Abstract
Aim of the study
The present study aims to estimate the prevalence and distribution of HPV genotypes and identify related risk factors among Turkish women.
Material and methods
11 624 Turkish women attending our gynaecological clinic and expressing a desire for access to cervical cancer screening were assessed during the years 2014–2016. Cervical specimens were collected and transported using the HC2 HPV DNA Collection Device (consisting of a cervical brush and digene Specimen Transport Medium).
Results
Among these 11 624 individuals, positive HPV test results were obtained for 325 (2.79%), and negative results were observed for 11 299 (97.2%). The vast majority of patients were between the 3rd and 5th decades and the mean age of the patients was 44 ±9.12 (range 27–66). Among the HPV-positive women, 205 were positive for a single HPV type (205/325 = 63.1% of HPV infections; 205/11624 = 1.76% of all samples) and 120 were positive for multiple types (120/325 = 36.9% of HPV infections; 120/11624 = 1.03% of all samples). The four most prevalent high-risk types were HPV 16, 31, 51 and 52, with frequencies of 11.25%, 7.83%, 6.06% and 3.16%, respectively.
Conclusions
There appears to be geographic variation in the distribution of HPV genotypes. In this study, the four most prevalent high-risk types were HPV 16, 31, 51 and 52, with frequencies of 11.25%, 7.83%, 6.06% and 3.16%, respectively.
Keywords: human papillomavirus DNA tests, cervical smears, HPV genotyping,
Introduction
The presence of certain human papillomavirus (HPV) types in the female genital tract is associated with a number of diseases, including condyloma, Bowenoid papulosis, cervical, vaginal, and vulvar intraepithelial neoplasia and carcinoma. The outcome of HPV infection depends on its oncogenic type. It is generally accepted that these viruses are predominantly sexually transmitted and that high-risk HPV types are the major recognized risk factor for development of cervical cancer. HPV types are divided into two groups: high-risk (HR) and low-risk (LR). HR HPV includes types 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, and 68, whereas LR HPV includes types 6, 11, 40, 42, 43, 44, 53, 54, 61, 72, 73, and 81 [1].
HPV DNA has been shown to be present in approximately 10% of women with normal cervical epithelium. However, the actual prevalence in specific groups of women is strongly influenced by age and other demographic variables [2]. Prevalence and distribution of HPV types differ around the world, and the importance of different HPV types varies by region. The highest regional prevalence was found to be in Africa, where 22% of women had evidence of HPV infection [2]. In the United States and Europe, four types are most often found in patients with cervical cancer, with type 16 accounting for approximately 50% of cases, followed by types 18, 31 and 45 [3]. There appears to be geographic variation in the distribution of HPV genotypes. For example, in an HPV prevalence study conducted in 13 areas from 11 countries (Nigeria, India, Vietnam, Thailand, Korea, Colombia, Argentina, Chile, the Netherlands, Italy and Spain) HPV-positive women in Europe were significantly more likely to be infected with HPV 16 than were those in sub-Saharan Africa (OR 2.6) [4]. In contrast, in a study of HPV prevalence performed in Uganda, the prevalence of HPV infections was 75%. Among high-risk types, the most frequently detected were HPV 52 (13%), HPV 51 (12%), HPV 18 (11%), and HPV 16 (11%) [5].
Knowledge of geographic variation in the prevalence of carcinogenic types and HPV genotype distribution is essential in order to assess the impact of HPV prophylactic vaccines. The present study aims to estimate the prevalence and distribution of HPV genotypes and identify related risk factors among Turkish women.
Material and methods
11 624 Turkish women attending the gynaecological clinic of Erzincan University and expressing a desire for access to cervical cancer screening were assessed between 2014 and 2016. The inclusion criteria were as follows: women 1) with a history of current or past sexual activity; 2) who were not pregnant at the time of enrolment; 3) with no history of total uterus or cervical resection; and 4) who provided agreement to undergo an HPV test and participate in the present study. The protocol of the study was approved by the Faculty of Medicine, Erzincan University Ethics Committee, prior to its start. Written informed consent was obtained from each participant involved in the investigation. The methods of this investigation were in accordance with the approved guidelines and the principles expressed in the Declaration of Helsinki.
