Abstract
Background:
Eruca sativa Mill. (Brassicaceae) is commonly utilized as an ingredient in salads and also as a folk remedy to treat various diseases.
Objective:
The objective of this study was to establish the contribution of the glucosinolate (GLS) fraction to the overall antioxidant, cytoprotection against oxidative insult and antimicrobial properties of the hydro-alcoholic extract of E. sativa leaves from Sicily (Italy), characterized phytochemically.
Materials and Methods:
The antioxidant activity was evaluated by different in vitro systems. The cytoprotective effect against hydrogen peroxide (H2O2)-induced oxidative stress was tested in human peripheral blood mononuclear cells (PBMCs). The antimicrobial potential against bacteria and fungi was assayed by standard methods.
Results:
E. sativa extract exhibited both radical scavenging (50% inhibitory concentration [IC50] 1.04 ± 0.04 mg/mL) and ferrous ions-chelating activity (IC50 0.327 ± 0.0032 mg/mL) and mild reducing power; the GLS fraction showed chelating ability only (IC50 0.225 ± 0.009 mg/mL). In the experimental model of H2O2-induced oxidative stress in human PBMCs, a significant cytoprotective effect and a suppression of reactive oxygen species production by both extract and GLS fraction were observed (P < 0.001). E. sativa extract displayed moderate antimicrobial activity against Gram-positive bacteria, and Staphylococcus aureus was the most sensitive strain (minimum inhibitory concentration 0.125 mg/mL), whereas the GLS fraction was not active.
Conclusion:
GLSs are not involved in the primary antioxidant activity of E. sativa leaf extract but they are, almost in part, responsible for its ferrous ion-chelating properties. Iron-chelating compounds in E. sativa extract may protect cells under conditions of oxidative stress, and GLSs might play a chief role in this effect.
SUMMARY
Eruca sativa Mill. leaf extract exhibited antioxidant activity in different in vitro systems, whereas the glucosinolate (GLS) fraction showed Fe2+-chelating ability only
A significant cytoprotective effect and a suppression of intracellular reactive oxygen species production by both extract and GLS fraction were observed in human peripheral blood mononuclear cells
E. sativa extract displayed moderate antimicrobial activity against Gram-positive bacteria, whereas the GLS fraction was not active.
Abbreviations used: GLS: Glucosinolate; H2O2: Hydrogen peroxide; PBMCs: Peripheral blood mononuclear cells; IC50: 50% inhibitory concentration; MIC: Minimum inhibitory concentration.
Keywords: Antimicrobial activity, antioxidant potential, Eruca sativa Mill., glucosinolate fraction, H2O2-induced oxidative stress
INTRODUCTION
Eruca sativa Mill. (Brassicaceae) is an annual plant, up to 1 m high. Leaves are dark green and <20 cm long. The basal leaves occur in a rosette and are lobed to pinnatifid; leaves on the upper parts of the plant are pinnatifid, with long-oblong terminal lobes, and are either coarsely toothed or lobed.[1]
This species is cultivated throughout the Mediterranean area, has gradually spread to other latitudes, and is used for its pungent flavor as an ingredient in green leafy salads.[2] E. sativa, commonly referred to as rocket, is widely utilized in folk medicine; traditionally, its use as astringent, diuretic, digestive, emollient, tonic, depurative, laxative, rubefacient, stimulant, as well as antimicrobial is documented.[3,4]
In terms of antioxidant compounds, it represents a good source of vitamins, such as Vitamin C, carotenoids, and polyphenols, which play a very important role among natural antioxidants.[5] Moreover, it is characterized by high glucosinolate (GLS) content, such as other cruciferous vegetables.
Recently, the direct antioxidant effects of the GLSs were reported even if, according to some authors, these compounds possess rather low direct antioxidant activity.[6,7,8] Cabello-Hurtado et al.[9] evaluated the radical scavenging activity of the main cauliflower GLSs by different in vitro models, demonstrating that it was highly dependent on the antioxidant assay used. It has been shown that purified glucoraphanin, after oral administration in rats, is absorbed intact and undergoes enterohepatic circulation; in addition, it is converted into the reduced analog glucoerucin in the body.[10] Thus, it was suggested that GLSs could directly exert their antioxidant activity (if any) into the circulation.[11]
This study aimed to establish the contribution of the GLS fraction to the overall antioxidant properties of the hydro-alcoholic extract of E. sativa leaves from Sicily (Italy). This extract has been fully characterized for its phytochemical content and its antioxidant activity tested in in vitro cell-free assays and in an ex vivo model of hydrogen peroxide (H2O2)-induced oxidative stress in human peripheral blood mononuclear cells (PBMCs).
