Abstract
Background:
Caowu (Radix Aconiti kusnezoffii, CW), the root of Aconitum kusnezoffii Reichb., has widely used clinically in rheumatic arthritis, painful joints, and tumors for thousands of years. However, the toxicity of heart and central nervous system induced by CW still limited the application.
Materials and Methods:
Metabolomics was performed to identify the sensitive and reliable biomarkers and to characterize the phenotypically biochemical perturbations and potential mechanisms of CW-induced toxicity, and the detoxification by combinatorial intervention of CW with Gancao (Radix Glycyrrhizae) (CG), Baishao (Radix Paeoniae Alba) (CB), and Renshen (Radix Ginseng) (CR) was also analyzed by pattern recognition methods.
Results:
As a result, the metabolites were characterized and responsible for pentose and glucuronate interconversions, tryptophan metabolism, amino sugar and nucleotide sugar metabolism, taurine and hypotaurine metabolism, fructose and mannose metabolism, and starch and sucrose metabolism, six networks of which were the same to the metabolic pathways of Chuanwu (Radix Aconiti, CHW) group. The ascorbate and aldarate metabolism was also characterized by CW group. The urinary metabolomics also revealed CW-induced serious toxicity to heart and liver. Thirteen significant metabolites were identified and had validated as phenotypic toxicity biomarkers of CW, five biomarkers of which were commonly owned in Aconitum. The changes of toxicity metabolites obtained from combinatorial intervention of CG, CB, and CR also were analyzed to investigate the regulation degree of toxicity biomarkers adjusted by different combinatorial interventions at 6th month.
Conclusion:
Metabolomics analyses coupled with pattern recognition methods in the evaluation of drug toxicity and finding detoxification methods were highlighted in this work.
SUMMARY
Metabolomics was performed to characterize the biochemical potential mechanisms of Caowu toxicity
Thirteen significant metabolites were identified and validated as phenotypic toxicity biomarkers of Caowu
-
Metabolite changes of toxicity obtained can be adjusted by different combinatorial interventions.
Pattern recognition plot reflects the toxicity effects tendency of the urine metabolic fluctuations according to time after treatment of herbal Caowu.
Abbreviations used: CW: Caowu (Radix Aconiti kusnezoffii); CHW: Chuanwu (Radix Aconiti); TCM: Traditional Chinese Medicine; CG: Caowu and Gancao; CB: Caowu and Baishao; CR: Caowu and Renshen; QC: Quality control; UPLC: Ultra performance liquid chromatography; MS: Mass spectrometry; PCA: Principal component analysis; PLS-DA: Partial least squares-discriminant analysis; OPLS: Orthogonal projection to latent structures analysis.
Keywords: Aconitum, biomarkers, detoxification, mass spectrometry, metabolomics, toxicity, traditional Chinese Medicine
INTRODUCTION
Aconitum herbs have a long history of clinical use, including Chuanwu (Radix Aconiti, CHW), Caowu (Radix Aconiti kusnezoffii, CW), and Fuzi (Radix Aconiti Lateralis Preparata, FZ). Traditional Chinese Medicine (TCM) Aconitum herbs have the potency of high toxicity, and modern research has shown that Aconitum alkaloids are not the active ingredients but the toxic components. The main effective and toxic components of CW are diester diterpenoid alkaloids, including aconitine, mesaconitine, hypaconitine, neoline, talatizamine, beiwutine, and deoxyaconitine.[1] Therefore, the identification, determination, and toxicity evaluation of Aconitum alkaloids were studied by many scholars. The lacks of sensitive and reliable biomarkers of its toxicity are always the main obstacles.[2] CW comes from the root of A. kusnezoffii Reichb., which has the effects of relieving the pain, diuretic and promoting metabolism. It is commonly used in the clinical treatment of various diseases, such as collapse, syncope, rheumatic arthritis, painful joints, tumors, and sciatica.[3,4] The problem of toxicity is still a constraint of effective application of CW and sometimes even life-threatening.[5] The heart and the central nervous system are the primary toxicity targets. Cardiac toxicity is the most important manifestation of CW, mainly due to clinical poisoning death. There are many cases about cardiac toxicity caused by swallowing the decoction of CW were reported, which usually presents various types of arrhythmia by interfering with sodium channels.[6,7,8] Toxicity from chronic or cumulative dosing is difficult to detect in routine clinical examination. Therefore, a clear understanding of the toxicity mechanism of CW and the influences on the metabolome are essential for safety clinically.
At present, the processing methods for detoxication of CW had many applications but more directed to reduce the toxicity of the main ingredients to achieve the purpose of reducing toxicity, which often resulted in not only reducing efficiency but also decreasing toxicity. Combinatorial intervention is the main form of TCM medication, which can play a role of detoxification or increasing efficiency through proper compatibility.[9,10,11] Gancao (Radix Glycyrrhizae) is the root of Glycyrrhiza uralensis Fisch., Baishao (Radix Paeoniae Alba) comes from the root of Paeonia lactiflora Pall., and Renshen (Radix Ginseng) is from the root of Panax ginseng C. A. Mey. Combinatorial intervention of Caowu and Gancao (CG), combinatorial intervention of Caowu and Baishao (CB), and combinatorial intervention of Caowu and Renshen (CR) were the most extensive used combinatorial intervention drugs in clinical. After compatibility, the content of ester alkaloids showed descended tendency which decreases the toxicity of Aconitum plants. However, it only limited the evaluation of the major toxic ingredients before and after combinatorial intervention and less studies of detoxification mechanism, which need to further research. In this study, combinatorial intervention of CG (Caowu: Gancao is 1:2), CB (Caowu: Baishao is 1:2) and CR (Caowu: Renshen is 1:2) were chosen to analyze the detoxification mechanism against CW.
The important point of metabolomics is that the approach has the potential power to reveal novel biochemical results of toxicant administration which can lead to mechanistic insights and identification of biomarkers of cause and/or result.[12,13,14] Urine is an ideal biomedium for disease studies because it is obtained easily, available readily, and fewer complexes than other body fluids. Ease of urine collection allows for serial sampling to monitor disease, toxicity, and therapeutic response.[15] Therefore, we determined the significant toxicity point in time of rats after administration of CW and used metabolomics to study changes in metabolic profiling of urine from CW administered orally to rats during 0–6 months. Through metabolic markers associated with toxicity in vivo and dynamic changes of metabolic pathways, the mechanism of CW toxicity was explored from the level of endogenous small molecule metabolites. After combinatorial intervention of CB, CG and CR were administrated to rats, starting from expression levels changes of these locked toxic markers in the metabolic process, to discuss the therapeutic effect and significance of compatibility detoxification of CB, CG, and CR. We try to establish common technology of toxic substances found from toxic drugs and establishment of detoxification methods and improve the effectiveness and safety of clinical medication.
