Abstract
Objective:
To investigate the effect of berberine (BBR) on intestinal barrier function in nonalcoholic fat liver disease (NAFLD) in rats.
Materials and Methods:
Rats were divided into three groups: normal diet group (control group [CON group]), high-fat diet feeding group (HFD group), and HFD with BBR group. After 8 weeks of HFD feeding, rats in the BBR group were given BBR intragastrically at a dose of 150 mg/kg daily for 4 weeks. The same volume of normal saline was given to the CON and HFD groups. Liver index was detected, and Sudan black B staining was used to study fatty degeneration, also the expression level of occluding and intestinal flora was analyzed.
Results:
BBR administration significantly reduced HFD-induced increase in body weight (CON group: 379.83 ± 61.51 g, HFD group: 485.24 ± 50.15 g, and BBR group: 428.60 ± 37.37 g). It obviously alleviated the HFD-induced liver fatty degeneration and histopathological changes of intestinal mucosa according to liver index low-density lipoprotein-cholesterol, high-density lipoprotein-cholesterol, and total cholesterol (P < 0.05). The triglyceride, alanine transaminase, and aspartate aminotransferase levels were greatly elevated after BBR treatment (P < 0.05); while endotoxin, intestinal fatty acid-binding protein, and tumor necrosis factor-α were significantly reduced (P < 0.05). Moreover, we found that BBR could obviously elevate the level of occludin and decrease the level of Faecalibacterium prausnitzii and upregulate the level of bacteroides.
Conclusion:
BBR provides significant protection in NAFLD through ameliorating intestinal barrier function.
SUMMARY
Berberine (BBR), an alkaloid that can be isolated from many plants, has been medically used for its wide range of antimicrobial and anti-inflammatory effects
This is a study of BBR on liver function and intestinal barrier function in nonalcoholic fat liver disease (NAFLD)
BBR treatment for NAFLD could significantly restore the liver function and provide significant protection in NAFLD through ameliorating intestinal barrier function.
Abbreviations used: BBR: Berberine, NAFLD: Nonalcoholic fat liver disease, ALT: Alanine transaminase, AST: Aspartate aminotransferase, TG: Triglyceride, I-FABP: Intestinal-fatty acid-binding protein, IBD: Inflammatory bowel disease.
Keywords: Bacteroides, berberine hydrochloride, Faecalibacterium prausnitzii, liver function test
INTRODUCTION
With the growing global waistline, the rate of metabolic syndrome has been greatly increased; along with the increased risk of diabetes, hyperlipidemia, and cardiovascular disorders. Lipid accumulation in the liver exerts a crucial role in the pathogenic mechanism of metabolic syndrome. The nonalcoholic fatty liver disease (NAFLD) is considered a general chronic liver disorder currently, and has gradually become a serious threat to human health. It is characterized by steatohepatitis and hepatomegaly in the defect of alcohol consumption timely. The most common causative factors for developing NAFLD include high-sugar/high-fat diet (HFD), metabolic syndrome, and physical inactivity.[1,2,3] In China, the popularity of NAFLD has been almost doubled in the last 10–15 years.[2] A large number of researches have reported on NAFLD treatment. Various therapies have been developed that aim at body weight modification by diet and lifestyle changes and specific drugs,[4,5,6] or modification of mediators of liver damage such as interleukin 10 (IL-10), IL-6, and tumor necrosis factor-α (TNF-α).
The large surface area of intestinal mucosa is under constant exposure to pathogens and dietary antigens. The intestinal mucosal immune system together with non-specific barriers like the commensal microflora is believed to provide protection for the host. It exerts critical functions in nutrient absorption and waste secretion. The single-cell epithelial layer lining the gut lumen is interconnected by tight junctions, and is able to control the uptake of molecules, thus preventing toxic substances such as endotoxin entering into the human body.
The intestinal microbiota serves as an important part of the intestinal barrier, and its effect in health of human being has drawn a great attention currently. It has been reported to closely correlated with metabolic syndrome and subsequent insulin resistance.[7] The relationship between gut microbiota and obesity has also been widely studied.[8,9] Ley et al.[10] reported that the diversity of microbiota might be influenced by obesity. The amount of Faecalibacterium prausnitzii has been reported to be dramatically elevated in obese children compared to children with normal weight in India. Modulation of gut microbiota might be an innovative therapeutic method for NAFLD treatment through improving intestinal microenvironment.