Besides, since E. sativa is traditionally used for its antiseptic action, it seemed interesting to extend our study to the evaluation of the antimicrobial activity against bacteria and fungi.
MATERIALS AND METHODS
Chemicals and reagents
Lymphoprep™ was obtained from Axis-Shield (Scotland). CellTiter-Blue® was supplied by Promega (USA). Unless indicated otherwise, all chemicals were purchased from Sigma-Aldrich (Milan, Italy).
Plant material and procedure of extraction
E. sativa Mill. leaves were supplied by a small farm located in Messina (Sicily, Italy). Plant material was collected in summer (July); leaves were freeze-dried immediately, extracted with 70% (v/v) aqueous methanol for 30 min at 70°C, and evaporated to dryness under vacuum on a rotary evaporator at 40°C. The yield was 23.05%.
Separation of glucosinolate fraction
GLS fraction was separated from E. sativa extract according to the method reported by Rochfort et al.,[12] with slight modifications. The GLS fraction was evaporated to dryness under a stream of nitrogen at room temperature.
Phytochemical investigations
Total phenolic content
The total phenolic content of E. sativa extract was measured using Folin–Ciocalteu method.[13] Total phenolics were expressed as mg gallic acid equivalents (GAE)/g extract (dw) ± standard deviation (SD). The results were obtained from the average of three independent experiments.
Identification of phenolic compounds by high-performance liquid chromatography-DAD
Phenolic compounds contained in E. sativa extract were identified by high-performance liquid chromatography (HPLC)-DAD analysis according to Tang et al.,[14] with some modifications, by comparison of retention time and ultraviolet spectra of pure standards. The quantitative determination of each compound was carried out using the external standard method.
Identification of desulfated-glucosinolates by liquid chromatography-mass spectrometry
To determine the major GLSs present in E. sativa extract, the desulfated-GLSs (DS-GLSs) were obtained according to the method reported by Barillari et al.,[8] with slight modifications. The DS-GLSs were analyzed by liquid chromatography-mass spectrometry (LC-MS) with positive ion atmospheric pressure chemical ionization (APCI+) and an ion trap detection and quantified by the addition of sinigrin as the internal standard.[15]
Antioxidant activity
The antioxidant activity was evaluated by different in vitro systems. E. sativa extract was tested at different concentrations (0.07–2 mg/mL) and GLS fraction at the dose corresponding to 0.07–2 mg/mL extract, calculated on the basis of the yield. The results were obtained from the average of three independent experiments.
The free radical scavenging activity was determined using the 1,1-diphenyl-2-picrylhydrazyl (DPPH) method.[13] Butylated hydroxytoluene (BHT) was used as reference. The results are reported as mean radical scavenging activity percentage (%) ± SD and mean 50% inhibitory concentration (IC50) ± SD.
The reducing power was evaluated by spectrophotometric detection of Fe3+-Fe2+ transformation method.[13] Ascorbic acid and BHT were used as reference. The results are expressed as mean absorbance values ± SD and ascorbic acid equivalent (ASE/mL).
The Fe2+-chelating activity was estimated by measuring the formation of the Fe2+- ferrozine complex, according to the method previously reported.[13] Ethylenediaminetetraacetic acid (EDTA) was used as reference. The results are reported as mean inhibition of the ferrozine-(Fe2+) complex formation (%) ± SD and IC50 ± SD.
Cytoprotective effects against hydrogen peroxide insult
Effects on hydrogen peroxide-induced cytotoxicity in human peripheral blood mononuclear cells
Peripheral blood samples were drawn from consenting healthy adult donors (20–38 years of age), and human PBMCs were isolated by Lymphoprep™ density gradient centrifugation. PBMCs were re-suspended in RPMI 1640 supplemented with 10% fetal calf serum, penicillin and streptomycin (100 U/mL each), 2 mM glutamine and 1% minimum essential medium nonessential amino acids, and vitamins and cultured at a density of 1 × 106 cells/mL (96-well plate) at 37°C and 5% CO2. PBMCs were treated with E. sativa extract or GLS fraction (0.07–2 mg/mL and dose corresponding to 0.07–2 mg/mL extract, respectively) for 24 h to determine cell viability by the CellTiter-Blue® assay. Fluorescence was measured on a multifunction plate reader (Synergy HT, BioTek), using 560 nm and 590 nm as excitation and emission wavelength, respectively.