Cervicovaginal swabs were collected from all participants by a gynaecologist according to the standard operation procedure for sampling at the recruitment sites. A slide was prepared for conventional Pap cytology and the cytobrush was then placed in specimen transport medium and transported. Cervical specimens were collected and transported using the HC2 HPV DNA (Qiagen Gaithersburg, Inc. USA) Collection Device (consisting of a cervical brush and digene Specimen Transport Medium). The swab was kept in 3 ml of sample transport medium for the HC2 HPV DNA Test. According to HC2 HPV DNA Test kit guidance, cervical specimens must be collected prior to the application of acetic acid or iodine if colposcopic examination is being performed. Specimens may be held for up to two weeks at room temperature and shipped without refrigeration to the testing laboratory. Specimens should be shipped in an insulated container using either an overnight or 2-day delivery vendor. At the testing laboratory, specimens should be stored at 2–8°C if the assay is to be performed within one week. If the assay will be performed later than one week, store specimens at –20°C for up to 3 months. HPV DNA detection and genotyping were performed by Hybrid Capture-II and PCR as described previously and only patients who underwent genotype identification were included this analysis [6]. HPV DNA detection in cervical swabs was conducted using real-time polymerase chain reaction (PCR) with a commercial kit (Fluorion, Iontek, Turkey). For DNA extraction, the QIAamp DNA Mini Kit (Qiagen, Hilden, Germany) was used in accordance with the instructions of the manufacturer. A 150 bp fragment of the L1 gene was amplified using GP5 and GP6 primers. An amplified gene product was identified via melting curve analysis and visualized by incorporation of SYBR Green dye during amplification. HPV genotyping was performed with DYEnamic ET Terminator Cycle Sequencing Kit (Amersham Biosciences Corp., NJ, USA) and ABI PRISM 310 Genetic Analyzer at Iontek Ltd, Turkey.
Cytological findings were classified in line with the 2004 Bethesda classification system, as follows: (1) within normal limits or reactive cellular changes (normal), (2) atypical squamous cells (a) low-grade squamous intraepithelial lesion (LSIL), (b) high-grade squamous intraepithelial lesion (HSIL) and (c) atypical squamous cells of undetermined significance (ASC-US). Colposcopy was performed in suspicious lesions. Colposcopy and tissue biopsies were taken from suspicious lesions on colposcopic examination. DNA extraction: from cervical samples using (HC2 HPV DNA Test kit), according to the manufacturer’s instructions.
According to suggestions of the International Agency for Research on Cancer and the US Food and Drug Administration (FDA), 14 HPV types (HPV 16, 18, 31, 33, 35, 39, 45, 51, 52, 56, 58, 59, 66, and 68) were classified as high-risk. Data were fed to the computer and analyzed using SPSS 22 (Statistical Package for the Social Sciences, Chicago, US). Qualitative data were described using frequencies and percentages. Quantitative data were described using the range (minimum and maximum), mean, and standard deviation. Comparison between different groups regarding categorical variables was tested using the chi-square test. A binomial 95% confidence interval (95% CI) was estimated for each calculation to obtain the prevalence of HPV. All genotypes from single and multiple infections were computed individually. These data were also stratified by age (≤ 29 years, 30–39 years, 40–49 years, 50–59 years and ≥ 60 years). P < 0.05 was considered statistically significant.
Results
A total of 11 624 healthy women were enrolled in this study. Table 1 shows the demographic, clinical and laboratory characteristics of the studied group. Among these 11 624 individuals, positive HPV test results were obtained for 325 (2.79%), and negative results were observed for 11 299 (97.2%). Seventy-four point eight percent of the patients were married, while the remaining 13.5% were single and 11.7% were divorced. Twenty-one point five percent of the study population consisted of nulliparous women and the remaining 78.46% had at least one delivery. The vast majority of patients were between the 3rd and 5th decades, and the mean age of the patients was 44 ±9.12 (range 27–66). Among the HPV-positive women, 205 were positive for a single HPV type (205/325 = 63.1% of HPV infections; 205/11 624 = 1.76% of all samples) and 120 were positive for multiple types (120/325 = 36.9% of HPV infections; 120/11 624 = 1.03% of all samples), 44 (13.5%) had dual infections, 49 (15.1%) had triple infections, and 27 (8.3%) had four or more infections. A maximum of six types were seen together. High and low-risk HPV types were also seen together in 15.4% of patients. Among the individuals who tested positive for a single HPV type 85.5% were at high risk of HPV infection. The four most prevalent high-risk types were HPV 16, 31, 51 and 52, with frequencies of 11.25%, 7.83%, 6.06% and 3.16%, respectively (Table 2).