MATERIALS AND METHODS
Chemicals and materials
Acetonitrile (high performance liquid chromatography [HPLC] grade) was purchased from Merck (Darmstadt, Germany). Distilled water was obtained from Watson's Food and Beverage Co., Ltd., (Guangzhou, China). Formic acid (HPLC grade) was supplied by the Beijing Reagent Company (Beijing, China). Leucine enkephalin was purchased from Sigma-Aldrich (MO, UK), and pentobarbital sodium was obtained from the Shanghai Chemical Agent Company of China Medicine Clique (Shanghai, China). Other chemicals were of analytical grade. Caowu was collected from Shengli village in Mianyang city of Sichuan Province (South, China). Baishao, Gancao, and Renshen were purchased from Harbin Tongrentang Drug Store (Harbin, China). All the crude drugs were authenticated by Prof. Xijun Wang, Department of Pharmacognosy of Heilongjiang University of Chinese Medicine (Harbin, China).
Animal handling and sample preparation
Male Wistar rats (clean grade, weighing 200 ± 20 g) were purchased from the Vital River Laboratory Animal Technology Co., Ltd. (Beijing, China) and were fed in the good laboratory practice Laboratory of Heilongjiang University of Chinese Medicine (Harbin, China). The animals were housed in controlled environments (temperature is 23°C ± 2°C; relative humidity is 60% ± 5%, kept on a light/dark cycle of 12 h alternating and with free access to standard food and water). All the rats were randomly divided into five groups of 48 rats each group as follows: control group and CW group (0.108 g/100 g body weight, is equivalent to 4 times the clinical dose), CG group, CB group, and CR group (0.324 g/100 g body weight). The animals from which the urine samples were prepared to be obtained were allowed to acclimatize in the metabolism cages for 1 week before treatment. The rats in the control group were administrated with distilled water and the rats in the drug groups were administrated with CW 0.108 g/100 g and CG, CB, and CR 0.324 g/100 g (with 1 ml/100 g) each day, respectively, throughout the whole procedure for 6 months. The experiments were all performed in accordance with the approved animal protocols and guidelines which were established by the Medicine Ethics Review Committee for Animal Experiments of Heilongjiang University of Chinese Medicine.
Specimens of Radix A. Kusnezoffii were collected from Shaojia County (Heilongjiang, China), Radix Paeoniae Alba, Radix Glycyrrhizae, and Radix Ginseng were purchased from Harbin Tongrentang Drug Store (Harbin, China). They were all authenticated by Prof. Xijun Wang, Department of Pharmacognosy of Heilongjiang University of Chinese Medicine (Harbin, China). The crude CW was cut into small pieces, and then CW, CG (Caowu: Gancao is 1:2), CB (Caowu: Baishao is 1:2), and CR (Caowu: Renshen is 1:2) were immersed in distilled water (10 fold) for 60 min and then boiled for 90 min. After that, the extracted solution was filtered through 16 layers of gauze, and the filter residue was boiled with distilled water (8-fold) again for another 60 min. Finally, the two parts of the filtrate were collected and combined, and then the decoction was transformed into the powder by freeze-drying. The frozen powder was dissolved in distilled water and made up to the concentration of crude drug and stored at 4°C for usage.
Sample collection
The rat urine (4 ml) was collected in every week from the metabolism cages and centrifuged at 12,000 rpm for 10 min at 4°C, and then the supernatants were collected and frozen at −80°C until analysis. After all the urine samples had been collected completely, the quality control (QC) sample was prepared to optimize the conditions of UPLC-Q-TOF-HDMS as it contained most information of whole urine samples.
Chromatographic and mass spectrometry conditions
Chromatographic analysis was performed in a Waters ACQUITY ultra performance liquid chromatography (UPLC) system controlled with MassLynx (V4.1, Waters Corporation, Milford, USA). An aliquot of 3 μl of urine sample solution was injected onto an ACQUITY UPLC HSS T3 (100 mm × 2.1 mm, 1.8 μm) (Waters Corporation, Milford, USA) at 45°C and the flow rate was 0.5 ml/min. The optimal mobile phase consisted of a linear gradient system is listed in Table S1 (291.8KB, tif) . Every time after the instrument was calibrated, the QC sample was analyzed to test the stability of the instrument first to ensure the consistent performance of the system. Mass spectrometry (MS) detection was performed by Waters Micromass Q-TOF Microt Synapt High Definition Mass Spectrometer (Manchester, UK) which was equipped with electrospray ionization (ESI). The optimal conditions of analysis were as follows. the source temperature was set at 110°C, desolvation gas temperature was 300°C, cone gas flow was 50 L/h, and desolvation gas flow was 600 L/h. In positive ion mode, the capillary voltage was 2.5 kV, the sampling cone voltage was 30.0 V, and the extraction cone voltage was 4.0 V. For negative ion mode, the capillary voltage was 2.3 kV, the sampling cone voltage was 30.0 V, and the extraction cone voltage was 3.5 V. Data were centroided and the mass was corrected during acquisition using an external reference (Lock-Spray) which consisted of a 0.2 ng/ml solution of leucine enkephalin infused at a flow rate of 100 μl/min at a concentration of 200 pg/ml, generating a reference ion for positive ion mode ([M+H]+ = 556.2771) and for negative ion mode ([M−H]− = 554.2614) to ensure accuracy during the MS analysis. During metabolite profiling, centroid data were acquired using MassLynx (V4.1) software for each sample from 50 to 1000 Da with a 0.3 s scan time and a 0.1 s interscan delay over a 12 min run time in both positive and negative ion mode.
The linear gradient system of mobile phase for urine samples
Multivariate data analysis
All the mass data were imported and recorded to MarkerLynx Application Manager and MassLynx V4.1 software (Waters Corp., Milford, USA) for peak detection and alignment. The retention time and m/z data for each peak also were determined. The resultant data matrices were introduced to EZinfo software 2.8 for the principal component analysis (PCA), partial least squares-discriminant analysis (PLS-DA), and orthogonal projection to latent structures (OPLS) analysis. PCA is used for variable reduction and separation into classes. To maximize class discrimination and biomarkers, the data should be further analyzed by OPLS-DA. S-plots were then calculated to visualize the relationship between covariance and correlation within the OPLS-DA results.
Biomarkers identification
Variables that had significant contributions to discrimination between groups were considered as potential biomarkers and were subjected to further identification for the molecular formula. Metabolite peaks were assigned by MS/MS analysis or interpreted with available biochemical databases, including ChemSpider (http://www.chemspider.com), Human Metabolome Database (HMDB, http://www.hmdb.ca/), and MassBank.jp (http://www.massbank.jp).
Metabolic pathway construction
The pathway analysis of potential biomarkers was performed with metabolomics pathway analysis (MetPA) Software (http://metpa.metabolomics.ca), which was based on the database source including KEGG (http://www.genome.jp/kegg/), SMPD (http://www.smpdb.ca/), METLIN (http://metlin.scripps.edu/), and HMDB (http://www.hmdb.ca/) to identify the metabolic pathway. The possible biological roles were evaluated by the enrichment analysis of MetaboAnalyst.
