Abstract
Background:
Forsythiae Fructus (FF) is a well-known medicinal herb derived from the dried fruits of Forsythia suspensa (Thunb.) Vahl. (Oleaceae). Recently, bioactive compounds isolated from hydrophobic solvent fractions of FF have been reported to have anti-oxidant, antibacterial, and anti-cancer effects.
Objective:
Almost all herbal medicines are derived from water extracts, which suggests different extraction methods might enhance the practical efficacies of herbal medicines. In this study, the authors further investigated the most potential anti-cancer fraction, that is, the hexane fraction (FFH) of the methanol extract (FFM) of the dried fruits of Forsythia suspensa.
Materials and Methods:
FFH was investigated by measuring its effects on the viability and apoptotic death of PC-3 cells (a prostate cancer cell line), on the expression levels of Bcl-2, Bax, cytochrome c, procaspase-9, procaspase-3 and PARP, and caspase-3 activity.
Results:
FFH significantly accelerated apoptotic cell death and decreased the protein levels of Bcl-2, procaspase-9, and procaspase-3.
Conclusion:
FFH can act as a pro-oxidative agent and induce the apoptosis of prostate cancer cells.
SUMMARY
Hexane fraction of the methanol extract of Forsythiae Fructus (FFH) at a concentration more than 50 µg/mL significantly reduced PC-3 cell viability
FFH time and dose dependently elevated intracellular ROS levels and increased the proportion of cells arrested in the G0/G1 phase
FFH significantly accelerated apoptotic cell death and diminished the protein expression levels of Bcl-2, procaspase-9, and procaspase-3
The protein expression levels of Bax, cytochrome c, and cleaved PARP were increased by FFH, and so was the caspase-3 activity.
Abbreviations used: FF: Forsythiae Fructus; FFM: Methanol extract of Forsythiae Fructus; FFH: Hexane fraction of the methanol extract; DCFH-DA: 2’,7’-dichlorodihydro-fluorescein diacetate.
Keywords: anti-cancer effect, Forsythiae Fructus, PC-3 cells
INTRODUCTION
Prostate cancer is one of the most commonly diagnosed cancers and the second leading cause of cancer-related death in men in the United States and in Western Europe. Jemal et al. reported 220,000 men were newly diagnosed and that 15% of prostate cancer patient population died in the United States in 2010.[1]
Various types of therapies, such as, hormone, surgery, radiation, and chemotherapy, have been used to treat prostate cancer,[2,3,4,5,6,7,8,9,10,11] but all are limited by serious side effects, which has encouraged others to develop chemotherapeutic agents that are effective and relatively safe. Furthermore, many scientists now recognize that herbal medicines represent a potential source of these chemotherapeutics.[12,13]
Forsythiae Fructus (FF) has been reported to have anti-cancer effects in various cancer cell lines.[14,15,16] In our preliminary study, the hexane fraction (FFH) of the methanolic extract of FF was found to show more apoptotic activity in cancer cell lines than in other solvent fractions.
Apoptosis is can occur through a death receptor mediated (extrinsic) pathway or a mitochondrially mediated (intrinsic) pathway. The extrinsic pathway is induced by death receptors, whereas the intrinsic pathway is initiated by stress inducers, cytokines, reactive oxygen species (ROS), protein degradation, nuclear condensation, and DNA fragmentation.[11,17,18,19,20,21,22]
Bcl-2 family members play central roles in the regulations of cell survival and death. This family is composed of antiapoptotic (Bcl-2) and proapoptotic (Bax) molecules. Bid translocation to mitochondria is induced by interactions between Bcl-2 family members, and this, translocation can induce cytochrome c release to cytosol. On the other hand, NF-κB activation can block this cytochrome c release and result in cell death signaling through, for example, the caspase cascade.[23,24,25,26]
In the present study, the anti-cancer effects of FFH were investigated by measuring its effects on intracellular ROS production, cell cycle distribution, apoptotic cell levels, and caspase-3 activity, and Bcl-2, Bax, cytochrome c, caspase 9, caspase 3, and PARP protein levels.
MATERIALS AND METHODS
The hexane fractionation of FF
FF was purchased from Hwalim Natural Herb Company (Busan, Korea), and was authenticated by one of the authors (Suin Cho, an experienced pharmacognocist). Specimens were deposited in the School of Korean Medicine, Pusan National University. Dried FF (500 g) was extracted three times by maceration in methanol (5L × 3) for 48 h at 25°C. The methanol extract (FFM) so obtained was then filtered and the methanol was evaporated using a rotary evaporator and speed vacuum concentrator (final yield of FFM 89 g). FFM was then suspended in water and partitioned sequentially to produce the following fractions: hexane (FFH, 11.50 g), chloroform (FFC, 5.31 g), ethyl acetate (FFE, 1.82 g), butanol (FFB, 11.06 g), and aqueous residue (FFW, 30.04 g). These materials were stored in sterile microtubes in a refrigerator at -20°C.
