Abstract
BACKGROUND
Previous results from an interim analysis of an open-label, randomized, phase 3 study demonstrated that bortezomib combined with pegylated liposomal doxorubicin (PLD) was superior to bortezomib monotherapy in patients with relapsed/refractory multiple myeloma who had previously received one or more lines of therapy. Protocol-defined final survival data from that study are provided here.
METHODS
Patients were randomized (1:1) to receive either bortezomib alone (1.3 mg/m2 intravenously on days 1, 4, 8, and 11 of every 21-day cycle) or bortezomib-PLD (bortezomib plus PLD 30 mg/m2 intravenously on day 4). The primary endpoint was the time to progression. Secondary efficacy endpoints included overall survival (OS), progression-free survival, and the overall response rate.
RESULTS
In total, 646 patients (bortezomib-PLD, n = 324; bortezomib alone, n = 322) were randomized between December, 2004, and March, 2006. On the clinical cutoff date (May 16, 2014) for the final survival analysis, at a median follow-up of 103 months, 79% of patients had died (bortezomib-PLD group: 253 of 324 patients; 78%; bortezomib alone group: 257 of 322 patients; 80%). The median OS in the bortezomib-PLD group was 33 months (95% confidence interval [CI], 28.9–37.1) versus 30.8 months (95% CI, 25.2–36.5) in the bortezomib alone group (hazard ratio, 1.047; 95% CI, 0.879–1.246; P = .6068). Salvage therapies included conventional and novel drugs, which were well balanced between the two treatment groups.
CONCLUSIONS
Despite inducing a superior time to progression, long-term follow-up revealed that PLD-bortezomib did not improve OS compared with bortezomib alone in patients with relapsed/refractory multiple myeloma. The inability to sustain the early observed survival advantage may have been caused by the effects of subsequent lines of therapy, and underscores the need for long-term follow-up of phase 3 trials while recognizing the challenge of having adequate power to detect long-term differences in OS.
Keywords: bortezomib, doxorubicin, multiple myeloma, pegylated liposomal doxorubicin, survival
INTRODUCTION
Multiple myeloma (MM), the second most common hematologic malignancy, affects terminally differentiated plasma cells,1 and is characterized by the overproduction of monoclonal immunoglobulins, osteolytic bone lesions, renal disease, and immunodeficiency.2 Although patients with MM often respond to initial therapy, the disease eventually relapses and becomes refractory to further treatment. However, with the advent of new and more effective drugs for the treatment of MM, both alone and in combination with established anti-MM agents, there is now a rapid increase in the number of therapeutic options available to patients with MM, particularly in the relapsed/refractory setting.
The combination of bortezomib and pegylated liposomal doxorubicin (PLD) is considered an important therapeutic option for patients with relapsed or refractory MM who have received 1 or more prior therapies. Initial therapy with novel combinations that included bortezomib and/or PLD demonstrated high overall response rates (which ranged from 80% to 100%), complete response rates (range, 20%–30%), and very good partial response rates (range, 30%–50%) in previously untreated patients with MM.3–7
The results from the interim analysis of the study demonstrated that the bortezomib-PLD combination significantly reduced the risk of developing disease progression by 45% and prolonged the median time to progression (TTP) by 3 months in patients with relapsed or refractory MM.8 An early overall survival (OS) benefit from treatment with bortezomib-PLD was observed compared with bortezomib monotherapy (hazard ratio [HR], 1.41; 95% confidence interval [CI], 1.002–1.97; P = .0476).8 Here, we report results from the protocol-defined, long-term follow-up for survival analysis of the study. The study is registered at clinicaltrials.gov as National Clinical Trial NCT00103506.
MATERIALS AND METHODS
Patients
Patients (aged ≥18 years) with confirmed MM whose disease had progressed after an initial response to at least 1 line of prior therapy or had been refractory to initial treatment were eligible. Patients who had an Eastern Cooperative Oncology Group performance status of 0 or 1, a life expectancy of at least 3 months, platelets ≥75,000/mm3, hemoglobin ≥8.0 g/dL, an absolute neutrophil count ≥1000/mm3, creatinine clearance ≥30 mL/minute, total bilirubin ≤1.5 times the upper limit of normal, and corrected serum calcium <12 mg/dL (3.0 mM/L) or ionized calcium <6.5 mg/dL (1.6 mmol/L) were enrolled in the study. Patients were bortezomib-naive and were excluded if they had previous disease progression while receiving anthracycline-containing therapy. Additional exclusion criteria included prior doxorubicin or other anthracycline exposure >240 mg/m2, clinically significant cardiac disease, a left ventricular ejection fraction less than institutional normal limits, and grade 2 or higher peripheral neuropathy.
