Abstract
Importance
Multiple myeloma has been classified as exhibiting “limited or suggestive evidence” of an association with exposure to herbicides in Vietnam Veterans. Occupational studies have shown that other pesticides (i.e., insecticides, herbicides, fungicides) are associated with excess risk of multiple myeloma (MM) and its precursor state, monoclonal gammopathy of undetermined significance (MGUS); however no studies have uncovered such an association in Vietnam Veterans.
Objective
To examine the relationship between MGUS and exposure to Agent Orange, including its contaminant 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), in Vietnam Veterans.
Design
Our study is a prospective cohort study, testing for MGUS in serum specimens collected and stored in 2002 by the Air Force Health Study (AFHS). The relevant exposure data collected by the AFHS was also used. We tested all specimens in 2013 without knowledge of the exposure status. The AFHS included former US Air Force personnel who participated in Operation Ranch Hand (Ranch Hand Veterans) and other US Air Force personnel who had similar duties in Southeast Asia during the same time period but were not involved in herbicide spray missions (comparison Veterans).
Setting
Agent Orange was used by the US Air Force personnel who conducted aerial spray missions of herbicides (Operation Ranch Hand) in Vietnam from 1962–1971.
Participants
We included 479 Ranch Hand Veterans and 479 comparison Veterans who participated in the 2002 follow-up examination of AFHS.
Exposure
Agent Orange and 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD, a contaminant of Agent Orange). Serum TCDD levels were measured in 1987, 1992, 1997, and 2002.
Main Outcomes and Measures
Risk of MGUS measured by prevalence, odds ratios (OR), and 95% confidence intervals (CI).
Results
The prevalence of MGUS in Ranch Hand Veterans (7.1%) was higher than in comparison Veterans (3.1%) (adjusted OR=2.37, 95% CI, 1.27–4.44; P=0.007). The cohort status was significantly (P=0.0001) associated with TCDD levels: 47% of Ranch Hand Veterans had serum TCDD levels >10.92 ppt compared to 2.5% of comparison Veterans.
Conclusions and Relevance
Ranch Hand Veterans have a significantly increased risk of MGUS, supporting an association between Agent Orange exposure and multiple myeloma.
INTRODUCTION
Over 22,000 Americans are diagnosed annually with multiple myeloma.1 The estimated U.S. prevalence count was 51,930 in 20032 and 83,367 in 20111; this rising trend is likely to continue due to improved diagnostics and therapeutics.3 The median age at diagnosis is 69 years,1 but it can occur in individuals as young as 30 years. Evidence from a large, prospective population-based cancer screening trial show that multiple myeloma is consistently preceded by a precursor state, monoclonal gammopathy of undetermined significance (MGUS).4
Although the cause of MGUS and multiple myeloma remain largely unclear, previous cohort 5–12 and case-control studies 13–26 have reported an elevated risk of multiple myeloma among farmers and other agricultural workers. More specifically, pesticides (i.e., insecticides, herbicides, fungicides) have been hypothesized as the basis for these associations.27–30 In the first prospective cohort study estimating MGUS risk in relation to pesticide exposure in a sample of 678 male pesticide applicators, a 2-fold significantly increased prevalence of MGUS was observed among pesticide applicators, adding support to the hypothesis that pesticides are linked to myelomagenesis.31
To expand our knowledge on the association between herbicides and MGUS, a precursor state of multiple myeloma, we assayed 958 serum samples obtained from U.S. Air Force (USAF) personnel who conducted aerial herbicide spray missions of Agent Orange in Vietnam from 1962–1971 (Operation Ranch Hand) and controls.32 Aims of our study were to determine the prevalence of MGUS among Ranch Hand Veterans versus controls and to assess the risk of MGUS in relation to the body burden of 2,3,7,8-tetrachlorodibenzo-p-dioxin (TCDD), an Agent Orange contaminant that has been classified as a human carcinogen.33
METHODS
Study Population
The study base population comprised 1,951 USAF personnel who participated in the 2002 follow-up examination of the Air Force Health Study (AFHS): 777 who conducted aerial herbicide spray missions of Agent Orange in Vietnam from 1962–1971 (Ranch Hand Veterans) and 1174 who had similar duties in Southeast Asia during the same time period, but were not involved in herbicide spray missions (comparison Veterans). The original Ranch Hand and comparison Veterans were identified from historical data sources, including morning reports, military personnel records, and historical computer tapes at the National Personnel Records Center and the USAF Human Resources Laboratory at Brooks Air Force Base.34 The AFHS conducted six follow-ups from 1982 to 2002. At each follow-up, serum specimens were collected and stored at −70°C.
The Veterans eligible for this study were at least 50 years of age at the 2002 follow-up, had a saved serum specimen collected at the 2002 follow-up, and consented to the use of their data and specimen for future research studies. A total of 1349 Veterans met the eligibility criteria, including 560 Ranch Hand and 789 comparison Veterans. Because the prevalence of MGUS increases rapidly with age, we systematically selected the oldest 480 of the 560 eligible Ranch Hand Veterans for our study. Subsequently, we selected 480 of the 789 comparison Veterans based on a stratified random sampling scheme determined by the age distribution (categories 50–59, 60–69, 70–79, and ≥80 years in 2002) of the selected Ranch Hand Veterans. Following the diagnostic criteria for MGUS,35 we excluded individuals with a prior history of multiple myeloma (ICD9 203.0), Waldenström’s macroglobulinemia (ICD9 273.3), solitary plasmacytoma (ICD9 238.6), or amyloidosis (ICD9 277.30, 277.39). After applying the MGUS criteria, we excluded one Ranch Hand and one comparison Veterans with multiple myeloma, resulting in a final sample size of 958 Veterans (479 Ranch Hand and 479 comparison Veterans).