Reagents
Thiazolyl blue tetrazolium bromide (MTT), 2″,7″-dichlorodihydro-fluorescein diacetate (DCFH-DA) and other reagents were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA). Tris was purchased from Duchefa Biochemie (BH Haarlem, The Netherlands), dimethyl sulfoxide (DMSO) from Junsei Chemical Co. (Tokyo, Japan). Fetal bovine serum (FBS) and penicillin-streptomycin (P/S) were purchased from Gibco (Los Angeles, CA, USA). Dulbecco's Modified Eagle's Medium (DMEM) was purchased from Welgene Inc. (Gyeongsangbuk-do, Korea). BCA reagent and albumin standard were from Thermo Scientific (Waltham, MA, USA). Primary Bcl-2 antibody was purchased from Oncogene (Bracknell, England). Bax and PAPR antibodies were obtained from Cell Signaling (Danvers, MA, USA) and cytochrome c, caspase-9, and β-actin antibodies from Santa Cruz (Paso Robles, CA, USA). Caspase-3 antibody was obtained from EMD Millipore (Billerica, MA, USA). Secondary antibodies goat anti-rabbit IgG, pAb, goat anti-mouse IgG, pAb, and caspase-3 activity assay kit were obtained from Enzo Life Sciences (Farmingdale, NY, USA). West-Q chemiluminescent substrate was purchased from GenDEPOT (Katy, TX, USA). The solvents used were of HPLC grade, unless stated otherwise.
Cell line and cell culture
PC-3 cells (a human prostate cancer cell line) were obtained from ATCC (American Type Culture Collection, Tockville, MA, USA). Cells were cultured in DMEM media supplemented with 10% FBS and 1% P/S and maintained in a 95% air/5% CO2 atmosphere at 37°C.
Cell viability assay and cell morphology
Cell viabilities were measured using a modified version of the 3-4,5-dimethylthiazol-2-yl]-2,5-diphenyltetrazolium bromide (MTT) method.[27] Briefly, PC-3 cells (5×103 per well) were incubated at 37°C with 5% CO2 for 18 h, and then with various concentrations of FFH for 24 h. After incubation, MTT solution (0.5 mg/mL) was added for 2 h and then the culture medium was removed. Absorbance was measured at 570 nm using a spectrophotometer (Tecan, Infinite® M200, Switzerland).
The effects of FFH on cell morphology and density were assessed. Cells were seeded at a density of 5×104 per well and incubated at 37°C in 5% CO2 for 18 h in the same manner as described above. Morphologic changes were examined using an inverted microscope (Nikon Eclipse, TS100F, Japan) at a magnification of 100×.
Intracellular ROS generation
Intracellular ROS levels were measured using DCFH-DA (a fluorescent dye). Briefly, PC-3 cells (5×104 per well) were incubated at 37°C in 5% CO2 for 18 h and then with various concentrations of FFH for 24 h. After incubation, DCFH-DA was added to a final concentration of 5 μM and cells were incubated for a further 30 min. They were then washed three times in PBS. Fluorescence was measured at an excitation wavelength of 485 nm and an emission wavelength of 530 nm using a fluorescence reader (Tecan, Infinite® M200, Switzerland). Intracellular ROS levels were analyzed by flow cytometry (BD FACS Canto II, USA) using the procedure described above.
Cell cycle analysis
PC-3 were seeded at 1×106 per well and incubated at 37°C in 5% CO2 for 18 h. They were then incubated with various concentrations of FFH for 24 h, fixed with 80% ethanol, and stored at –20°C overnight. Cells were then suspended in propidium iodide (PI) staining solution (0.01 mg/mL PI and 0.1 mg/mL RNase A) and incubated at 37°C for 30 min in the dark. Cell cycle distributions were analyzed by flow cytometry (BD FACS Canto II, USA).
Annexin-V/PI double staining
To determine whether cell death was due to apoptosis, flow cytometry was used to determine proportions of apoptotic cells induced by FFH treatment. Briefly, PC-3 cells were seeded at 1×106 per well and incubated at 37°C in 5% CO2 for 18 h. They were then incubated with various concentrations of FFH for 24 h when attached cells were gently trypsinized. After washing with PBS, cells were suspended in binding buffer, treated with 5 μL of annexin-V/FITC and 10 μL of PI solution, incubated at 37°C for 30 min in the dark, and immediately analyzed by flow cytometry (BD FACS Canto II, USA).
