Abstract
Background:
Oxidative stress and nonenzymatic protein glycation lead to serious diabetic complications that increase the risk of mortality. Rhinacanthus nasutus leaf crude extracts are previously reported for their antidiabetic, antiglycation, and antioxidant potential.
Objective:
The present study was performed to prepare a standardized rhinacanthins-rich extract (RRE) and evaluate its superoxide scavenging and antiglycation effects as compared to its marker compounds, namely, rhinacanthin-C (RC), rhinacanthin-D (RD), and rhinacanthin-N (RN).
Materials and Methods:
RRE was obtained by microwave-assisted green extraction along with a simple step of fractionation using Amberlite® column. RC, RD, and RN were isolated from the RRE using silica gel column chromatography. Superoxide scavenging activity was performed by cyclic voltammetry, and fructose-mediated human serum albumin glycation model was used for antiglycation activity. In silico studies were conducted to identify the structure-activity relationships of rhinacanthins.
Results:
On the basis of kinetic measurements, RRE exhibited the most potent antioxidant activity via ErCi mechanism, with a 50% inhibitory concentration (IC50) value of 8.0 μg/mL, antioxidant capacity of 39439 M−1, and binding constant of 45709 M−1. Antiglycation assay showed that RRE exhibited almost equivalent glycation inhibitory effect to that of RC, with IC50 values of 39.7 and 37.3 μg/mL, respectively, but higher than that of RD (IC50 of 50.4 μg/mL), RN (IC50 of 89.5 μg/mL), as well as the positive control, rutin (IC50 of 41.5 μg/mL).
Conclusions:
The potent superoxide scavenging and albumin glycation inhibitory effect of RRE rationalized its therapeutic application in various chronic diseases, especially in the complications of diabetes.
SUMMARY
Rhinacanthins-rich extract (RRE) exhibited potent superoxide scavenging activity
RRE and rhinacanthin-C showed remarkable and comparable antiglycation effect
Rhinacanthins exhibited antiglycation activity by masking specific residues of albumin.
Abbreviations used: RRE: Rhinacanthins-rich extract; RC: Rhinacanthin-C; RD: Rhinacanthin-D; RN: Rhinacanthin-N; IC50: 50% inhibitory concentration; Kao: Antioxidant activity coefficient; Kb: Binding constant; ErCi: Reversible electron transfer followed by an irreversible chemical reaction; DM: Diabetes mellitus; AGEPs: Advanced glycation end products; NMR: Nuclear magnetic resonance; HPLC: High-performance liquid chromatography; CV: Cyclic voltammetry; DMSO: Dimethyl sulfoxide; Ipa: Anodic peak current; Ipc: Cathodic peak current; HSA: Human serum albumin; MOE: Molecular operating environment; PASSonline: Online prediction of activity spectra for substances.
Keywords: Antiglycation, antioxidant, rhinacanthins-rich extract, Rhinacanthus nasutus
INTRODUCTION
Type-2 diabetes mellitus (DM) is a serious global health concern. The international prevalence of DM increased from 4.7% to 8.5% during the last three decades, and the number of diabetic patients was estimated as 422 million in 2014.[1] Oxidative stress caused by an imbalance of free radicals is implicated in various chronic disease conditions including DM. Chronic DM leads to serious complications including nephropathy, neuropathy, retinopathy, and cardiovascular problems and increases the risk of mortality. The overproduction of free radicals can be attenuated by intake of antioxidants.[2] Diabetic complications are instigated by “macromolecule aging” phenomena involving nonenzymatic glycation reactions such as nucleophilic addition reactions between the amino groups of proteins and the carbonyl groups of reducing sugars in chronic hyperglycemic conditions.[3,4,5] Hyperglycemia and oxidative stress are the major factors in accelerating the formation of early glycation products that subsequently rearrange and dehydrate into more stable compounds known as advanced glycation end products (AGEPs).[3,6] Formation of AGEPs acts as positive feedback for oxidative stress that further damages cells and intensifies diabetic complications.[7]
