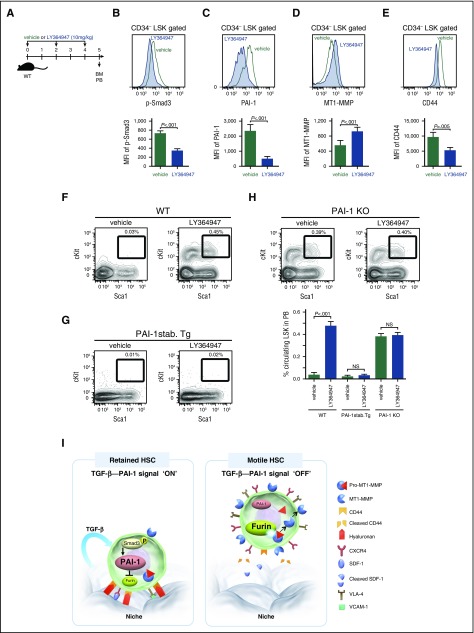
Figure 7.
TGF-β–induced iPAI-1 activation is responsible for the retention of HSPCs in the BM niche. (A) Schema for in vivo experiments. Representative flow cytometric profiles and MFI (n = 6) for p-Smad3 (B), iPAI-1 (C), MT1-MMP (D), and CD44 (E) expressions in BM LSK CD34− cells isolated from vehicle- or LY364947-treated WT mice. (F-G) Representative flow cytometric profiles and percentages (n = 6) of mobilized LSK cells in PB of vehicle- or LY364947-treated WT (F), stable PAI-1 transgenic mouse (PAI-1 stab. Tg) (G), or PAI-1 KO (H) mice. Means ± SD are shown. (I) A proposed working model schematically representing the main message of this work. In a steady state, TGF-β signaling stays active in niche-residing HSPCs. TGF-β–induced iPAI-1 inhibits Furin-dependent maturation of MT1-MMP, thereby causing HSPCs to remain within the BM niche. Conversely, when the TGF-β–iPAI-1 signal is suppressed, for example, in response to environmental stimuli, Furin-dependent MT1-MMP maturation becomes enhanced. The enhanced expression of MT1-MMP results in MT1-MMP–mediated CD44 cleavage as well as CXCR4 and VLA-4 activation, which in turn stimulates detachment of HSPCs from the niche and active trafficking into the bloodstream. VCAM-1, vascular cell adhesion molecule-1.