Caspase-3 activity assay
The effects of FFH on in vitro caspase-3 activity were determined using a caspase-3 activity assay kit (Enzo Life Sciences, Farmingdale, NY, USA). Briefly, PC-3 cells (1×106 per well) were incubated at 37°C in 5% CO2 for 18 h and then with various concentrations of FFH for 24 h. Collected cells were lysed and cell lysates were quantified using the BCA method. Cell lysates (100 μg), fluorogenic peptide substrate (200 μM), and DEVD-p-nitroaniline (pNA; final concentration 200 μM) were then added in 96-well plate and incubated at 37°C in 5% CO2 for 1 h. Absorbance was measured at 405 nm using a spectrophotometer (Tecan, Infinite® M200, Switzerland).[28]
Western blot analysis
The expression levels of Bcl proteins, cytochrome c, caspases, and PARP were assessed by Western blotting. Briefly, PC-3 cells were seeded at 1×106 per well, incubated at 37°C in 5% CO2 for 18 h, and with various concentrations of FFH for 24 h. Attached cells were then washed twice with ice-cold PBS and total proteins were isolated using protein extraction solution (pro-prep, iNtRON, Gyeonggi-do, Korea). Cell lysates were obtained by centrifugation at 13,250g for 10 min at 4°C. Proteins were separated using sodium-dodecyl sulfate polyacrylamide gel, transferred to PVDF membranes (Millipore, Darmstadt, Germany), which were then blocked by 5% skim milk in TBST buffer for 1 h at room temperature and then incubated for 4°C overnight with specific antibodies for Bcl-2 (1:500), Bax (1:500), cytochrome c (1:200), caspase-9 (1:200), caspase-3 (1:200), PARP (1:500), and β-actin (1:500). After this overnight incubation, horseradish peroxidase (HRP) conjugated goat anti-rabbit IgG, pAb (1:5000), and HRP-conjugated goat anti-mouse IgG pAb (1:3000) were added for 2 h. Membranes were then treated with ECL solution (Gen DEPOT, Houston, TX, USA) and protein expression levels were determined using a photosensitive luminescent analyzer system (Amersham™ Imager 600, UK) and the Image J program (NIH, MA, USA). Values are presented as ratios of densities of respective protein bands to β-actin.
Statistical analysis
One-way ANOVA was used to determine the significances of differences between the experimental groups and the control group. Results are presented as mean ± standard deviation (mean ± SD). SIGMAPLOT ver. 12.0 was used for reasons of the statistical analysis, and P values of 0.05 or less were considered statistically significant.
RESULTS AND DISCUSSION
Cell viabilities and morphologic changes
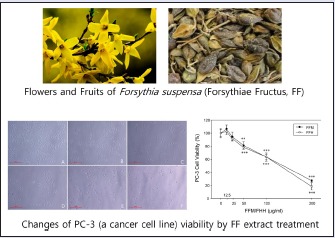
The viabilities of PC-3 cells in the presence of 12.5-200 μg/mL FFM or FFH for 24 h were measured using the MTT assay. At concentrations higher than 50 μg/mL, FFM and FFH both significantly lowered cell viabilities [Figure 1]; thus, FFH was administered to cells at concentrations up to 200 μg/mL during our study of anti-cancer properties. Cell viability was found to decrease in a dose-dependent manner [Figure 1B], which was in line with the observed effects of FFM or FFH on cell viability [Figure 1A]. Morphologically, treatment with FFM or FFH was found to result in shrunken, bulging cells indicative of apoptosis and necrosis.
Figure 1.

Effects of FFM and FFH on the viability and morphology of PC-3 cells. Cell viabilities were measured using a MTT assay as described in Materials and Methods section. (A) Values are the mean ± SD of three independent experiments. **P < 0.01 and ***P < 0.001 vs. the nontreated control. (B) The effects of FFH on the density and morphology of PC-3 cells were observed using an inverted microscope (original magnification 100×; scale bar = 500 pixels). (A) Nontreated control; (B) 12.5 μg/mL; (C) 25 μg/mL; (D) 50 μg/mL; (E) 100 μg/mL; (F) 200 μg/mL of FFH administered
Measurements of intracellular ROS
ROS are known to be closely related to the initiation of the apoptotic cascade.[29,30,31] Therefore, we investigated the effect of FFH on intracellular ROS levels in PC-3 cells. At concentrations up to 100 μg/mL FFH significantly elevated intracellular ROS levels in a dose- and time-dependent manner [Figure 2]. Intracellular ROS levels as determined by flow cytometry showed a similar increasing tendency on increasing FFH concentration [Figure 1]. In a preliminary study, we found the free radical scavenging activities of FFH were lower than those of the other solvent fractions (data not shown). Considering preliminary and present results, it would appear FFH induces PC-3 cell death by acting as a pro-oxidative agent.
Figure 2.