To evaluate the cytoprotective effects against H2O2 insult, after 24 h incubation in the presence or absence of extract and GLS fraction, H2O2 was added to induce oxidative stress. Cell viability after exposure of PBMCs to H2O2 (10 mM) for 30 min was determined by the CellTiter-Blue® assay. H2O2 only treated cells were used as the positive control. The results were obtained from the average of three independent experiments, and data were expressed as mean cell viability (%) ± SD.
Effects on hydrogen peroxide-induced intracellular reactive oxygen species production in peripheral blood mononuclear cells
Intracellular reactive oxygen species (ROS) were measured using the oxidant-sensitive probe 2’,7’-dichlorofluorescin diacetate as previously described.[16] PBMCs were pretreated with E. sativa extract and GLS fraction as reported above, followed by exposure to H2O2 (10 mM) for 30 min. Fluorescence was measured using a multifunction plate reader (Synergy HT, BioTek) using 485 nm as λecc and 530 nm as λem. All data were analyzed and expressed as mean ± SD of three independent determinations.
Statistical analysis
A one-way ANOVA followed by Dunnett's posttest was performed to determine the significance of differences between the H2O2-exposed group and treated groups.
Antimicrobial activity
Microbial strains and culture conditions
The following strains were used as indicators for the antimicrobial testing and were obtained from the Department of Scienze Chimiche, Biologiche, Farmaceutiche ed Ambientali, University of Messina, in-house culture collection (Messina, Italy): seven Gram-positive standard strains, Bacillus subtilis ATCC 6633, Enterococcus durans V3 (wild-type strain), Enterococcus hirae ATCC 10541, Listeria monocytogenes ATCC 7644, Staphylococcus aureus ATCC 6538P, methicillin-resistant S. aureus (MRSA) ATCC 43300, and Staphylococcus epidermidis ATCC 12228; six Gram-negative, Escherichia coli ATCC 25922, Proteus mirabilis (wild-type strain), Proteus vulgaris (wild-type strain), Pseudomonas aeruginosa ATCC 27853, Serratia marcescens AM1 (wild-type strain), and Salmonella typhi ATCC 0901; and three fungi, Aspergillus niger ATTC 16404, Candida albicans ATCC 10231, and Candida parapsilosis ATCC 29947. Furthermore, 12 clinical isolates of S. aureus from specimens of skin infections were also assayed.
Antimicrobial testing
The minimum inhibitory concentration (MIC) and minimum bactericidal concentration (MBC) and minimum fungicidal concentration values of E. sativa extract and GLS fraction were determined using the broth microdilution method according to the protocols recommended by the Clinical and Laboratory Standards Institute.[17,18]
The MICs were also performed in the Bioscreen C (Labsystems Oy, Helsinki, Finland) for all strains, as previously reported.[19] The tested concentrations ranged from 4 to 0.0039 mg/mL. All experiments were performed in triplicate on 3 independent days. Positive and negative controls with selected antibiotics (ofloxacin, tetracycline, and ampicillin) and solvents (dimethyl sulfoxide [DMSO]) were included in each assay.
RESULTS
Phytochemical investigations
The total phenolic content of E. sativa hydro-alcoholic extract was 42.05 ± 0.35 mg GAE/g (dw).
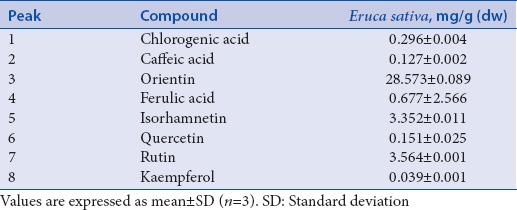
HPLC/DAD analysis of E. sativa extract allowed the identification and quantification of five flavonoids (35.678 mg/g dw) and three hydroxycinnamic acids (1.099 mg/g dw). Orientin turned out to be the main flavonoid detected; among hydroxycinnamic acids, ferulic acid was found to be the most abundant one [Table 1].
Table 1.
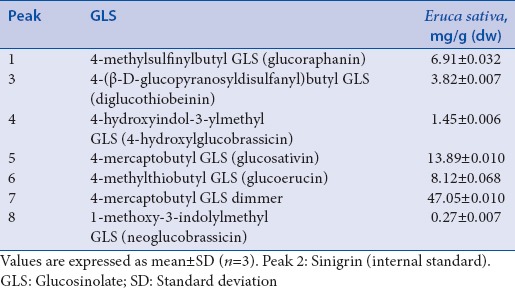
Quantitative determination of phenolic compounds detected by high-performance liquid chromatography-DAD analysis in Eruca sativa leaves
LC-MS analysis led to identification of the major DS-GLSs present in E. sativa extract. Three aliphatic compounds, namely, DS-glucoraphanin, DS-glucosativin, DS-glucoerucin, two indoles, namely, DS-GLSs 4-hydroxylglucobrassicin desulfated and DS-neoglucobrassicin, DS-diglucothiobeinin, and a dimeric 4-mercaptobutyl GLS desulfated were identified by their target ion and further MS/MS measurements after fragmentation of (M+ H)+. DS-GLSs content was 81.51 ± 0.14 mg/g (dw) [Table 2].