A number of compounds have been used to inhibit AGEPs formation such as amino guanidine, but toxicity and adverse effects limit the use of these agents.[8] Plant extracts and isolated phytochemicals are recognized as highly valuable sources of novel therapeutic molecules which offer a potential alternative to currently used drugs that may be associated with side effects. Various plant extracts and phytochemicals have been reported to offer potential as antidiabetic drugs, which function via antioxidant and anti-AGEPs mechanisms.[9,10] In particular, Rhinacanthus nasutus (L.) Kurz (family Acanthaceae), a medicinal herb native to Thailand and Southeast Asia, has traditionally been used in the treatment of various disorders including DM.[11] In China and Taiwan, R. nasutus has been consumed as an herbal drink.[12,13] Methanol extracts of R. nasutus leaf have been investigated extensively for antidiabetic activity.[14,15,16,17,18] Ethanol and aqueous extracts of R. nasutus leaves have also been reported to exhibit antioxidant and antiglycation activities.[19,20] Rhinacanthin-C (RC), a major phytochemical of R. nasutus leaf, has recently been shown to elicit antidiabetic, hyperlipidemic, and pancreatic protection effects in diabetic rats.[21] However, the multistage and high-cost purification process of RC hinders drug development. Rhinacanthins-rich extract (RRE) is a semipurified extract obtained from R. nasutus leaf that contains almost 70% w/w rhinacanthins in total, with 60% w/w of RC as the major constituent.[22] In the present study, RRE was obtained using a simple, environment-friendly, “green” extraction method to investigate its superoxide scavenging and AGEPs inhibitory activity. RRE offers significant advantages as an alternative to RC in terms of lower production cost and potentially equivalent or higher bioactivity due to synergism between RRE components.[23,24] In silico studies were conducted to identify the relationships between rhinacanthins structure and antiglycation activity and to predict their antioxidant potential.
MATERIALS AND METHODS
Chemicals
Tetrabutylammonium perchlorate was obtained from TCI, Japan, and dimethyl sulfoxide (DMSO) of high-performance liquid chromatography (HPLC) grade was obtained from Merck, Germany. Human serum albumin (HSA), fraction V was purchased from Advent Bio, USA. Rutin was obtained from Alfa Aesar, Germany. All other chemicals used were of analytical grade.
Plant material source, extraction, and isolation
The fresh leaves of R. nasutus were collected from the Botanical Garden of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Hat Yai Campus, Thailand, and the voucher specimen (No. 0011814) was filed in the herbarium of the Faculty of Pharmaceutical Sciences, Prince of Songkla University, Thailand. Leaves were washed with tap water and dried at 60°C for 24 h in a hot air oven and reduced to powders using a grinder, which were passed through a No. 45 sieve.
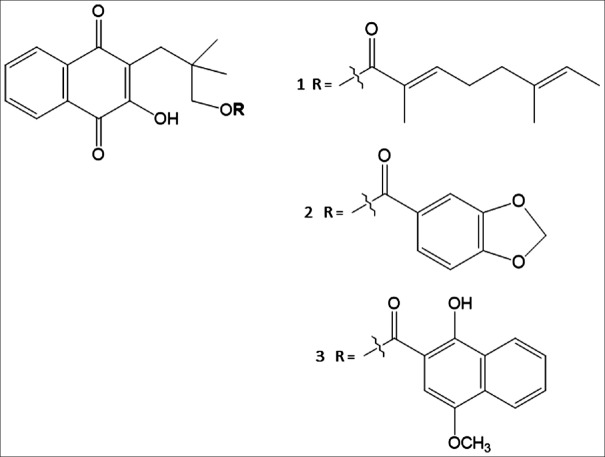
RRE was prepared using ethanol by previously described method,[22] with some modifications using green extraction process. RC, rhinacanthin-D (RD), and rhinacanthin-N (RN) were purified from RRE using a silica gel column eluted by hexane and ethyl acetate (99:1, v/v). The structures of all three compounds [Figure 1] were confirmed by comparing the 1H and 13C-NMR spectral data with those from the literature.[25,26]
Figure 1.
Chemical structures of rhinacanthin-C (1), rhinacanthin-D (2), and rhinacanthin-N (3)
High-performance liquid chromatography analysis of rhinacanthins-rich extract
HPLC analysis of RRE was performed as previously described[22] using an Ultra-Fast Liquid Chromatograph Shimadzu system incorporating a Discovery® C18 (5 μm, 4.6 × 150 mm) column (Supelco, PA, USA) equipped with a photodiode array detector and autosampler (Shimadzu Corp. Kyoto, Japan).