Berberine (BBR, C20H18NO4) is an alkaloid which can be obtained from many species of plants, such as goldenseal (Hydrastis canadensis), goldenthread (Coptis chinensis), and Oregon grape (Berberis aquifolium). It has been medically used for a long history in China for its wide range of antimicrobial and anti-inflammatory effects.[11] It has been reported recently that BBR treatment could lower body weight, increase insulin sensitivity, also alleviate dyslipidemia.[12,13] However, the influence of BBR on the intestinal barrier has rarely been reported. Therefore, our study aimed to demonstrate the effect of BBR on intestinal barrier function in NAFLD rats.
MATERIALS AND METHODS
The experimental procedures were approved by the Ethic Committee of Affiliated Hospital of Hebei University of Engineering (Protocol Number: 2015023). Six-week-old Sprague-Dawley male rats weighing 180–200 g were housed at room temperature (23°C ± 1°C) with a light and darkness cycle of 12 h for a week. The rats were randomly assigned (8 rats each): (1) control group (CON group): fed with normal diet that composed of carbohydrate 67%, fat 10%, protein 23%; (2) model group with HFD group:[14] fed with basal feeding stuff supplemented with 2% cholesterol and 10% lard; (3) BBR treatment group, which is composed of HFD and BBR. Each group of rats was fed with equal amount of food and provided water ad libitum. After 8 weeks of feeding, BBR (150 mg/kg) was daily administered to the rats of BBR group by intragastric administration for 4 weeks, while an equal amount of normal saline was intragastrically administered to the CON and HFD groups.
Blood sampling
Blood samples were obtained after 12 h of fasting. In brief, the rats were in anesthesia by intraperitoneal injection with pentobarbital sodium (1%, 50 mg/kg), and blood samples were collected from aorta abdominalis. Triglyceride (TG), alanine transaminase (ALT), and aspartate aminotransferase (AST) levels in serum were measured using a Hitachi 7600 analyzer (Japan).
Sampling of liver tissue and small intestine
After blood sampling, the liver was obtained, and the wet weight was measured. Liver index was obtained according to the following formula: liver index = liver wet weight/body weight × 100%. Then, several pieces of liver tissue were taken from the same sites and were instant frozen in liquid nitrogen with temperature at −80°C. Partial liver tissue was used for Sudan black B (SBB) staining and hematoxylin and eosin (H and E) staining.
Several segments of ileum (about 2.0 cm each) were dissected 1.5 cm away from ileocecal junction for immunohistochemistry (IHC) assay and H and E staining.
Collection of fecal samples
Fresh fecal samples were collected immediately after the rats were sacrificed. Feces (2 g) were collected around the ileocecal junction and stored at −80°C immediately for the detection of intestinal flora.
Measurement of endotoxin
Plasma endotoxin was detected using the Chromogenic TAL Endotoxin Assay Kit (Zhanjiang A & C Biological Ltd.; China), according to manufacturer's instructions. It was based on the correlation between the concentration of endotoxin and the color of the solution.
Enzyme-linked immunosorbent assay
Expression of intestinal fatty acid-binding protein (I-FABP) and TNF-α in serum was measured using enzyme-linked immunosorbent assay (ELISA) following the manufacturers’ guidance. The kits used were Rat I-FABP ELISA Kit (Westang Biotechnology, Ltd., Shanghai, China) and Rat TNF-α ELISA Kit (BlueGene Biotech Co., Ltd., Shanghai, China) for plasma I-FABP and serum TNF-α measurement, respectively.
Sudan black B staining
Lipids in liver tissue were detected by SBB staining.[15] Briefly, frozen sections with 8–15 μm in thickness were washed by distilled water and 70% ethanol. The sections were then immersed into SBB dye solution (saturated SBB in 70% ethanol) for 10–35 min, followed by differentiation in 70% ethanol. Counterstain was performed using hematoxylin, and the sections were mounted in glycerin-gelatin. The levels of liver fatty degeneration were categorized into five levels (F0–F4) according to the percentage of fatty liver cells as follows: F0, <5%; F1, 5%–30%; F2, 31%~50%; F3, 51%–75%; and F4 >75%.
