Abstract
Male infertility affects up to 12% of the world’s male population and is linked to various environmental and medical conditions. Manual microscope-based testing and computer-assisted semen analysis (CASA) are the current standard methods to diagnose male infertility; however, these methods are labor-intensive, expensive, and laboratory-based. Cultural and socially dominated stigma against male infertility testing hinders a large number of men from getting tested for infertility, especially in resource-limited African countries. We describe the development and clinical testing of an automated smartphone-based semen analyzer designed for quantitative measurement of sperm concentration and motility for point-of-care male infertility screening. Using a total of 350 clinical semen specimens at a fertility clinic, we have shown that our assay can analyze an unwashed, unprocessed liquefied semen sample with <5-s mean processing time and provide the user a semen quality evaluation based on the World Health Organization (WHO) guidelines with ~98% accuracy. The work suggests that the integration of microfluidics, optical sensing accessories, and advances in consumer electronics, particularly smartphone capabilities, can make remote semen quality testing accessible to people in both developed and developing countries who have access to smartphones.
INTRODUCTION
More than 45 million couples worldwide are affected by infertility, and more than 40% of these cases include some component of male infertility. It is estimated that, on a global scale, up to 12% of men (>30 million) will have fertility issues during their lifetime (1). Sperm abnormalities are definitive markers for male infertility and have been linked to varicocele, hormonal imbalances, and genetic reasons such as Y-deletions (2–4), but other medical conditions can also influence semen quality (5). Sperm abnormalities may be correlated with lifestyle and environmental effects as well (6). Semen analysis is considered the cornerstone in male infertility evaluation, but men often feel embarrassed to go to urologists, and women carry the weight with regard to infertility most of the time (7, 8). Furthermore, health care disparities related to economic, cultural, societal, geographical, and religious factors are major impediments to accessing infertility care worldwide, especially in developing countries, making it one of the most underestimated and neglected health care issues (9). Here, we present a true point-of-care smartphone-based semen analyzer that is easy to use, rapid, automated, and inexpensive. It provides sperm concentration, motility, and linear and curvilinear velocities by performing an on-phone image analysis using a semen sample loaded into a low-cost disposable microfluidic device. Along with a modular wireless weight scale, it also provides semen volume, total sperm count, and total motile sperm count.
The critical components for determining seminal quality are sperm concentration, motility, and morphology. Manual microscope-based testing and computer-assisted semen analysis (CASA) systems are the current clinical standard methods; however, these methods are labor-intensive, expensive, and laboratory-based. CASA-based techniques require highly trained technicians for producing reliable and repeatable results. CASA techniques also require bulky microscope-based image analysis systems that greatly limit their point-of-care applications. Many fertility clinics and small hospitals are believed to not have CASA-based platforms and so use a time-consuming manual method for semen analysis (10). Manual test results are subjective, therefore making it difficult to compare results from different clinics (11).
Turbidimetry (12), photon correlation spectroscopy (13), laser Doppler velocimetry (14), impedance-based analysis (15), lensless imaging (16), paper-based colorimetric detection (17), and ball lens–based imaging (18) are some of the other methods developed for semen analysis. However, these methods have not completely addressed all of the requirements for a true point-of-care, inexpensive, rapid, easy-to-use, automated diagnostic assay for semen analysis that can be used by an inexperienced user. Some of these methods cannot provide direct estimates of sperm concentration and motility, and almost all of the methods require expensive, bulky components for data collection and separate computational units for analysis of the collected data. Paper-based quantification assays may offer low-cost alternatives for routine semen analysis. One study made use of the color change resulting from the reactivity of yellow tetrazolium dye in the presence of the diaphorase flavoprotein enzyme present in human sperm to form purple formazan for the quantification of live and motile sperm concentrations (17). Although the paper-based colorimetric analysis does not require expensive and bulky components, it requires a desktop scanner connected to a computer for measuring live and motile sperm concentrations with a limit of detection of 8.46 million live sperm/ml. Similarly, although lensless imaging techniques benefit from large field-of-view (FOV) imaging, they are limited by the requirement of a separate computational unit for relatively time-consuming and computationally intensive hologram-based image reconstruction. The evaluation results demonstrated by the lensless imaging for semen analysis showed that accurate quantification of sperm concentration using this method is limited to 8.3 million immotile sperm/ml and 6.5 million motile sperm/ml, suggesting its technical limitation in evaluating undiluted, unwashed human semen samples with clinically relevant sperm concentrations (16). More recent work on point-of-care semen analysis using a ball lens–cellphone setup only magnifies the image of a semen sample pipetted onto polyethylene sheets that needs to be transferred to a separate monitor for manual analysis (18). An expert technician can then manually count the number of sperm per FOV and evaluate the motility of the sample. The reported sperm motility results using this ball lens–cellphone setup suggest that this system may be susceptible to a high frequency of false negatives. Therefore, this ball lens–cellphone setup is not appropriate for home-based semen analysis.
Current home-based male infertility assays on the market are FertilMARQ and SpermCheck, which use a chemical staining approach for detecting sperm-specific proteins on the sperm head. Trak, another U.S. Food and Drug Administration–approved product, uses centrifugal force to estimate sperm concentration on a microfluidic device (19). The assay requires a small centrifuge for analysis and classifies semen samples based on sperm concentration criterion of 15 million sperm/ml with >94% accuracy. However, all of these assays can only measure sperm concentration and not motility.
Microfluidics as an enabling biotechnology has shown great promise in the development of biomedical devices with broad applications in medicine and biology, such as low-cost biosensing assays, single-cell analysis, gene expression profiling, and DNA sequencing (20). The application of microfluidics for sperm analysis has garnered considerable attention over the past decade, and several advanced platforms have been developed, such as high-throughput sperm genomics (21). On the other hand, advances in consumer electronics and particularly in mobile processing power, memory size, power consumption, and functionality have paved the way for their applications in health care (22). Numerous health care applications have stemmed from supplementing external attachments to smartphones (23–25), with multiple point-of-care diagnostic devices using the on-board smartphone camera (25–27). Integrating microfluidics, optical sensing modality, and smartphones can provide a powerful platform to develop affordable and easily accessible point-of-care fertility diagnostic assays.
Here, we present an automated smartphone-based diagnostic assay, which uses a small volume of semen sample (<35 μl) loaded into a disposable microfluidic device for measuring sperm concentration and motility (Fig. 1). We have evaluated the assay using fresh, unwashed, unprocessed patient semen samples by comparing the results obtained by the smartphone-based assay performed by trained and untrained users with the results obtained by a CASA-based platform. We showed that the smartphone-based platform could detect abnormal semen samples with regard to sperm concentration (<15 million sperm/ml) and motility (<40%) among samples with sperm concentrations between 0 and 408 million sperm/ml with 97.71% accuracy. The quantitative results on patient semen samples showed an excellent agreement between the smartphone-based platform and CASA using Passing-Bablok regression analysis and Bland-Altman analysis.
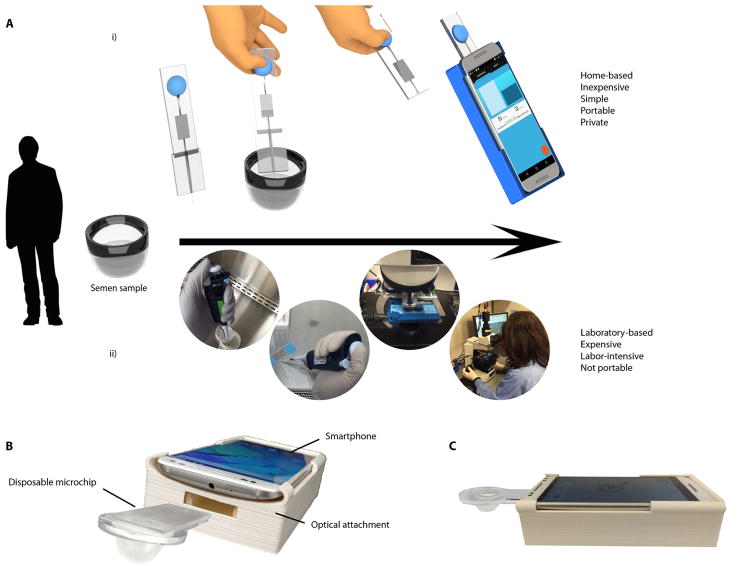
Fig. 1. The process flow for semen analysis using the smartphone-based device and conventional methods.