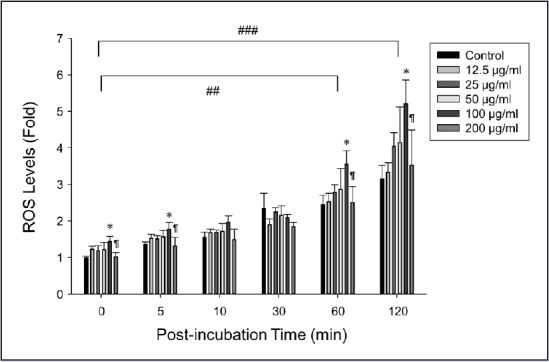
Effects of FFH on ROS levels in PC-3 cells. Amounts of ROS were determined using DCFH-DA. Results are the mean ± SD of three independent experiments. ##P < 0.01 and ###P < 0.001 vs. 100 μg/mL FFH at baseline (zero) time and *P < 0.05 vs. the nontreated control at each time point. ¶P < 0.05 vs. 100 μg/mL FFH
Effect of FFH on G0/G1 phase cell cycle arrest
Cell cycle distributions were evaluated by flow cytometry after PI staining. The percentage of PC-3 cells in the G0/G1 phase in 50 μg/mL FFH-treated group (69%) was significantly greater than that for untreated PC-3 cells (54%). Percentages in the S and G2/M phases decreased from 12 to 6% (S phase) and from 30 to 19% (G2/M phase) on treating cells with 50 μg/mL FFH [Figure 3]. These results suggest FFH causes PC-3 cell death mediated by G0/G1 phase cell cycle arrest.
Figure 3.
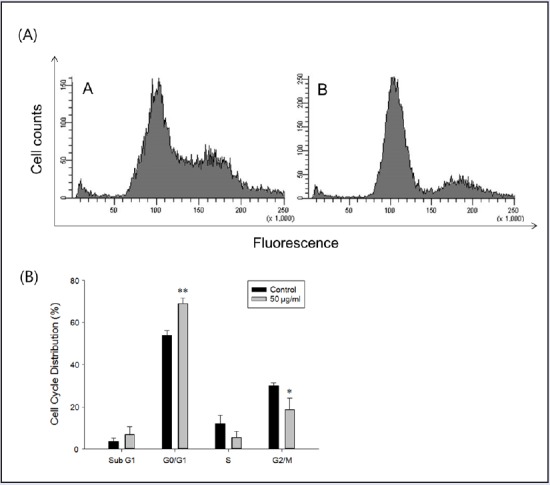
Effects of FFH on the cell cycle distribution. (A) Cell cycle distributions were analyzed by staining intracellular DNA with PI. PC-3 cells were treated with 50 μg/mL of FFH for 24 h. (A) Control; (B) Cells treated with 50 μg/mL of FFH. (B) Percentages of cells in the different phases are shown. Results are the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01 vs. the nontreated control
Effect of FFH on PC-3 apoptosis
During the early stage of apoptosis, phosphatidylserine (PS) residues are exposed on cell surfaces, and annexin-V conjugates appear with these residues.[32] To quantify the apoptotic effect of FFH treatment, flow cytometric analysis was performed using annexin-V/PI double staining. The results obtained showed FFH increased the population of cells both of early and late apoptosis in a dose-dependent manner [Figure 4]. These results suggest that the antitumor effect of FFH is related to the induction of apoptosis in PC-3 cells.
Figure 4.
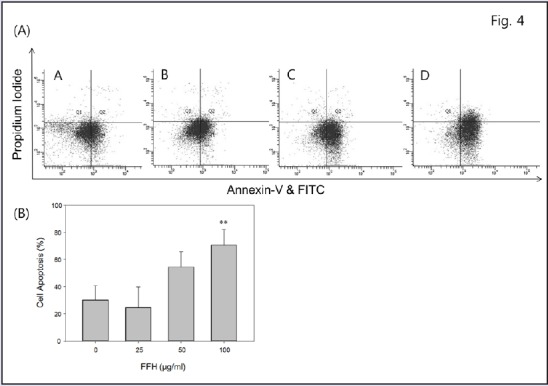
Effect of FFH on apoptosis in PC-3 cells. (A) Percentages of apoptotic cells were determined by annexin-V and PI double staining. PC-3 cells were treated with 25, 50, or 100 μg/mL of FFH for 24 h. (A) Control; (B) 25 μg/mL; (C) 50 μg/mL; (D) 100 μg/mL of FFH administered. (B) The percentages of cells located in four different areas from Q1 to Q4 are shown. Results are the mean ± SD of three independent experiments. **P < 0.01 vs. the nontreated control
Effects of FFH on protein expressions and caspase-3 activity
The protein expressions of apoptosis pathway intermediates were examined in FFH-treated PC-3 cells. Apoptosis was partially regulated by Bcl-2 family protein, and the Bcl-2 family contains antiapoptotic (Bcl-2) and proapoptotic (Bax) molecules.[33] Our study demonstrates that the Bcl-2 protein levels decreased dose dependently [Figure 5], but Bax levels increased dose dependently [Figure 6].
Figure 5.