The study protocol was approved by an Institutional Review Board (IRB) of the U.S. Centers for Disease Control and Prevention. Access to the AFHS data and stored serum specimens for this study was approved by the Institute of Medicine, which serves as a custodian for AFHS resources. As for the protein assays conducted at the National Institutes of Health (NIH), an exemption from IRB review was obtained from the NIH Office of Human Subjects Research.
Clinical, Laboratory, and Exposure data
Following the study protocol, we obtained the AFHS questionnaire, physical examination, and laboratory data for the selected Veterans. All data were obtained with coded ID numbers that were different from the original ID numbers of the AFHS participants. No personal identifying information was obtained. In brief, we obtained information regarding race, birth year, military occupation (enlisted ground, enlisted flyer, officer), body mass index (weight in kilograms divided by height in meters squared), smoking history (pack-years), drinking history (drink-years), lipid-adjusted TCDD levels, serum creatinine levels, history of radiation- or chemo-therapy for cancer treatments (yes, no), and coded diagnoses (ICD-9). One pack-year of smoking was defined as 365 packs of cigarettes smoked during a single year. One drink-year was the equivalent of drinking 1.5 ounces of an 80 proof alcoholic beverage, one 12-ounce beer, or one 5-ounce glass of wine per day for 1 year.36 We also obtained multiple causes of death for deceased Veterans. Lipid-adjusted serum TCDD concentrations were measured by using high resolution gas chromatographic/high resolution mass spectrometric analysis, as previously described.37 TCDD concentrations were either measured in 1987 or reconstructed.36 The 1987 reference point was chosen because the majority of participants had a TCDD measurement in 1987.36 When a participant did not have a TCDD measurement in 1987, the measurement existing in the nearest subsequent follow-up was chosen (1992, 1997, 2002 follow-ups); for the Ranch Hand Veterans whose chosen measurement exceeded 10 ppt, their 1987 level was reconstructed by extrapolating the measurement using a first-order elimination model with a half-life of 7.6 years.36 Dioxin results below the limit of detection were estimated as the limit of detection divided by the square root of 2.38
Serum Specimen and Laboratory Methods
A 1 ml aliquot from the serum collected by the AFHS during the 2002 examination was obtained for each Veteran selected for this study. Each aliquot tube was labeled only with the participant’s coded ID number and the specimen collection year. All specimens were shipped on dry ice to the Multiple Myeloma Research Laboratory at the National Cancer Institute, where protein assays were performed. The samples from the two cohorts (Ranch Hand and Comparison) were tested concurrently in a blinded fashion by R.C., D.B. and O.L. In brief, serum specimens were first analyzed using conventional agarose-gel electrophoresis to determine the occurrence and pattern of M-protein bands, as described previously.31 Samples observed with an M-protein band, equivocal band pattern, or abnormal free light chain (FLC) ratio were further analyzed by immunofixation to characterize the heavy and light chain isotypes of the M protein. Serum protein electrophoresis and immunofixation were performed using the SPIFE 3000 (Helena Laboratories, Beaumont, TX). The FLC levels in all serum specimens were determined using a turbitimetric assay (Freelite®; The Binding Site, Birmingham, UK) performed on a SPAPLUS® Automated Analyzer for Specialist Protein Analysis.39 The Freelite assay is comprised of two separate measurements: one to detect free-κ light chains (normal range, 3.3–19.4 mg/L), and the other to detect free-λ light chains (normal range, 5.7–26.3 mg/L).40 We assessed monoclonality based on the κ/λ FLC-ratios.39 In accord with the literature,40 a normal FLC-ratio was defined as 0.26–1.65 and 0.37–3.1 for individuals with estimated glomerular filtration rates (eGFR) of ≥60 mL/min/1.73 m2 and <60 mL/min/1.73m2, respectively. Using the Modification of Diet in Renal Disease Study equation (http://nkdep.nih.gov/lab-evaluation/gfr/estimating.shtml), we calculated eGFR from serum creatinine. Based on current diagnostic criteria, MGUS was defined as either the presence of an M-protein band detected by immunofixation or an abnormal FLC-ratio with an increased total concentration of the involved light chain.41 Light-chain MGUS was defined as the presence of a free light chain band without heavy chain expression in immunofixation or presence of an abnormal FLC-ratio with an increased level of the involved light chain.41
Statistical Analyses
We examined the differences in the demographic, exposure, and clinical characteristics between Ranch Hand and comparison Veterans by using Fisher’s exact test for categorical variables and Kruskal-Wallis test for continuous variables. The relationship between MGUS status (present versus absent) and cohort status (Ranch Hand Veteran versus comparison Veteran) was assessed without adjustment by using Fisher’s exact test. Our study would achieve a power of 80% to detect a doubling of the MGUS prevalence in the Ranch Hand cohort relative to the comparison cohort, assuming an MGUS prevalence of 5% in the comparison cohort, with 474 Veterans per cohort employing two-sided testing with a significance level of 5%. Our study included 479 Veterans in each cohort. An adjusted contrast was carried out with a logistic regression model of MGUS status in terms of the cohort status and covariates. The relationship between MGUS status and TCDD body burden was assessed using a logistic regression model of MGUS in terms of TCDD. We used the lipid-adjusted TCDD concentrations of all Veterans in our study categorized into 4 levels (cut points: 25th, 50th, 75th percentiles). We carried out logistic regression analyses for cohort status and TCDD body burden separately because they were highly correlated. Adjusted odds ratios (OR) and 95% confidence intervals (95% CI) were calculated by including the following covariates in all models: age, race, body mass index at the 2002 examination, and changes in the body mass index between 2002 and the time of blood draw for the TCDD measurement. These covariates were chosen because age and race are established risk factors for MGUS,42 while body mass index is a known determinant of TCDD half-life43 and also a suggested risk factor for multiple myeloma.44 We also examined the following variables, individually or collectively, as potential additional covariates judged by the Wald test P-values (<=0.3) and changes in odds ratios (>15%): military occupation, smoking history, drinking history, history of radiation- or chemo-therapy for cancer treatments. We did not assess interaction effects between the covariates. All analyses were carried out by using SAS Version 9.3 for Windows (SAS Institute, Cary, North Carolina). Statistical significance was assessed using a two-sided P value of 0.05.