(A) (i) Process of operation: A small volume of an unwashed, unprocessed semen sample is loaded into a microfluidic device that has a capillary-based disposable tip at the inlet and a rubber bulb at the outlet to create negative pressure in the microchannel for manual, power-free sample loading. The user manually detaches the capillary disposable tip from the microfluidic device through a simple snap-off mechanism and inserts the clean microfluidic device into the smartphone optical attachment. The user then initializes the smartphone application, which analyzes the sample with <5-s mean video processing time. (ii) Conventional method of semen analysis performed in a laboratory setting. The sample is drawn using a pipette and is loaded onto a semen glass slide counting chamber. The glass slide is placed under a desktop microscope, and a technician performs the analysis either manually or using a CASA system. (B) Actual image of the smartphone accessory and the microchip (C) along with its side view.
RESULTS
Optical modality design for low-power testing
The hardware component of the smartphone-based platform consists of an optical attachment for image magnification and device positioning and a disposable microfluidic device for semen sample handling. The optical attachment is a lightweight (~75 g), compact (15.2 × 8.2 × 3.4 cm), three-dimensionally (3D) printed housing that contains an inexpensive white light-emitting diode (LED) used for transillumination, two aspheric lenses, a small and low-cost battery, an electronic switch, and wiring components to connect the electronics. The optical attachment was custom-designed to align its optical axis to the smartphone camera through a simple slide-on mechanism. The optical attachment was designed to have an opening to pass the microchip through for chip positioning on the attachment. The need for manual focusing was eliminated by positioning the microchip slot at a specific distance from the lens arrangement such that the sample contained in the microchip is in optimal focus. The device was calibrated using a micrometer scale (fig. S1), and the resolving power of the attachment was tested using micropolystyrene beads (3 to 10 μm), such that it was observed to be capable of visualizing and detecting sperm heads (fig. S2 and movie S1).
The disposable microfluidic device for semen sample handling was designed on the basis of a power-free mechanical pumping mechanism using a polydimethylsiloxane (PDMS) bulb for easy-to-use, on-chip semen sample loading through creating a negative pressure chamber at the end of the device (fig. S3 and Fig. 1). The total material cost to fabricate the smartphone accessory and the disposable microfluidic device was $4.45, including $3.59 for the optical attachment and $0.86 for the microfluidic device, in comparison to tens of thousands of dollars for a typical CASA-based platform (table S1).
The software (smartphone application) of the technology was designed with a user-friendly interface to guide the user through each step of testing (fig. S4). The test results are stored locally on the phone for semen quality monitoring over time. The developed assay was able to record sample videos at a rate of 30 frames per second (fps) with a maximum effective FOV of 886 × 886 pixels for the smartphone used in this study (Motorola Moto X). The smartphone application was able to image, automatically analyze, and report the results at an average time of 4.48 s (table S2).
Preclinical testing: Detecting samples with low sperm count
To evaluate the performance of the smartphone-based semen analyzer in classifying samples based on their concentrations at the lower range, in a double-blinded approach, we measured sperm concentrations for 56 cryopreserved semen samples with sperm concentrations below and above 100,000 sperm/ml (table S3). A separate technician performed a parallel microscope-based manual sperm count using a standard hemocytometer. This evaluation showed the ability of the assay to be used for monitoring patients who undergo vasectomy. Men who undergo vasectomy are required to monitor their semen quality for 3 to 6 months after the procedure to verify whether the procedure was successful (sperm concentration, ≤100,000 sperm/ml) (28).
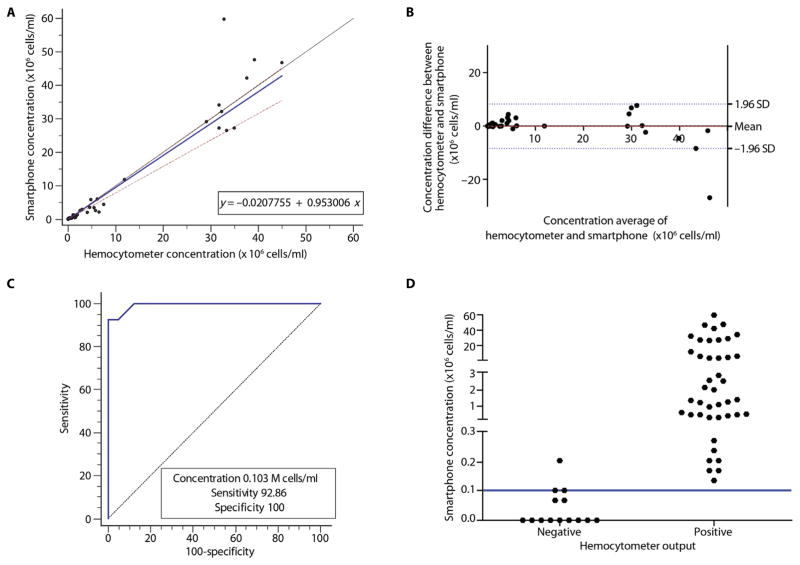
In Fig. 2A, good agreement between the two methods can be observed for the tested sperm concentration range. Passing-Bablok regression analysis (29) (n = 56) showed an A intercept value of −0.02078 with a confidence interval (CI) of −0.06186 to −0.001318 and a B slope value of 0.9530 with a CI of 0.7913 to 1.0032. The cusum test for linearity showed no significant deviation from linearity with P = 0.52. The Bland-Altman analysis (30) (n = 56) comparing the smartphone-based sperm count and manual microscope-based sperm count showed a mean bias of −0.07612 million sperm/ml with an SD of 4.270 million sperm/ml (Fig. 2B). The limits of agreement (LOAs) ranged from −8.446 to 8.293 million sperm/ml. These statistical analyses showed that the difference between manual and smartphone-based semen analyses increased as sperm concentration increased in the semen samples. However, we did not observe a fixed difference in measuring sperm concentration between the two methods. To establish a threshold for optimum sensitivity and specificity, we performed receiver operating characteristic (ROC) analysis. ROC analysis plot along with a vertical scatterplot illustrated the sensitivity and specificity of the device (Fig. 2, C and D). The ROC analysis (n = 56) showed that the sperm concentration threshold value of 0.103 × 106 sperm/ml yields an optimum sensitivity of 92.86% with a CI of 66.1 to 99.8% and a specificity of 100% with a CI of 91.6 to 100.0% for the smartphone-based device to identify semen samples with actual sperm concentrations below and above the threshold of 100,000 sperm/ml measured by the microscope-based method. The accuracy was 98.21%, and the area under the curve (AUC) was 0.9930 with a binomial exact CI ranging from 0.9230 to 1.0000.
Fig. 2. Device evaluation using cryopreserved semen samples based on sperm concentration criterion of 100,000 sperm/ml.
(A) Direct comparison of sperm concentrations calculated by the smartphone-based device and manual microscope-based method. The solid blue line represents the regression line, the solid black line represents the identity line, and two dashed red lines represent the confidence band (n = 56). (B) Bland-Altman analysis to compare the results obtained by the smartphone-based semen analyzer and manual microscope-based method. The red dashed line is the mean difference of the methods, and the blue dotted lines represent the 95% LOAs. ROC curves (C) along with a vertical scatterplot (D) show the sensitivity and specificity of the smartphone-based device in detecting samples with sperm concentrations at ~100,000 sperm/ml. In (D), “Negative” and “Positive” represent sperm concentrations below and above 100,000 cells/ml, respectively, indicated by the blue line. Manual analysis was used as the reference method.
Clinical semen specimen testing performed by trained users
To evaluate the performance, sensitivity, specificity, and accuracy of the smartphone-based semen analyzer, we tested 164 semen samples from patients at the Massachusetts General Hospital (MGH) fertility clinic and compared the results with the results obtained by a CASA-based platform for semen analysis (table S4). The overall repeatability of CASA for measuring sperm concentration and motility was above 90% (tables S5 and S6).