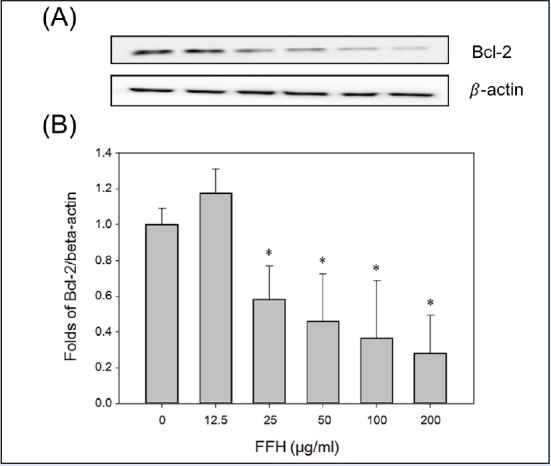
Effects of FFH on Bcl-2 expression in PC-3 cells. (A) Bcl-2 expression levels were evaluated by Western blotting using 30 μg of cell lysates. (B) Protein expression were normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. the nontreated control
Figure 6.
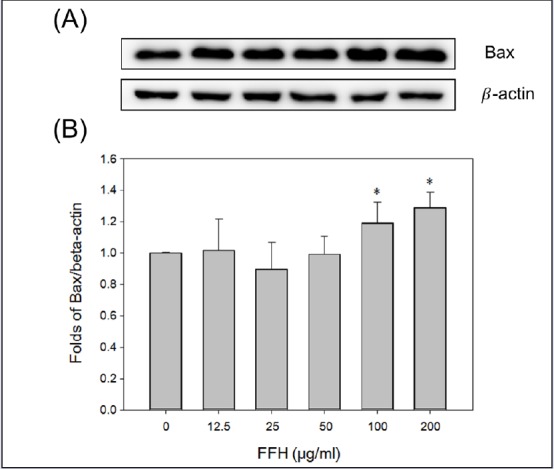
Effects of FFH on Bax expression in PC-3 cells. (A) Bax expression levels were evaluated by Western blotting using 30 μg of cell lysates. (B) Protein expression was normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. the nontreated control
Cytochrome c is mediator of apoptosis caused by DNA damage. Bcl-2 protein has been found to block apoptotic mechanisms downstream of cytochrome c release and depolarize the inner mitochondrial membrane.[34] In the present study, cytochrome c protein expression was increased dose dependently when FFH was treated with PC-3 cells [Figure 7].
Figure 7.
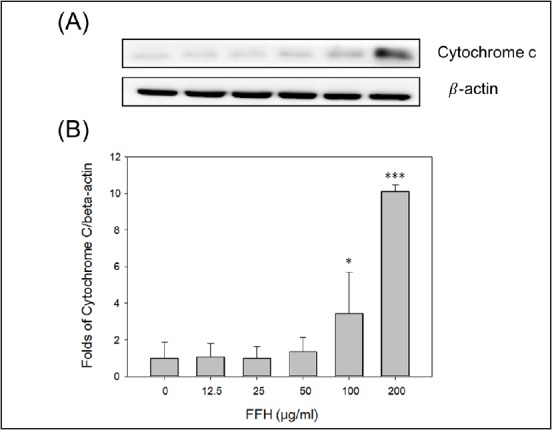
Effects of FFH on cytochrome c expression in PC-3 cells. (A) Cytochrome c levels were evaluated by Western blotting using 30 μg of cell lysates. (B) Protein expression was normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. the nontreated control
In apoptotic pathway, cytoplasm emission increases when cysteine protease caspase-9 is activated; then activated caspase-3 leads to apoptosis.[35] When PC-3 cells were treated with FFH, the activities of the pro-form of caspase-9 and -3 were decreased [Figures 8 and 9] and the expression of the cleaved form of activated caspase-3 expression increased [Figure 10].
Figure 8.
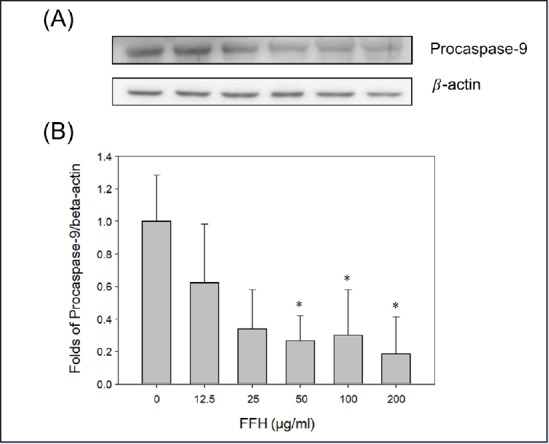
Effects of FFH on procaspase-9 expression in PC-3 cells. (A) Procaspase-9 expression was evaluated by Western blotting using 30 μg of cell lysates. (B) Protein expression was normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 and **P < 0.01 vs. the nontreated control
Figure 9.