RESULTS
As shown in Table 1, the 479 Ranch Hand Veterans and 479 comparison Veterans had similar demographic characteristics, life-style characteristics and medical histories. All Veterans were men. The median age in 2002 was 65 years for both Ranch Hand Veterans (range, 55–89 years) and comparison Veterans (range, 55–84 years); less than 5% of the Veterans were black in both cohorts (Table 1). The cohort status was significantly (P=0.0001) associated with the TCDD levels (≤3.65, 3.66–5.80, 5.81–10.92, >10.92 ppt) from the 1987 reference year. This is illustrated most obviously by the increased percentage of men with TCDD>10.92 ppt in the Ranch Hand Veterans (47.5%) relative to those in the comparison Veterans (2.5%) (Table 1). For the majority of the Ranch Hand Veterans (417, or 87%) and comparison Veterans (354, or 74%), the 1987 reference year level was determined by taking the direct measurement made in 1987. For the remaining Veterans without a TCDD measurement made in 1987, the reference year value was determined as described in the Methods section; a total of 19 Ranch Hand Veterans had been assigned a reconstructed 1987 value by extrapolation.
Table 1.
Characteristics of Ranch Hand and Comparison Veterans Selected for the Study
| No. (%) |
P Valueb | ||
|---|---|---|---|
| Ranch Hand (n=479)a | Comparison (n=479)a | ||
| Age in 2002, y | |||
| Median (IQR) | 65.0 (58.0–70.0) | 65.0 (59.0–70.0) | .76 |
| 55–59 | 143 (29.9) | 144 (30.1) | |
| 60–64 | 91 (19.0) | 90 (18.8) | |
| 65–69 | 120 (25.1) | 120 (25.1) | |
| 70–74 | 97 (20.3) | 98 (20.5) | |
| 75–89 | 28 (5.8) | 27 (5.6) | |
| Men | 479 (100) | 479 (100) | |
| Race | |||
| Black | 23 (4.8) | 20 (4.2) | .75 |
| Non-black | 456 (95.2) | 459 (95.8) | |
| Body mass indexc | |||
| Median (IQR) | 28.2 (25.7–31.2) | 28.4 (26.1–31.0) | |
| Under weight | 1 (0.2) | 0 (0) | |
| Normal | 90 (18.8) | 77 (16.1) | .36 |
| Over weight | 225 (47.1) | 246 (51.4) | |
| Obese | 162 (33.9) | 156 (32.6) | |
| Received treatment for cancer within 5 yearsc | 9 (1.9) | 7 (1.5) | .80 |
| Tested positive for HIV infectionc | 1 (0.2) | 0 (0) | |
| Occupation group | |||
| Officer | 236 (49.3) | 228 (47.6) | |
| Enlisted flyer | 94 (19.6) | 78 (16.3) | .18 |
| Enlisted ground | 149 (31.1) | 173 (36.1) | |
| Lifetime cigarette smoking, pack-yrsc | |||
| Median (IQR) | 8.0 (0–29.3) | 7.5 (0–24.0) | .28 |
| Lifetime drinking, drink-yrsc | |||
| Median (IQR) | 21.6 (7.4–48.9) | 23.0 (9.1–53.5) | .30 |
| Lipid-adjusted TCDD, pptd | |||
| Median (IQR) | 10.5 (6.2–21.4) | 4.1 (2.9–5.8) | |
| ≤3.65 | 45 (9.4) | 196 (40.9) | |
| 3.66–5.80 | 68 (14.2) | 169 (35.3) | <.0001 |
| 5.81–10.92 | 138 (28.9) | 102 (21.3) | |
| >10.92 | 227 (47.5) | 12 (2.5) | |
Abbreviations: IQR, interquartile range; HIV, human immunodeficiency virus; TCDD, 2,3,7,8-tetrachlorodibenzo-p-dioxin.
Numbers for some variables do not sum to group totals (n=479) because of missing data.
Kruskal-Wallis test for continuous variables: Fisher’s exact test for categorical variables.
Data was obtained during the AFHS 2002 physical examination. One pack-yr smoking was defined as 365 packs of cigarettes smoked during a single year; One drink-yr was the equivalent of drinking 1.5 ounces of an 80 proof alcoholic beverage, one 12-ounce beer, or one 5-ounce glass of wine per day for 1 year36.