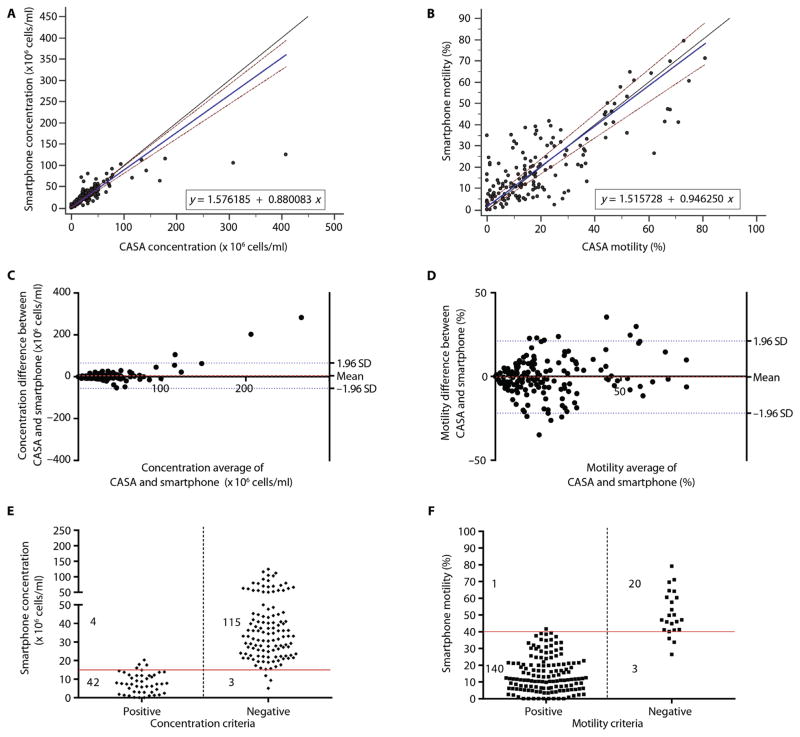
Passing-Bablok analysis (n = 164) was performed to evaluate the performance of the smartphone-based platform in calculating sperm concentration in the patient semen samples as compared to CASA (Fig. 3A). The A and B values were 1.5762 (CI, 0.4678 to 2.8996) and 0.8801 (CI, 0.8106 to 0.9588), respectively. The cusum test for linearity showed no significant deviation from linearity with P = 0.69. These results may suggest that there was a saturation point for sperm concentration measurement in the current version of the smartphone-based system for samples with high sperm concentrations (>100 million sperm/ml). However, the device saturation point did not have any significant effect on the diagnostic performance of the smartphone-based platform to accurately detect abnormal semen samples with sperm concentrations below the World Health Organization (WHO) threshold (15 million sperm/ml) for male semen quality classification (31). Here, we also report the Passing-Bablok analysis for patient semen samples with sperm concentrations below 100 million sperm/ml (n = 156) to evaluate the performance of the smartphone-based semen analyzer within the clinically relevant range (fig. S5A). The A and B values were 1.3173 (CI, 0.0990 to 2.0584) and 0.9348 (CI, 0.8604 to 1.006), respectively. The cusum test results showed no significant deviation from linearity with P = 0.80. A relatively better agreement between CASA and the smartphone-based semen analyzer was observed for samples with sperm concentrations below 100 million sperm/ml. We also performed Passing-Bablok analysis on sperm motility measurements for 164 patient semen samples obtained using the smartphone-based semen analyzer and CASA (Fig. 3B). The A and B values were 1.5157 (CI, 0 to 2.8432) and 0.9463 (CI, 0.8428 to 1.0504), respectively. The cusum test results showed no significant deviation from linearity with P = 0.69.
Fig. 3. On-site device evaluation performed by trained users using unwashed, unprocessed semen samples.
Passing-Bablok analysis to compare (A) sperm concentration and (B) sperm motility as measured by the smartphone-based semen analyzer and CASA for all patient semen samples. The solid blue line represents the regression line, the solid black line represents the identity line, and two dashed red lines represent the confidence band in the Passing-Bablok figures. Bland-Altman analysis to compare (C) sperm concentration and (D) sperm motility as measured by the smartphone-based semen analyzer and CASA for all patient semen samples. The red dashed line in the Bland-Altman figures is the mean difference of the methods, and the blue dotted lines represent the 95% LOAs. Scatterplots (E and F) represent the qualitative performance of the device in measuring sperm concentration and motility, respectively. The solid red lines represent the threshold values for each criterion. “Positive” and “Negative” in (E) represent sperm concentration below and above 15 million sperm/ml measured by CASA, respectively. “Positive” and “Negative” in (F) represent sperm motility below and above 40% measured by CASA, respectively.
We performed Bland-Altman analysis (n = 164) to evaluate sperm concentration results obtained by the smartphone-based semen analyzer as compared to CASA (Fig. 3C). The mean bias was 5.068 million sperm/ml with an SD of 31.00 million sperm/ml (LOA, −55.68 to 65.82 million sperm/ml). We also performed Bland-Altman analysis for samples with sperm concentrations below 100 million sperm/ml (fig. S5B; n = 156). The mean bias was 0.4309 million sperm/ml with an SD of 10.90 million sperm/ml (LOA, −20.93 to 21.79 million sperm/ml). Figure 3D shows the Bland-Altman analysis for sperm motility measurements (n = 164) using the smartphone-based semen analyzer and CASA. The mean bias was −0.3232% with an SD of 10.98% (LOA, −21.84% to 21.20%). Collectively, Passing-Bablok and Bland-Altman analyses showed that the difference between CASA and the smartphone-based device in measuring sperm concentration and motility increased as sperm concentration and motility increased in the samples. These differences, however, did not have a significant effect on the ability of the smartphone-based semen analyzer to identify abnormal semen samples.
We evaluated the performance of the smartphone-based device to detect semen samples with sperm concentrations below and above the WHO threshold of 15 million sperm/ml (Fig. 3E; n = 164) (31). The sensitivity and specificity of the smartphone-based device to detect abnormal semen samples based on sperm concentration criterion were 91.30% (CI, 79.21 to 97.58%) and 97.46% (CI, 92.75 to 99.47%), respectively, and the accuracy was 95.73%. The observed AUC was 0.94 (binomial exact CI, 0.90 to 0.97). The performance of the smartphone-based device to detect abnormal semen samples with sperm motility below the WHO threshold of 40% (31) was also evaluated (Fig. 3F; n = 164). The sensitivity and specificity of the device based on sperm motility criterion were 99.29% (CI, 96.11 to 99.98%) and 86.96% (CI, 66.41 to 97.22%), respectively, and the accuracy was 97.56%. The observed AUC was 0.93 (binomial exact CI, 0.88 to 0.96). We also evaluated the sensitivity, specificity, and accuracy of the smartphone-based semen analyzer as a qualitative test to detect abnormal semen samples when both sperm concentration (<15 million sperm/ml) and sperm motility (<40%) criteria were used together for semen analysis (n = 164) (table S7). These results showed that the sensitivity and specificity of the smartphone-based semen analyzer to detect abnormal semen samples using both sperm concentration and motility parameters were 99.31% (CI, 96.22 to 99.98%) and 89.47% (CI, 66.86 to 98.70%), respectively, with an accuracy of 98.17%. The observed AUC was 0.94 (binomial exact CI, 0.90 to 0.97).
The smartphone-based semen analyzer was able to rapidly and accurately calculate the average linear and curvilinear velocities of sperm in an undiluted, unwashed semen sample. Bland-Altman analyses (n = 24) were performed to evaluate the smartphone-based device compared to CASA and manual microscope-based methods for calculating the sperm linear and curvilinear velocities (fig. S6 and table S4). These results suggest high comparability of the smartphone-based device with CASA for sperm velocity measurement. The smartphone-based semen analyzer also has the potential to measure total sperm count and total motile sperm count based on the WHO guidelines (31). We developed a separate compact custom-designed modular wireless weight scale for semen sample volume estimation (fig. S7). The performance of the weight scale module was first evaluated by comparing its results with the results obtained by a conventional weighing scale (Ohaus, EP64 Explorer Pro). An excellent agreement with a Pearson’s correlation coefficient of r = 1 (n = 27) was observed between the two scales (fig. S8A). Our preliminary experimental results showed a strong correlation between the smartphone-based semen analyzer and CASA in measuring the total sperm count (r = 0.9089) and total motile sperm count (r = 0.9294) (fig. S8, B and C; n = 14).
The current version of the smartphone-based semen analyzer uses the smartphone camera for sample imaging and can be used with different cellphones with minor modifications in the hardware optical attachment depending on the physical dimensions of the cellphone. To evaluate the performance of our system, we used three different smart-phones (Moto X, Moto G4, and LG G4) for semen analysis (fig. S9 and table S8). No changes were made to manually tune the optical focus. Fine optical focus was achieved and controlled by the smartphone’s autofocusing ability. The Bland-Altman analysis for sperm concentration measurements using (i) Moto X and Moto G4 smartphones (fig. S9A) and (ii) Moto X and LG G4 smartphones (fig. S9B) was performed for 13 unprocessed semen samples. The mean bias between Moto X and Moto G4 smartphones was 0.7692 million sperm/ml with an SD of 3.166 million sperm/ml, and the LOA ranged from −5.437 million to 6.975 million sperm/ml. The mean bias between Moto X and LG G4 smartphones was 4.462 million sperm/ml with an SD of 6.851 million sperm/ml, and the LOA ranged from −8.966 million to 17.89 million sperm/ml. We also performed Bland-Altman analysis for sperm motility measurements for the 13 semen samples using (i) Moto X and Moto G4 smartphones (fig. S9C) and (ii) Moto X and LG G4 smartphones (fig. S9D). The mean bias between Moto X and Moto G4 smartphones was 0.2308% with an SD of 2.833%, and the LOA ranged from −5.322 to 5.783%. The mean bias between Moto X and LG G4 smartphones was 0.7692% with an SD of 6.954%, and the LOA ranged from −12.86 to 14.40%. These results showed that there was no difference between the three smartphones in identifying undiluted, unwashed abnormal semen samples based on the WHO criteria on sperm concentration and motility. Minor differences were observed in terms of quantitative measurements. The sample processing time was also dependent on the smartphone’s processing power (table S8).