Table 2.
Quantitative determination of glucosinolates detected by liquid chromatography-mass spectrometry analysis in Eruca sativa leaves
Antioxidant activity
The antioxidant activity of E. sativa extract and GLS fraction was determined by DPPH test, reducing power assay, and ferrous ions (Fe2+)-chelating activity.
The extract exhibited radical scavenging activity, dose-dependent, which reached the 71% of inhibition at the higher tested concentration (2 mg/mL); nonetheless, it showed an activity lower than BHT, as indicated also by IC50 values (1.04 ± 0.04 mg/mL and 0.12 ± 0.01 mg/mL, respectively). GLS fraction did not display any radical scavenging activity [Figure 1].
Figure 1.
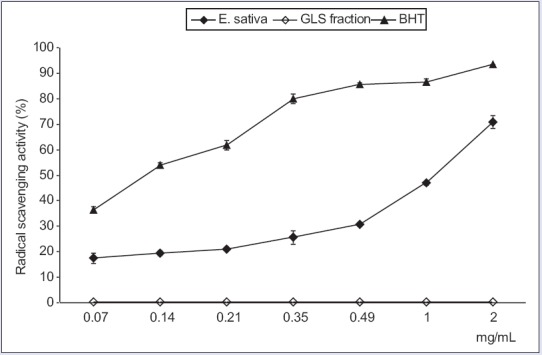
Free radical scavenging activity of Eruca sativa leaf extract and glucosinolate fraction, measured by the 1,1-diphenyl-2-picrylhydrazyl method. The results are expressed as the mean percentage (%) ± standard deviation (n = 3)
The reducing power of the E. sativa extract increased in a dose-dependent manner and was mild, compared to the standard BHT, as confirmed by ASE values (20.27 ASE/mL and 1.75 ASE/mL, respectively). GLS fraction did not show any reducing power (data not shown).
Both the extract and GLS fraction showed good Fe2+-chelating ability, dose-dependent, with an IC50 value of 0.327 ± 0.0032 mg/mL and 0.225 ± 0.009 mg/mL, respectively. Nonetheless, their activity was lower than EDTA [Figure 2].
Figure 2.
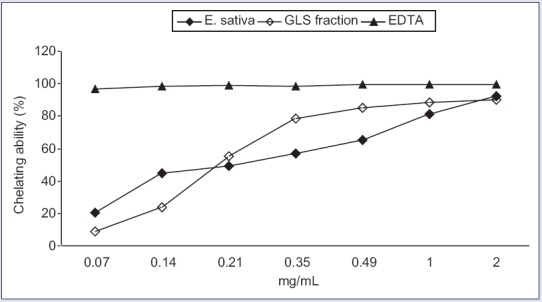
Ferrous ions (Fe2+)-chelating activity of Eruca sativa leaf extract and glucosinolate fraction, measured by inhibition of ferrozine-Fe2+ complex formation. The results are expressed as the mean percentage (%) ± standard deviation (n = 3)
Cytoprotective effects against hydrogen peroxide insult
Effects on hydrogen peroxide-induced cytotoxicity in human peripheral blood mononuclear cells
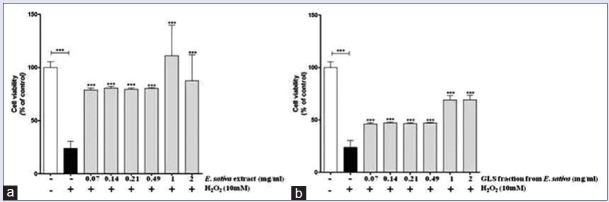
The treatment of PBMCs with E. sativa extract and GLS fraction showed no significant alteration in cell viability (data not shown). On the other hand, H2O2 at a concentration of 10 mM caused more than 70% inhibition of PBMCs viability (P < 0.001). Interestingly, when PBMCs were pretreated for 24 h with E. sativa extract and GLS fraction, the H2O2-induced cytotoxic effect was reduced. At all concentrations tested, cell viability increased by approximately 60% with the extract and 20% with GLS fraction, compared to the PBMCs incubated with H2O2 alone (P < 0.001) [Figure 3].