Assay for antioxidant activity using cyclic voltammetry
Cyclic voltammograms were obtained using an Autolab PGSTAT 302 Potentiostat in combination with software GPES 4.9 (Eco Chemie, Utrecht, The Netherland). The electrochemical experiments were carried out in a conventional three electrodes comprising glassy carbon as the working electrode, saturated calomel as the reference electrode, and platinum wire as the counter electrode. The working electrode was polished with alumina before making cyclic voltammetry (CV) measurements. All CV runs were carried out at 25 ± 1°C at 25 mV/s sweep rate.[27]
To assess the antioxidant capacity of the target compounds, CV was performed in 0.1 M tetrabutylammonium perchlorate in DMSO.[27] CV was first carried out at 25 mV/s scan rate without any sample or blank solution (0.1 M tetrabutylammonium perchlorate in DMSO) to produce the superoxide. Aliquots (μL) of samples (4 mg/mL) were then added to DMSO and CV measurements were recorded together with the response of anodic and cathodic peaks. An addition of further aliquots of the sample was terminated when the anodic peak was diminished. The percent superoxide scavenging effect was calculated as follows.
Percentage inhibition = Ip° − Ip × 100/Ip°
Where Ip and Ip° are the anodic peak currents of superoxide with and without sample.
Kinetic parameters were also measured, namely, antioxidant activity coefficient (Kao), binding constant (Kb), and spontaneity of the interaction (−ΔG) of RRE and its marker compounds.[28,29]
Antiglycation assay
Fructose-mediated HSA glycation inhibitory activity of REE, RC, RD, and RN was performed as previously described.[30] Rutin was used as a positive control. The percent protein glycation inhibition of the test compounds and positive control was calculated as follows:
Percentage inhibition = (1 − fluorescence of test sample/fluorescence of the control) × 100
Molecular docking simulations
Molecular docking simulations of RC, RD, and RN were carried out to help rationalize the observed antiglycation activity of the REE and its marker compounds. RC, RD, and RN were docked against HSA, which contains multiple binding sites having preference for specific chemotypes. Molecular docking simulations focused on two sites having significant correlation with drug activity. Site I was identified using the coordinates of warfarin (PDB:2BXD) while Site II was characterized by ibuprofen binding (PDB:2BXG). Both the structures were subjected to protein correction module in MOE2015.10 before docking. Protonate 3D was used to add missing hydrogen atoms. Docking was carried out using the default rigid receptor protocol. The interaction pattern was observed by protein–ligand interaction profiler, a web server for automatic detection of protein–ligand interactions.[31] All the visuals were rendered using Chimera[32] and molecular operating environment.[33]
In silico bioprediction
In silico bioactivity screening of RC, RD, and RN was performed using the prediction of activity spectra for substances (PASSonline) webserver based on their chemical structure.[34]
RESULTS AND DISCUSSION
Determination of rhinacanthins contents in rhinacanthins-rich extract
On the basis of HPLC analysis, RRE used in this study contained RC as a major constituent (62.2% w/w), while RD (7.9% w/w) and RN (3.6% w/w) were present as minor components.
Antioxidant activity of rhinacanthins-rich extract and its marker compounds
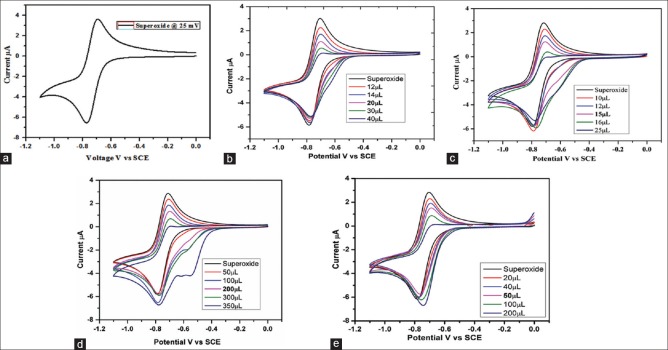
A reversible cyclic voltammogram was obtained with cathodic peak current (Ipc) -4.69 μA and anodic peak current (Ipa) 4.59 μA [Figure 2a]. The forward to reverse peak current ratio of almost unity confirmed the generation of superoxide free radical which is decreased in the presence of scavenging agents, resulting in a reduction of anodic peak current.[27] The scavenging ability of RRE and its marker compounds was assessed by adding increasing volumes of RRE (10–25 μL), RC (12–40 μL), RD (50–350 μL), and RN (20–200 μL) solutions to react with the electrochemically generated superoxide [Figure 2b-2e]. RRE and RC exhibited the highest and almost equivalent superoxide scavenging activity with 50% inhibitory concentration (IC50) values of 8.0 and 9.6 μg/mL, respectively. These results are significantly higher than the minor naphthoquinone esters, RD (IC50 value 91.4 μg/mL) and RN (IC50 value 45.1 μg/mL) [Table 1]. In the present study, the added volume of the different compounds reflected their scavenging impact. RRE was found to be the most powerful scavenger, and interestingly, the data revealed that the scavenging potency of RRE is identical to the combined activity of its specific chemical composition. These in vitro findings support a previous in vivo study on enhanced antioxidative enzymes in the liver and pancreas of diabetic rats by R. nasutus leaf methanol extract and RC.[16,21]
Figure 2.