Clinical semen specimen testing performed by untrained users
To evaluate the usability and simplicity of the smartphone-based male infertility screening device, we recruited untrained users and performed a double-blinded evaluation of semen analysis using the smartphone-based platform and CASA (Fig. 4 and table S9). None of the developers of the smartphone-based semen analyzer were present during the double-blinded usability experiments.
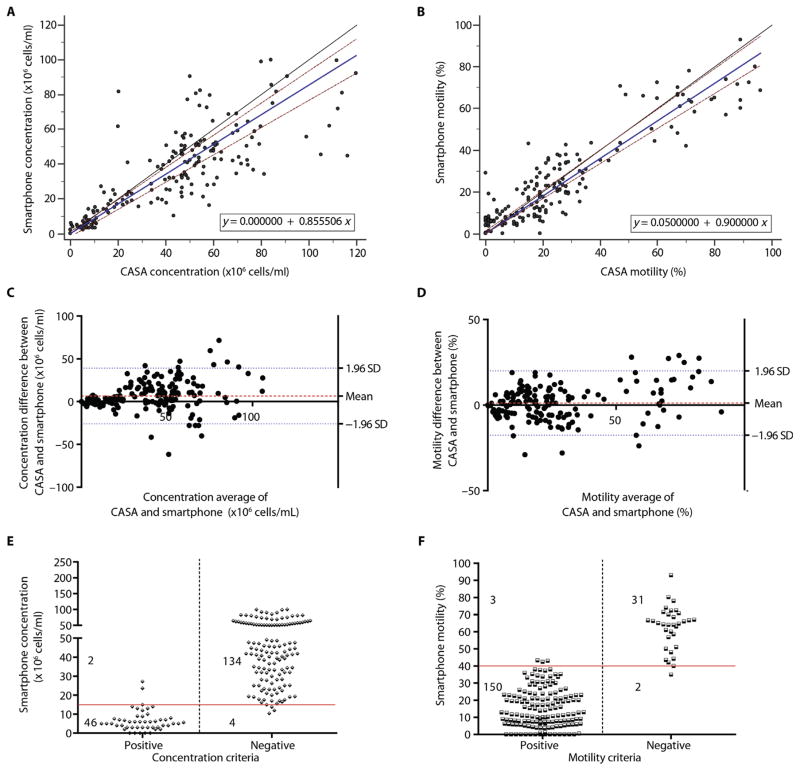
Fig. 4. On-site device evaluation performed by 10 untrained users using unwashed, unprocessed semen samples.
Passing-Bablok analysis to compare (A) sperm concentration and (B) sperm motility as measured by the smartphone-based semen analyzer and CASA for all patient semen samples. The solid blue line represents the regression line, the solid black line represents the identity line, and two dashed red lines represent the confidence band in the Passing-Bablok figures. Bland-Altman analysis to compare (C) sperm concentration and (D) sperm motility as measured by the smartphone-based semen analyzer and CASA for all patient semen samples. The red dashed line in the Bland-Altman figures is the mean difference of the methods, and the blue dotted lines represent the 95% LOAs. Scatterplots (E and F) represent the qualitative performance of the device in measuring concentration and motility. The solid red lines represent the threshold values for each criterion. “Positive” and “Negative” in (E) represent sperm concentration below and above 15 million sperm/ml measured by CASA, respectively. “Positive” and “Negative” in (F) represent sperm motility below and above 40% measured by CASA, respectively.
A Passing-Bablok analysis (n = 186) was performed to evaluate the performance of the smartphone-based semen analyzer in calculating sperm concentrations in semen samples tested by the untrained users as compared to CASA (Fig. 4A). The A and B values were 0 (CI, −2.2148 to 1.1984) and 0.8555 (CI, 0.7870 to 0.9235), respectively. The cusum test results showed no significant deviation from linearity with P = 0.27. These results showed that the difference between CASA and smartphone-based device in measuring sperm concentration increased with an increase in sperm concentration of the semen samples. Figure 4B shows a Passing-Bablok analysis of sperm motility measurements for 186 patient semen samples obtained using the smartphone-based device and CASA. The A and B values were 0.05 (CI, 0 to 1.2745) and 0.9 (CI, 0.8410 to 0.9737), respectively. The cusum test results showed no significant deviation from linearity with P = 0.31. There was an increase in the difference between CASA and the smartphone-based system in measuring sperm motility with an increase in the motility of the semen samples. We did not observe a fixed difference between CASA and the smartphone-based system in measuring sperm concentration and motility of the semen samples.
Through a Bland-Altman analysis, we observed that the mean bias in measuring the sperm concentration of the samples using the smartphone-based device and CASA was 6.657 million sperm/ml with an SD of 16.65 million sperm/ml (LOA, −25.98 to 39.29 million sperm/ml) (Fig. 4C; n = 186). We also performed Bland-Altman analysis for sperm motility measurements of the 186 patient samples measured using the smartphone-based device and CASA (Fig. 4D). The mean bias was −0.2450% with an SD of 10.28% (LOA, −20.40 to 19.91%). Collectively, Passing-Bablok and Bland-Altman analyses results showed that there were no fixed differences between CASA and the smartphone-based semen analyzer in measuring sperm concentration and motility. However, the difference between the two systems in measuring sperm concentration and motility increased as sperm concentration and motility of the semen samples increased.
The performance of the smartphone-based device as a qualitative assay to detect semen samples with sperm concentrations below and above the WHO threshold of 15 million sperm/ml was evaluated (Fig. 4E; n = 186). The sensitivity and specificity of the smartphone-based device to identify abnormal semen samples based on sperm concentration criterion were 95.83% (CI, 85.75 to 99.49%) and 97.10% (CI, 92.74 to 99.20%), respectively, and the accuracy was 96.77%. The observed AUC was 0.96 (binomial exact CI, 0.93 to 0.99). The sensitivity and specificity of the smartphone-based device identifying abnormal semen samples based on sperm motility criterion were 98.04% (CI, 94.38 to 99.59%) and 93.94% (CI, 79.77 to 99.26%), respectively, and the accuracy was 97.31% (Fig. 4F; n = 186). The observed AUC was 0.96 (binomial exact CI, 0.92 to 0.98). We also evaluated the sensitivity, specificity, and accuracy of the smartphone-based semen analyzer as a qualitative test to detect abnormal semen samples when both sperm concentration (<15 million sperm/ml) and sperm motility (<40%) were used together for semen analysis (table S10; n = 186). These results showed that the sensitivity and specificity of the smartphone-based semen analyzer to detect abnormal semen samples using both sperm concentration and motility parameters were 98.15% (CI, 94.68 to 99.62%) and 91.67% (CI, 73.00 to 98.97%), respectively, with an accuracy of 97.31% (table S10; n = 186). The observed AUC was 0.95 (binomial exact CI, 0.91 to 0.98).
Comparison of the results obtained by the trained and untrained users
To evaluate the sensitivity, specificity, and accuracy of the smartphone-based semen analyzer, we combined all the results obtained by untrained and trained study participants using 350 patient semen samples. The results obtained by the smartphone-based semen analyzer were compared with the results obtained by CASA. We performed Passing-Bablok analysis (n = 350) to evaluate the performance of the smartphone-based semen analyzer in calculating sperm concentration in patient semen samples as compared to CASA (fig. S10A). The A intercept value was 0.8146 with a CI of 0.0041 to 1.9313, and the B slope value was 0.8495 with CI ranging from 0.8039 to 0.8975. The cusum test results showed no significant deviation from linearity with P = 0.80. Figure S10B shows a Passing-Bablok analysis of sperm motility measurements for 350 patient semen samples obtained using the smartphone-based semen analyzer and CASA. The A intercept value was 0.4171 with a CI of 0 to 1.5431, and the B slope value was 0.9095 with CI ranging from 0.8596 to 0.9700. The cusum test results showed no significant deviation from linearity with P = 0.16.