Agent Orange and MGUS prevalence
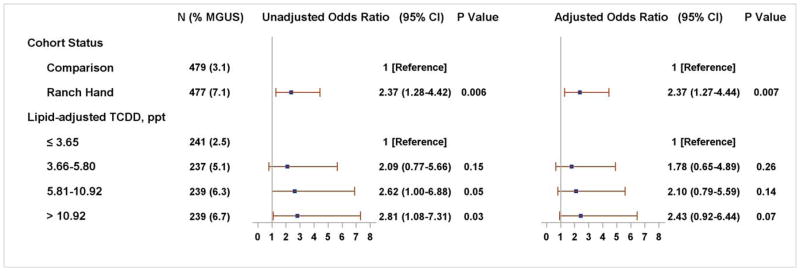
The crude prevalence of overall MGUS was 7.1% (34/479) in Ranch Hand Veterans and 3.1% (15/479) in comparison Veterans (Table 2). This translated into a 2.4-fold higher MGUS prevalence in Ranch Hand Veterans than comparison Veterans after adjusting for age, race, body mass index in 2002, the change in body mass index between 2002 and the time of blood draw for TCDD measurement (adjusted OR=2.37, 95% CI, 1.27–4.44; P=0.007) (Figure 1). The risk of MGUS was significantly increased in Veterans <70 years of age (OR=3.4, 95% CI 1.46–8.13, P=0.004), whereas no significant increase in the risk was seen in the ≥70 years group (OR=1.4, 95% CI 0.55–3.63, P=0.63). Although a logistic model of MGUS in terms of cohort (Ranch Hand, Comparison), age (<70, ≥70), and the cohort by age interaction term revealed a non-significant interaction term (P=0.17), small cell counts may have contributed to the lack of significance. When compared to the Veterans in the lowest TCDD level (≤3.65 ppt), the crude ORs for having MGUS were 2.09 (95% CI, 0.77–5.66; P=0.15), 2.62 (95% CI, 1.00–6.88; P=0.05), and 2.81 (95% CI, 1.08–7.31; P=0.03) for Veterans with TCDD levels of 3.66–5.80 ppt, 5.81–10.92 ppt, and >10.92 ppt, respectively (Figure 1). After adjusting for covariates, the TCDD effect observed at the >10.92 ppt level did not reach the conventional significance level at P=0.05 (adjusted OR=2.43, 95% CI 0.92–6.44; P=0.07).
Table 2.
Age-Specific Prevalence of MGUS among Ranch Hand and Comparison Veterans
| Age Range, y | MGUS N/Total N Prevalence
% (95% CI) |
P Value a | |||
|---|---|---|---|---|---|
| Ranch Hand Veterans | Comparison Veterans | ||||
| 55–59 | 5/143 | 3.5 (1.1–8.0) | 0/144 | 0 | |
| 60–69 | 18/211 | 8.5 (5.1–13.2) | 7/210 | 3.3 (1.4–6.8) | |
| ≥70 | 11/125 | 8.8 (4.5–15.2) | 8/125 | 6.4 (2.8–12.2) | |
| All | 34/479 | 7.1 (5.0–9.8) | 15/479 | 3.1 (1.8–5.1) | .008 |
P value by Fisher’s exact test for the null hypothesis that there is no association between cohort status and MGUS.
Figure 1.
Adjusted Odds Ratios of MGUS among US Veterans Associated with TCDD Exposure and Operation Ranch Hand
Abbreviations: OR, odds ratio; 95% CI, 95% confidence interval; MGUS, monoclonal gammopathy of undetermined significance; TCDD, 2,3,7,8- tetrachlorodibenzo-p-dioxin.
Adjusted ORs were estimated for cohort status and for 2,3,7,8-TCDD in separate logistic regression models. Both models included the following covariates: age in 2002 in 5 year increments, race, body mass index in 2002 in increments of 2 units, and change in body mass index between 2002 and at the time of blood draw for the TCDD measurement. Lipid-adjusted TCDD concentrations were either measured in 1987 or, if not measured, reconstructed for the 1987 level using TCDD concentrations measured between 1992 and 2002.36 Dioxin results below the limit of detection were calculated as the limit of detection divided by the square root of 2.38 Two Ranch Hand Veterans who had missing BMI or lipid-adjusted TCDD were excluded, leaving 956 Veterans in the analysis.
MGUS characteristics
The most common heavy chain isotypes among MGUS cases were IgG and IgM, followed by IgA in both Ranch Hand Veterans and comparison Veterans (Table 3). The Ranch Hand Veterans had a slightly higher (P=0.19) M-protein concentration (median, 0.48 g/dL; interquartile range [IQR], 0.32–0.76) than comparison Veterans (median, 0.35 g/dL; IQR, 0.20–0.47). The prevalence of MGUS expressing heavy chains was higher than that of LC-MGUS: 4.8% vs 2.3% in Ranch Hand Veterans and 2.3% vs 0.8% in comparison Veterans (Table 3). In a subgroup analysis, the risk for heavy chain MGUS remained significant in the Ranch Hand cohort compared to the comparison cohort (adjusted OR=2.2, 95% CI, 1.06–4.55; P=0.04) (data not shown). In both Ranch Hand Veterans and comparison Veterans the prevalence of MGUS increased with age (Table 2).
Table 3.