Cyclic voltammogram of 0.1 M tetrabutylammonium perchlorate in DMSO (a) with different concentration of rhinacanthins-rich extract (b), rhinacanthin-C (c), rhinacanthin-D (d), and rhinacanthin-N (e) at the glassy carbon electrode (25 mV/s scan rate)
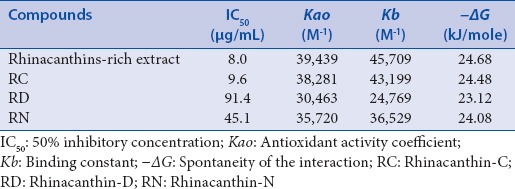
Table 1.
Superoxide scavenging effect and kinetic parameters of rhinacanthins-rich extract, rhinacanthin-C, rhinacanthin-D, and rhinacanthin-N in cyclic voltammetry of 0.1 M tetrabutylammonium perchlorate in dimethyl sulfoxide at glassy carbon electrode, at 25 mV/s scan rate
The relative superoxide scavenging capacity of the test compounds was expressed as the antioxidant activity coefficient (Kao),[29] which is the ratio of current density, in the presence and absence of substrate to the electrochemically generated superoxide free radicals. The relative antioxidant activity of each compound was quantified using the following equation:
Kao = ΔJ/([Jo − Jres] ΔC)
Where ΔJ is the change in oxygen current density in the presence of analyte, Jo is the limiting current density of oxygen in the absence of analyte, Jres is the residual current density of dissolved oxygen, and ΔC is the change in the concentration of the analyte in mol/L. The equation is valid only for the region in which there is a linear change in the value, i.e., at low sample concentration. The antioxidant activity of the compounds was determined using a modified expression where ΔC is replaced by ΔVext. The tabulated data showed that the antioxidant activity of the samples is RRE > RC > RN > RD [Table 1].
The degree of interaction between the superoxide anionic radical and each test compound was expressed in terms of the binding constant “Kb”[28,29] derived from the reduction in peak current and determined using the following equation:
log (1/[AO]) = log Kb + log [Ip/Ip° − Ip]
Where Ip and Ip° are the peak currents of electrochemically generated superoxide anion radical in the presence or absence of the test compound, respectively, AO is the compound concentration which was replaced by the volume of the compound ΔVext. Since the volume of the solution containing O2•− is fixed, volumetric addition of the samples is proportional to their number of moles, i.e., concentration. Compounds resulting in higher Kb values show enhanced interaction with the free radical. As shown in Table 2, it is evident that the Kb values follow the order: RRE > RC > RN > RD. Moreover, the Kb values reveal strong interaction even at very low concentration. The ΔG values calculated using the following equation and displayed in Table 1 indicate the degree of spontaneity of the interaction between the superoxide free radicals and the test compounds and support the scavenging capacity as measured by Kao. Together with the Kb value, a threshold “ΔG” value may provide a useful factor to classify sample scavenging ability.
Table 2.
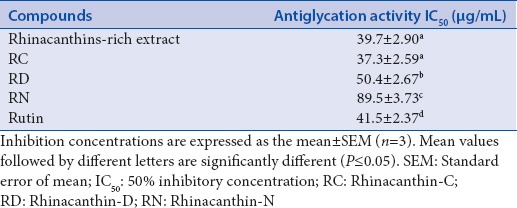
Antiglycation activity of rhinacanthins-rich extract and its marker compounds
ΔG = −RT lnKb
Based on the irreversible scavenging of superoxide [Figure 2b] and the values of kinetic parameters [Table 1], RRE may be classed as a potent superoxide scavenger, operating via ErCi mechanism and probably involving a synergistic effect due to the combination of rhinacanthins.