We also performed Bland-Altman analysis (n = 350) to evaluate sperm concentration results obtained by the smartphone-based semen analyzer as compared to CASA (fig. S10C). The mean bias was 5.912 million sperm/ml with an SD of 24.42 million sperm/ml, and the LOA ranged from −41.95 to 53.77 million sperm/ml. Figure S10D shows the Bland-Altman analysis for sperm motility measurements of the 350 patient semen samples measured using the smartphone-based semen analyzer and CASA. The mean bias was 0.5% with an SD of 10.27%, and the LOA ranged from −19.63% to 20.64%.
Overall, the smartphone-based semen analyzer performed well when operated by trained and untrained users in measuring sperm concentration and motility (Table 1 and fig. S10, E and F; n = 350). The sensitivity and specificity of the smartphone-based semen analyzer in detecting abnormal semen samples based on the WHO criterion for sperm concentration were 93.62% (CI, 86.62 to 97.62%) and 97.27% (CI, 94.45 to 98.89%), respectively, and the accuracy was 96.29%. The observed AUC was 0.95 (binomial exact CI, 0.93 to 0.97). We also evaluated the performance of the smartphone-based semen analyzer in identifying abnormal semen samples based on sperm motility criterion (motility, <40%) (fig. S10F; n = 350). The sensitivity and specificity of the smartphone-based semen analyzer based on the sperm motility criterion were 98.64% (CI, 96.55 to 99.63%) and 91.07% (CI, 80.38 to 97.04%), respectively, and the accuracy was 97.43%. The observed AUC was 0.95 (binomial exact CI, 0.92 to 0.97). The sensitivity, specificity, and accuracy of the smartphone-based semen analyzer as a qualitative test to detect abnormal semen samples with sperm concentrations of less than 15 million sperm/ml or sperm motilities of less than 40% were 98.70% (CI, 96.70 to 99.64%), 90.70% (CI, 77.86 to 97.41%), and 97.71%, respectively. The observed AUC was 0.95 (binomial exact CI, 0.92 to 0.97). Collectively, Passing-Bablok and Bland-Altman analyses on the results obtained from both trained and untrained users showed that there were no fixed differences between CASA and the smartphone-based semen analyzer in measuring sperm concentration and motility. However, the difference between the two systems in measuring sperm concentration and motility increased with an increase in sperm concentration and motility of the semen samples. These combined results showed that the smartphone-based semen analyzer performed well in qualitatively identifying abnormal semen samples based on the WHO thresholds on sperm concentration (<15 million sperm/ml) and sperm motility (<40%).
Table 1. Contingency table comparing CASA and smartphone-based testing.
“Negative” indicates semen samples with sperm concentration and motility above the WHO threshold (sperm concentration of >15 million sperm/ml and sperm motility of >40%). “Positive” indicates semen samples with sperm concentration or motility below the WHO threshold (sperm concentration of <15 million sperm/ml or sperm motility of <40%).
| Smartphone | ||||
|---|---|---|---|---|
| Negative | Positive | |||
| CASA | Negative | 39 | 4 | Specificity, 90.70% |
| Positive | 4 | 303 | Sensitivity, 98.70% | |
| Accuracy, 97.71% | ||||
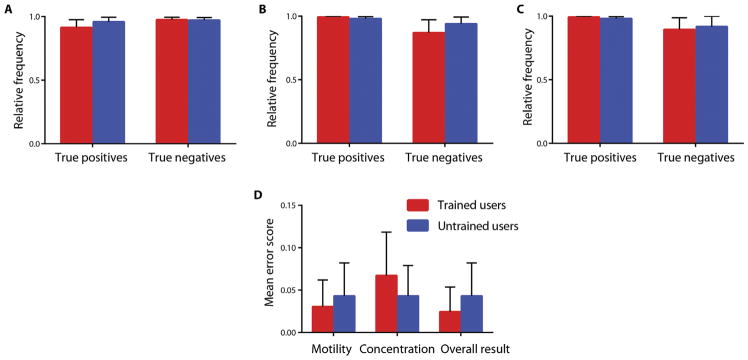
To further evaluate the performance of the smartphone-based device, we compared the results obtained by trained and untrained users using the Holm-Sidak statistical analysis (Fig. 5). Figure 5A compares the frequency of correctly classifying patient semen samples based on the WHO guidelines on sperm concentration (below and above 15 million sperm/ml) when trained and untrained users performed the semen analysis. No statistical significance was observed for identifying semen samples with sperm concentration below the WHO criterion (15 million sperm/ml) (P = 0.43) and samples with sperm concentrations above the WHO threshold (P = 0.88) (Fig. 5A). Similarly, there was no statistically significant difference between the sample classification results obtained by trained and untrained users for correctly classifying semen samples with sperm motility below (P = 0.45) and above (P = 0.41) the WHO threshold (40% motility) (Fig. 5B). We measured the frequency of correctness in semen sample classification by trained and untrained users when both sperm concentration and motility were considered for the detection of abnormal semen samples (Fig. 5C). No statistical significance was observed for identifying abnormal (sperm concentration of <15 million/ml or motility of <40%) (P = 0.47) and normal (sperm concentration of >15 million/ml and motility of >40%) (P = 0.83) semen samples when trained and untrained users performed the tests.
Fig. 5. Usability of the smartphone-based semen analyzer by trained and untrained users.
The accuracy of correctly classifying the patient semen samples using the smartphone-based semen analyzer based on the WHO criteria for (A) sperm concentration, (B) sperm motility, and (C) sperm concentration and motility using 164 samples tested by trained users and 186 samples tested by 10 untrained users. Relative frequency represents the rate of correctness of the trained and untrained user groups. (D) A comparison of the mean error score as obtained for the groups of trained and untrained users. The mean error scale ranges from 0 to 2. All error bars represent the 95% CIs.
The mean error score of both user groups were also tested (Fig. 5D). There was not a statistically significant difference between the error scores estimated for the trained and untrained users. The two groups were tested for statistically significant difference with the Holm-Sidak method of analysis. No statistically significant difference was observed in the error scores of motility (P = 0.63), concentration (P = 0.44), and combined sperm concentration and motility results (P = 0.46) between the two groups. The smartphone-based semen analyzer did not show any statistically significant difference when used by trained or untrained users.
Here, we have tested 350 unprocessed semen samples, 95 of which had sperm concentrations of less than 15 million sperm/ml based on CASA results and 88 of which were correctly identified by our system. Furthermore, of the 295 samples that had sperm motilities of less than 40% based on CASA, 290 were correctly classified as abnormal semen samples using the smartphone-based system. Considering both sperm concentration and motility criteria, 307 semen samples were categorized as abnormal (sperm concentration of <15 million sperm/ml or motility of <40%) using CASA, and 303 of these samples were identified correctly by the smartphone-based semen analyzer.
DISCUSSION
We have demonstrated that the smartphone-based semen analyzer can accurately measure sperm concentration, motility, total sperm count, total motile sperm count, and linear and curvilinear velocities using a small volume (<35 μl) of an unwashed, unprocessed semen sample loaded into a disposable microchip. We developed an automated smartphone-based diagnostic assay with the potential to make male fertility testing as accessible, easy, fast, and private as pregnancy tests.
Our technology evaluation results using preclinical cryopreserved and fresh, undiluted, unprocessed clinical patient semen samples showed that the smartphone-based semen analyzer performed well compared to the conventional method of semen analysis, which is the manual microscope-based analysis method. Device variability, coefficient of variation (CV), in preclinical samples was 4.47%. The optical attachment and the smartphone application were designed such that minimal input from an untrained user was required. The optical focus and lighting intensity were optimized and fixed in the smartphone accessory to eliminate the need for manual adjustment by the user and to reduce the errors in image processing. Professional training and routine quality control exercises are critical to minimize errors in CASA-based and manual microscope-based semen analysis methods. Our easy-to-use platform eliminates the need for such exercises and may aid in the standardization of semen tests because variability in semen analysis is still relatively high (11). Furthermore, infertility in resource-limited settings is also a global issue, and the ability to provide routine, point-of-care, low-cost, reliable semen analysis testing is needed (9). CASA systems are relatively expensive, and even in the United States, many clinics and fertility centers use manual semen analysis methods, resulting in poor standardization (10, 32). Our system, with its low manufacturing costs (<$5 material cost), can potentially introduce a better standardization of care in regions of the world that require it the most.