The study protocol was approved by local independent ethics committees, and the study was conducted in accordance with the ethical principles originating in the Declaration of Helsinki, the International Conference on Harmonization Good Clinical Practice guidelines, applicable regulatory requirements, and in compliance with the protocol. All participants provided written informed consent to participate in the study.
Study Design and Treatment
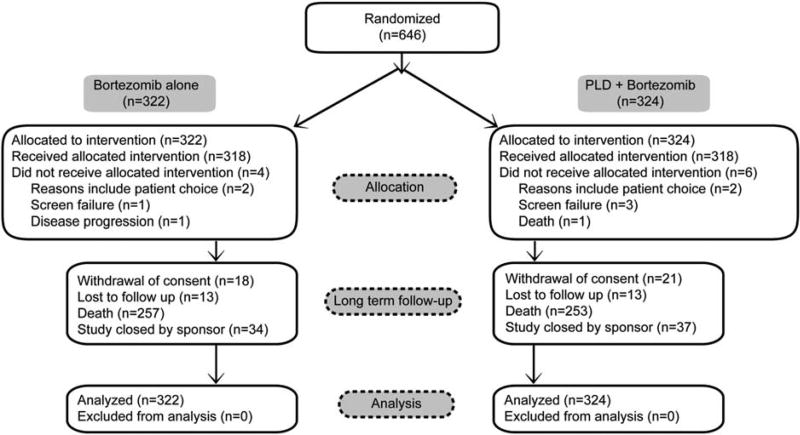
This was a phase 3, open-label, randomized, active-controlled, multicenter study. The eligible patients were randomized (1:1) to receive either bortezomib monotherapy (1.3 mg/m2 intravenously on days 1, 4, 8, and 11 of every 21-day cycle; n = 322) or bortezomib-PLD combination therapy (the same bortezomib monotherapy with PLD, 30 mg/m2 as a 1-hour intravenous infusion on day 4 of each 21-day cycle; n = 324) (Fig. 1). Before randomization, patients were stratified according to their serum β2 microglobulin levels (≤2.5, >2.5 and ≤5.5, or >5.5 mg/L) and response to previous treatment (response followed by progression or primary refractory disease). Study treatment was continued until disease progression, unacceptable treatment-related toxicity, or up to 8 cycles. Patients who were still responding after 8 cycles and had acceptable tolerability to the drug continued the treatment. Crossover to combination therapy from monotherapy was disallowed. Details of the study, including results from the interim analysis data, were published in 2007.8 The final survival analysis presented here was based on the clinical cutoff date (May 16, 2014) upon final study closure.
Figure 1.
This is a Consolidated System for Reporting Trials (CONSORT) diagram for the intent-to-treat analysis set. PLD indicates pegylated liposomal doxorubicin.
Endpoint
OS was defined as the interval from randomization to death from any cause. Data were censored at the last date the patient was known to be alive (for patients who were not known to have died).
Statistical Method
Long-term follow-up survival data were collected until approximately 80% of enrolled patients had died. The distribution of OS was estimated for each treatment group using the Kaplan-Meier method. The log-rank test, stratified by enrollment strata, was used to compare the 2 treatment groups. A stratified Cox proportional-hazards model was used to estimate HRs (bortezomib monotherapy vs bortezomib-PLD: HR >1 indicates a treatment effect in favor of bortezomib-PLD) and 95% CIs. The effects of other prognostic factors were examined in a separate Cox proportional-hazards model. Subgroup analysis was performed to obtain HR estimates in each subgroup along with associated 95% CIs.