RESULTS AND DISCUSSION
Acquisition of data
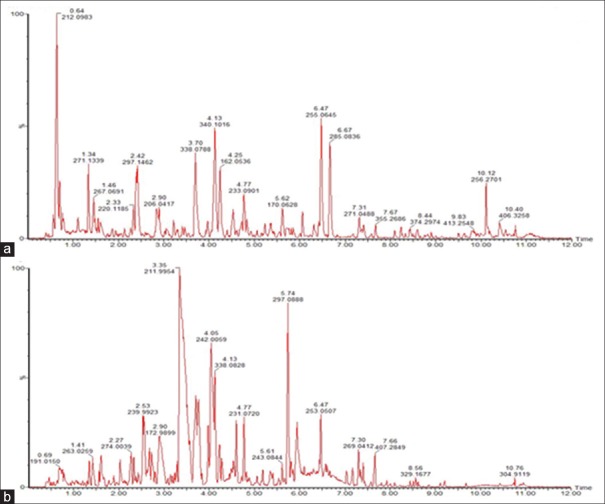
Global metabolic profiling in both positive ion mode and negative ion mode was analyzed by UPLC-Q-TOF-HDMS. Ions including (6052) ESI+ and (3371) ESI− ions were obtained. Using the optimal conditions of UPLC-Q-TOF-HDMS described at the beginning of this article, the typical total ion current and the representative-based peak intensity chromatograms for the QC samples of urine in both positive ion mode and negative ion mode are presented in Figure 1a and b, respectively. As Figure 1 reported, the low molecular mass metabolites could be separated well in 10 min. To visualize the subtle similarities and differences among these complex data better, multiple pattern recognition methods were used to phenotype the urine metabolome of CW-induced toxicity in rats. The methods of PCA, PLS, and OPLS-DA were used here to classify the metabolic phenotypes and identify the differentiating metabolites.
Figure 1.
The representative base peak intensity chromatogram of rat urine samples analyzed by UPLC-TOF-HDMS in (a) positive electrospray ionization mode and (b) negative electrospray ionization mode
Metabolic changes by principal component analysis and partial least squares-discriminant analysis
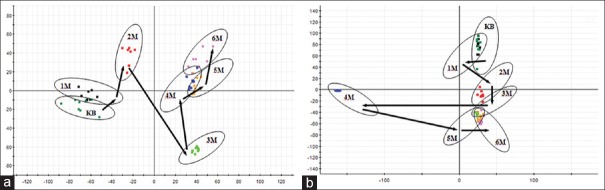
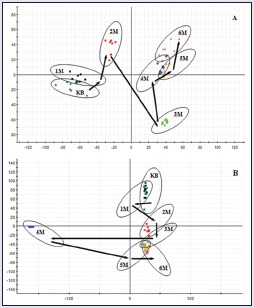
In the PCA scores, every point represented an individual urine sample. As score plots indicate the scatter of the samples, the PCA results indicated the similar metabolomic compositions when the data were clustered together and compositionally different metabolomes when dispersed. The PCA scores plot could divide the different time-point urine samples into different blocks in the group, which suggested that the metabolic profiles had changed due to the treatment. The dispersion degree of different time-point urine samples in the group was obvious. The supervised PLS-DA analysis revealed greater difference of metabolic signature in the urine samples from CW groups, compared with the control group and combinatorial intervention groups. It was noted that the change was in a time-dependent manner. The urine metabolic patterns of CW groups at the 1st, 2nd, 3rd, 4th, 5th, and 6th month were plotted by the PLS-DA method, in which the arrow depicted the variable trend of metabolic patterns at different times [Figure 2]. With the passage of time, the data sets of rat urine samples in CW group changed toward a direction away from the data sets in the control group, and the degree of deviation was most notable at the 6th month. Therefore, it was concluded that CW group had altered the urine metabolic profiles of normal rats, with the strongest perturbations occurring at 6th month, suggesting that the metabolism turbulence and the significant pathobiological had changed.
Figure 2.
The score plot reflecting the tendency of the urine metabolic fluctuations according to time after treatment of CW. (a) Electrospray ionization + mode, (b) electrospray ionization − mode. KB: Control group, 1M: 1st month, 2M: 2nd month, 3M: 3rd month, 4M: 4th month, 5M: 5th month, 6M: 6th month
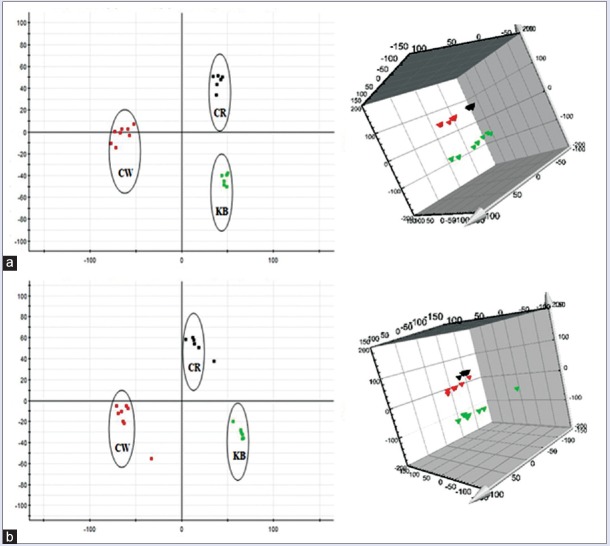
Urine metabolic profiling data of rats in 6th month of the control group (KB), CW group, and different combinatorial intervention groups (CG, CB, and CR) were imported MarkerLynx software for data dimensionality reduction and mass matrix information acquisition, respectively. Further, EZinfo software modules were used to do the unsupervised PCA and PLS-DA for the data of CW, KB, and different combinatorial intervention groups. The results from Figures 3-5 showed that the urine data in 6th month of different combinatorial intervention groups were close to that of the control group, while longer distance with CW group. It was concluded that the expression level of endogenous metabolites in different combinatorial intervention groups was close to that of the control group in the 6th month of treatment. In other words, the rats in combinatorial intervention groups (CG, CB, and CR) had no toxic reaction in 6th month of the administration, which mean the detoxification against CW.
Figure 3.