The smartphone-based semen analyzer may also be used for home-based monitoring of men who undergo vasectomy. It is estimated that worldwide more than 33 million couples prefer to perform vasectomy as a safer, less expensive, and simpler method compared to female sterilization for contraception (33). There are more than 20 million men who underwent vasectomy in China and India alone (34). The number of vasectomy procedures per year in the United States has been estimated to be between 175,000 and 550,000 procedures per year (35, 36). Although vasectomy failure is lower than 1%, it is highly recommended that men perform a follow-up semen analysis to confirm the success of the procedure. As per guidelines, sperm concentration in semen of patients should be less than 100,000 sperm/ml between 8 and 16 weeks after the vasectomy procedure (28). It has been reported that patient compliance for postvasectomy follow-up semen analysis has been extremely poor (37). The smartphone-based semen analyzer has the potential to improve patient compliance for postvasectomy semen analysis by providing a rapid, easy-to-use, affordable, and private method for accurate sperm concentration and motility measurement. Our results demonstrated the ability of the smartphone-based semen analyzer to accurately classify semen samples with sperm counts below and above the 100,000 sperm/ml threshold set for postvasectomy monitoring.
One of the limitations of automated optical-based semen analyzers is measuring sperm concentration with high accuracy in samples with higher-than-average numbers of nonsperm cells such as white blood cells. We have designed our sperm detection and tracking algorithm to perform size-based differentiation of sperm heads from other larger cells such as white blood cells. However, although our single parameter–based detection algorithm is effective at reducing processing time, it is prone to misidentifications when nonsperm cells or debris of similar size to the sperm head are present in the FOV of the smartphone-based semen analyzer. This limitation may be overcome with a more computationally intensive set of image processing algorithms at the expense of processing time or with relatively more expensive optical sensing–based hardware. Similarly, our current version of the smartphone-based semen analyzer cannot evaluate sperm morphology; however, its ability to accurately identify abnormal semen samples based on sperm concentration and motility can potentially shift the paradigm in male infertility management in both developed and developing countries.
A rapid, point-of-care diagnostic assay for semen analysis can also have an important application in animal breeding. Currently, animal breeders immediately transfer collected semen samples to an equipped laboratory for semen analysis through a sample transfer process with a controlled exposure to environmental effects such as cold, excessive heat, light, shock, and physical trauma. The sample container must be prewarmed at 37° or 38°C and kept at this temperature during the transfer. Additionally, sperm maturation and development can take a few weeks (~65 days for stallions, depending on the species) (38). If any unintended event/disease occurs for the male animal during the sperm maturation process, the previously produced and stored mature sperm may not be negatively affected, and only sperm being produced would be harmed. Therefore, a regular point-of-care semen analysis could have an important role in the efficient and cost-effective management of animal breeding. Considering the differences between human semen and animal semen in terms of sperm concentration, motility, and sample volume, the current version of the smartphone-based semen analyzer would need to be augmented for applications in animal breeding. We envision that animal semen sample dilution would be required before semen analysis testing using the current version of the smartphone-based platform.
The advances in microtechnologies and the surge in consumer electronics have paved a solid foundation for developing mobile health (mhealth) technologies with the potential to transform the current paradigm in global health. The work reported here is an example of how smartphones can be seamlessly integrated with hardware, software, and microfluidics to develop a point-of-care diagnostic device to address clinical gaps in male infertility management.
MATERIALS AND METHODS
Study design
The goal of this study was to develop and evaluate a smartphone-based diagnostic assay for point-of-care semen analysis through accurate sperm concentration and motility measurement using a small undiluted, unprocessed semen sample loaded into a disposable microchip. We had initially set a target of ~50 samples based on a power analysis for AUC with a null hypothesis value of 50%, an estimated AUC value of 80%, an α value of 0.05, and a power of 95. The disease prevalence was set at 50% because those visiting the clinic have ~50% chance of showing signs of infertility. We further increased the number of samples to more than 150 to achieve greater clinical relevance with more statistical power. We had originally set a target of ~120 samples to be tested by untrained users. This sample size was increased to 186 to collect data from patient samples with a broad range of sperm concentrations and motilities below and above the WHO criteria. Initially, we wanted to evaluate the ability of the smartphone-based semen analyzer to accurately detect semen samples with sperm concentrations below and above 100,000 sperm/ml, a threshold criterion for postvasectomy failure. In an attempt to prepare semen samples with sperm concentrations as low as <100,000 sperm/ml, we prepared 61 serially diluted cryopreserved semen samples. Three repeated measurements were made for each sample to measure the coefficient of variability. Five cryopreserved semen samples were excluded from this study because of microfluidic device contamination by debris in the channels, which caused false detection. Precautionary methods were in place when chips were fabricated for the clinical assessment by taking steps to fabricate these devices in a sterile environment.
For the assessment of infertility diagnosis based on sperm concentration and motility, we carried out our evaluation using 164 undiluted, unprocessed, fresh semen samples received from patients at the MGH fertility clinic, and the smartphone-based results were compared with the results calculated by a CASA-based platform (Hamilton Thorne, HTM-CEROS II). Ten untrained users who were completely unfamiliar with the smartphone-based semen analyzer were recruited to perform semen analysis tests with 186 unwashed, undiluted semen samples. The untrained users ranged from administrative staff with no background in science to clinical nurses. The users were between 25 and 45 years old. The results obtained by the untrained users were compared with the results obtained by CASA. An instruction sheet was provided to the users, and the entire set of experiments was carried out in a double-blinded manner. The original developers of the device were not present during the device usability testing with the untrained users. No patient selection criteria were in place for the trained user study. For the untrained user study, we collected data from patient samples with a broad range of sperm concentrations and motilities below and above the WHO criterion of 15 million sperm/ml. No samples were excluded from this clinical evaluation study. During this study, we have also evaluated the performance of a modular weight scale attachment in estimating the total sperm count and total motile sperm count.
Optical attachment
The optical attachment, which housed a pair of lenses obtained from pick-up heads of a DVD and CD drive, a small battery, and an LED, was 3D printed using Ultimaker 2 Extended with Ultimaker PLA (poly-lactic acid) as the printing material. The lenses were positioned inside the optical attachment aligned to the optical axis of the smartphone’s rear camera. The 3D model of the attachment was designed in SolidWorks for a Moto X smartphone (Motorola, XT1575). Sample fine focus was achieved through the smartphone’s autofocus capability. A broadband white LED (Microtivity, IL041) was used for transillumination of the sample powered by a 3-V battery (Panasonic, CR1620).
Microchip design
The microfluidic devices were fabricated using plain glass slides (VWR, 48300-025), double-sided adhesives (DSAs) (30 μm; 3M, 82603), poly(methyl methacrylate) (PMMA) (3.175 mm; McMaster-Carr, 8560K239), and PDMS (Dow Corning, Sylgard 184). PMMA and DSA sheets were cut using a laser cutter (Universal Laser Systems, VLS 2.30). The rubber bulb used in the microfluidic devices for electric-free on-chip sample loading was made out of PDMS on a 3D printed master mold composed of acrylonitrile butadiene styrene (Ultimaker). The bulb was attached to PMMA with PDMS. The PMMA was attached to the microchip using DSA. Before use, the inlet was covered with a latex fabric for hermetic sealing and was bonded to the microchip using super glue (3M, B004OKKWGE), and a small incision was made on the PDMS bulb. An extender cap, holding a plastic microhematocrit capillary tube (Fisherbrand, 22-315791) as an extender capillary tip, was 3D printed with PLA. The extender cap was placed at the inlet of the microfluidic device.
Smartphone application
Once the microchip containing the sample is loaded into the attachment, the smartphone application records 1-s duration videos (30 fps) and processes each frame to obtain the various semen parameters. The image processing, sperm detection and tracking, and data analysis were all performed on-phone, which was running on Android 6.0. A custom Android application was developed using Android Studio. We used the sdk and ndk libraries provided with the Android development tools as well as other libraries including OpenCV (version 2.4.8) and JavaCV (version 1.1) for the image processing calculations in the smartphone application. The application was designed to process data obtained by the smartphone camera using the optical attachment. The analysis was performed in two steps: (i) processing individual frames of the recorded video and extracting coordinates of the sperm observed in the FOV and (ii) tracking the observed positions of sperm across frames by mapping their paths. We used an adaptive thresholding algorithm as part of the sperm detection method for sperm concentration and motility analysis.
Sample preparation and preclinical testing
Cryopreserved semen samples were obtained from California Cryobank and New England Cryobank and were stored in liquid nitrogen. The samples were thawed before use by placing them in an incubator at 37°C for 14 min. The samples were serially diluted with phosphate-buffered saline at multiple ratios. The samples were loaded into the microchips and were tested using the smartphone-based semen analyzer. In parallel, 10 μl of each sample was loaded into a Neubauer hemocytometer (Sigma, Z359629) and was counted under a phase-contrast optical microscope (Zeiss, Observer D1). At least four different FOVs were analyzed for each sample, and three repeated measurements were made using the smartphone application.