RESULTS
Patients and Treatments
Of 646 enrolled patients, 324 were randomized to the bortezomib-PLD group and 322 were randomized to the bortezomib monotherapy group. The baseline demographics and other characteristics were well balanced, and the groups were similar in the number and type of prior systemic therapy for MM.8
OS Analysis
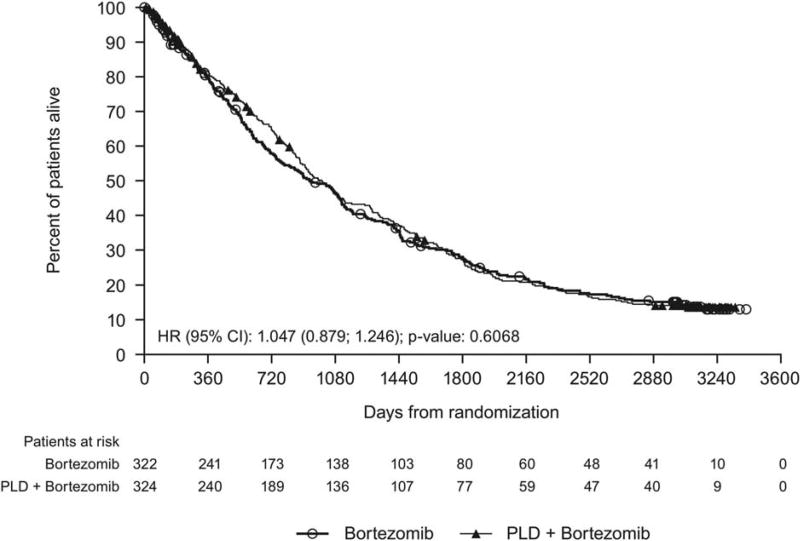
At the clinical cutoff, 79% of patients had died (bortezomib-PLD, 253 patients [78%]; bortezomib, 257 patients [80%]), 6% withdrew consent, 4% were lost to follow-up, and 11% were still alive (bortezomib-PLD, 37 patients [11%]; bortezomib, 34 patients [11%]). The median follow-up for survival was 103 months (8.6 years). The median OS for patients in the bortezomib-PLD group was 33.0 months (95% CI, 28.9–37.1 months) versus 30.8 months (95% CI, 25.2–36.5 months) for those in the bortezomib monotherapy group (HR, 1.047; 95% CI, 0.879–1.246; P = .6068). This 2-month difference in median survival in favor of the bortezomib-PLD group over the bortezomib monotherapy group was not statistically significant (Fig. 2).
Figure 2.
This Kaplan-Meier curve illustrates overall survival in the intent-to-treat analysis set. CI indicates confidence interval; HR, hazard ratio; PLD, pegylated liposomal doxorubicin.
Subgroup Analysis
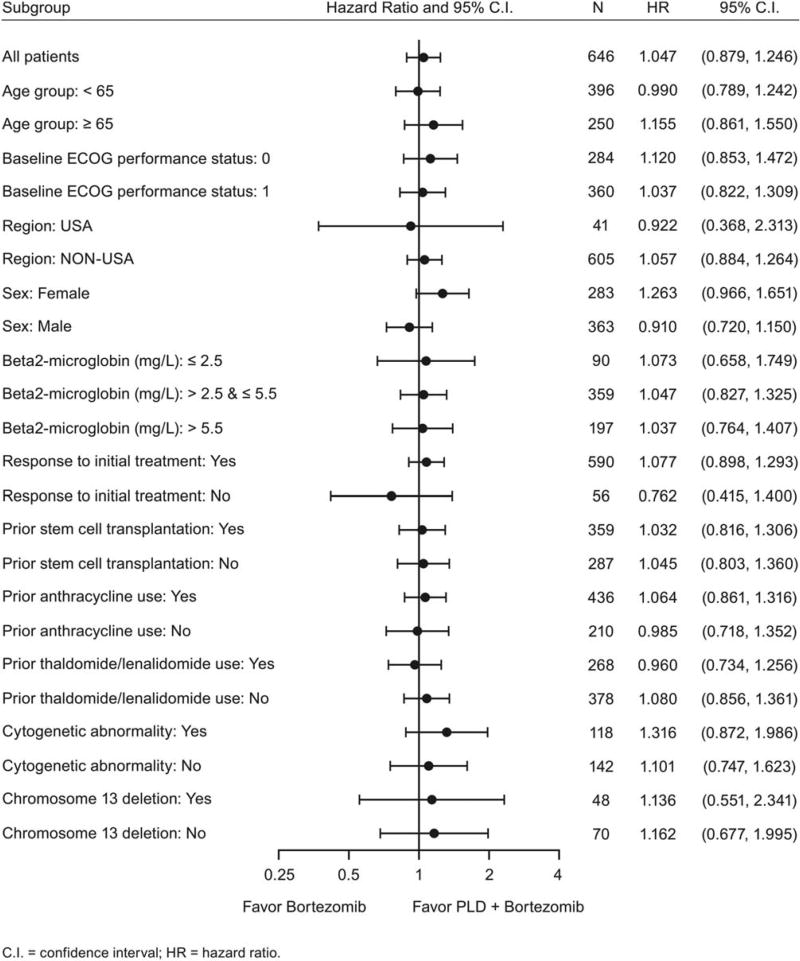
Subgroup analyses based on baseline variables were performed to evaluate their impact on the overall results. The results from the survival analysis by subgroup were generally consistent with the overall results except among those patients who had no response to initial treatments (Fig. 3).