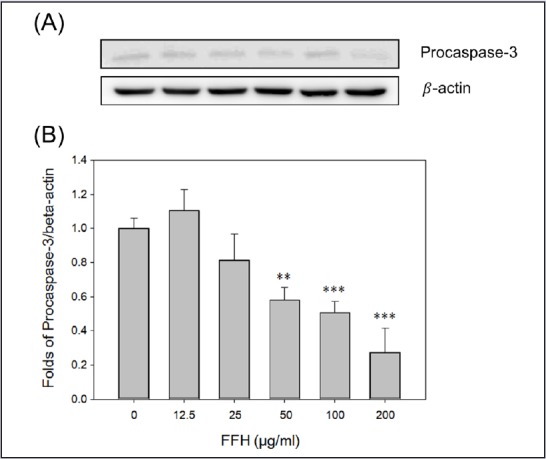
Effects of FFH on procaspase-3 expression in PC-3 cells. (A) Procaspase-3 expression was evaluated by Western blot method using 30 μg of cell lysates. (B) Protein expression was normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. **P < 0.01 and ***P < 0.001 vs. the nontreated control
Figure 10.
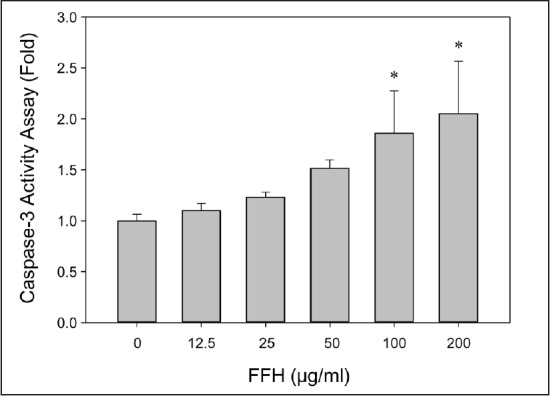
Effects of FFH on caspase-3 activity in PC-3 cells. Caspase-3 activity assay was determined using a colorimetric method. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. the nontreated control
Caspase-3 catalases PARP cleavage, which is considered a hallmark of apoptosis.[17,36,37,38] In the present study, cleaved PARP expression increased dose dependently when PC-3 cells were treated with FFE [Figure 11]. Furthermore, caspase-3 activation and cleaved PARP expression were found to correspond to apoptosis Figure 10 and Figure 11.
Figure 11.
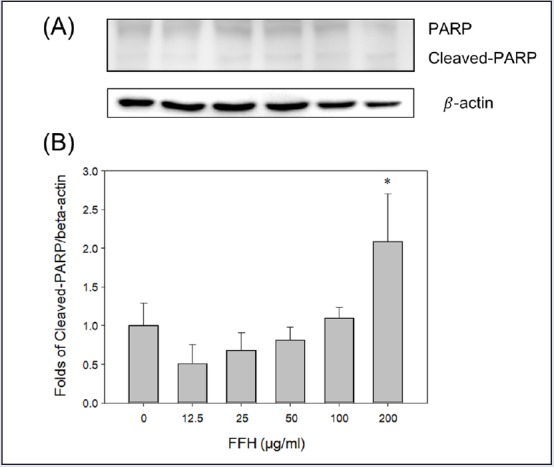
Effects of FFH on PARP expression in PC-3 cells. (A) PARP expression was evaluated by Western blotting using 30 μg of cell lysates. (B) Protein expression was normalized vs. β-actin. Results are presented as the mean ± SD of three independent experiments. *P < 0.05 vs. nontreated control
The above results suggest that FFH controls the protein levels of Bcl-2 family members, activates caspases, and increases cleaved PARP protein levels, and, thus, induces apoptosis via the death receptor dependent pathway in prostate cancer (PC-3) cells.
CONCLUSION
In this study, the anti-cancer effects of FFH were investigated with respect to cell viability, intracellular ROS production, cell cycle distribution, and proportions of apoptotic cells in PC-3 prostate cancer cells. In addition, the effects of FFH on the protein levels of Bcl-2, Bax, cytochrome c, procaspase-9, procaspase-3, and PARP, and on caspase-3 activity were assessed. When administered at a concentration higher than 50 μg/mL FFH significantly and dose dependently reduced cell viability. In addition, FFH time and dose dependently elevated intracellular ROS levels and increased the proportion of cells arrested in the G0/G1 phase. Annexin-V and PI double staining showed that FFH significantly accelerated apoptotic cell death and diminished the protein expression levels of Bcl-2, procaspase-9, and procaspase-3. In addition, the protein expression levels of Bax, cytochrome c, and cleaved PARP were increased by FFH, and so was caspase-3 activity. These results indicate that the anti-cancer effects of FFH on PC-3 prostate cancer cells are closely related to the apoptotic cascade. Taken together, we suggest FFH acts as a pro-oxidative agent that induces apoptosis in prostate cancer cells and that it should be viewed as a potential agent for the treatment of prostate cancer.