Hematoxylin and eosin staining and immunohistochemistry assay
Liver tissues were fixed with formalin (10%), routinely embedded in paraffin. 4–6 μm tissue sections were prepared, with H and E staining[16] or used for IHC assay.[17] The changes of intestinal mucosal structure and intestinal villus were observed using a microscope at ×400 magnification. Mice-anti-rat occludin monoclonal antibody was used to detect the level of occludin in intestinal epithelium. The expression of occludin was analyzed using Image-Pro Plus (Version 6.0, Media Cybernetic, Rockville, USA). The results were expressed as the average optical density of three randomly selected areas in the slides.
Detection of intestinal flora
The total DNA extractions of feces were analyzed using a HiPure Stool DNA Kit (Magen, GuangZhou, China) according to the instructions of manufacturer. Specific primers targeted for F. prausnitzii 16S rDNA were 5’-GGA GGA AGA AGG TCT TCGG-3’ (forward) and 5’-AAT TCC GCC TAC CTC TGC ACT-3’ (reverse); for bacteroides were 5’-CTG AAC CAG CCA AGT AGCG-3’ (forward) and 5’-CCG CAA ACT TTC ACA ACT GAC TTA-3’ (reverse). The levels of F. prausnitzii and bacteroides were determined by real-time polymerase chain reaction (PCR) as previously described. All PCR experiments were conducted in triplicate with a reaction volume (20 μL), including 10 μL of SybrGreen Quantitative PCR Master Mix (Applied Biosystems, Carlsbad, CA, USA), 1 μL of forward and reverse primer each, and 2 μL of fecal DNA template. Amplification program was composed of an initial denaturing step for 3 min at temperature 95°C, followed by 40 cycles of 95°C for 15 s, 57°C for 20 s, and 72°C for 45 s, and then with a final extension step at 72°C for 5 min.
Statistical analysis
Data were presented as mean ± standard deviation. A significant difference was analyzed using one-way analysis of variance and Newman Keuls Multiple Comparison Test using SPSS (version 17.0, IBM, New York, USA), followed by post hoc test if the null hypothesis was rejected.
RESULTS
Effect of berberine on body weight, liver index, and related serum lipid parameters
Rats fed with HFD tended to develop obesity in both HFD and BBR groups. After 4 weeks of BBR treatment, the rats’ weight in the BBR group was dramatically decreased when compared with the HFD group, and the data were slightly higher than that of the CON group with normal diet, but no significant difference was observed [Figure 1]. In 12 weeks, compared with the CON group, rats with HFD had slower response and dull hair, and showed less activity and feeding times. By contrast, the rats in the BBR group had soft and shiny hair and were more sensitive to the reaction [Table 1]. Our results suggest that BBR treatment could significantly reduce body weight of rats feeding with HFD. As shown in Table 1, liver index was significantly increased in HFD group (3.67 ± 0.22) when compared with CON group (2.56 ± 0.18). BBR treatment significantly decreased HFD-induced increase in liver index (2.91 ± 0.27) compared with HFD group after 12 weeks. The serum levels of TC, TG, and low-density lipoprotein-cholesterol in HFD group were higher than those of CON group. However, BBR was also shown to alleviate the lipid levels [Table 2].
Figure 1.
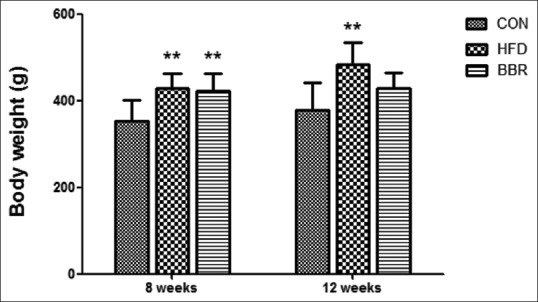
Effect of berberine on body weights at 8 and 12 weeks
Table 1.