Figure 3.
Protective effects of Eruca sativa leaf extract (a) and glucosinolate fraction (b) on hydrogen peroxide-induced cytotoxicity in human peripheral blood mononuclear cells as measured by the CellTiter-Blue® assay. Data shown are mean ± standard deviation of three independent experiments. ***P < 0.001 (one-way ANOVA followed by Dunnett's post hoc test)
Effects on hydrogen peroxide-induced intracellular reactive oxygen species production in peripheral blood mononuclear cells
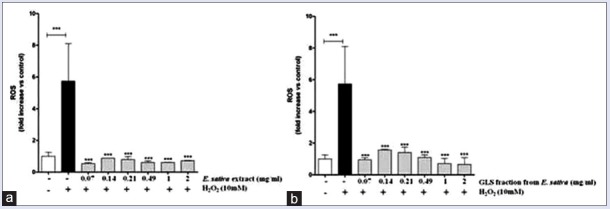
Treatment with E. sativa extract and GLS fraction alone did not alter significantly ROS levels compared to untreated control cells (data not shown). The treatment of PBMCs with H2O2 (10 mM) induced approximately 5-fold increase in ROS production compared to the control (P < 0.001). In contrast, H2O2-induced ROS production was suppressed by both E. sativa extract and GLS fraction at all concentrations tested (P < 0.001) [Figure 4].
Figure 4.
Effects of Eruca sativa leaf extract (a) and glucosinolate fraction (b) on hydrogen peroxide-induced intracellular reactive oxygen species production in human peripheral blood mononuclear cells. Data shown are mean ± standard deviation of three independent experiments. ***P < 0.001 (one-way ANOVA followed by Dunnett's post hoc test)
Antimicrobial activity
E. sativa extract displayed antimicrobial activity against Gram-positive bacteria only; S. aureus ATCC 6538P was the most susceptible strain (MIC 0.125 mg/mL), followed by S. aureus (MRSA) ATCC 43300 (MIC 0.250 mg/mL), B. subtilis, E. durans, E. hirae, L. monocytogenes, and S. epidermidis (MIC 0.5 mg/mL). A weak bactericidal activity was highlighted exclusively for S. aureus ATCC 6538P and B. subtilis (MBC 0.5 mg/mL). Concerning the 12 clinical isolates of S. aureus from specimens of skin infections, E. sativa extract inhibited the growth of 50% (MIC50) and 90% (MIC90) of the strains at the concentration of 0.25 mg/mL and 0.5 mg/mL, respectively.
The GLS fraction did not show antimicrobial effects against any of the Gram-positive and negative bacteria or the fungi tested in this study (MIC and MBC values >4 mg/mL). The results of negative controls containing DMSO indicate the complete absence of inhibition of all the strains tested (data not shown).
DISCUSSION
The role of GLSs as natural antioxidants is debated. A direct antioxidant activity has been reported for the 4-(b-D-glucopyranosyldisulfanyl) butyl-GLS (diglucothiobeinin), which exerted higher antioxidant activity compared to the other disulfide GLSs.[7] Barillari et al.[8] have demonstrated that the 4-methylthiobutyl GLS, commonly known as glucoerucin, exhibited a good direct antioxidant activity. On the contrary, other studies have reported that GLSs are unlikely to account for the direct antioxidant effects of extracts of species of Brassicaceae, considering the weak antioxidant properties showed by purified GLSs.[6]
Germanò et al.[20] have shown that bud extract of Capparis spinosa L., also belonging to the Brassicaceae family, maintained its antioxidant properties after GLSs removal as well.
Recently, two different research groups evaluated the antioxidant capacity of pure GLS compounds by different in vitro radical scavenging assays.[9,11] The results of these works highlighted a weak radical scavenging activity in the DPPH test for all the GLSs assayed, whereas only glucobrassicin, glucoiberin, and gluconapin displayed antioxidant activity in the oxygen radical absorbance capacity and in the superoxide radical scavenging activity assays. Further, 2,2-azino-bis (3-ethyl-benzothiazoline-6-sulfonic acid) assay was carried out by both these research groups, but discordant results were found.
The antioxidant properties of E. sativa have been previously reported.[21,22] In the current study, the role of GLSs in the antioxidant activity of E. sativa leaf hydro-alcoholic extract has been established by different in vitro methods. E. sativa extract showed primary antioxidant properties, as highlighted both in the DPPH test and in reducing power assay, whereas the GLS fraction was completely inactive under the same experimental conditions. Our data are in accordance with those obtained by Cabello-Hurtado et al.[9] and clearly indicate that other bioactives are involved in the primary antioxidant properties of the extract.