Financial support and sponsorship
Nil
Conflicts of interest
There are no conflicts of interest
REFERENCES
- 1.Jemal A, Siegel R, Xu J, Ward E. Cancer statistics, 2010. CA Cancer J Clin. 2010;60:277–300. doi: 10.3322/caac.20073. [DOI] [PubMed] [Google Scholar]
- 2.Culig Z, Hobisch A, Bartsch G, Klocker H. Expression and function of androgen receptor in carcinoma of the prostate. Microsc Res Tech. 2000;51:447–55. doi: 10.1002/1097-0029(20001201)51:5<447::AID-JEMT7>3.0.CO;2-M. [DOI] [PubMed] [Google Scholar]
- 3.Petrylak DP. The current role of chemotherapy in metastatic hormone-refractory prostate cancer. Urology. 2005;65:5 Suppl7. doi: 10.1016/j.urology.2005.03.053. [DOI] [PubMed] [Google Scholar]
- 4.Rathkopf D, Scher HI. Androgen receptor antagonists in castration-resistant prostate cancer. Cancer J. 2013;19:43–9. doi: 10.1097/PPO.0b013e318282635a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.hu ML, Kyprianou N. Role of androgens and the androgen receptor in epithelial-mesenchymal transition and invasion of prostate cancer cells. FASEB J. 2010;24:769–77. doi: 10.1096/fj.09-136994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Scher HI, Sawyers CL. Biology of progressive, castration-resistant prostate cancer: directed therapies targeting the androgen-receptor signaling axis. J Clin Oncol. 2005;23:8253–61. doi: 10.1200/JCO.2005.03.4777. [DOI] [PubMed] [Google Scholar]
- 7.Joyce R, Fenton MA, Rode P, Constantine M, Gaynes L, Kolvenbag G, et al. High dose bicalutamide for androgen independent prostate cancer: effect of prior hormonal therapy. J Urol. 1998;59:149–53. doi: 10.1016/s0022-5347(01)64039-4. [DOI] [PubMed] [Google Scholar]
- 8.Oh WK, Kantoff PW. Management of hormone refractory prostate cancer: current standards and future prospects. J Urol. 1998;160:1220–9. [PubMed] [Google Scholar]
- 9.Oefelein MG, Agarwal PK, Resnick MI. Survival of patients with hormone refractory prostate cancer in the prostate specific antigen era. J Urol. 2004;171:1525–8. doi: 10.1097/01.ju.0000118294.88852.cd. [DOI] [PubMed] [Google Scholar]
- 10.Chen CD, Welsbie DS, Tran C, Baek SH, Chen R, Vessella R, et al. Molecular determinants of resistance to antiandrogen therapy. Nat Med. 2004;10:33–9. doi: 10.1038/nm972. [DOI] [PubMed] [Google Scholar]
- 11.Lu W, Ogasawara MA, Huang P. Models of reactive oxygen species in cancer. Drug Discov Today Dis Models. 2007;4:67–73. doi: 10.1016/j.ddmod.2007.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.van Die MD, Bone KM, Emery J, Williams SG, Pirotta MV, Paller CJ. Phytotherapeutic interventions in the management of biochemically recurrent prostate cancer: a systematic review of randomised trials. BJU Int. 2016;117(Suppl 4):17–34. doi: 10.1111/bju.13361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chaudhary T, Chahar A, Sharma JK, Kaur K, Dang A. Phytomedicine in the treatment of cancer: a health technology assessment. J Clin Diagn Res. 2015;9:XC04–9. doi: 10.7860/JCDR/2015/15701.6913. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Hausott B, Greger H, Marian B. Naturally occurring lignans efficiently induce apoptosis in colorectal tumor cells. J Cancer Res Clin Oncol. 2003;129:569–76. doi: 10.1007/s00432-003-0461-7. [DOI] [PubMed] [Google Scholar]
- 15.Sun J, Liu BR, Hu WJ, Yu LX, Qian XP. In vitro anticancer activity of aqueous extracts and ethanol extracts of fifteen traditional Chinese medicines on human digestive tumor cell lines. Phytother Res. 2007;21:1102–4. doi: 10.1002/ptr.2196. [DOI] [PubMed] [Google Scholar]
- 16.Zhao L, Yan X, Shi J, Ren F, Liu L, Sun S, et al. Ethanol extract of Forsythia suspensa root induces apoptosis of esophageal carcinoma cells via the mitochondrial apoptotic pathway. Mol Med Rep. 2015;11:871–80. doi: 10.3892/mmr.2014.2874. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gadjev I, Stone JM, Gechev TS. Programmed cell death in plants: new insights into redox regulation and the role of hydrogen peroxide. Int Rev Cell Mol Biol. 2008;270:87–144. doi: 10.1016/S1937-6448(08)01403-2. [DOI] [PubMed] [Google Scholar]
- 18.Khandrika L, Kumar B, Koul S, Maroni P, Koul HK. Oxidative stress in prostate cancer. Cancer Lett. 2009;282:18. doi: 10.1016/j.canlet.2008.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Minelli A, Bellezza I, Conte C, Culig Z. Oxidative stress-related aging: a role for prostate cancer? Biochim Biophys Acta. 2009;1795:83–91. doi: 10.1016/j.bbcan.2008.11.001. [DOI] [PubMed] [Google Scholar]