MGUS and FLC-MGUS among Ranch Hand Veterans and Comparison Veterans
| Ranch Hand (n=479) | Comparison (n=479) | |
|---|---|---|
| MGUS, Overalla, n (prevalence %) | 34 (7.1) | 15 (3.1) |
| MGUS with heavy chain | 23 (4.8) | 11 (2.3) |
| IgG | 12 (2.5) | 8 (1.7) |
| IgA | 3 (0.6) | 1 (0.2) |
| IgM | 6 (1.3) | 2 (0.4) |
| Biclonalb | 2 (0.4) | 0 (0.0) |
| LC-MGUS | 11 (2.3) | 4 (0.8) |
| M-protein concentrationc, Median (IQR), g/dL | 0.48 (0.32–0.76) | 0.35 (0.20–0.47) |
| Free-κ light chain concentrationd, Median (IQR), mg/L | 24.3 (11.5–50.6) | 17.1 (13.9–20.6) |
| Free-λ light chain concentrationd, Median (IQR), mg/L | 27.0 (14.6–208.0) | 18.4 (13.3–37.9) |
| dFLC concentrationd, Median (IQR), mg/L | 37.5 (11.3–191.7) | 4.3 (0.31–24.4) |
| FLC Ratiod, Median (IQR) | 1.28 (0.14–2.02) | 0.98 (0.60–1.35) |
Abbreviations: dFLC, difference between involved and uninvolved FLCs; LC-MGUS, light chain monoclonal gammopathy of undetermined significance.
Includes heavy chain MGUS, biclonal MGUS, and FLC MGUS.
One Veteran had two separate bands of IgG, and the other had one IgG band and one IgA band.
Based on the 21 Ranch Hand and 10 comparison Veterans whose M-protein concentration was quantifiable.
Based on the 11 Ranch Hand and 4 comparison Veterans who had LC-MGUS as described in the Methods section.
DISCUSSION
Agent Orange was used by the USAF personnel who conducted aerial spray missions of herbicides (Operation Ranch Hand) in Vietnam from 1962–1971.32 The main ingredients of Agent Orange were 2,4-dichlorophenoxyacetic acid (2,4-D) and 2,4,5-trichlorophenoxyacetic acid (2,4,5-T), but the human carcinogen TCDD33 was also present in variable amounts as a contaminant.45 The measurement of serum TCDD levels in the Ranch Hand personnel confirmed TCDD exposure, raising concerns regarding long-term health effects from Agent Orange and TCDD.32 The AFHS that began in 1982 included multiple myeloma and other relatively rare cancers as end points, but it lacked statistical power to assess the excess risk associated with Agent Orange/TCDD exposure in the Ranch Hand cohort. A series of reports from the Institute of Medicine (IOM) from 1994 to 2013 addressed these concerns by reviewing available literature and drawing conclusions from the overall evidence.45 The IOM reports identified seven specific types of cancer for which the evidence of a positive association with Agent Orange/TCDD exposure was considered sufficient or at least limited/suggestive. Four of these were B-cell lymphoid malignancies; the evidence was sufficient for chronic lymphocytic leukemia, Hodgkin lymphoma, and non-Hodgkin lymphoma, while the evidence was only limited/suggestive for MM.45 All seven types of cancer were recognized by the US Department of Veterans Affairs and federal law as presumptive conditions for the purposes of health care and disability compensation. Our findings provide the first direct scientific evidence for an association between MM and Agent Orange/TCDD exposure among the Ranch Hand Veterans.
Using 958 stored serum samples obtained from Ranch Hand Veterans and comparison Veterans, we found the prevalence of MGUS in Ranch Hand Veterans to be twice that of comparison Veterans. Our observations are important in that they add support to a previous finding that certain pesticides play a role in the development of MGUS.31 In our study, the odds of having MGUS increased with increasing body burden of TCDD, although the trend was not statistically significant.
In a prior investigation based on 77,469 healthy adults enrolled in a U.S. nationwide population-based prospective cancer screening trial, 71 persons developed multiple myeloma during the course of the study.4 In that study, with the use of serially collected pre-diagnostic serum samples obtained up to almost 10 years before multiple myeloma diagnosis, all multiple myeloma cases were found to be preceded by the premalignant plasma cell disorder MGUS.4 These findings establish a key role for MGUS in the pathway to multiple myeloma. The current findings are also in accord with a prospective cohort study showing a 2-fold higher prevalence of MGUS among 678 private and commercial applicators licensed to apply restricted-use pesticides in Iowa or North Carolina.31 A large (n=12,482) population-based MGUS screening study representative of the US population recently found striking geographical variations; the prevalence of MGUS was 3.1% and 2.1% (P=0.052) for the North/Midwest versus South/West regions of the country, respectively.42 Future studies are needed to determine whether these geographic differences are attributable to environmental exposures associated with pesticide use.
In our study, the prevalence of LC-MGUS was 2.3% among Ranch Hand Veterans and 0.8% among comparison Veterans. The prevalence in comparison Veterans is similar to the age-standardized prevalence for men reported in a population screening study in the United States (prevalence=1.0%, 95% CI, 0.8%–1.2%).46 The underlying mechanisms for the higher prevalence of LC-MGUS in the Ranch Hand Veterans remain to be better understood. Prospective data show that approximately 20% of all myeloma patients have LC-myeloma and that LC-myeloma is preceded by LC-MGUS.47 Although the association is less well defined, it should be mentioned that both heavy chain MGUS and LC-MGUS can precede the development of chronic lymphocytic leukemia, non-Hodgkin’s lymphoma, and other B-cell malignancies.48–49 We also observed an apparent excess of IgM MGUS in our study population. Based on prior studies, there is an excess risk of Waldenström’s macroglobulinemia and other B cell lymphomas among individuals with IgM MGUS.49 In our study population, no Veterans were diagnosed with these conditions by 2002.