Figure 3.
Overall survival analysis is illustrated by subgroup (intent-to-treat analysis set). ECOG indicates Eastern Cooperative Oncology Group; PLD, pegylated liposomal doxorubicin.
Subsequent Therapy
Patients in both groups received salvage therapies (78% in the bortezomib-PLD group vs 80% in the bortezomib monotherapy group), which were well balanced (Table 1). The most frequent salvage therapies (received by >10% patients in any group) in the bortezomib-PLD group versus the bortezomib monotherapy group included dexamethasone (47% vs 51%), thalidomide (31% vs 31%), cyclophosphamide (26% vs 31%), melphalan (24% vs 22%), lenalidomide (23% vs 21%), bortezomib (23% vs 18%), and doxorubicin (6% vs 11%). No tumor assessment data were collected after subsequent therapy.
TABLE 1.
Subsequent Antimyeloma Therapy by Therapeutic Subgroup in >5% of Patients (Intentto-Treat Analysis Set)
| No. of Patients (%) | |||
|---|---|---|---|
|
|
|||
| Variable | Bortezomib | Bortezomib 1 PLD |
Total |
| No. of patients | 322 | 324 | 646 |
| Total no. patients who received subsequent therapy | 256 (80) | 252 (78) | 508 (79) |
| Dexamethasone | 164 (51) | 153 (47) | 317 (49) |
| Prednisone | 50 (16) | 57 (18) | 107 (17) |
| Prednisolone | 20 (6) | 24 (7) | 44 (7) |
| Cyclophosphamide | 99 (31) | 85 (26) | 184 (29) |
| Melphalan | 70 (22) | 78 (24) | 148 (23) |
| Bortezomib | 58 (18) | 74 (23) | 132 (20) |
| Doxorubicin | 34 (11) | 18 (6) | 52 (8) |
| Vincristine | 31 (10) | 19 (6) | 50 (8) |
| Thalidomide | 101 (31) | 99 (31) | 200 (31) |
| Lenalidomide | 66 (21) | 75 (23) | 141 (22) |
| Radiotherapy | 19 (6) | 21 (7) | 40 (6) |
Abbreviation: PLD, pegylated liposomal doxorubicin.
DISCUSSION
Results from this randomized, phase 3, final survival analysis report suggest a similar OS for the bortezomib-PLD combination therapy versus bortezomib monotherapy after a long-term median follow-up of 8.6 years. At the clinical cutoff of May 16, 2014, for the current analysis, a mortality rate of 79% was reported. This study represents the first long-term follow-up report of this combination regimen in patients with relapsed/refractory MM. The median OS for patients who received bortezomib-PLD patients was 33.0 months compared with 30.8 months for those who received bortezomib monotherapy.