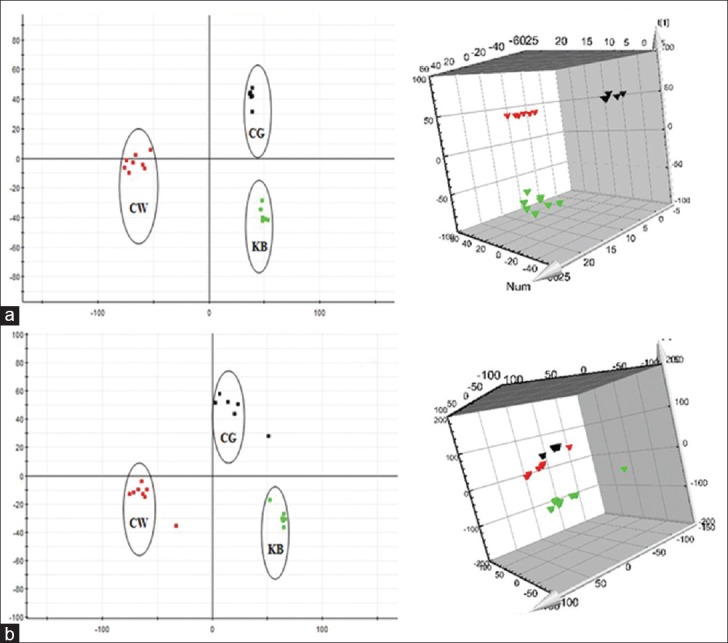
Principal component analysis score plots of urine samples collected from CW group, CG group and KB group of rats in the 6th month. (a) Electrospray ionization + mode, (b) Electrospray ionization mode ( CW group;
CW group;  CG group;
CG group;  KB group). KB: Control group
KB group). KB: Control group
Figure 5.
Principal component analysis score plots of urine samples collected from CW group, CR group and KB group of rats in the 6th month. (a) Electrospray ionization + mode, (b) electrospray ionization mode ( CW group;
CW group;  CR group;
CR group;  KB group). KB: Control group
KB group). KB: Control group
Figure 4.
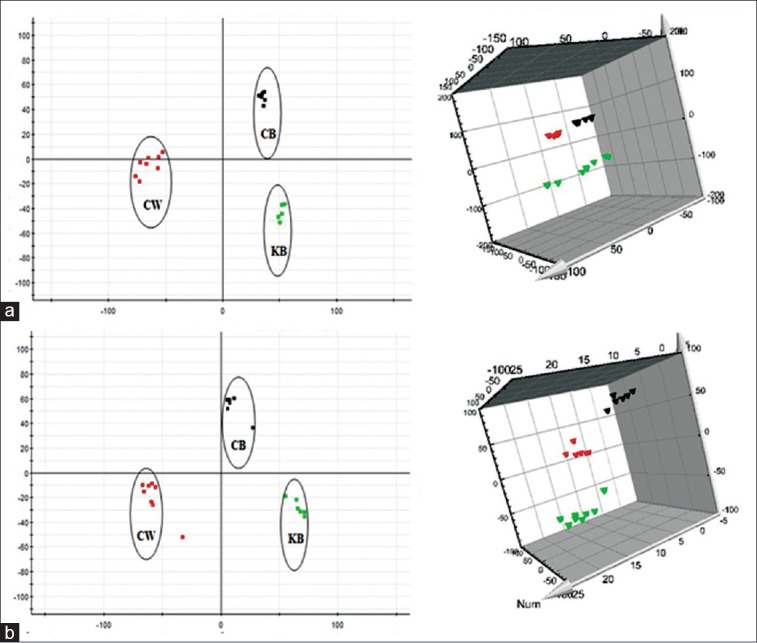
Principal component analysis score plots of urine samples collected from CW group, CB group and KB group of rats in the 6th month. (a) Electrospray ionization + mode, (b) Electrospray ionization mode ( CW group;
CW group;  CB group;
CB group;  KB group). KB: Control group
KB group). KB: Control group
The MS signals which were responsible for the differentiation were characterized by the variable importance plot (VIP) after PLS-DA analysis [Figure 6]. In VIP scattergram, ion debris was V-shaped arrangement. The VIP values of the bottom ions were small and show the contribution rate of metabolic trajectory change was small. Conversely, the VIP values of scarce top-ion debris were big, and the contribution rate of the metabolic profiling trajectory changes was great. It meant the farther away from the origin points, the higher value of the ions in VIP score plot. The potential markers were extracted from S-plots constructed following the OPLS analysis, and markers were chosen based on their contributions to the change and correlation within the data sets.
Figure 6.
Scatter plot of the variable importance plot in urine metabolites of treatment with CW group. (a) Electrospray ionization + mode, (b) Electrospray ionization − mode
Identification of the endogenous metabolites
The robust UPLC-QTOF-HDMS segregation analysis that was performed using the aforementioned protocol showed the retention time and the precise molecular mass and supplied the MS/MS data which were necessary for the structural identification of the biomarkers. The precise molecular mass was determined within a reasonable degree of measurement error (<5 ppm) using Q-TOF, and the potential element composition, degree of unsaturation, and fractional isotope abundance of the compounds were also obtained. We found the presumed molecular formula in the ChemSpider, HMDB, MassBank.jp, and other databases to confirm possible chemical compositions, and then, the MS/MS data were screened to determine the potential structures of the ions. All the detected ions were arranged according to the VIP value (>3). What followed was a description of the identification procedure of one positive ion as an example. According to the protocol detailed above in this article, the ion at Rt = 1.34 and (M+H)+ =271.1406 had a high VIP value. Its molecular formula was speculated to be C11H19N4O4 based on the analysis of its elemental composition and fractional isotope abundance. The main fragment ions analyzed by the MS/MS screening were observed at m/z 229.1482, 184.1267, 166.1154, 114.0705, and 70.0793, which could be the (M+H)+ of lost –CHNO, –C2H4N2O2, –C3H6O4, –C6H10N2O3, and –C8H16N3O3, respectively. Finally, based on a database search, the ion was determined to be 2-(3-carboxy-3-(methylammonio) propyl)-L-histidine. Its mass spectrum and proposed fragmentation pathway were displayed in Figure 7. According to the protocol above, 13 biomarkers were identified in the positive and negative ion detection mode in CW group, of which eight potential biomarkers were identified in positive mode and six potential biomarkers were identified in negative mode. Only one potential biomarker was in both modes [Tables S2 (1.1MB, tif) and S3 (751.9KB, tif) ]. Compared with the other drugs of Aconitum (including FZ and CHW), five biomarkers were commonly owned in Aconitum drugs, which were 5-hydroxy-6-methoxyindole glucuronide, 4,6-dihydroxyquinoline, 5-L-glutamyl-taurine, 3-methyldioxyindole, and palmitoyl glucuronide. The other eight biomarkers were also identified by CW group including L-phenylalanyl-L-hydroxyprolin, 2-(3-carboxy-3-(methylammonio) propyl)-L-histidine, 2-methylbutyroylcarnitine, 3-oxohexadecanoic acid, 2-phenylethanol glucuronide, D-glucuronic acid 1-phosphate, guanosine diphosphate (GDP)-L-fucose, and 3-indol carboxylic acid glucuronide.
Figure 7.