Comparison of body weight (g) and liver index (%) of each group (n=8) in 12 weeks

Table 2.
Comparison of serum lipid parameters of each group after 12 weeks’ feeding
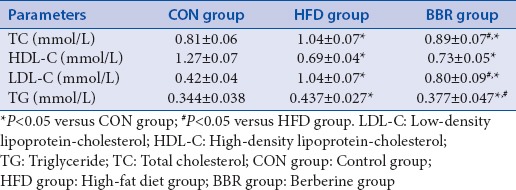
Effect of berberine on serum levels of alanine transaminase and aspartate aminotransferase
Serum levels of ALT and AST were also measured using a Hitachi 7600 analyzer. Our results showed that the serum levels of ALT (115.58 ± 22.54) and AST(203.35 ± 28.23) were all significantly elevated in HFD group after 12 weeks of HFD, when compared with CON group (ALT: 35.96 ± 12.69 and AST: 125.60 ± 23.76) [Table 3]. Compared with HFD group, BBR treatment for 4 weeks obviously reduced the serum levels of ALT (80.94 ± 21.16) and AST (155.79 ± 35.95).
Table 3.
Comparison of alanine transaminase and aspartate aminotransferase parameters of each group after 12 weeks’ feeding (mean±standard deviation, n=8)

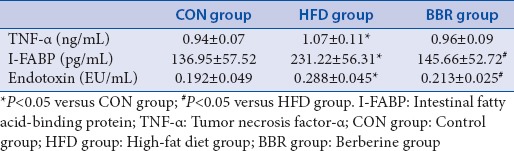
Effect of berberine on endotoxin, tumor necrosis factor-α, and intestinal fatty acid-binding protein
As shown in Table 4, the levels of endotoxin (0.288 ± 0.045 ng/mL) and I-FABP (231.22 ± 56.31 pg/mL) in rat plasma in HFD group were significantly superior to those in CON group (endotoxin: 0.192 ± 0.049 ng/mL; I-FABP: 0.192 ± 0.049 pg/mL). Moreover, the levels of plasma endotoxin and I-FABP were significantly decreased with the intragastric administration of BBR. The level of TNF-α was greatly increased in HFD group when compared with CON group. The data were decreased to some extent in the BBR group when compared with the group of HFD, though the difference was not statistically significant [Table 4].
Table 4.
Comparison of levels of tumor necrosis factor-α, intestinal fatty acid-binding protein, and endotoxin of each group after 12 weeks’ feeding (mean±standard deviation, n=8)
Histological observations
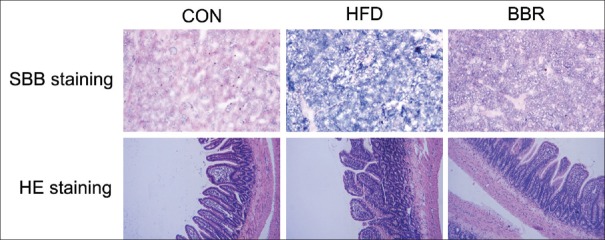
Lipids in liver tissue detected by SBB staining were shown in Figure 2. Lipid droplets were not found in CON group with normal diet. Fatty degeneration was found in both HFD and BBR groups, and was less severe in BBR group. No rat in any group developed hepatic fibrosis [Table 5]. The H and E staining of intestinal mucosa showed that BBR treatment obviously alleviated the HFD-induced histopathological changes of intestinal mucosa [Figure 2].
Figure 2.
Berberine alleviated high-fat diet-induced histological changes of intestinal mucosa as analyzed by Sudan black B and hematoxylin and eosin staining. The changes of intestinal mucosal structure and intestinal villus were observed under microscope (×400). Control represents the control group with normal diet, high-fat diet represents the model group with high-fat feeding, and berberine represents model group fed with berberine
Table 5.