The observed antioxidant ability could depend mainly on some flavonoids, i.e., orientin, rutin, and isorhamnetin, contained in higher amounts. Literature data report the marked free radical properties of isorhamnetin and rutin; moreover, it has been shown that orientin is an efficient radical scavenger, too.[23,24,25] However, other constituents such as Vitamin C could also contribute to these antioxidant properties.[5]
Secondary antioxidant properties are generally determined by measuring the ability to chelate transition metal ions, especially Fe2+ but also Cu2+ and Zn2+.[21,26,27] Natella et al.[11] previously investigated the ability of pure GLSs in protecting LDL from copper-catalyzed oxidation, highlighting a good activity for gluconasturtiin only. We investigated whether E. sativa exerts a secondary antioxidant activity by measuring the ability to chelate transition metal ions such as Fe2+. Both E. sativa extract and GLS fraction showed a marked Fe2+-chelating ability, indicating a significant role of GLSs in the strong chelating ability of E. sativa leaf extract.
Aimed at establishing the efficacy of E. sativa extract and GLS fraction to counteract oxidative stress in a more complex biological setting, a cell culture model was used to evaluate cytotoxicity and intracellular ROS production induced by H2O2. In the experimental model of H2O2-induced oxidative stress in human PBMCs, both the extract and GLS fraction showed a statistically significant cytoprotective effect. The present findings indicate that the modulation of intracellular ROS production by E. sativa extract could represent a mechanism of action by which it exerts the cytoprotective effects observed in our ex vivo model of oxidative stress.
As reported above, E. sativa extract displayed both primary and secondary antioxidant properties, whereas GLS fraction showed Fe2+-chelating ability only; hence, the obtained results suggest that iron-chelating compounds contained in E. sativa extract may protect cells under conditions of oxidative stress, and GLSs might play a chief role in this effect. Nonetheless, polyphenols may be involved in the cytoprotective effect of E. sativa extract, too. Phenolic compounds, acting as antioxidants, may function as terminators of free radical chains and as chelators of redox-active metal ions that are capable of catalyzing lipid peroxidation.
Concerning the antimicrobial activity, the obtained results showed that E. sativa extract was effective against Gram-positive bacteria, giving support to the ethnopharmacological use of this species. In contrast, the GLS fraction did not show any activity against the bacteria and fungi tested; thus, it can be hypothesized that other phytochemicals are involved in the antibacterial properties of the crude extract. The in vitro antimicrobial activity of some polyphenol classes has been widely shown by the scientific literature published over the past two decades. The antibacterial properties highlighted could depend, almost in part, on phenolic compounds contained in E. sativa extract, i.e., flavonoids and phenolic acids. Among flavonoids, orientin, the main compound identified in the extract, quercetin, and kaempferol were previously found to be active against S. aureus; on the contrary, no antimicrobial effects were observed for isorhamnetin and rutin against the same strain.[28,29,30] Daglia[31] reported the antibacterial activity of ferulic and caffeic acids, against S. aureus and L. monocytogenes, whereas chlorogenic acid showed no activity against Gram-positive bacteria.
CONCLUSION
Our findings showed that GLSs are not involved in the antibacterial and primary antioxidant activities of E. sativa leaf extract but they contribute to its ferrous ion-chelating properties. E. sativa extract seems to be able to protect human mononuclear cells against H2O2 insult by increasing cells resistance to oxidative stress, and GLSs might play a key role in this effect.
Financial support and sponsorship
Nil.
Conflicts of interest
There are no conflicts of interest.