Antiglycation activity of rhinacanthins-rich extract and its marker compounds
The fructose-mediated HSA glycation inhibitory activity of RRE and its marker compounds was evaluated to explore their potential role in treating diabetic complications. Previous reports rationalized the anti-AGEPs activity of RRE, RC, RD, and RN on the basis of their 1,4-naphthoquinone skeletal structure.[20,35] In the present study, both RRE and RC were found to exhibit significant glycation inhibitory activity with IC50 values of 39.7 and 37.3 μg/mL, respectively, that were slightly higher than that of the positive control, rutin (41.5 μg/mL) [Table 2]. RRE and RC showed almost equivalent antiglycation activity, similar to the previously reported anti-inflammatory and antimicrobial activities.[23,24] Furthermore, the antiglycation activity of RC supports the findings of a study by Adam et al.,[21] in which RC caused a reduction of glycated hemoglobin levels in diabetic rats. RD and RN, the minor naphthoquinone compounds of RRE, also showed impressive antiglycation activity with IC50 values of 50.4 and 89.5 μg/mL, respectively [Table 2]. Diabetes and age-related diseases including neurotoxic disorders are mainly caused by the unusual protein aggregation.[7] Thus, the potent anti-AGEPs activity of RRE measured in this study recommends further evaluation of these compounds as therapeutics for the treatment of a range of conditions of major clinical and global significance.
Molecular interaction studies
Molecular docking studies are commonly performed in the process of drug discovery to predict the occurrence of protein–ligand binding and its possible lead to therapeutic effect. We applied molecular docking protocols to explore binding between HSA, the major transport protein in the circulatory and lymphatic system,[36] and RRE and its marker compounds (RC, RN, and RD) and thereby help explain their observed antiglycation activity. The nonenzymatic glycation of lysine and arginine residues in HSA, in the case of diabetes, impairs the transport of several moieties leading to detrimental physiological effects.[37] Masking of the lysine and arginine residues has therefore been proposed as an effective strategy to inhibit nonenzymatic glycation of HSA. There are two main sites in the HSA structure which offer opportunities for drug action, Sudlow's Site I and Site II. Docking simulations were performed using both sites to investigate ligand binding. Sudlow's Site I was identified using the coordinates of warfarin from the PDB:2BXD while ibuprofen from PDB: 2BXG identified Sudlow's Site II.[38] The docking scores of each compound calculated for the Sudlow's Site I and II of HSA are presented in Table 3.
Table 3.
The docking scores of the top ranked pose of rhinacanthin-C, rhinacanthin-D and rhinacanthin-N with the druggable sites in human serum albumin

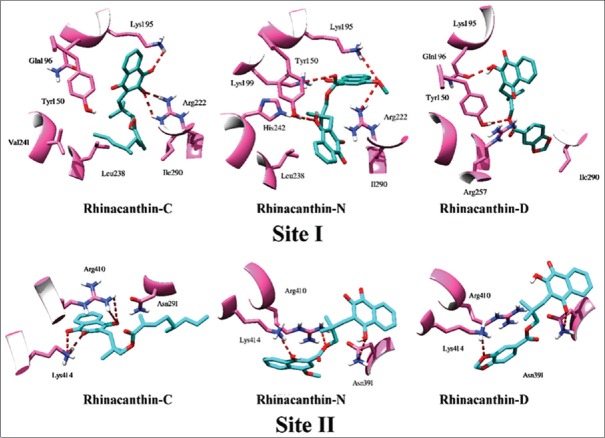
The binding mode of RC, RD, and RN in each site is presented in Figure 3. The compounds establish polar contacts with surrounding arginine and lysine residues. In Sudlow's Site I, RC forms hydrogen bonds with Lys195 and Arg222, RD binds to Lys199 and Arg257, and RN interacts with Lys195, Lys199, Arg218, and Arg222. The rhinacanthins are also involved in the formation of salt bridges. In the case of Sudlow's Site II, RC, RD, and RN were found to interact with Arg410 and Lys 414. The complexes are also stabilized by Van der Waal's forces between the ligands and other amino acid residues at Sudlow's Site I, namely, Tyr150, Leu238, and Leu260. Residues Ile388, Asn391, and Phe403 lining Sudlow's Site II provided anchorage for the ligands via formation of hydrophobic and aromatic contacts. Interestingly, the binding pattern of RC, RD, and RN in this analysis is consistent with docking studies of cinnamic acid reported earlier.[39]
Figure 3.