Table 1.
Demographic characteristics, clinical and laboratory findings
| Characteristics | n = 325 (%) |
|---|---|
| Age (years) mean ± SD | 44 ±9.12 |
| Parity | |
| Nullipara | 69 (21.5) |
| Multipara | 256 (78.5) |
| Residence | |
| Urban | 192 (59.1) |
| Rural | 133 (40.9) |
| Occupation | |
| Housewife | 181 (55.7) |
| Worker | 144 (44.3) |
| Marital status | |
| Single | 44 (13.5) |
| Married | 243 (74.8) |
| Divorced | 38 (11.7) |
| Educational status | |
| Illiterate | 40 (12.3) |
| Primary education | 137 (42.2) |
| High school | 106 (32.6) |
| University | 42 (12.9) |
| Smoking | |
| No | 190 (58.5) |
| Yes | 135 (41.5) |
| Contraception method | |
| None | 147 (45.2) |
| IUCDs | 103 (31.7) |
| Injectables | 15 (4.6) |
| Condoms | 33 (10.2) |
| COCs | 27 (8.3) |
| Gynaecological findings | |
| Normal | 232 (71.4) |
| Genital warts | 45 (13.8) |
| Intermenstrual bleeding | 22 (6.8) |
| Post-coital bleeding | 26 (8 %) |
| Pap smear & biopsy results | |
| Normal | 240 (73.8%) |
| ASC-US | 31 (9.5%) |
| LSIL | 51 (15.7%) |
| HSIL | 3 (0.9) |
| Colposcopy results | |
| Normal | 196 (60.3) |
| Acetowhite epithelium | 87 (26.8) |
| Leukoplakia | 12 (3.7) |
| Atypical vascularization | 8 (2.5) |
| Mosaicism | 8 (2.5) |
| Punctuation | 14 (4.3) |
| HPV DNA | |
| Negative | 11299 (97.2) |
| Positive | 325 (2.79) |
| Multiple infections | |
| 1 type | 205 (63.1) |
| 2 types | 44 (13.5) |
| 3 types | 49 (15.1) |
| 4 types | 19 (5.8) |
| 5 types | 7 (2.2) |
| 6 types | 1 (0.3) |
IUCDs – intra-uterine contraception device; COCs – combined oral contraceptive pills; LSIL – low-grade squamous intra-epithelial lesion; HSIL – high-grade squamous intraepithelial lesion; ASC-US – atypical squamous cells of undetermined significance
Table 2.
Distribution of HPV types in the study population
| HPV types | Frequency (n) | Percent (%) |
|---|---|---|
| 16 | 89 | 11.25 |
| 31 | 62 | 7.83 |
| 51 | 48 | 6.06 |
| 52 | 25 | 3.16 |
| 58 | 24 | 3.03 |
| 39 | 23 | 2.90 |
| 68 | 21 | 2.65 |
| 18 | 18 | 2.27 |
| 56 | 18 | 2.27 |
| 59 | 18 | 2.27 |
| 35 | 17 | 2.15 |
| 33 | 14 | 1.77 |
| 45 | 14 | 1.77 |
| 53 | 4 | 0.50 |
| 82 | 3 | 0.38 |
| 62 | 1 | 0.12 |
| 83 | 1 | 0.12 |
| 81 | 1 | 0.12 |
| 66 | 1 | 0.12 |
| Unclassified | 389 | 49.17 |
| Total | 791 | 100.0 |
Table 3 investigates the relation between the risk factors and HPV infection. It was found that HPV positive cases were mainly in the age groups 30–39 years (37.2%) and 40-49 years (31.5%), but this distribution was statistically insignificant (p = 0.249). Reproductive history, residence, occupation, smoking habit, use of contraception and history of irregular genital bleeding of the studied group showed a statistically insignificant difference between the most common types of HPV and the others. Twenty-six point seven percent of patients with the most common types HPV showed normal cytology, while 73.3% of them showed cytological changes: 6.46% with LSIL, 0.30% with HSIL and 4.3% with ASC-US. Human papillomavirus DNA positivity among patients with abnormal and normal cytology was 26.1% and 73.8%, respectively. HPV was positive in 15.7%, 0.92% and 9.53% of the LSIL, HSIL, and ASC-US patients, respectively. The most common HPV types in our study were as follows: HPV 16 (11.25%), HPV 31 (7.83%), HPV 51 (6.06%), and HPV 52 (3.16%).