On the basis of observations from in vitro studies of synergistic activity between bortezomib and anthracyclines9 and phase 1 data,10 a phase 3 study compared single-agent bortezomib with bortezomib plus PLD in patients with relapsed/refractory MM.8 The results from the study demonstrated that bortezomib-PLD was superior to bortezomib monotherapy in treating patients with relapsed or refractory MM whose disease had failed 1 or more lines of prior therapy. Both the TTP and the overall response rate were in favor of the combination therapy compared with monotherapy.8 Furthermore, an interim analysis also demonstrated a trend toward an OS benefit favoring bortezomib-PLD combination therapy.8 A secondary analysis of that phase 3 study demonstrated that the therapeutic advantage of prolonged TTP attributed to the PLD-bortezomib combination, compared with bortezomib as a single agent, was maintained regardless of exposure to immunomodulatory drug therapy in prior lines of treatment.11 Bortezomib-PLD was also active in patients whose disease was refractory to other antimyeloma agents11 and was safe and effective in patients with renal compromise.12
However, despite the superiority in TTP and an early trend in OS favoring the combination therapy in the interim analysis, the long-term follow-up results revealed similar OS for bortezomib-PLD combination therapy and bortezomib monotherapy. Results from a subgroup analysis are generally consistent with the overall finding, while patients who were aged ≥65 years, who had Eastern Cooperative Oncology Group performance status of 0, who had cytogenetic abnormalities, or who were women appeared to have outcomes in favor of the combination therapy.
The lack of any difference in the results from the final OS analysis could be because the majority of patients in this study received subsequent therapy. The advancements in treatment options for MM and the availability of new therapies, such as immunomodulators (eg, lenalidomide), other proteasome inhibitors, etc, have led to longer life expectancy for patients with MM. Indeed, the original design assumed that median survival in the bortezomib group would be only 20 months, which was exceeded by 50%, suggesting the benefits of novel agents. With an armamentarium of treatment options available, it would be unlikely to observe a difference in survival between the treatment groups in this study after prolonged follow-up. Several studies demonstrating a survival advantage in patients with myeloma have used the approach of continuing therapies until disease progression; whereas, in this study, the median number of treatment cycles received by patients was only 5 in both groups. Therefore, in retrospect, it is perhaps overly optimistic to expect that 5 cycles of PLD given over approximately 3.5 months would induce a sustained improvement in long-term OS. Results from this update underscore a general need for the long-term follow-up of phase 3 oncology trials and the practical challenge of having adequate power for long-term survival as a primary endpoint if effective subsequent therapies are available.
Regardless of the significant improvement in patient’s overall outcomes with recent advances in treatment options, MM remains incurable in the majority of patients, prompting a continued search for additional therapeutic options, including multidrug-combinations. A prospective comparison of bortezomib-thalidomide-dexamethasone versus thalidomide-dexamethasone in patients who relapsed after high-dose therapy plus autologous stem cell transplantation demonstrated that the median TTP was significantly longer with bortezomib-thalidomide-dexamethasone than with thalidomide-dexamethasone, with a trend in favor of a survival benefit.13 This study demonstrated that a triplet bortezomib-based combination was superior to a 2-drug thalidomide-based regimen for patients with relapsed MM in terms of significantly greater response rate and longer TTP. Currently, other novel agents are being evaluated in combination with bortezomib in phase 3 trials with longer follow-up. Multiple other bortezomib-based combinations have also been studied in clinical trials, with steroids plus alkylators, immunomodulatory drugs, monoclonal antibodies, heat-shock protein-90, histone deacetylase inhibitors, pan-Bcl-2 family inhibitors, and other classes of targeted inhibitors.14–16 If promising results are confirmed with longer follow-up, then these combinations can bring about a shift in the treatment paradigm, maximizing their synergism while minimizing toxicities.
In summary, the current results demonstrate that, despite the superior TTP observed and the early trend in favor of OS with the combination therapy, long-term follow-up revealed that treatment with the bortezomib-PLD combination did not improve OS compared with bortezomib monotherapy in patients with relapsed or refractory MM. The inability to sustain the observed early survival advantage may have been caused by the effects of subsequent lines of therapy, and underscores the need for long-term follow-up of phase 3 trials while recognizing the challenge of having adequate power to detect differences in long-term OS.
Acknowledgments
We thank all the patients for their participation in this study and acknowledge the collaboration and commitment of all investigators and their staff. We also thank Dr. Shalini Nair (SIRO Clinpharm Pvt Ltd) for providing writing assistance and Dr. Namit Ghildyal (Janssen Research & Development, LLC) for providing additional editorial support for this article.
FUNDING SUPPORT
This study was funded by a grant from Janssen Research & Development, LLC. R.Z.O. would like to acknowledge support from the National Cancer Institute (P50 CA142509, U10 CA032102, P30 CA016672, R01 CA184464, and R01 CA194264).