Visualization of the binding mode of rhinacanthin-C, rhinacanthin-D, and rhinacanthin-N in the Sudlow's sites of human serum albumin
In silico predictions of bioactivity
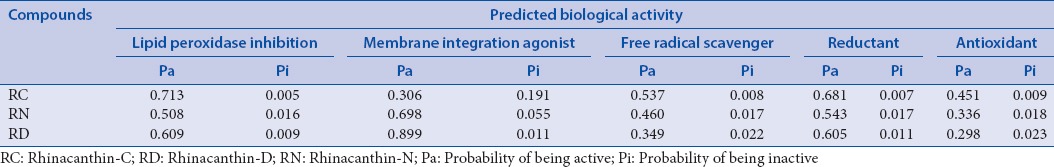
PASSonline is used to predict the potential targets and pharmacological effect of the marker compounds of RRE based on structural information. The analysis presents the ratio of probability of being active (Pa) or inactive (Pi) with regard to a particular biological effect.[40] The antioxidant potential and selection of predicted biological activities for the marker compounds of RRE presented in Table 4 provide additional, strong support for the superoxide scavenging activity of RRE.
Table 4.
The pharmacological activities prediction of rhinacanthin-C, rhinacanthin-D and rhinacanthin-N by Prediction of Activity Spectra for Substances Online
CONCLUSIONS
This is the first report on antioxidant and antiglycation potential of RRE and its marker compounds, RC, RD, and RN. The docking studies determined the binding mode of rhinacanthins with respect to HSA. Rhinacanthins exhibited antiglycation activity by masking different residues of albumin. The potent superoxide scavenging and remarkable protein glycation inhibitory effects of RRE further rationalized its therapeutic application in various chronic diseases, especially in the complications of diabetes.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This research was financially supported by Thailand Education Hub for ASEAN Countries PhD Award (2014) and Thesis Support Grant (2016) from the Graduate School, Prince of Songkla University, Hat-Yai, Songkhla, Thailand. The authors wish to thank Mr. John Constable and Prof. Allan Coombes for assistance with English editing of the manuscript.
REFERENCES
- 1.WHO. Global Report on Diabetes. Geneva: World Health Organisation; 2016. [Cited on 2017 Mar 08]. Available from: http://www.who.int/diabetes/publications/grd-2016/en/ [Google Scholar]
- 2.Asmat U, Abad K, Ismail K. Diabetes mellitus and oxidative stress – A concise review. Saudi Pharm J. 2016;24:547–53. doi: 10.1016/j.jsps.2015.03.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Xi M, Hai C, Tang H, Chen M, Fang K, Liang X. Antioxidant and antiglycation properties of total saponins extracted from traditional Chinese medicine used to treat diabetes mellitus. Phytother Res. 2008;22:228–37. doi: 10.1002/ptr.2297. [DOI] [PubMed] [Google Scholar]
- 4.Singh VP, Bali A, Singh N, Jaggi AS. Advanced glycation end products and diabetic complications. Korean J Physiol Pharmacol. 2014;18:1–14. doi: 10.4196/kjpp.2014.18.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Abdallah HM, El-Bassossy HM, Mohamed GA, El-Halawany AM, Alshali KZ, Banjar ZM. Mangostanaxanthones III and IV: Advanced glycation end-product inhibitors from the pericarp of Garcinia mangostana. J Nat Med. 2017;71:216–26. doi: 10.1007/s11418-016-1051-8. [DOI] [PubMed] [Google Scholar]
- 6.Yamagishi S, Maeda S, Matsui T, Ueda S, Fukami K, Okuda S. Role of advanced glycation end products (AGEs) and oxidative stress in vascular complications in diabetes. Biochim Biophys Acta. 2012;1820:663–71. doi: 10.1016/j.bbagen.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 7.Li J, Liu D, Sun L, Lu Y, Zhang Z. Advanced glycation end products and neurodegenerative diseases: Mechanisms and perspective. J Neurol Sci. 2012;317:1–5. doi: 10.1016/j.jns.2012.02.018. [DOI] [PubMed] [Google Scholar]
- 8.Peng X, Zheng Z, Cheng KW, Shan F, Ren GX, Chen F, et al. Inhibitory effect of mung bean extract and its constituents vitexin and isovitexin on the formation of advanced glycation endproducts. Food Chem. 2008;106:475–81. [Google Scholar]