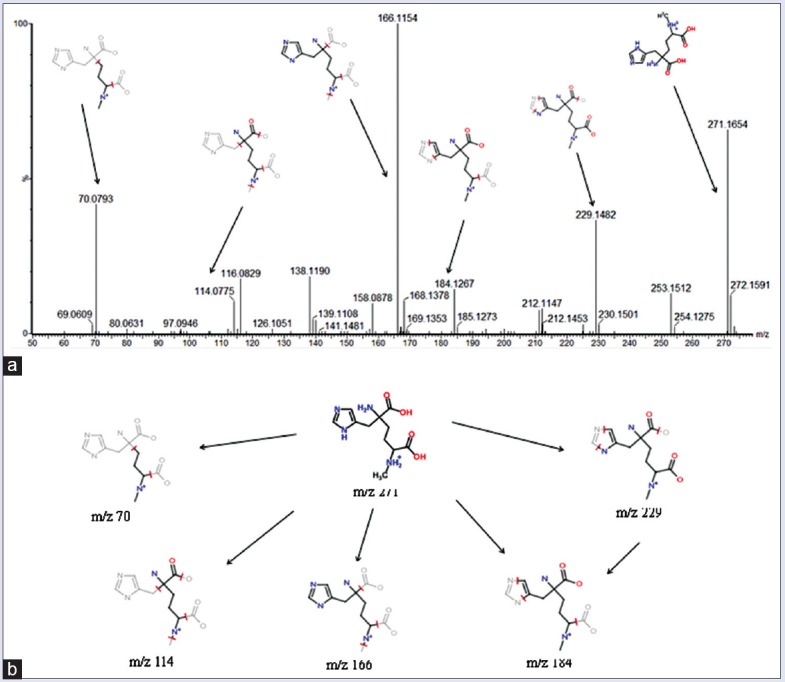
(a) Mass spectra of 2-(3-Carboxy-3-(methylammonio) propyl)-L-histidine in positive mode. (b) Proposed fragmentation pathway of 2-(3-Carboxy-3-(methylammonio) propyl)-L-histidine
Potential biomarkers identified in electrospray ionization positive mode
Potential biomarkers identified in electrospray ionization negative mode
Analysis of biological pathway
Further analysis of pathways and networks affected by the treatment of CW was performed by ingenuity network analysis. MetPA was a web-based and easy using method. MetPA was able to combine results from analysis of powerful pathway enrichment with the pathway topology analysis. It could help identify the most relevant pathway involved in the conditions under the study. MetPA used the advanced-quality analysis progress or software such as KEGG, Pathway Studio, MetaCore, and Ingenuity Pathway Analysis (IPA) metabolic pathway as the backend knowledgebase. In this study, we only considered the out-degree as node important measurements. Biological pathway analysis revealed that metabolites which were identified together were important for the host response to toxicity. They were responsible for pentose and glucuronate interconversions, tryptophan metabolism, amino sugar and nucleotide sugar metabolism, taurine and hypotaurine metabolism, ascorbate and aldarate metabolism, fructose and mannose metabolism, and starch and sucrose metabolism [Table S4 (345.6KB, tif) and Figure 8]. IPA generated seven kinds of networks. The results indicated that these endogenous metabolites showed the marked perturbations over the whole time-course of the sampling and could further contribute to the development research of CW toxicity. Compared with the results of CHW study, six networks were the same metabolic pathways between CW group and CHW group,[16] such as pentose and glucuronate interconversions, tryptophan metabolism, amino sugar and nucleotide sugar metabolism, taurine and hypotaurine metabolism, fructose and mannose metabolism, and starch and sucrose metabolism. Therefore, it was deduced that the six kinds of metabolic pathways might be the main networks responded to the toxicity of Aconitum drugs.
Figure 8.
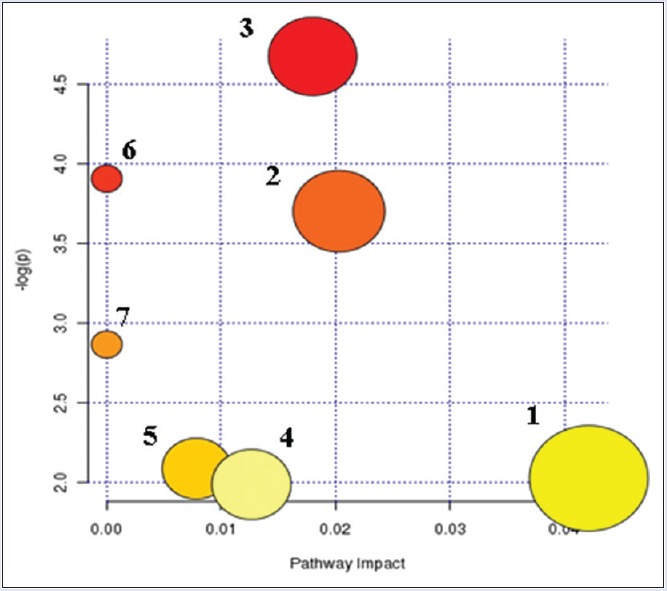
Summary of pathway analysis with metabolomics pathway analysis. (1) Pentose and glucuronate interconversions. (2) Tryptophan metabolism. (3) Amino sugar and nucleotide sugar metabolism. (4) Taurine and hypotaurine metabolism. (5) Ascorbate and aldarate metabolism. (6) Fructose and mannose metabolism. (7) Starch and sucrose metabolism
Results from Ingenuity Pathway Analysis with Metabolomics Pathway Analysis
Expression levels in toxic biomarkers
In the present study, the changes of expression levels in metabolic biomarkers of CW group were inferred in the toxicity rats based on changes in the intermediates during substance metabolism, and the regulations of expression levels in toxic biomarkers of CG group, CB group, and CR group were analyzed by comparing with that of CW group [displayed in Figure 9]. Glycolic acid was used to connect the liposoluble toxic substances with glycosidic bond, and the glucuronides, which was highly hydrophilic, was excreted by the kidney. The acidifying effects were the important indicators used to evaluate the ability of the liver to remove toxic substances or drugs outside.[17,18,19] In this study, the change of glucuronidation products, such as 5-hydroxy-6-methoxyindole glucuronide, 3-indole carboxylic acid glucuronide, D-glucuronic acid 1-phosphate, 2-phenylethanol glucuronide, and palmitoyl glucuronide, indicated that the glucuronidation process in liver was influenced, and the normal functioning of liver detoxification was depressed, which was induced by the treatment of CW. Tryptophan was an essential amino acid, which was not only one of the amino acid compositions of the protein but also involved in the mediation of protein synthesis. In the protein synthesis of the liver, it had been proved that tryptophan could affect the metabolism of liver RNA and protein.[20] Tryptophan metabolism levels were reported to associate with depression, mental retardation, high blood pressure, and thyroid dysfunction and some other diseases.[21] 3-Methyldioxyindole was an important urinary metabolite and oxidation product of 3-methylindole which was a metabolic product of tryptophan. The level of 3-methyldioxyindole in the urine of rats was increased significantly after treatment of CW, which might induce the disorders of tryptophan metabolism. After combinatorial intervention, the expression level of 3-methyldioxyindole in CG group, CB group, and CR group were all regulated to be close to that of control group. 4,6-Dihydroxyquinoline was the metabolic product of 5-hydroxykynurenamine, which was the immediate precursor of neurotransmitter serotonin by the enzyme monoamine oxidase from the metabolism of 5-hydroxytryptophan. In this study, it was observed that the urine level of 4, 6-dihydroxyquinoline increased after administration of CW, indicating that the neural system was abnormal. However, the levels were adjusted to return to normal in the three combinatorial intervention groups. It was known that taurine was an essential amino acid, which was abundant in the brain, heart, breast, gallbladder, and kidney organs. Taurine had many diverse biological functions, such as a neurotransmitter in the brain, a stabilizer of cell membranes, and a facilitator in the transport of ions. The expression levels of taurine in the body associated with a variety of diseases, such as depression and Parkinson's and kidney failure, which induced taurine level significantly lower. Taurine levels in the body of patients with heart failure and epilepsy were significantly higher.