Footnotes
Presented as a poster at the 56th Annual Meeting and Exposition of the American Society of Hematology; December 6–9, 2014; San Francisco, CA.
CONFLICT OF INTEREST DISCLOSURES
Robert Z. Orlowski reports research funding from Janssen-Cilag and Janssen Research &Development, LLC (Janssen R&D); grants from Amgen, Array BioPharma Inc, Bristol-Myers Squibb, Celgene Corporation, Janssen R&D, Millennium Pharmaceuticals, Onyx Pharmaceuticals, and Spectrum Pharmaceuticals; and personal fees from Abbott Laboratories, Amgen, Array BioPharma Inc, Bio-Theryx, Bristol-Myers Squibb, Celgene Corporation, Cephalon Inc, Forma Therapeutics, Genentech Inc, Incyte, Janssen-Cilag, Janssen R&D, Millennium Pharmaceuticals, and Novartis. Pieter Sonneveld reports grants from Celgene Corporation, Janssen-Cilag, Amgen, Takeda, and Karyopharm Therapeutics, Inc., and personal fees from Celgene Corporation, Janssen-Cilag, and Ortho Biotech. Joan Bladé reports grants from Janssen-Cilag and Celgene Corporation and personal fees from Amgen, Binding Site, Celgene Corporation, Janssen-Cilag, Janssen R&D, and Takeda Pharmaceuticals. Roman Hajek reports research funding and personal fees from Janssen R&D. Andrew Spencer is a consultant for Janssen-Cilag. Tadeusz Robak reports research funding from Janssen R&D. Anna Dmoszynska reports research funding from Janssen R&D. Noemi Horvath reports research funding and personal fees from Janssen R&D. Ivan Spicka reports grants from Celgene Corporation; personal fees from Amgen, Bristol-Myers Squibb, Janssen-Cilag, and Janssen R&D; and nonfinancial support from Celgene Corporation and Janssen-Cilag. Heather J. Sutherland reports research funding from Janssen R&D and personal fees from Orthobiotech. Liang Xiu is an employee of Janssen R&D. Andrew Cakana is a contract worker for Janssen Pharmaceuticals. Jesús F. San-Miguel reports personal fees from Janssen-Cilag. Trilok Parekh is an employee of Johnson & Johnson and owns stock in the company.
AUTHOR CONTRIBUTIONS
Robert Z. Orlowski, Trilok Parekh, Liang Xiu, and Andrew Cakana were responsible for the design, conduct, and data analysis and interpretation for the study. Trilok Parekh was also responsible for the collection and assembly of data and additional administrative support. Robert Z. Orlowski, Arnon Nagler, Pieter Sonneveld, Joan Bladé, Roman Hajek, Andrew Spencer, Jesús F. San-Miguel, Tadeusz Robak, Anna Dmoszynska, Noemi Horvath, Ivan Spicka, Heather J. Sutherland, and Alexander N. Suvorov were responsible for the provision of study materials or patient enrollment. All authors had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis. All authors meet International Committee of Medical Journal Editors criteria, and all those who fulfilled those criteria are listed as authors. All authors provided direction and comments on the article, made the final decision about where to publish these data, and approved submission to the journal.