Our study, using the serum specimens and data collected by the AFHS, has several important strengths. It includes both Ranch Hand Veterans and other AFHS participants who had similar duties in Southeast Asia during the same period but were not involved in herbicide spray missions. We used the objective measurements of serum TCDD levels as a marker of exposure for both cohorts. Other strengths include 25 years of follow-up time over which the cohort has been monitored for a wide range of health effects and availability of multiple causes of death information. Furthermore, we used established and standardized protein assays that allowed comparison with other population-based studies.42,50
Our study also has limitations. We had no objective measurements of exposure to phenoxy herbicides (2,4-D and 2,4,5-T), so cohort status was used as a surrogate for phenoxy herbicide exposure. The first TCDD measurements were made in 1987, up to 25 years after their Agent Orange exposure in Vietnam, and we could not account for individual variations in whole body elimination of TCDD. Furthermore, a higher proportion of Ranch Hand Veterans (86.6%) had a TCDD level measured in 1987 than did comparison Veterans (74.1%), which could have introduced a bias in our results. Other limitations include a limited demographic spectrum that excludes women and the potential confounding by unknown and uncontrolled confounders (e.g., family medical history and civilian occupation). Despite the fact that we had access to a large cohort with stored serum samples and risk factor data, we were unable to assess the independent effects of TCDD and cohort status in relation to MGUS risk due to multicollinearity (i.e. TCDD levels and cohort status were correlated). Because of these weaknesses, we cannot discriminate between the phenoxy herbicides, the TCDD contaminant, or other cohort factors as the underlying causes of the increased prevalence of MGUS in Ranch Hand Veterans.
In conclusion, using stored serum samples obtained from USAF personnel who conducted aerial herbicide spray missions of Agent Orange in Vietnam from 1962–1971 (Operation Ranch Hand) and matched controls, we screened 958 Veterans for MGUS and found the prevalence to be 2-fold higher among Ranch Hand Veterans. The cohort status was significantly (P=0.0001) associated with TCDD levels (≤3.65, 3.66–5.80, 5.81–10.92, >10.92), illustrated most obviously by the increased percentage of men with TCDD>10.92 ppt in the Ranch Hand Veterans (47.5%) relative to those in the comparison Veterans (2.5%). The MGUS prevalence was 2.43-fold higher (95%CI, 0.92–6.44; P=0.07) among Veterans with TCDD levels>10.92 ppt, compared to Veterans with TCDD levels ≤3.65 ppt. Our finding that exposure to Agent Orange is associated with an increased risk of MGUS supports the association with multiple myeloma.
Acknowledgments
The authors would like to acknowledge the contribution of the Medical Follow-Up Agency of the Institute of Medicine, which prepared the AFHS data used throughout this article. This study was supported by the Intramural Program at the Agency for Toxic Substances and Disease Registry, the Intramural Program at the National Cancer Institute, and the Air Force Health Study Assets Research Program at the Institute of Medicine through an award to the CDC Foundation. The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Agency for Toxic Substances and Disease Registry, Centers for Disease Control and Prevention, National Institutes of Health, or Food and Drug Administration. No relevant conflict of interest exist for any of the authors.
Footnotes
Author Contributions: All authors contributed to the manuscript writing. OL, YS, JM, KC, NC, ER, DM, GM, and RV contributed to the study design. OL, YS, NK, and JM oversaw the overall conduct of data collection and performed final data analysis. OL, RC, DB, and KC oversaw and performed laboratory testing.
Government statement on copyright: The publisher acknowledges the right of the US government to retain a non-exclusive royalty-free license in and to any copyright covering the article.
References
- 1.SEER Stat Fact Sheets: Myeloma. 2014 ( http://seer.cancer.gov/statfacts/html/mulmy.html.)
- 2.SEER Cancer Statistics Review, 1975–2003. ( http://seer.cancer.gov/archive/csr/1975_2003/)
- 3.Turesson I, Velez R, Kristinsson SY, Landgren O. Patterns of Improved Survival in Patients With Multiple Myeloma in the Twenty-First Century: A Population-Based Study. J Clin Oncol. 2009;28:830–4. doi: 10.1200/JCO.2009.25.4177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Landgren O, Kyle RA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance (MGUS) consistently precedes multiple myeloma: a prospective study. Blood. 2009;113:5412–7. doi: 10.1182/blood-2008-12-194241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Alavanja MC, Blair A, Merkle S, Teske J, Eaton B. Mortality among agricultural extension agents. Am J Ind Med. 1988;14:167–76. doi: 10.1002/ajim.4700140207. [DOI] [PubMed] [Google Scholar]