[22,23] The product 5-L-glutamyl-taurine was produced from taurine via the enzyme gamma-glutamyl-transpeptidase in the urine, the level of which suggested an increased taurine metabolism rate and potentially explained why amino acid metabolism was disrupted by CW. The urine levels of 5-L-glutamyl-taurine of CG group, CB group and CR group had no change compared with that of control group. Fatty acid was a carboxylic acid having a long hydrocarbon chain, which was an important energy source for the body.[24] Liver and muscle were the most active organization of fatty acid oxidation, and the most important oxidized form was β-oxidation. After the treatment of CW, the level of long-chain fatty acids such as 3-oxo-hexadecanoic acid was increased obviously, which was induced by abnormal fatty acid metabolism. However, the expression levels of 3-oxo-hexadecanoic acid were not influenced after administration with CG, CB, and CR. Hydroxyproline was the product of proline hydroxylated and also the main component of the collagen. Hydroxyproline and proline played a key role in the stability of collagen. Studies had shown that hydroxyproline levels in the urine related to the liver fibrosis.[25,26,27] L-phenylalanyl-L-hydroxyproline was the hydrolyzate of the hydroxyproline in urine, which was produced by proteolytic breakdown of collagen. Therefore, metabolic disorders affecting collagen metabolism in CW-treated rats contributed to the decreases in L-phenylalanyl-L-hydroxyproline levels. Fucose played a very important role in the complex sugars and glycoprotein metabolism, and it had close ties with cell identification, protein regulating, adhesion of albumin cells and endothelial cell. Through the role of GDP-L-fucose pyrophosphorylase in the synthesis of glycolipids, oligosaccharides, and glycoproteins, fucose and GDP-L-fucose transformed with each other. Reports had confirmed that changes in the level of fucose were also associated with liver cancer, breast cancer, and leukoencephalopathy in mammals.[28,29,30] The GDP-L-fucose content was increased after administration of CW, demonstrating that a metabolic disorder of GDP-L-fucose was induced by CW in rat livers. The urine expression of L-phenylalanyl-L-hydroxyproline and GDP-L-fucose in combinatorial intervention groups was both close to the level of control group. The regulating effectiveness of expression levels of toxic biomarkers which were produced by the combinatorial intervention group (CG, CB, and CR) indicated that the damages to body caused by the toxicity of CW were decreased after combinatorial intervention. CG, CB, and CR had the effective of detoxification against CW.
Figure 9.
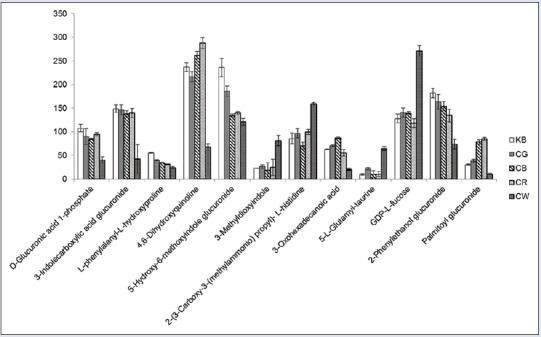
Urine level variation in KB, Caowu and Renshen and Caowu groups of identified biomarkers
Along with the variability of medical model and changes in the spectrum of disease, the medical model of prevention and health care has brought a new opportunity of development for the ancient Chinese medicine. In recent years, the application field and range of TCM have gradually expanded and extended, and the academic status has significantly improved. Due to the frequent occurrence of toxicity and adverse reactions of traditional Chinese medicine, the internationalization process has been hindered and the modernization of traditional Chinese medicine has been slow.[5] It is because toxic material foundation of toxic drugs is unknown, toxic mechanism is unclear, and thus, there is no effective detoxification method. Conventional studies of toxicity mechanism were limited to conventional toxicology methods, such as biochemical examinations and pathological observations. However, they could neither interpret the toxic processes nor clarify the mechanism of toxicity.[31] With the continuous development and improvement of metabolomics technology, the advantages of metabolomics in the field of drug safety evaluation and toxicity prediction were gradually emerged. The core of metabolomics is quantitative determination of multi-parameter response by the pathological and physiological stimuli, genetic-inducted or time-related factors. Qualitative and quantitative analysis of all small molecule metabolites is simultaneously processed. Pattern recognition methods are used to identify relevant biomarkers and associated with biological events in vivo, which can access to the information of toxicity sites, pathways, and strength.[32] The small molecule biomarkers have the contributions to the understanding of the mechanisms responsible for toxicity of TCM. Metabolomic technologies can provide diagnostic and prognostic biomarkers specific for early stages of toxicity and define patterns of toxicity for early identification of TCM.
CONCLUSION
The toxicity of CW is reported to be mostly caused by excessive or wrong medication, so the clinical practice of CW is still limited due to the lack of sensitive and reliable biomarkers. Metabolomics has brought great opportunities to improve the detection of toxicity, discovery of biomarkers, and detoxification against toxicity of CW. Clinical, biochemical parameters and histopathology have been done as the preliminary works. The serum samples were detected at different time points of 6 months from rats administered with CW, CB, CG, and CR. The results showed that cardiac-related functions, lactate dehydrogenase, Na-K-ATP, CK activity, and liver-related aspartate transaminase, alanine transaminase, alkaline phosphatase activity had significantly changed in the latter part of the administration with CW compared with KB (the control group), but no change between CB and KB, CG and KB, CR and KB. Histopathology of the tissue (heart, liver, spleen, lungs, and kidney) exposed to CW was examined. As a result, only the heart and liver from CW groups produced pathological changes after 6 months, but the other groups had no variation. It suggested that if the rats were long-term administrated with CW, the heart and liver were mainly damaged, and the toxicity was seen in the administration of the first 5–6 months. However, after combinatorial intervention, Baishao, Gancao, and Renshen had detoxification against CW.