Assessment with patients’ semen samples (trained and untrained users)
Specimens analyzed were from patients seeking treatment from the Vincent Andrology laboratory at the MGH. The use of the discarded specimens was approved by the Human Studies Institutional Review Boards of the MGH. Semen samples were obtained on-site by masturbation and collected into a sterile plastic container. This study was performed with compliance to previously published guidelines (31, 39). Men were instructed to abstain from ejaculation for at least 48 hours before the sample was produced. Although the sample liquefaction can be done at room temperature according to the WHO fifth edition guidelines (31), we liquefied the samples used for device evaluation by trained users at 37°C for 20 min before analysis. Semen sample volume was calculated by measuring the sample weight using a standard laboratory-based weight scale. To measure both sperm concentration and motility, 6 μl of semen from each sample was placed into a prewarmed (37°C) Leja counting chamber (Hamilton Thorne) and analyzed using a CASA system (HTM-CEROS II). A minimum of 200 sperm cells from at least four different FOVs were analyzed from each specimen. If the sperm concentrations exceeded 100 million sperm/ml, standard dilutions (1:10 or 1:20) were performed, and the samples were reanalyzed using CASA to obtain accurate measurements. Similarly, samples with sperm concentrations less than 5 million sperm/ml were verified with a manual assessment before the final CASA output was recorded. Patient specimens were loaded into the microchip and tested using the smartphone-based semen analyzer in parallel to the CASA system. Single measurements were made for each sample tested with the smartphone-based setup regardless of the concentration.
To further evaluate the performance and ease of use of the smartphone-based semen analyzer, untrained users (n = 10) who were completely unfamiliar with the platform were recruited at the MGH fertility clinic to perform 186 semen analysis tests. The participants were only given the device, disposables, and a simple instruction (fig. S11) to perform the tests. The developers of the smartphone-based semen analyzer were not present at the site during the double-blinded semen analysis tests by untrained users. Triplicate CASA measurement results on 112 semen samples were used for CASA repeatability calculations (tables S5, S6, and S11). To evaluate the reliability of our CASA system for measuring sperm concentration, we tested and analyzed 1067 semen samples using both CASA and manual analysis before the initiation of the study. Similarly, we tested 1243 samples to evaluate the accuracy of the CASA system for motility measurement in comparison to manual analysis. The mean % variations between the two methods are reported in table S12.
Statistical analysis
Passing-Bablok regression analysis (29), coefficient of variation, and ROC analysis were performed using MedCalc 14.8.1. Bland-Altman analysis (30) was performed using GraphPad Prism version 6. Sample size estimation was performed using MedCalc. Stata 13 was also used to calculate the classification accuracy reported for sensitivity and specificity of the device.
Mean error was calculated by assigning weights for each of the classifications as obtained by both trained and untrained user groups. If the device classified the tested samples correctly relative to the CASA-based method, a score of 0 was assigned. Scores of 1 and 2 were assigned for false positives and false negatives, respectively. Arithmetic mean scores were obtained for sperm concentration, sperm motility, and overall results for both trained users and untrained users. Statistical significance was determined using t tests for multiple comparisons with Holm-Sidak corrections and 5% α.
Supplementary Material
Acknowledgments
We would like to thank J. E. Chavarro, M. N. Kathrins, R. Singh, A. Vasan, and S. Kallakuri for fruitful discussions and their help in microchip fabrications. Funding: This work is supported by the Brigham Research Institute Pilot Grant, the Bright Futures Prize, the Innovation Evergreen Fund, and the Fund to Sustain Research Excellence (Brigham Research Institute, Brigham and Women’s Hospital, Harvard Medical School), NIH grants (1R01AI118502 and P30ES000002), a Harvard National Institute of Environmental Health Sciences Grant (Harvard T.H. Chan School of Public Health, Harvard Center for Environmental Health), and an American Society of Reproductive Medicine Award (American Board of Obstetrics and Gynecology, American College of Obstetricians and Gynecologists, American Society for Reproductive Medicine, and Society for Reproductive Endocrinology and Infertility).
Footnotes
www.sciencetranslationalmedicine.org/cgi/content/full/9/382/eaai7863/DC1
Materials and Methods
Fig. S1. Image of a micrometer scale taken with the smartphone-based semen analyzer.
Fig. S2. Images of microbeads and sperm taken with a laboratory-based microscope and the smartphone-based semen analyzer.
Fig. S3. Process flow of microchip fabrication.
Fig. S4. Smartphone application flow.
Fig. S5. Device performance for samples with concentrations below 100 million sperm/ml.
Fig. S6. Bland-Altman analysis for the measurement of sperm curvilinear (VCL) and linear velocities (VSL).
Fig. S7. Weight scale schematic and design.
Fig. S8. Weight scale performance in estimating the total count and total motile count.
Fig. S9. Bland-Altman analysis of the three phones.
Fig. S10. Overall agreement and diagnostic performance of the smartphone-based semen analyzer.
Fig. S11. Steps of operation for semen testing using the smartphone-based semen analyzer.
Table S1. Table of costs.
Table S2. Processing times for the 350 samples tested by trained and untrained users.
Table S3. Raw data of cryopreserved samples collected for measuring sperm concentration.
Table S4. Raw data collected from clinical specimens at MGH fertility clinic tested by trained users.
Table S5. Repeatability measures for the concentration parameter.
Table S6. Repeatability measures for the motility parameter.
Table S7. Diagnostic results for samples tested by trained users.
Table S8. Comparison of the performance of three different phones with the smartphone-based semen analyzer.
Table S9. Raw data collected from clinical specimens at MGH fertility clinic tested by untrained users.
Table S10. Diagnostic results for samples tested by untrained users.
Table S11. CASA triplicate measurements at MGH fertility clinic for 112 samples.
Table S12. Measurement of CASA accuracy against manual analysis.
Movie S1. Video of a semen sample recorded with the smartphone-based semen analyzer.
Competing interests: H.S. is the inventor of patent application PCT/US2016/038739 submitted by the Brigham and Women’s Hospital that covers home evaluation of the quality of semen samples.
Author contributions: H.S. designed the study. M.K.K., A.S., M.S., and H.S. designed the smartphone optical attachment. M.K.K., A.S., and H.S. designed the microfluidic device for semen sample handling. M.K.K. developed the smartphone code for sperm detection and monitoring. M.K.K. and C.P. developed the graphical user interface of the smartphone application. A.S., M.V., and P.T. performed the experiments. P.T. designed and developed the miniaturized weight scale. C.L.B. assisted in clinical validation field testing using patient semen samples and performed all the CASA tests. J.C.P. provided clinical advice and support in developing and validating the smartphone-based assay. M.K.K., A.S., and M.S.D. analyzed the data. M.K.K. and H.S. wrote the manuscript. All coauthors edited the manuscript.