- 20.Priault M, Chaudhuri B, Clow A, Camougrand N, Manon S. Investigation of bax-induced release of cytochrome c from yeast mitochondria permeability of mitochondrial membranes, role of VDAC and ATP requirement. Eur J Biochem. 1999;260:684–91. doi: 10.1046/j.1432-1327.1999.00198.x. [DOI] [PubMed] [Google Scholar]
- 21.Reed JC, Jurgensmeier JM, Matsuyama S. Bcl-2 family proteins and mitochondria. Biochim Biophys Acta. 1998;1366:127–37. doi: 10.1016/s0005-2728(98)00108-x. [DOI] [PubMed] [Google Scholar]
- 22.Van Breusegem F, Dat JF. Reactive oxygen species in plant cell death. Plant Physiol. 2006;141:384–90. doi: 10.1104/pp.106.078295. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Miyamoto S, Verma IM. Rel/NF-kappa B/I kappa B story. Adv Cancer Res. 1995;66:255–92. [PubMed] [Google Scholar]
- 24.Wang CY, Mayo MW, Korneluk RG, Goeddel DV, Baldwin AS. NF-kappaB antiapoptosis: induction of TRAF1 and TRAF2 and c-IAP1 and c-IAP2 to suppress caspase-8 activation. Science. 1998;281:1680–3. doi: 10.1126/science.281.5383.1680. [DOI] [PubMed] [Google Scholar]
- 25.Kaur M, Agarwal R. Transcription factors: molecular targets for prostate cancer intervention by phytochemicals. Curr Cancer Drug Targets. 2007;7:355–67. doi: 10.2174/156800907780809732. [DOI] [PubMed] [Google Scholar]
- 26.Green DR, Reed JC. Mitochondria and apoptosis. Science. 1998;281:1309–12. doi: 10.1126/science.281.5381.1309. [DOI] [PubMed] [Google Scholar]
- 27.Carmichael J, DeGraff WG, Gazdar AF, Minna JD, Mitchell JB. Evaluation of a tetrazolium-based semiautomated colorimetric assay: assessment of radiosensitivity. Cancer Res. 1987;47:943–6. [PubMed] [Google Scholar]
- 28.Gurtu V, Kain SR, Zhang G. Fluorometric and colorimetric detection of caspase activity associated with apoptosis. Anal Biochem. 1997;251:98–102. doi: 10.1006/abio.1997.2220. [DOI] [PubMed] [Google Scholar]
- 29.Trachootham D, Alexandre J, Huang P. Targeting cancer cells by ROS-mediated mechanisms: a radical therapeutic approach? Nat Rev Drug Discov. 2009;8:579–91. doi: 10.1038/nrd2803. [DOI] [PubMed] [Google Scholar]
- 30.Simon HU, Haj-Yehia A, Levi-Schaffer F. Role of reactive oxygen species (ROS) in apoptosis induction. Apoptosis. 2000;5:415–8. doi: 10.1023/a:1009616228304. [DOI] [PubMed] [Google Scholar]
- 31.Liou GY, Storz P. Reactive oxygen species in cancer. Free Radic Res. 2010;44:479–96. doi: 10.3109/10715761003667554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Lecoeur H, Prevost MC, Gougeon ML. Oncosis is associated with exposure of phosphatidylserine residues on the outside layer of the plasma membrane: a reconsideration of the specificity of the annexin V/propidium iodide assay. Cytometry. 2001;44:65–72. doi: 10.1002/1097-0320(20010501)44:1<65::aid-cyto1083>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 33.van Delft MF, Huang DC. How the Bcl-2 family of proteins interact to regulate apoptosis. Cell Res. 2006;16:203–13. doi: 10.1038/sj.cr.7310028. [DOI] [PubMed] [Google Scholar]
- 34.Kluck RM, Bossy-Wetzel E, Green DR, Newmeyer DD. The release of cytochrome c from mitochondria: a primary site for Bcl-2 regulation of apoptosis. Science. 1997;275:1132–6. doi: 10.1126/science.275.5303.1132. [DOI] [PubMed] [Google Scholar]
- 35.Zuo Y, Xiang B, Yang J, Sun X, Wang Y, Cang H, et al. Oxidative modification of caspase-9 facilitates its activation via disulfide-mediated interaction with Apaf-1. Cell Res. 2009;19:449–57. doi: 10.1038/cr.2009.19. [DOI] [PubMed] [Google Scholar]
- 36.Los M, Mozoluk M, Ferrari D, Stepczynska A, Stroh C, Renz A, et al. Activation and caspase-mediated inhibition of PARP: a molecular switch between fibroblast necrosis and apoptosis in death receptor signaling. Mol Biol Cell. 2002;13:978–88. doi: 10.1091/mbc.01-05-0272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Elmore S. Apoptosis: a review of programmed cell death. Toxicol Pathol. 2007;35:495–516. doi: 10.1080/01926230701320337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Soldani C, Scovassi AI. Poly(ADP-ribose) polymerase-1 cleavage during apoptosis: an update. Apoptosis. 2002;7:321–8. doi: 10.1023/a:1016119328968. [DOI] [PubMed] [Google Scholar]