- 6.Baris D, Silverman DT, Brown LM, et al. Occupation, pesticide exposure and risk of multiple myeloma. Scand J Work Environ Health. 2004;30:215–22. doi: 10.5271/sjweh.782. [DOI] [PubMed] [Google Scholar]
- 7.Cerhan JR, Cantor KP, Williamson K, Lynch CF, Torner JC, Burmeister LF. Cancer mortality among Iowa farmers: Recent results, time trends, and lifestyle factors (United States) Cancer Causes Control. 1998;9:311–9. doi: 10.1023/a:1008877204830. [DOI] [PubMed] [Google Scholar]
- 8.Lee E, Burnett CA, Lalich N, Cameron LL, Sestito JP. Proportionate mortality of crop and livestock farmers in the United States, 1984–1993. Am J Ind Med. 2002;42:410–20. doi: 10.1002/ajim.10131. [DOI] [PubMed] [Google Scholar]
- 9.Nandakumar A, English DR, Dougan LE, Armstrong BK. Incidence and outcome of multiple myeloma in Western Australia, 1960 to 1984. Aust N Z J Med. 1988;18:774–9. doi: 10.1111/j.1445-5994.1988.tb00178.x. [DOI] [PubMed] [Google Scholar]
- 10.Pukkala E, Notkola V. Cancer incidence among Finnish farmers, 1979–93. Cancer Causes Control. 1997;8:25–33. doi: 10.1023/a:1018474919807. [DOI] [PubMed] [Google Scholar]
- 11.Stark AD, Chang HG, Fitzgerald EF, Riccardi K, Stone RR. A retrospective cohort study of cancer incidence among New York State Farm Bureau members. Arch Environ Health. 1990;45:155–62. doi: 10.1080/00039896.1990.9936709. [DOI] [PubMed] [Google Scholar]
- 12.Steineck G, Wiklund K. Multiple myeloma in Swedish agricultural workers. Int J Epidemiol. 1986;15:321–5. doi: 10.1093/ije/15.3.321. [DOI] [PubMed] [Google Scholar]
- 13.Boffetta P, Stellman SD, Garfinkel L. A Case-Control Study of Multiple-Myeloma Nested in the American-Cancer-Society Prospective-Study. Int J Cancer. 1989;43:554–9. doi: 10.1002/ijc.2910430404. [DOI] [PubMed] [Google Scholar]
- 14.Brownson RC, Reif JS, Chang JC, Davis JR. Cancer risks among Missouri farmers. Cancer. 1989;64:2381–6. doi: 10.1002/1097-0142(19891201)64:11<2381::aid-cncr2820641131>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 15.Burmeister LF. Cancer in Iowa farmers: recent results. Am J Ind Med. 1990;18:295–301. doi: 10.1002/ajim.4700180309. [DOI] [PubMed] [Google Scholar]
- 16.Cantor KP, Blair A. Farming and mortality from multiple myeloma: a case-control study with the use of death certificates. J Natl Cancer Inst. 1984;72:251–5. [PubMed] [Google Scholar]
- 17.Costantini AS, Miligi L, Kriebel D, et al. A multicenter case-control study in Italy on hematolymphopoietic neoplasms and occupation. Epidemiology. 2001;12:78–87. doi: 10.1097/00001648-200101000-00014. [DOI] [PubMed] [Google Scholar]
- 18.Cuzick J, De Stavola B. Multiple myeloma--a case-control study. Br J Cancer. 1988;57:516–20. doi: 10.1038/bjc.1988.118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Demers PA, Vaughan TL, Koepsell TD, et al. A case-control study of multiple myeloma and occupation. Am J Ind Med. 1993;23:629–39. doi: 10.1002/ajim.4700230410. [DOI] [PubMed] [Google Scholar]
- 20.Eriksson M, Karlsson M. Occupational and other environmental factors and multiple myeloma: a population based case-control study. Br J Ind Med. 1992;49:95–103. doi: 10.1136/oem.49.2.95. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Figgs LW, Dosemeci M, Blair A. Risk of multiple myeloma by occupation and industry among men and women: a 24-state death certificate study. JOM. 1994;36:1210–21. doi: 10.1097/00043764-199411000-00007. [DOI] [PubMed] [Google Scholar]
- 22.Franceschi S, Barbone F, Bidoli E, et al. Cancer risk in farmers: results from a multi-site case-control study in north-eastern Italy. Int J Cancer. 1993;53:740–5. doi: 10.1002/ijc.2910530506. [DOI] [PubMed] [Google Scholar]
- 23.Heineman EF, Olsen JH, Pottern LM, Gomez M, Raffn E, Blair A. Occupational risk factors for multiple myeloma among Danish men. Cancer Causes Control. 1992;3:555–68. doi: 10.1007/BF00052753. [DOI] [PubMed] [Google Scholar]
- 24.La Vecchia C, Negri E, D’Avanzo B, Franceschi S. Occupation and lymphoid neoplasms. Br J Cancer. 1989;60:385–8. doi: 10.1038/bjc.1989.290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Milham S., Jr Leukemia and multiple myeloma in farmers. Am J Epidemiol. 1971;94:507–10. [PubMed] [Google Scholar]
- 26.Pearce N, Reif JS. Epidemiologic studies of cancer in agricultural workers. Am J Ind Med. 1990;18:133–48. doi: 10.1002/ajim.4700180206. [DOI] [PubMed] [Google Scholar]
- 27.Blair A, Dosemeci M, Heineman EF. Cancer and other causes of death among male and female farmers from twenty-three states. Am J Ind Med. 1993;23:729–42. doi: 10.1002/ajim.4700230507. [DOI] [PubMed] [Google Scholar]
- 28.Blair A, Zahm SH, Pearce NE, Heineman EF, Fraumeni JF., Jr Clues to cancer etiology from studies of farmers. Scand J Work Environ Health. 1992;18:209–15. doi: 10.5271/sjweh.1578. [DOI] [PubMed] [Google Scholar]
- 29.Brown LM, Burmeister LF, Everett GD, Blair A. Pesticide Exposures and Multiple-Myeloma in Iowa Men. Cancer Causes Control. 1993;4:153–6. doi: 10.1007/BF00053156. [DOI] [PubMed] [Google Scholar]