REFERENCES AND NOTES
- 1.Agarwal A, Mulgund A, Hamada A, Chyatte MR. A unique view on male infertility around the globe. Reprod Biol Endocrinol. 2015;13:37. doi: 10.1186/s12958-015-0032-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Diamond DA, Zurakowski D, Bauer SB, Borer JG, Peters CA, Cilento BG, Jr, Paltiel HJ, Rosoklija I, Retik AB. Relationship of varicocele grade and testicular hypotrophy to semen parameters in adolescents. J Urol. 2007;178:1584–1588. doi: 10.1016/j.juro.2007.03.169. [DOI] [PubMed] [Google Scholar]
- 3.de Kretser DM. Endocrinology of male infertility. Br Med Bull. 1979;35:187–192. doi: 10.1093/oxfordjournals.bmb.a071568. [DOI] [PubMed] [Google Scholar]
- 4.Reijo R, Lee TY, Salo P, Alagappan R, Brown LGJ, Rosenberg M, Rozen S, Jaffe T, Straus D, Hovatta O, de la Chapelle A, Silber S, Page DC. Diverse spermatogenic defects in humans caused by Y chromosome deletions encompassing a novel RNA-binding protein gene. Nat Genet. 1995;10:383–393. doi: 10.1038/ng0895-383. [DOI] [PubMed] [Google Scholar]
- 5.Eisenberg ML, Li S, Behr B, Pera RR, Cullen MR. Relationship between semen production and medical comorbidity. Fertil Steril. 2015;103:66–71. doi: 10.1016/j.fertnstert.2014.10.017. [DOI] [PubMed] [Google Scholar]
- 6.Skakkebaek NE, Rajpert-De Meyts E, Buck Louis GM, Toppari J, Andersson A-M, Eisenberg ML, Jensen TK, Jørgensen N, Swan SH, Sapra KJ, Ziebe S, Priskorn L, Juul A. Male reproductive disorders and fertility trends: Influences of environment and genetic susceptibility. Physiol Rev. 2016;96:55–97. doi: 10.1152/physrev.00017.2015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Carmeli YS, Birenbaum-Carmeli D. The predicament of masculinity: Towards understanding the male’s experience of infertility treatments. Sex Roles. 1994;30:663–677. [Google Scholar]
- 8.Inhorn MC. Masturbation semen collection and men’s IVF experiences: Anxieties in the Muslim world. Body Soc. 2007;13:37–53. [Google Scholar]
- 9.Ombelet W. Global access to infertility care in developing countries: A case of human rights equity and social justice. Facts Views Vis Obgyn. 2011;3:257–266. [PMC free article] [PubMed] [Google Scholar]
- 10.Amann RP, Katz DF. Reflections on CASA after 25 years. J Androl. 2004;25:317–325. doi: 10.1002/j.1939-4640.2004.tb02793.x. [DOI] [PubMed] [Google Scholar]
- 11.Punjabi U, Wyns C, Mahmoud A, Vernelen K, China B, Verheyen G. Fifteen years of Belgian experience with external quality assessment of semen analysis. Andrology. 2016;4:1084–1093. doi: 10.1111/andr.12230. [DOI] [PubMed] [Google Scholar]
- 12.Halangk W, Bohnensack R. Quantification of sperm motility by a turbidimetric assay. Correlation to cellular respiration. Biomed Biochim Acta. 1986;45:331–341. [PubMed] [Google Scholar]
- 13.Frost J, Cummins HZ. Motility assay of human sperm by photon correlation spectroscopy. Science. 1981;212:1520–1522. doi: 10.1126/science.7233239. [DOI] [PubMed] [Google Scholar]
- 14.Earnshaw JC, Munroe G, Thompson W, Traub AI. Automated laser light scattering system for assessment of sperm motility. Med Biol Eng Comput. 1985;23:263–268. doi: 10.1007/BF02446869. [DOI] [PubMed] [Google Scholar]
- 15.Segerink LI, Sprenkels AJ, ter Braak PM, Vermes I, van den Berg A. On-chip determination of spermatozoa concentration using electrical impedance measurements. Lab Chip. 2010;10:1018–1024. doi: 10.1039/b923970g. [DOI] [PubMed] [Google Scholar]
- 16.Su TW, Erlinger A, Tseng D, Ozcan A. Compact and light-weight automated semen analysis platform using lensfree on-chip microscopy. Anal Chem. 2010;82:8307–8312. doi: 10.1021/ac101845q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Nosrati R, Gong MM, San Gabriel MC, Pedraza CE, Zini A, Sinton D. Paper-based quantification of male fertility potential. Clin Chem. 2016;62:458–465. doi: 10.1373/clinchem.2015.250282. [DOI] [PubMed] [Google Scholar]
- 18.Kobori Y, Pfanner P, Prins GS, Niederberger C. Novel device for male infertility screening with single-ball lens microscope and smartphone. Fertil Steril. 2016;106:574–578. doi: 10.1016/j.fertnstert.2016.05.027. [DOI] [PubMed] [Google Scholar]
- 19.Schaff UY, Fredriksen LL, Epperson JG, Quebral TR, Naab S, Sarno MJ, Eisenberg ML, Sommer GJ. Novel centrifugal technology for measuring sperm concentration in the home. Fertil Steril. 2016;107:358–364.e4. doi: 10.1016/j.fertnstert.2016.10.025. [DOI] [PubMed] [Google Scholar]
- 20.Weibel DB, Whitesides GM. Applications of microfluidics in chemical biology. Curr Opin Chem Biol. 2006;10:584–591. doi: 10.1016/j.cbpa.2006.10.016. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Fan HC, Behr B, Quake SR. Genome-wide single-cell analysis of recombination activity and de novo mutation rates in human sperm. Cell. 2012;150:402–412. doi: 10.1016/j.cell.2012.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Steinhubl SR, Muse ED, Topol EJ. The emerging field of mobile health. Sci Transl Med. 2015;7:283rv283. doi: 10.1126/scitranslmed.aaa3487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Laksanasopin T, Guo TW, Nayak S, Sridhara AA, Xie S, Olowookere OO, Cadinu P, Meng F, Chee NH, Kim J, Chin CD, Munyazesa E, Mugwaneza P, Rai AJ, Mugisha V, Castro AR, Steinmiller D, Linder V, Justman JE, Nsanzimana S, Sia SK. A smartphone dongle for diagnosis of infectious diseases at the point of care. Sci Transl Med. 2015;7:273re271. doi: 10.1126/scitranslmed.aaa0056. [DOI] [PubMed] [Google Scholar]
- 24.Wojtczak J, Bonadonna P. Pocket mobile smartphone system for the point-of-care submandibular ultrasonography. Am J Emerg Med. 2013;31:573–577. doi: 10.1016/j.ajem.2012.09.013. [DOI] [PubMed] [Google Scholar]
- 25.D’Ambrosio MV, Bakalar M, Bennuru S, Reber C, Skandarajah A, Nilsson L, Switz N, Kamgno J, Pion S, Boussinesq M, Nutman TB, Fletcher DA. Point-of-care quantification of blood-borne filarial parasites with a mobile phone microscope. Sci Transl Med. 2015;7:286re284. doi: 10.1126/scitranslmed.aaa3480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Berg B, Cortazar B, Tseng D, Ozkan H, Feng S, Wei Q, Chan RYL, Burbano J, Farooqui Q, Lewinski M, Di Carlo D, Garner OB, Ozcan A. Cellphone-based hand-held microplate reader for point-of-care testing of enzyme-linked immunosorbent assays. ACS Nano. 2015;9:7857–7866. doi: 10.1021/acsnano.5b03203. [DOI] [PubMed] [Google Scholar]
- 27.Ming K, Kim J, Biondi MJ, Syed A, Chen K, Lam A, Ostrowski M, Rebbapragada A, Feld JJ, Chan WCW. Integrated quantum dot barcode smartphone optical device for wireless multiplexed diagnosis of infected patients. ACS Nano. 2015;9:3060–3074. doi: 10.1021/nn5072792. [DOI] [PubMed] [Google Scholar]
- 28.Sharlip ID, Belker AM, Honig S, Labrecque M, Marmar JL, Ross LS, Sandlow JI, Sokal DC. Vasectomy: AUA guideline. J Urol. 2012;188:2482–2491. doi: 10.1016/j.juro.2012.09.080. [DOI] [PubMed] [Google Scholar]
- 29.Passing H, Bablok A new biometrical procedure for testing the equality of measurements from two different analytical methods. Application of linear regression procedures for method comparison studies in clinical chemistry, Part I. J Clin Chem Clin Biochem. 1983;21:709–720. doi: 10.1515/cclm.1983.21.11.709. [DOI] [PubMed] [Google Scholar]
- 30.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 31.World Health Organization; World Health Organization, editor. WHO Laboratory Manual for the Examination and Processing of Human Semen. 2010. p. 5. [Google Scholar]
- 32.Walczak-Jedrzejowska R, Marchlewska K, Oszukowska E, Filipiak E, Bergier L, Slowikowska-Hilczer J. Semen analysis standardization: Is there any problem in Polish laboratories? Asian J Androl. 2013;15:616–621. doi: 10.1038/aja.2013.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Department of Economic and Social Affairs. World Contraceptive Use 2009. United Nations: 2009. [Google Scholar]
- 34.Pile JM, Barone MA. Demographics of vasectomy–USA and international. Urol Clin North Am. 2009;36:295–305. doi: 10.1016/j.ucl.2009.05.006. [DOI] [PubMed] [Google Scholar]
- 35.Eisenberg ML, Lipshultz LI. Estimating the number of vasectomies performed annually in the United States: Data from the National Survey of Family Growth. J Urol. 2010;184:2068–2072. doi: 10.1016/j.juro.2010.06.117. [DOI] [PubMed] [Google Scholar]
- 36.Kogan P, Wald M. Male contraception: History and development. Urol Clin North Am. 2014;41:145–161. doi: 10.1016/j.ucl.2013.08.012. [DOI] [PubMed] [Google Scholar]
- 37.Duplisea J, Whelan T. Compliance with semen analysis. J Urol. 2013;189:2248–2251. doi: 10.1016/j.juro.2013.01.062. [DOI] [PubMed] [Google Scholar]
- 38.Morel MCGD. CABI, editor. Equine Reproductive Physiology, Breeding and Stud Management. 2015. p. 4. [Google Scholar]
- 39.Björndahl L, Barratt CLR, Mortimer D, Jouannet P. How to count sperm properly: Checklist for acceptability of studies based on human semen analysis. Hum Reprod. 2016;31:227–232. doi: 10.1093/humrep/dev305. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.