- 9.Chinchansure AA, Korwar AM, Kulkarni MJ, Joshi SP. Recent development of plant products with anti-glycation activity: A review. RSC Adv. 2015;5:31113–38. [Google Scholar]
- 10.Suantawee T, Cheng H, Adisakwattana S. Protective effect of cyanidin against glucose- and methylglyoxal-induced protein glycation and oxidative DNA damage. Int J Biol Macromol. 2016;93(Pt A):814–21. doi: 10.1016/j.ijbiomac.2016.09.059. [DOI] [PubMed] [Google Scholar]
- 11.Brimson JM, Tencomnao T. Medicinal herbs and antioxidants: Potential of Rhinacanthus nasutus for disease treatment? Phytochem Rev. 2014;13:643–51. [Google Scholar]
- 12.Huang RT, Lu YF, Inbaraj BS, Chen BH. Determination of phenolic acids and flavonoids in Rhinacanthus nasutus (L.) kurz by high-performance-liquid-chromatography with photodiode-array detection and tandem mass spectrometry. J Funct Foods. 2015;12:498–508. [Google Scholar]
- 13.Li DL, Zheng XL, Duan L, Deng SW, Ye W, Wang AH, et al. Ethnobotanical survey of herbal tea plants from the traditional markets in Chaoshan, China. J Ethnopharmacol. 2017;205:195–206. doi: 10.1016/j.jep.2017.02.040. [DOI] [PubMed] [Google Scholar]
- 14.Rao PV, Naidu MD. Antidiabetic effect of Rhinacanthus nasutus leaf extract in streptozotocin induced diabetic rats. Libyan Agr Res Center J Int. 2010;1:310–2. [Google Scholar]
- 15.Rao PV, Madhavi K, Naidu MD. Hypolipidemic properties of Rhinacanthus nasutus in streptozotocin induced diabetic rats. J Pharmacol Toxicol. 2011;6:589–95. [Google Scholar]
- 16.Rao PV, Sujana P, Vijayakanth T, Naidu MD. Rhinacanthus nasutus-its protective role in oxidative stress and antioxidant status in streptozotocin induced diabetic rats. Asian Pac J Trop Dis. 2012;2:327–30. [Google Scholar]
- 17.Rao PV, Madhavi K, Naidu MD, Gan SH. Rhinacanthus nasutus ameliorates cytosolic and mitochondrial enzyme levels in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 2013. 2013:1–6. doi: 10.1155/2013/486047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Rao PV, Madhavi K, Naidu MD, Gan SH. Rhinacanthus nasutus improves the levels of liver carbohydrate, protein, glycogen, and liver markers in streptozotocin-induced diabetic rats. Evid Based Complement Alternat Med 2013. 2013:1–7. doi: 10.1155/2013/102901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Thephinlap C, Pangjit K, Suttajit M, Srichairatanakool S. Anti-oxidant properties and anti-hemolytic activity of Psidium guajava, Pandanous odorus and Rhinacanthus nasutus. J Med Plants Res. 2013;7:2001–9. [Google Scholar]
- 20.Sompong W, Adisakwattana S. Inhibitory effect of herbal medicines and their trapping abilities against methylglyoxal-derived advanced glycation end-products. BMC Complement Altern Med. 2015;15:394. doi: 10.1186/s12906-015-0897-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Adam SH, Giribabu N, Rao PV, Sayem AS, Arya A, Panichayupakaranant P, et al. Rhinacanthin C ameliorates hyperglycaemia, hyperlipidemia and pancreatic destruction in streptozotocin-nicotinamide induced adult male diabetic rats. Eur J Pharmacol. 2016;771:173–90. doi: 10.1016/j.ejphar.2015.12.028. [DOI] [PubMed] [Google Scholar]
- 22.Panichayupakaranant P, Charoonratana T, Sirikatitham A. RP-HPLC analysis of rhinacanthins in Rhinacanthus nasutus: Validation and application for the preparation of rhinacanthin high-yielding extract. J Chromatogr Sci. 2009;47:705–8. doi: 10.1093/chromsci/47.8.705. [DOI] [PubMed] [Google Scholar]
- 23.Bhusal N, Panichayupakaranant P, Reanmongkol W. In vivo analgesic and anti-inflammatory activities of a standardized Rhinacanthus nasutus leaf extract in comparison with its major active constituent rhinacanthin-C. Songklanakarin J Sci Tech. 2014;36:326–31. [Google Scholar]
- 24.Puttarak P, Charoonratana T, Panichayupakaranant P. Antimicrobial activity and stability of rhinacanthins-rich Rhinacanthus nasutus extract. Phytomedicine. 2010;17:323–7. doi: 10.1016/j.phymed.2009.08.014. [DOI] [PubMed] [Google Scholar]