- 30.Khuder SA, Mutgi AB. Meta-analyses of multiple myeloma and farming. Am J Ind Med. 1997;32:510–6. doi: 10.1002/(sici)1097-0274(199711)32:5<510::aid-ajim11>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 31.Landgren O, Kyle RA, Hoppin JA, et al. Pesticide exposure and risk of monoclonal gammopathy of undetermined significance in the Agricultural Health Study. Blood. 2009;113:6386–91. doi: 10.1182/blood-2009-02-203471. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Institute of Medicine. Disposition of the Air Force Health Study. National Academy Press; Washington, DC: 2006. Available from http://www.nap.edu/catalog/11590.html. [Google Scholar]
- 33.International Agency for Research on Cancer. Chemical agents and related occupations. Vol 100F. A review of human carcinogens. WHO Press; Geneva, Switzerland: 2012. [Google Scholar]
- 34.Lathrop GD, Wolfe WH, Albanese RA, Moynahan PM. Epidemiologic investigation of health effects in Air Force personnel following exposure to herbicides: Baseline Morbidity Study Results. USAF School of Aerospace Medicine; Brooks Air Force Base, TX: 1984. [Google Scholar]
- 35.Landgren O. Monoclonal gammopathy of undetermined significance and smoldering multiple myeloma: biological insights and early treatment strategies. Hematology Am Soc Hematol Educ Program. 2013;2013:478–87. doi: 10.1182/asheducation-2013.1.478. [DOI] [PubMed] [Google Scholar]
- 36.Michalek JE, Robinson J, Fox K, et al. 2002 Follow-Up Examination Results. Springfield: National Technical Information Service; 2005. The Air Force Health Study: An Epidemiologic Investigation of Health Effects in Air Force Personnel Following Exposure to Herbicides. [Google Scholar]
- 37.Patterson DG, Jr, Hampton L, Lapeza CR, Jr, Belser WT, Green V, Alexander LR, Needham LL. High resolution gas chromatographic/high resolution mass spectrometric analysis of human serum on a whole weight and lipid weight basis for 2,3,7,8-tetrachlorodibenzo-p-dioxin. Annals of Chemistry. 1987;59:2000–5. doi: 10.1021/ac00142a023. [DOI] [PubMed] [Google Scholar]
- 38.Hornung RW, Reed DR. Estimation of average concentration in the presence of nondetectable values. Appl Occup Environ Hyg. 1990;5:46–51. [Google Scholar]
- 39.Katzmann JA, Clark RJ, Abraham RS, et al. Serum reference intervals and diagnostic ranges for free kappa and free lambda immunoglobulin light chains: relative sensitivity for detection of monoclonal light chains. Clin Chem. 2002;48:1437–44. [PubMed] [Google Scholar]
- 40.Hutchison CA, Landgren O. Polyclonal immunoglobulin free light chains as a potential biomarker of immune stimulation and inflammation. Clin Chem. 2011;57:1387–9. doi: 10.1373/clinchem.2011.169433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Korde N, Kristinsson SY, Landgren O. Monoclonal gammopathy of undetermined significance (MGUS) and smoldering multiple myeloma (SMM): novel biological insights and development of early treatment strategies. Blood. 2011;117:5573–81. doi: 10.1182/blood-2011-01-270140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Landgren O, Graubard BI, Katzmann JA, et al. Racial disparities in the prevalence of monoclonal gammopathies: a population-based study of 12,482 persons from the National Health and Nutritional Examination Survey. Leukemia. 2014;28:1537–42. doi: 10.1038/leu.2014.34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Wolfe WH, Michalek JE, Miner JC, et al. Determinants of TCDD half-life in veterans of operation ranch hand. J Toxicol Environ Health. 1994;41:481–8. doi: 10.1080/15287399409531858. [DOI] [PubMed] [Google Scholar]
- 44.Wallin A1, Larsson SC. Body mass index and risk of multiple myeloma: a meta-analysis of prospective studies. Eur J Cancer. 2011;47(11):1606–15. doi: 10.1016/j.ejca.2011.01.020. [DOI] [PubMed] [Google Scholar]
- 45.Institute of Medicine. Veterans and Agent Orange: Update 2012. National Academy Press; Washington, DC: 2013. [PubMed] [Google Scholar]
- 46.Dispenzieri A, Katzmann J, Kyle R, et al. Prevalence and risk of progression of light-chain monoclonal gammopathy of undetermined significance: a retrospective population-based cohort study. Lancet. 2010;375:1721–8. doi: 10.1016/S0140-6736(10)60482-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Weiss BM, Abadie J, Verma P, Howard RS, Kuehl WM. A monoclonal gammopathy precedes multiple myeloma in most patients. Blood. 2009;113(22):5418–22. doi: 10.1182/blood-2008-12-195008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Tsai H-T, Caporaso NE, Kyle RA, et al. Evidence of serum immunoglobulin abnormalities up to 9.8 years prior to diagnosis of chronic lymphocytic leukemia: a prospective study. Blood. 2009;114:4928–432. doi: 10.1182/blood-2009-08-237651. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Turesson I, Kovalchik SA, Pfeiffer RM, et al. Monoclonal gammopathy of undetermined significance and risk of lymphoid and myeloid malignancies: 728 cases followed up to 30 years in Sweden. Blood. 2014;123:338–45. doi: 10.1182/blood-2013-05-505487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Wu SP, Minter A, Costello R, et al. MGUS prevalence in an ethnically Chinese population in Hong Kong. Blood. 2013;121:2363–4. doi: 10.1182/blood-2012-11-466011. [DOI] [PMC free article] [PubMed] [Google Scholar]