In this study, the metabolomics was based on the UPLC-QTOF-HDMS technique, IPA, and pattern recognition analyses and was applied to investigate metabolic changes caused by toxicity and biomarkers identification in the urine of rats following treatment with CW. Metabolomic data also were analyzed and the results suggested that the toxicity caused by CW group was greatly elevated with the time-response, and the accumulation of long-term toxicity in urine of rats in CW group contributed to the obvious separation from that of rats in control group. In addition, urine metabolomic profiling had also provided 13 sensitive biomarkers identifications, five biomarkers of which were commonly owned in Aconitum drugs. These biomarkers were responsible by IPA and MetPA for pentose and glucuronate interconversions, tryptophan metabolism, amino sugar and nucleotide sugar metabolism, taurine and hypotaurine metabolism, ascorbate and aldarate metabolism, fructose and mannose metabolism, and starch and sucrose metabolism, of which six networks were the same to CHW group. It was deduced that these metabolic pathways might be the main networks responded to the toxicity of Aconitum drugs. Our works not only improved the understanding of toxicity mechanisms induced by CW but also provided an experimental foundation for studies of reducing the toxicity of CW. The urine metabolomics can systematically study the relationship between the toxicity and metabolite in vivo, conducive to a more comprehensive understanding and evaluating the toxicity of drugs. The changes of toxicity metabolites obtained from combinatorial intervention of CG, CB, and CR also were analyzed to investigate the regulation degree of toxicity biomarkers adjusted by different combinatorial intervention groups in 6th month. The results indicated that the three combinatorial intervention drugs could effectively regulate the expression level of toxic biomarkers to normal, and thus, the three combinatorial intervention drugs could be determined as the effective antidotes against the toxicity of CW.
The research determined the main target organs of toxicity for the heart and liver after long-term administration of CW. At the time point of CW with significant toxicity, it was found that CW mainly affected glucuronate interconversions, nucleotide sugar metabolism, fatty acid metabolism, and proline metabolism associated with the liver; affected taurine transformation, amino sugar metabolism associated with the heart; and affected tryptophan metabolism associated with the nervous system. It made the related metabolic products or intermediate products arise from abnormal expression of toxicity. After administration of CG, CB, and CR, the metabolic markers of metabolic pathways and metabolic trajectory associated with toxicity of CW occurred callback, which had no toxic effects on the body. It played on the role of detoxification against the toxicity of CW and provided evidence to establish the clinical use of toxic drugs.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
Acknowledgements
This work was supported by grants from the Key Program of Natural Science Foundation of State (Grant No. 81302905, 81373930, 81430093, 81673586), National Key Subject of Drug Innovation (Grant No. 2015ZX09101043-005, 2015ZX09101043-011), TCM State Administration Subject of Public Welfare of (Grant No. 2015468004), Natural Science Foundation of Heilongjiang Province of China (H2015038), University Nursing Program for Young Scholars with Creative Talents in Heilongjiang Province (UNPYSCT-2015118).
REFERENCES
- 1.Sun H, Wang M, Zhang A, Ni B, Dong H, Wang X. UPLC-Q-TOF-HDMS analysis of constituents in the root of two kinds of Aconitum using a metabolomics approach. Phytochem Anal. 2013;24:263–76. doi: 10.1002/pca.2407. [DOI] [PubMed] [Google Scholar]
- 2.Wang X, Wang H, Zhang A, Lu X, Sun H, Dong H, et al. Metabolomics study on the toxicity of aconite root and its processed products using ultraperformance liquid-chromatography/electrospray-ionization synapt high-definition mass spectrometry coupled with pattern recognition approach and ingenuity pathways analysis. J Proteome Res. 2012;11:1284–301. doi: 10.1021/pr200963e. [DOI] [PubMed] [Google Scholar]
- 3.Kawasaki R, Motoya W, Atsumi T, Mouri C, Kakiuchi N, Mikage M. The relationship between growth of the aerial part and alkaloid content variation in cultivated Aconitum carmichaeli Debeaux. J Nat Med. 2011;65:111–5. doi: 10.1007/s11418-010-0466-x. [DOI] [PubMed] [Google Scholar]
- 4.Singhuber J, Zhu M, Prinz S, Kopp B. Aconitum in traditional Chinese medicine: A valuable drug or an unpredictable risk? J Ethnopharmacol. 2009;126:18–30. doi: 10.1016/j.jep.2009.07.031. [DOI] [PubMed] [Google Scholar]
- 5.Shaw D. Toxicological risks of Chinese herbs. Planta Med. 2010;76:2012–8. doi: 10.1055/s-0030-1250533. [DOI] [PubMed] [Google Scholar]
- 6.Fu M, Wu M, Wang JF, Qiao YJ, Wang Z. Disruption of the intracellular Ca2+ homeostasis in the cardiac excitation-contraction coupling is a crucial mechanism of arrhythmic toxicity in aconitine-induced cardiomyocytes. Biochem Biophys Res Commun. 2007;354:929–36. doi: 10.1016/j.bbrc.2007.01.082. [DOI] [PubMed] [Google Scholar]
- 7.Fu M, Li RX, Fan L, He GW, Thornburg KL, Wang Z. Sarcoplasmic reticulum Ca2+ release channel ryanodine receptor (RyR2) plays a crucial role in aconitine-induced arrhythmias. Biochem Pharmacol. 2008;75:2147–56. doi: 10.1016/j.bcp.2008.02.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wright SN. Comparison of aconitine-modified human heart (hH1) and rat skeletal (mu1) muscle Na+ channels: An important role for external Na+ ions. J Physiol. 2002;538(Pt 3):759–71. doi: 10.1113/jphysiol.2001.012915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Xie S, Zhang G, Sun G, Zhu X, Chen R, Zhang S, et al. Detoxication experimental study on different compatibility proportion of aconiti lateralis radix praeparata and glycyrrhizae radix et rhizoma. Zhongguo Zhong Yao Za Zhi. 2012;37:2210–4. [PubMed] [Google Scholar]
- 10.Yan-Wen LI, Zhi-Yong LI, Hui-Yong LI, Peng TA, Ce YA, Ying-Kai ZH, Meng CU. New thought of traditional Chinese medicine in detoxification by compatibility. Chin J Exp Tradit Med Formul. 2012;18:321–4. [Google Scholar]