- 25.Sendl A, Chen JL, Jolad SD, Stoddart C, Rozhon E, Kernan M, et al. Two new naphthoquinones with antiviral activity from Rhinacanthus nasutus. J Nat Prod. 1996;59:808–11. doi: 10.1021/np9601871. [DOI] [PubMed] [Google Scholar]
- 26.Wu TS, Hsu HC, Wu PL, Leu YL, Chan YY, Chern CY, et al. Naphthoquinone esters from the root of Rhinacanthus nasutus. Chem Pharm Bull (Tokyo) 1998;46:413–8. doi: 10.1248/cpb.46.413. [DOI] [PubMed] [Google Scholar]
- 27.Muhammad H, Tahiri IA, Muhammad M, Masood Z, Versiani MA, Khaliq O, et al. A comprehensive heterogeneous electron transfer rate constant evaluation of dissolved oxygen in DMSO at glassy carbon electrode measured by different electrochemical methods. J Electroanal Chem. 2016;775:157–62. [Google Scholar]
- 28.Korotkova EI, Avramchik OA, Kagiya TV, Karbainov YA, Tcherdyntseva NV. Study of antioxidant properties of a water-soluble Vitamin E derivative-tocopherol monoglucoside (TMG) by differential pulse voltammetry. Talanta. 2004;63:729–34. doi: 10.1016/j.talanta.2003.12.030. [DOI] [PubMed] [Google Scholar]
- 29.Korotkova EI, Karbainov YA, Shevchuk AV. Study of antioxidant properties by voltammetry. J Electroanal Chem. 2002;518:56–60. [Google Scholar]
- 30.Khan KM, Shah Z, Ahmad VU, Khan M, Taha M, Rahim F, et al. Synthesis of 2,4,6-trichlorophenyl hydrazones and their inhibitory potential against glycation of protein. Med Chem. 2011;7:572–80. doi: 10.2174/157340611797928415. [DOI] [PubMed] [Google Scholar]
- 31.Salentin S, Schreiber S, Haupt VJ, Adasme MF, Schroeder M. PLIP: Fully automated protein-ligand interaction profiler. Nucleic Acids Res. 2015;43:443–7. doi: 10.1093/nar/gkv315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pettersen EF, Goddard TD, Huang CC, Couch GS, Greenblatt DM, Meng EC, et al. UCSF Chimera – A visualization system for exploratory research and analysis. J Comput Chem. 2004;25:1605–12. doi: 10.1002/jcc.20084. [DOI] [PubMed] [Google Scholar]
- 33.Molecular Operating Environment, Chemical Computing Group Inc. 2016. [cited on 2017 Mar 20]. Available from: http://www.chemcomp.com/
- 34.Parasuraman S. Prediction of activity spectra for substances. J Pharmacol Pharmacother. 2011;2:52–3. doi: 10.4103/0976-500X.77119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jung YS, Joe BY, Cho SJ, Konishi Y. 2,3-Dimethoxy-5-methyl-1,4-benzoquinones and 2-methyl-1,4-naphthoquinones: Glycation inhibitors with lipid peroxidation activity. Bioorg Med Chem Lett. 2005;15:1125–9. doi: 10.1016/j.bmcl.2004.12.029. [DOI] [PubMed] [Google Scholar]
- 36.Nicholson JP, Wolmarans MR, Park GR. The role of albumin in critical illness. Br J Anaesth. 2000;85:599–610. doi: 10.1093/bja/85.4.599. [DOI] [PubMed] [Google Scholar]
- 37.Iqbal S, Alam MM, Naseem I. Vitamin D prevents glycation of proteins: An in vitro study. FEBS Lett. 2016;590:2725–36. doi: 10.1002/1873-3468.12278. [DOI] [PubMed] [Google Scholar]
- 38.Ghuman J, Zunszain PA, Petitpas I, Bhattacharya AA, Otagiri M, Curry S. Structural basis of the drug-binding specificity of human serum albumin. J Mol Biol. 2005;353:38–52. doi: 10.1016/j.jmb.2005.07.075. [DOI] [PubMed] [Google Scholar]
- 39.Qais FA, Alam MM, Naseem I, Ahmad I. Understanding the mechanism of non-enzymatic glycation inhibition by cinnamic acid: An in vitro interaction and molecular modelling study. RSC Adv. 2016;6:65322–37. [Google Scholar]
- 40.Kadir FA, Kassim NM, Abdulla MA, Yehye WA. PASS-predicted Vitex negundo activity: Antioxidant and antiproliferative properties on human hepatoma cells-an in vitro study. BMC Complement Altern Med. 2013;13:1–13. doi: 10.1186/1472-6882-13-343. [DOI] [PMC free article] [PubMed] [Google Scholar]