Hepatocyte steatosis distribution and extent in the three groups
Immunohistochemistry assay
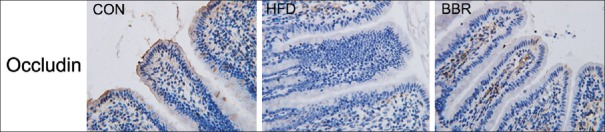
The level of occludin was detected by IHC assay. As shown in Figure 3, the occludin level of intestinal epithelial cells in HFD group was markedly decreased compared with the CON group. After BBR treatment, the level of occludin in BBR group was dramatically elevated compared to the HFD group.
Figure 3.
Berberine treatment increased the level of occludin. The occludin level was detected by immunohistochemistry assay. The results were observed under microscope (×400). Control represents the control group with normal diet, high-fat diet represents the model group with high-fat feeding, and berberine represents model group fed with berberine
Change of gut microbiota
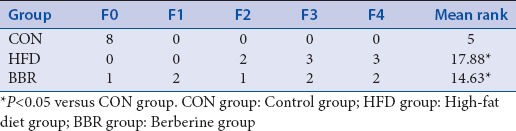
The relative levels of F. prausnitzii in HFD group were markedly elevated when compared with CON group [Table 6]. Administration of BBR could significantly decrease HFD-induced increase in the relative levels of F. prausnitzii. In contrast, the relative levels of bacteroides in HFD group were greatly lower than those in CON group. After intragastric administration of BBR, the level of bacteroides was significantly elevated compared to HFD group. No significant differences were detected between BBR and CON groups for both F. prausnitzii and bacteroides, suggesting that BBR treatment could effectively alleviate the pathological alterations of gut microbial ecology that is caused by HFD/obesity.
Table 6.
Comparison of relative levels of intestinal Faecalibacterium prausnitzii and Bacteroides in each group

DISCUSSION
HFD was used in this study to construct the rat model of NAFLD, which has similar pathology of human NAFLD. The advantage of this model is that it presents the characteristics of metabolic syndrome such as obesity and hyperlipidemia. It has been reported that animals with long-term HFD gradually develop insulin resistance and mild nonalcoholic steatohepatitis, but do not develop hepatic fibrosis and liver cancer.[18] So far, the most common recipes for HFD are composed of lard oil (10%–15%) and cholesterol (1%–2%). However, the disadvantage of this model is that it only develops slight degree of hepatic inflammation and fibrosis. In our study, saturated fatty acid and palmitic acid were used as energy source. After 8 weeks of HFD, liver index and serum TG level were significantly elevated; while the levels of aminotransferases did not exhibit a significant difference. The results of SBB showed the accumulation of lipid and fatty degeneration of liver cells. The results were similar to previous studies.[18]
BBR has been demonstrated to have a variety of bioactivities and pharmacological actions in the potential treatment of NAFLD. The study of Kim revealed that intraperitoneal injection of BBR (3 weeks) could alleviate fatty liver and hyperlipidemia.[19] BBR could reduce fat storage in livers of hyperlipidemic hamsters,[20] ameliorate liver steatosis, as well as reduce the lipid content of liver in mice with HFD.[21] In the present study, it was revealed that body weight, liver index, and the level of TG had been significantly reduced. The lipid accumulation and fatty degeneration of liver cells had also been significantly alleviated after administration of BBR. It has been reported that BBR supplement may downregulate the levels of ALT and AST in type 2 diabetes mellitus patients.[22,23] Our results also revealed that AST and ALT levels had been significantly reduced by BBR treatment, indicating the restoration of liver function.
Physical diffusion barriers play an important role in intestinal barrier, which consists of successive epithelial cells and tight junctions. The physical diffusion barriers serve as restriction in permeability of molecules. It has been reported that HFD could cause overgrowth of Gram-negative bacteria in intestine.[10] The change in intestinal microbiota could increase the intestinal permeability and cause intestinal inflammation.[24] Occludin is one of the key structural proteins for tight junctions. In our results, the abnormal expression pattern of occludin in HFD-fed rats suggested that the intestinal barrier dysfunction may exist in NAFLD patients with increased intestinal permeability. Our results also indicated that the decreased level of occludin could be partially rescued by BBR treatment. I-FABP is a sensitive diagnostic marker for identifying intestinal ischemia.[25] Normally, serum I-FABP cannot be detected in healthy people. However, when the intestinal barrier is damaged by intestinal ischemia, intestinal permeability increased leading to the rise of serum I-FABP.[26] In our study, the serum levels of endotoxin and I-FABP have been elevated with HFD; while BBR treatment could significantly decrease the levels of endotoxin and I-FABP. Our results suggest that BBR could effectively alleviate the lipid metabolism disorders and decrease the intestinal permeability that were abnormally increased by HFD.