REFERENCES
- 1.Hall MK, Jobling JJ, Rogers GS. Some perspectives on rocket as a vegetable crop: A review. Veget Crops Res Bull. 2012;76:21–41. [Google Scholar]
- 2.Bennett RN, Rosa EA, Mellon FA, Kroon PA. Ontogenic profiling of glucosinolates, flavonoids, and other secondary metabolites in Eruca sativa (salad rocket), Diplotaxis erucoides (wall rocket), Diplotaxis tenuifolia (wild rocket), and Bunias orientalis (Turkish rocket) J Agric Food Chem. 2006;54:4005–15. doi: 10.1021/jf052756t. [DOI] [PubMed] [Google Scholar]
- 3.Sarwar Alam M, Kaur G, Jabbar Z, Javed K, Athar M. Eruca sativa seeds possess antioxidant activity and exert a protective effect on mercuric chloride induced renal toxicity. Food Chem Toxicol. 2007;45:910–20. doi: 10.1016/j.fct.2006.11.013. [DOI] [PubMed] [Google Scholar]
- 4.Darwish RM, Aburjai TA. Effect of ethnomedicinal plants used in folklore medicine in Jordan as antibiotic resistant inhibitors on Escherichia coli. BMC Complement Altern Med. 2010;10:9. doi: 10.1186/1472-6882-10-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martínez-Sánchez A, Gil-Izquierdo A, Gil MI, Ferreres F. A comparative study of flavonoid compounds, Vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J Agric Food Chem. 2008;56:2330–40. doi: 10.1021/jf072975+. [DOI] [PubMed] [Google Scholar]
- 6.Plumb GW, Lambert N, Chambers SJ, Wanigatunga S, Heaney RK, Plumb JA, et al. Are whole extracts and purified glucosinolates from cruciferous vegetables antioxidants? Free Radic Res. 1996;25:75–86. doi: 10.3109/10715769609145657. [DOI] [PubMed] [Google Scholar]
- 7.Kim SJ, Jin S, Ishii G. Isolation and structural elucidation of 4-(beta-D-glucopyranosyldisulfanyl) butyl glucosinolate from leaves of rocket salad (Eruca sativa L.) and its antioxidative activity. Biosci Biotechnol Biochem. 2004;68:2444–50. doi: 10.1271/bbb.68.2444. [DOI] [PubMed] [Google Scholar]
- 8.Barillari J, Canistro D, Paolini M, Ferroni F, Pedulli GF, Iori R, et al. Direct antioxidant activity of purified glucoerucin, the dietary secondary metabolite contained in rocket (Eruca sativa Mill.) seeds and sprouts. J Agric Food Chem. 2005;53:2475–82. doi: 10.1021/jf047945a. [DOI] [PubMed] [Google Scholar]
- 9.Cabello-Hurtado F, Gicquel M, Esnault MA. Evaluation of the antioxidant potential of cauliflower (Brassica oleracea) from a glucosinolate content perspective. Food Chem. 2012;132:1003–9. [Google Scholar]
- 10.Bheemreddy RM, Jeffery EH. The metabolic fate of purified glucoraphanin in F344 rats. J Agric Food Chem. 2007;55:2861–6. doi: 10.1021/jf0633544. [DOI] [PubMed] [Google Scholar]
- 11.Natella F, Maldini M, Leoni G, Scaccini C. Glucosinolates redox activities: Can they act as antioxidants? Food Chem. 2014;149:226–32. doi: 10.1016/j.foodchem.2013.10.134. [DOI] [PubMed] [Google Scholar]
- 12.Rochfort S, Caridi D, Stinton M, Trenerry VC, Jones R. The isolation and purification of glucoraphanin from broccoli seeds by solid phase extraction and preparative high performance liquid chromatography. J Chromatogr A. 2006;1120:205–10. doi: 10.1016/j.chroma.2006.01.046. [DOI] [PubMed] [Google Scholar]
- 13.Miceli N, Trovato A, Marino A, Bellinghieri V, Melchini A, Dugo P, et al. Phenolic composition and biological activities of Juniperus drupacea Labill. berries from Turkey. Food Chem Toxicol. 2011;49:2600–8. doi: 10.1016/j.fct.2011.07.004. [DOI] [PubMed] [Google Scholar]
- 14.Tang D, Yang D, Tang A, Gao Y, Jiang X, Mou J, et al. Simultaneous chemical fingerprint and quantitative analysis of Ginkgo biloba extract by HPLC-DAD. Anal Bioanal Chem. 2010;396:3087–95. doi: 10.1007/s00216-010-3536-8. [DOI] [PubMed] [Google Scholar]
- 15.Saha S, Hollands W, Teucher B, Needs PW, Narbad A, Ortori CA, et al. Isothiocyanate concentrations and interconversion of sulforaphane to erucin in human subjects after consumption of commercial frozen broccoli compared to fresh broccoli. Mol Nutr Food Res. 2012;56:1906–16. doi: 10.1002/mnfr.201200225. [DOI] [PubMed] [Google Scholar]