- 11.Xue L, Zhang HY, Qin L, Wang XC, Wang L. Effect of chuanwu and baishao used separately or in combination on adjuvant arthritis in rats. Zhongguo Zhong Yao Za Zhi. 2000;25:175–8. [PubMed] [Google Scholar]
- 12.Zhang A, Sun H, Dou S, Sun W, Wu X, Wang P, et al. Metabolomics study on the hepatoprotective effect of scoparone using ultra-performance liquid chromatography/electrospray ionization quadruple time-of-flight mass spectrometry. Analyst. 2013;138:353–61. doi: 10.1039/c2an36382h. [DOI] [PubMed] [Google Scholar]
- 13.Dong W, Wang P, Meng X, Sun H, Zhang A, Wang W, et al. Ultra-performance liquid chromatography-high-definition mass spectrometry analysis of constituents in the root of radix stemonae and those absorbed in blood after oral administration of the extract of the crude drug. Phytochem Anal. 2012;23:657–67. doi: 10.1002/pca.2370. [DOI] [PubMed] [Google Scholar]
- 14.Robertson DG, Watkins PB, Reily MD. Metabolomics in toxicology: Preclinical and clinical applications. Toxicol Sci. 2011;120(Suppl 1):S146–70. doi: 10.1093/toxsci/kfq358. [DOI] [PubMed] [Google Scholar]
- 15.Zhang A, Sun H, Wu X, Wang X. Urine metabolomics. Clin Chim Acta. 2012;414:65–9. doi: 10.1016/j.cca.2012.08.016. [DOI] [PubMed] [Google Scholar]
- 16.Dong H, Zhang A, Sun H, Wang H, Lu X, Wang M, et al. Ingenuity pathways analysis of urine metabolomics phenotypes toxicity of Chuanwu in Wistar rats by UPLC-Q-TOF-HDMS coupled with pattern recognition methods. Mol Biosyst. 2012;8:1206–21. doi: 10.1039/c1mb05366c. [DOI] [PubMed] [Google Scholar]
- 17.Wang H, Yan G, Zhang A, Li Y, Wang Y, Sun H, et al. Rapid discovery and global characterization of chemical constituents and rats metabolites of Phellodendri amurensis cortex by ultra-performance liquid chromatography-electrospray ionization/quadrupole-time-of-flight mass spectrometry coupled with pattern recognition approach. Analyst. 2013;138:3303–12. doi: 10.1039/c3an36902a. [DOI] [PubMed] [Google Scholar]
- 18.Ma L, Sun J, Peng Y, Zhang R, Shao F, Hu X, et al. Glucuronidation of edaravone by human liver and kidney microsomes: Biphasic kinetics and identification of UGT1A9 as the major UDP-glucuronosyltransferase isoform. Drug Metab Dispos. 2012;40:734–41. doi: 10.1124/dmd.111.043356. [DOI] [PubMed] [Google Scholar]
- 19.Yin Q, Wang P, Zhang A, Sun H, Wu X, Wang X. Ultra-performance LC-ESI/quadrupole-TOF MS for rapid analysis of chemical constituents of Shaoyao-Gancao decoction. J Sep Sci. 2013;36:1238–46. doi: 10.1002/jssc.201201198. [DOI] [PubMed] [Google Scholar]
- 20.Sun H, Wu F, Zhang A, Wei W, Han Y, Wang X. Profiling and identification of the absorbed constituents and metabolites of schisandra lignans by ultra-performance liquid chromatography coupled to mass spectrometry. Biomed Chromatogr. 2013;27:1511–9. doi: 10.1002/bmc.2951. [DOI] [PubMed] [Google Scholar]
- 21.Guchhait RB, Janson C, Price WH. Validity of plasma factor in schizophrenia as measured by tryptophan uptake. Biol Psychiatry. 1975;10:303–14. [PubMed] [Google Scholar]
- 22.Nørrelund H, Wiggers H, Halbirk M, Frystyk J, Flyvbjerg A, Bøtker HE, et al. Abnormalities of whole body protein turnover, muscle metabolism and levels of metabolic hormones in patients with chronic heart failure. J Intern Med. 2006;260:11–21. doi: 10.1111/j.1365-2796.2006.01663.x. [DOI] [PubMed] [Google Scholar]
- 23.Rainesalo S, Keränen T, Palmio J, Peltola J, Oja SS, Saransaari P. Plasma and cerebrospinal fluid amino acids in epileptic patients. Neurochem Res. 2004;29:319–24. doi: 10.1023/b:nere.0000010461.34920.0c. [DOI] [PubMed] [Google Scholar]
- 24.Wang X, Zhang A, Sun H, Wu G, Sun W, Yan G. Network generation enhances interpretation of proteomics data sets by a combination of two-dimensional polyacrylamide gel electrophoresis and matrix-assisted laser desorption/ionization-time of flight mass spectrometry. Analyst. 2012;137:4703–11. doi: 10.1039/c2an35891c. [DOI] [PubMed] [Google Scholar]
- 25.Brown KE, Dennery PA, Ridnour LA, Fimmel CJ, Kladney RD, Brunt EM, et al. Effect of iron overload and dietary fat on indices of oxidative stress and hepatic fibrogenesis in rats. Liver Int. 2003;23:232–42. doi: 10.1034/j.1600-0676.2003.00832.x. [DOI] [PubMed] [Google Scholar]
- 26.Lee HS, Shun CT, Chiou LL, Chen CH, Huang GT, Sheu JC. Hydroxyproline content of needle biopsies as an objective measure of liver fibrosis: Emphasis on sampling variability. J Gastroenterol Hepatol. 2005;20:1109–14. doi: 10.1111/j.1440-1746.2005.03901.x. [DOI] [PubMed] [Google Scholar]
- 27.Yang KL, Hung KC, Chang WT, Li EI. Establishment of an early liver fibrosis model by the hydrodynamics-based transfer of TGF-beta1 gene. Comp Hepatol. 2007;6:9. doi: 10.1186/1476-5926-6-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Noda K, Miyoshi E, Gu J, Gao CX, Nakahara S, Kitada T, et al. Relationship between elevated FX expression and increased production of GDP-L-fucose, a common donor substrate for fucosylation in human hepatocellular carcinoma and hepatoma cell lines. Cancer Res. 2003;63:6282–9. [PubMed] [Google Scholar]
- 29.Renaud DL. Lysosomal disorders associated with leukoencephalopathy. Semin Neurol. 2012;32:51–4. doi: 10.1055/s-0032-1306386. [DOI] [PubMed] [Google Scholar]
- 30.Blixt O, Bueti D, Burford B, Allen D, Julien S, Hollingsworth M, et al. Autoantibodies to aberrantly glycosylated MUC1 in early stage breast cancer are associated with a better prognosis. Breast Cancer Res. 2011;13:R25. doi: 10.1186/bcr2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ganzera M. Recent advancements and applications in the analysis of traditional Chinese medicines. Planta Med. 2009;75:776–83. doi: 10.1055/s-0029-1185686. [DOI] [PubMed] [Google Scholar]
- 32.Arakaki AK, Skolnick J, McDonald JF. Marker metabolites can be therapeutic targets as well. Nature. 2008;456:443. doi: 10.1038/456443c. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
The linear gradient system of mobile phase for urine samples
Potential biomarkers identified in electrospray ionization positive mode
Potential biomarkers identified in electrospray ionization negative mode
Results from Ingenuity Pathway Analysis with Metabolomics Pathway Analysis