The anti-inflammatory effect of BBR has been widely reported. BBR could increase insulin sensitivity through alleviation of inflammation.[27] Jeong et al. reported that BBR could markedly downregulate the levels of pro-inflammatory cytokines in white adipose tissue, such as IL-1, TNF-α, and IL-6.[28] Furthermore, Zhang et al. indicated that BBR could prevent injuries of LPS-induced intestine, as well as decrease the levels of inflammatory cytokines.[29] Our study revealed that the level of TNF-α has been greatly elevated in rats with HFD; while this effect was significantly inhibited by BBR.
In the recent years, the effect of intestinal microbiota in the prevention of metabolic disorders has drawn increasing attention. Due to the poor absorption of BBR in the bloodstream from the gut, the possible mechanism of its antidiabetic and anti-hyperlipidemia effect may be related to the modulation of intestinal microbiota.[30,31] BBR treatment caused a significant decrease in the number of Firmicutes in the mice fed with HFD, as well as Bacteroidetes, as reported by Xie et al.[32] Cao et al. found that the level of F. prausnitzii in inflammatory bowel disease was significantly decreased compared with normal healthy controls.[33] In our study, we found that, in HFD-fed rats, the level of F. prausnitzii and bacteroides was dramatically elevated. BBR treatment could obviously decrease the level of F. prausnitzii while upregulating the level of bacteroides. Our results suggest that BBR may improve the intestinal microenvironment through modulation of gut microflora and therefore preserve intestinal barrier functions. However, only two representative species of intestinal microbiota were selected in this study. Further investigations are necessary for clarifying the effect of BBR on general gut microbiota.
CONCLUSION
BBR treatment could significantly restore the liver function, reduce body weight, and alleviate the histopathological changes of intestinal mucosa in mouse models of NAFLD. The protective effects of BBR might be exerted by improving intestinal barrier through elevating the level of occludin and modulating gut microflora.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Bellentani S, Scaglioni F, Marino M, Bedogni G. Epidemiology of non-alcoholic fatty liver disease. Dig Dis. 2010;28:155–61. doi: 10.1159/000282080. [DOI] [PubMed] [Google Scholar]
- 2.Fan JG, Saibara T, Chitturi S, Kim BI, Sung JJ, Chutaputti A, et al. What are the risk factors and settings for non-alcoholic fatty liver disease in Asia-Pacific? J Gastroenterol Hepatol. 2007;22:794–800. doi: 10.1111/j.1440-1746.2007.04952.x. [DOI] [PubMed] [Google Scholar]
- 3.Torres DM, Harrison SA. Diagnosis and therapy of nonalcoholic steatohepatitis. Gastroenterology. 2008;134:1682–98. doi: 10.1053/j.gastro.2008.02.077. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JB, Bhathal PS, Hughes NR, O’Brien PE. Nonalcoholic fatty liver disease: Improvement in liver histological analysis with weight loss. Hepatology. 2004;39:1647–54. doi: 10.1002/hep.20251. [DOI] [PubMed] [Google Scholar]
- 5.Gueugnon C, Mougin F, Simon-Rigaud ML, Regnard J, Nègre V, Dumoulin G, et al. Effects of an in-patient treatment program based on regular exercise and a balanced diet on high molecular weight adiponectin, resistin levels, and insulin resistance in adolescents with severe obesity. Appl Physiol Nutr Metab. 2012;37:672–9. doi: 10.1139/h2012-045. [DOI] [PubMed] [Google Scholar]