- 16.Melchini A, Catania S, Stancanelli R, Tommasini S, Costa C. Interaction of a functionalized complex of the flavonoid hesperetin with the AhR pathway and CYP1A1 expression: Involvement in its protective effects against benzo[a]pyrene-induced oxidative stress in human skin. Cell Biol Toxicol. 2011;27:371–9. doi: 10.1007/s10565-011-9194-6. [DOI] [PubMed] [Google Scholar]
- 17.Clinical and Laboratory Standards Institute, editor. Reference method for broth dilution antifungal susceptibility testing of yeasts, approved standard M27-A3. 3rd ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute (CLSI); 2008. [Google Scholar]
- 18.Clinical and Laboratory Standards Institute, editor. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, approved standard M07-A8. 8th ed. Wayne, PA, USA: Clinical and Laboratory Standards Institute (CLSI); 2009. [Google Scholar]
- 19.D’Arrigo M, Ginestra G, Mandalari G, Furneri PM, Bisignano G. Synergism and postantibiotic effect of tobramycin and Melaleuca alternifolia (tea tree) oil against Staphylococcus aureus and Escherichia coli. Phytomedicine. 2010;17:317–22. doi: 10.1016/j.phymed.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 20.Germanò MP, De Pasquale R, D’Angelo V, Catania S, Silvari V, Costa C. Evaluation of extracts and isolated fraction from Capparis spinosa L. buds as an antioxidant source. J Agric Food Chem. 2002;50:1168–71. doi: 10.1021/jf010678d. [DOI] [PubMed] [Google Scholar]
- 21.Heimler D, Isolani L, Vignolini P, Tombelli S, Romani A. Polyphenol content and antioxidative activity in some species of freshly consumed salads. J Agric Food Chem. 2007;55:1724–9. doi: 10.1021/jf0628983. [DOI] [PubMed] [Google Scholar]
- 22.Maia ML, Correia-Sá L, Coelho A, Barroso MF, Domingues VF, Delerue-Matos C. Eruca sativa: Benefits as antioxidants source versus risks of already banned pesticides. J Environ Sci Health B. 2015;50:338–45. doi: 10.1080/03601234.2015.1000178. [DOI] [PubMed] [Google Scholar]
- 23.Edenharder R, Grünhage D. Free radical scavenging abilities of flavonoids as mechanism of protection against mutagenicity induced by tert-butyl hydroperoxide or cumene hydroperoxide in Salmonella typhimurium TA102. Mutat Res. 2003;540:1–18. doi: 10.1016/s1383-5718(03)00114-1. [DOI] [PubMed] [Google Scholar]
- 24.Cheel J, Theoduloz C, Rodríguez J, Schmeda-Hirschmann G. Free radical scavengers and antioxidants from Lemongrass (Cymbopogon citratus (DC.) Stapf.) J Agric Food Chem. 2005;53:2511–7. doi: 10.1021/jf0479766. [DOI] [PubMed] [Google Scholar]
- 25.Bao M, Lou Y. Isorhamnetin prevent endothelial cell injuries from oxidized LDL via activation of p38MAPK. Eur J Pharmacol. 2006;547:22–30. doi: 10.1016/j.ejphar.2006.07.021. [DOI] [PubMed] [Google Scholar]
- 26.Gülçin I, Elmastas M, Aboul-Enein HY. Determination of antioxidant and radical scavenging activity of Basil (Ocimum basilicum L. Family Lamiaceae) assayed by different methodologies. Phytother Res. 2007;21:354–61. doi: 10.1002/ptr.2069. [DOI] [PubMed] [Google Scholar]
- 27.Karamac M. Chelation of Cu(II), Zn(II), and Fe(II) by tannin constituents of selected edible nuts. Int J Mol Sci. 2009;10:5485–97. doi: 10.3390/ijms10125485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Perera Córdova WH, González Mesa L, Payo Hill AL, Nogueiras Lima C, Delgado Lamas G, Oquendo Suárez M. Antimicrobial activity of crude extracts and flavonoids from leaves of Pluchea carolinensis (Jacq.) G. Don. Pharmacol Online. 2006;3:757–61. [Google Scholar]
- 29.Lee OH, Lee BY. Antioxidant and antimicrobial activities of individual and combined phenolics in Olea europaea leaf extract. Bioresour Technol. 2010;101:3751–4. doi: 10.1016/j.biortech.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 30.Kawashty SA, Hussein SR, Marzouk MM, Ibrahim LF, Helal MM, El Negomy SI. Flavonoid constituents from Morettia philaena (Del.) DC. and their antimicrobial activity. J Appl Sci Res. 2012;8:1484–9. [Google Scholar]
- 31.Daglia M. Polyphenols as antimicrobial agents. Curr Opin Biotechnol. 2012;23:174–81. doi: 10.1016/j.copbio.2011.08.007. [DOI] [PubMed] [Google Scholar]