretagogues. Biochimie. 2009;91:1493–8. doi: 10.1016/j.biochi.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 112.Enoki T, Ohnogi H, Nagamine K, Kudo Y, Sugiyama K, Tanabe M, et al. Antidiabetic activities of chalcones isolated from a Japanese Herb, Angelica keiskei. J Agric Food Chem. 2007;55:6013–7. doi: 10.1021/jf070720q. [DOI] [PubMed] [Google Scholar]
- 113.van Raalte DH, Verchere CB. Glucagon-like peptide-1 receptor agonists: Beta-cell protection or exhaustion? Trends Endocrinol Metab. 2016;27:442–5. doi: 10.1016/j.tem.2016.04.009. [DOI] [PubMed] [Google Scholar]
- 114.Hui H, Zhao X, Perfetti R. Structure and function studies of glucagon-like peptide-1 (GLP-1): The designing of a novel pharmacological agent for the treatment of diabetes. Diabetes Metab Res Rev. 2005;21:313–31. doi: 10.1002/dmrr.553. [DOI] [PubMed] [Google Scholar]
- 115.American Diabetes Association. Standards of medical care in diabetes-2012. Diabetes Care. 2012;35(Suppl 1):S11–63. doi: 10.2337/dc12-s011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Dalle S, Burcelin R, Gourdy P. Specific actions of GLP-1 receptor agonists and DPP4 inhibitors for the treatment of pancreatic β-cell impairments in type 2 diabetes. Cell Signal. 2013;25:570–9. doi: 10.1016/j.cellsig.2012.11.009. [DOI] [PubMed] [Google Scholar]
- 117.Wang X, Zhou J, Doyle ME, Egan JM. Glucagon-like peptide-1 causes pancreatic duodenal homeobox-1 protein translocation from the cytoplasm to the nucleus of pancreatic beta-cells by a cyclic adenosine monophosphate/protein kinase A-dependent mechanism. Endocrinology. 2001;142:1820–7. doi: 10.1210/endo.142.5.8128. [DOI] [PubMed] [Google Scholar]
- 118.Thorens B, Guillam MT, Beermann F, Burcelin R, Jaquet M. Transgenic reexpression of GLUT1 or GLUT2 in pancreatic beta cells rescues GLUT2-null mice from early death and restores normal glucose-stimulated insulin secretion. J Biol Chem. 2000;275:23751–8. doi: 10.1074/jbc.M002908200. [DOI] [PubMed] [Google Scholar]
- 119.Nibbs AE, Scheidt KA. Asymmetric methods for the synthesis of flavanones, chromanones, and azaflavanones. Eur J Org Chem. 2012;2012:449–62. doi: 10.1002/ejoc.201101228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Priscilla DH, Jayakumar M, Thirumurugan K. Flavanone naringenin: An effective antihyperglycemic and antihyperlipidemic nutraceutical agent on high fat diet fed streptozotocin induced type 2 diabetic rats. J Funct Foods. 2015;14:363–73. [Google Scholar]
- 121.Priscilla DH, Roy D, Suresh A, Kumar V, Thirumurugan K. Naringenin inhibits α-glucosidase activity: A promising strategy for the regulation of postprandial hyperglycemia in high fat diet fed streptozotocin induced diabetic rats. Chem Biol Interact. 2014;210:77–85. doi: 10.1016/j.cbi.2013.12.014. [DOI] [PubMed] [Google Scholar]
- 122.Zhang B, Chen T, Chen Z, Wang M, Zheng D, Wu J, et al. Synthesis and anti-hyperglycemic activity of hesperidin derivatives. Bioorg Med Chem Lett. 2012;22:7194–7. doi: 10.1016/j.bmcl.2012.09.049. [DOI] [PubMed] [Google Scholar]
- 123.Klein-Schwartz W, Stassinos GL, Isbister GK. Treatment of sulfonylurea and insulin overdose. Br J Clin Pharmacol. 2016;81:496–504. doi: 10.1111/bcp.12822. [DOI] [PMC free article] [PubMed] [Google Scholar]