- 6.Harrison SA, Fincke C, Helinski D, Torgerson S, Hayashi P. A pilot study of orlistat treatment in obese, non-alcoholic steatohepatitis patients. Aliment Pharmacol Ther. 2004;20:623–8. doi: 10.1111/j.1365-2036.2004.02153.x. [DOI] [PubMed] [Google Scholar]
- 7.Wu JC. Psychological co-morbidity in functional gastrointestinal disorders: Epidemiology, mechanisms and management. J Neurogastroenterol Motil. 2012;18:13–8. doi: 10.5056/jnm.2012.18.1.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Rajilić-Stojanović M, Biagi E, Heilig HG, Kajander K, Kekkonen RA, Tims S, et al. Global and deep molecular analysis of microbiota signatures in fecal samples from patients with irritable bowel syndrome. Gastroenterology. 2011;141:1792–801. doi: 10.1053/j.gastro.2011.07.043. [DOI] [PubMed] [Google Scholar]
- 9.McKernan DP, Gaszner G, Quigley EM, Cryan JF, Dinan TG. Altered peripheral toll-like receptor responses in the irritable bowel syndrome. Aliment Pharmacol Ther. 2011;33:1045–52. doi: 10.1111/j.1365-2036.2011.04624.x. [DOI] [PubMed] [Google Scholar]
- 10.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 11.Yu HH, Kim KJ, Cha JD, Kim HK, Lee YE, Choi NY, et al. Antimicrobial activity of berberine alone and in combination with ampicillin or oxacillin against methicillin-resistant staphylococcus aureus. J Med Food. 2005;8:454–61. doi: 10.1089/jmf.2005.8.454. [DOI] [PubMed] [Google Scholar]
- 12.Lee YS, Kim WS, Kim KH, Yoon MJ, Cho HJ, Shen Y, et al. Berberine, a natural plant product, activates AMP-activated protein kinase with beneficial metabolic effects in diabetic and insulin-resistant states. Diabetes. 2006;55:2256–64. doi: 10.2337/db06-0006. [DOI] [PubMed] [Google Scholar]
- 13.Kong W, Wei J, Abidi P, Lin M, Inaba S, Li C, et al. Berberine is a novel cholesterol-lowering drug working through a unique mechanism distinct from statins. Nat Med. 2004;10:1344–51. doi: 10.1038/nm1135. [DOI] [PubMed] [Google Scholar]
- 14.Xu P, Zhang XG, Li YM, Yu CH, Xu L, Xu GY, et al. Research on the protection effect of pioglitazone for non-alcoholic fatty liver disease (NAFLD) in rats. J Zhejiang Univ Sci B. 2006;7:627–33. doi: 10.1631/jzus.2006.B0627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Subramaniam HN, Chaubal KA. Evaluation of intracellular lipids by standardized staining with a Sudan black B fraction. J Biochem Biophys Methods. 1990;21:9–16. doi: 10.1016/0165-022x(90)90040-j. [DOI] [PubMed] [Google Scholar]
- 16.Yan L, Wu CR, Wang C, Yang CH, Tong GZ, Tang JG, et al. Effect of Candida albicans on intestinal ischemia-reperfusion injury in rats. Chin Med J (Engl) 2016;129:1711–8. doi: 10.4103/0366-6999.185862. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Ying C, Chunmin Y, Qingsen L, Mingzhou G, Yunsheng Y, Gaoping M, et al. Effects of simulated weightlessness on tight junction protein occludin and zonula occluden-1 expression levels in the intestinal mucosa of rats. J Huazhong Univ Sci Technolog Med Sci. 2011;31:26–32. doi: 10.1007/s11596-011-0145-5. [DOI] [PubMed] [Google Scholar]
- 18.Mow WS, Vasiliauskas EA, Lin YC, Fleshner PR, Papadakis KA, Taylor KD, et al. Association of antibody responses to microbial antigens and complications of small bowel Crohn's disease. Gastroenterology. 2004;126:414–24. doi: 10.1053/j.gastro.2003.11.015. [DOI] [PubMed] [Google Scholar]
- 19.Kim WS, Lee YS, Cha SH, Jeong HW, Choe SS, Lee MR, et al. Berberine improves lipid dysregulation in obesity by controlling central and peripheral AMPK activity. Am J Physiol Endocrinol Metab. 2009;296:E812–9. doi: 10.1152/ajpendo.90710.2008. [DOI] [PubMed] [Google Scholar]