References
- 1.Munshi NC, Anderson KC. New strategies in the treatment of multiple myeloma. Clin Cancer Res. 2013;19:3337–3344. doi: 10.1158/1078-0432.CCR-12-1881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Redic K. Carfilzomib: a novel agent for multiple myeloma. J Pharm Pharmacol. 2013;65:1095–1106. doi: 10.1111/jphp.12072. [DOI] [PubMed] [Google Scholar]
- 3.Barlogie B, Anaissie E, van Rhee F, et al. Incorporating bortezomib into upfront treatment for multiple myeloma: early results of total therapy 3. Br J Haematol. 2007;138:176–185. doi: 10.1111/j.1365-2141.2007.06639.x. [DOI] [PubMed] [Google Scholar]
- 4.Jakubowiak AJ, Kendall T, Al-Zoubi A, et al. Phase II trial of combination therapy with bortezomib, pegylated liposomal doxorubicin, and dexamethasone in patients with newly diagnosed myeloma. J Clin Oncol. 2009;27:5015–5022. doi: 10.1200/JCO.2008.19.5370. 2009;27:5015–5022. [DOI] [PubMed] [Google Scholar]
- 5.Offidani M, Corvatta L, Piersantelli MN, et al. Thalidomide, dexamethasone, and pegylated liposomal doxorubicin (ThaDD) for patients older than 65 years with newly diagnosed multiple myeloma. Blood. 2006;108:2159–2164. doi: 10.1182/blood-2006-03-013086. [DOI] [PubMed] [Google Scholar]
- 6.San-Miguel JF, Schlag R, Khuageva N, et al. Bortezomib plus melphalan and prednisone for initial treatment of multiple myeloma. N Engl J Med. 2008;359:906–917. doi: 10.1056/NEJMoa0801479. [DOI] [PubMed] [Google Scholar]
- 7.Voorhees PM, Orlowski RZ, Mulkey F, et al. Long-term outcomes for newly-diagnosed multiple myeloma patients treated with pegylated liposomal doxorubicin and bortezomib: final results of CLAGB (Alliance) 10303, a multicenter phase II study. Br J Haematol. 2015;171:373–377. doi: 10.1111/bjh.13592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Orlowski RZ, Nagler A, Sonneveld P, et al. Randomized phase III study of pegylated liposomal doxorubicin plus bortezomib compared with bortezomib alone in relapsed or refractory multiple myeloma: combination therapy improves time to progression. J Clin Oncol. 2007;25:3892–3901. doi: 10.1200/JCO.2006.10.5460. [DOI] [PubMed] [Google Scholar]
- 9.Mitsiades N, Mitsiades CS, Richardson PG, et al. The proteasome inhibitor PS-341 potentiates sensitivity of multiple myeloma cells to conventional chemotherapeutic agents: therapeutic applications. Blood. 2003;101:2377–2380. doi: 10.1182/blood-2002-06-1768. [DOI] [PubMed] [Google Scholar]
- 10.Orlowski RZ, Voorhees PM, Garcia RA, et al. Phase 1 trial of the proteasome inhibitor bortezomib and pegylated liposomal doxorubicin in patients with advanced hematologic malignancies. Blood. 2005;105:3058–3065. doi: 10.1182/blood-2004-07-2911. [DOI] [PubMed] [Google Scholar]
- 11.Sonneveld P, Hajek R, Nagler A, et al. Combined pegylated liposomal doxorubicin and bortezomib is highly effective in patients with recurrent or refractory multiple myeloma who received prior thalidomide/lenalidomide therapy. Cancer. 2008;112:1529–1537. doi: 10.1002/cncr.23326. [DOI] [PubMed] [Google Scholar]
- 12.Blade J, Sonneveld P, San Miguel JF, et al. Pegylated liposomal doxorubicin plus bortezomib in relapsed or refractory multiple myeloma: efficacy and safety in patients with renal function impairment. Clin Lymphoma Myeloma. 2008;8:352–355. doi: 10.3816/CLM.2008.n.051. [DOI] [PubMed] [Google Scholar]
- 13.Garderet L, Iacobelli S, Moreau P, et al. Superiority of the triple combination of bortezomib-thalidomide-dexamethasone over the dual combination of thalidomide-dexamethasone in patients with multiple myeloma progressing or relapsing after autologous transplantation: the MMVAR/IFM 2005-04 randomized phase III trial from the Chronic Leukemia Working Party of the European Group for Blood and Marrow Transplantation. J Clin Oncol. 2012;30:2475–2482. doi: 10.1200/JCO.2011.37.4918. [DOI] [PubMed] [Google Scholar]
- 14.Moreau P, Richardson PG, Cavo M, et al. Proteasome inhibitors in multiple myeloma: 10 years later. Blood. 2012;120:947–959. doi: 10.1182/blood-2012-04-403733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wright JJ. Combination therapy of bortezomib with novel targeted agents: an emerging treatment strategy. Clin Cancer Res. 2010;16:4094–4104. doi: 10.1158/1078-0432.CCR-09-2882. [DOI] [PubMed] [Google Scholar]
- 16.Lonial S, Mitsiades CS, Richardson PG. Treatment options for relapsed and refractory multiple myeloma. Clin Cancer Res. 2011;17:1264–1277. doi: 10.1158/1078-0432.CCR-10-1805. [DOI] [PubMed] [Google Scholar]