- 20.Brusq JM, Ancellin N, Grondin P, Guillard R, Martin S, Saintillan Y, et al. Inhibition of lipid synthesis through activation of AMP kinase: An additional mechanism for the hypolipidemic effects of berberine. J Lipid Res. 2006;47:1281–8. doi: 10.1194/jlr.M600020-JLR200. [DOI] [PubMed] [Google Scholar]
- 21.Chang X, Yan H, Fei J, Jiang M, Zhu H, Lu D, et al. Berberine reduces methylation of the MTTP promoter and alleviates fatty liver induced by a high-fat diet in rats. J Lipid Res. 2010;51:2504–15. doi: 10.1194/jlr.M001958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Di Pierro F, Villanova N, Agostini F, Marzocchi R, Soverini V, Marchesini G, et al. Pilot study on the additive effects of berberine and oral type 2 diabetes agents for patients with suboptimal glycemic control. Diabetes Metab Syndr Obes. 2012;5:213–7. doi: 10.2147/DMSO.S33718. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang H, Wei J, Xue R, Wu JD, Zhao W, Wang ZZ, et al. Berberine lowers blood glucose in type 2 diabetes mellitus patients through increasing insulin receptor expression. Metabolism. 2010;59:285–92. doi: 10.1016/j.metabol.2009.07.029. [DOI] [PubMed] [Google Scholar]
- 24.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 25.Sonnino R, Ereso G, Arcuni J, Franson R. Human intestinal fatty acid binding protein in peritoneal fluid is a marker of intestinal ischemia. Transplant Proc. 2000;32:1280. doi: 10.1016/s0041-1345(00)01225-2. [DOI] [PubMed] [Google Scholar]
- 26.Shi H, Wu B, Wan J, Liu W, Su B. The role of serum intestinal fatty acid binding protein levels and D-lactate levels in the diagnosis of acute intestinal ischemia. Clin Res Hepatol Gastroenterol. 2015;39:373–8. doi: 10.1016/j.clinre.2014.12.005. [DOI] [PubMed] [Google Scholar]
- 27.Hotamisligil GS. Inflammation and metabolic disorders. Nature. 2006;444:860–7. doi: 10.1038/nature05485. [DOI] [PubMed] [Google Scholar]
- 28.Jeong HW, Hsu KC, Lee JW, Ham M, Huh JY, Shin HJ, et al. Berberine suppresses proinflammatory responses through AMPK activation in macrophages. Am J Physiol Endocrinol Metab. 2009;296:E955–64. doi: 10.1152/ajpendo.90599.2008. [DOI] [PubMed] [Google Scholar]
- 29.Zhang Q, Piao XL, Piao XS, Lu T, Wang D, Kim SW, et al. Preventive effect of Coptis chinensis and berberine on intestinal injury in rats challenged with lipopolysaccharides. Food Chem Toxicol. 2011;49:61–9. doi: 10.1016/j.fct.2010.09.032. [DOI] [PubMed] [Google Scholar]
- 30.Han J, Lin H, Huang W. Modulating gut microbiota as an anti-diabetic mechanism of berberine. Med Sci Monit. 2011;17:RA164–7. doi: 10.12659/MSM.881842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Zhang X, Zhao Y, Zhang M, Pang X, Xu J, Kang C, et al. Structural changes of gut microbiota during berberine-mediated prevention of obesity and insulin resistance in high-fat diet-fed rats. PLoS One. 2012;7:e42529. doi: 10.1371/journal.pone.0042529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Xie W, Gu D, Li J, Cui K, Zhang Y. Effects and action mechanisms of berberine and Rhizoma Coptidis on gut microbes and obesity in high-fat diet-fed C57BL/6J mice. PLoS One. 2011;6:e24520. doi: 10.1371/journal.pone.0024520. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Cao Y, Shen J, Ran ZH. Association between Faecalibacterium prausnitzii reduction and inflammatory bowel disease: A meta-analysis and systematic review of the literature. Gastroenterol Res Pract 2014. 2014:872725. doi: 10.1155/2014/872725. [DOI] [PMC free article] [PubMed] [Google Scholar]