Table 3.
Relation and distribution of studied risk factors to HPV DNA status
| Most common type (types 16 and 31) | Other types | P value | |
|---|---|---|---|
| Age (years) mean ± SD | 42.83 ±8.66 | 44.71 ±9.34 | |
| Age group (n, %) | χ2 = 5.401 | ||
| < 29 | 2 (0.61) | 4 (1.23) | p = 0.249 |
| 30–39 | 50 (15.33) | 71 (22.15) | |
| 40–49 | 44 (13.5) | 59 (18.15) | |
| 50–59 | 21 (6.46) | 55 (16.92) | |
| > 60 | 6 (1.84) | 13 (4.0) | |
| Parity | χ2 = 0.662 | ||
| Nullipara | 27 (8.30) | 42 (12.92) | p = 0.718 |
| Multipara | 97 (29.84) | 159 (48.92) | |
| Residence | χ2 = 0,384 | ||
| Urban | 70 (2.15) | 122 (37.53) | p = 0.535 |
| Rural | 53 (16.30) | 80 (24.61) | |
| Occupation | χ2 = 2.983 | ||
| Housewife | 61 (18.71) | 120 (36.80) | p = 0.084 |
| Worker | 62 (19.07) | 82 (25.23) | |
| Marital status | χ2 = 1.218 | ||
| Single | 18 (5.53) | 26 (8) | p = 0.544 |
| Married | 88 (26.99) | 155 (47.69) | |
| Divorced | 17 (5.23) | 21 (6.46) | |
| Educational status | χ2 = 3.615 | ||
| Illiterate | 13 (4) | 27 (8.28) | p = 0.306 |
| Primary education | 48 (14.76) | 89 (27.38) | |
| High school | 41 (12.57) | 65 (20.0) | |
| University | 21 (6.46) | 21 (6.46) | |
| Smoking | χ2 = 1.297 | ||
| No | 67 (20.61) | 123 (68.61) | p = 0.255 |
| Yes | 56 (17.23) | 79 (24.30) | |
| Contraception method | χ2 = 6,889 | ||
| None | 47 (14.41) | 100 (30.76) | p = 0.142 |
| IUCDs | 43 (13.23) | 60 (18.40) | |
| Injectables | 8 (2.45) | 7 (2.14) | |
| Condoms | 11 (3.38) | 22 (6.76) | |
| COCs | 14 (4.29) | 13 (4.0) | |
| Gynaecological findings | χ2 = 1.337 | ||
| Normal | 91 (28) | 141 (43.38) | p = 0.720 |
| Genital warts | 16 (4.92) | 28 (8.92) | |
| Intermenstrual bleeding | 6 (1.84) | 16 (4.92) | |
| Post-coital bleeding | 10 (3.06) | 16 (4.92) | |
| Pap smear & biopsy results | χ2 = 1.232 | ||
| Normal | 87 (26.76) | 153 (47.07) | p = 0.745 |
| ASC-US | 14 (4.29) | 17 (5.21) | |
| LSIL | 21 (6.46) | 30 (9.23) | |
| HSIL | 1 (0.30) | 2 (0.61) | |
| Colposcopy results | χ2 = 4.601 | ||
| Normal | 67 (20.61) | 129 (39.69) | p = 0.467 |
| Acetowhite epithelium | 31 (9.53) | 51 (15.69) | |
| Leukoplakia | 7 (2.15) | 5 (1.53) | |
| Atypical vascularization | 3 (0.92) | 5 (1.53) | |
| Mosaicism | 3 (0.92) | 5 (1.53) | |
| Punctuation | 7 (2.15) | 7 (2.15) | |
Discussion
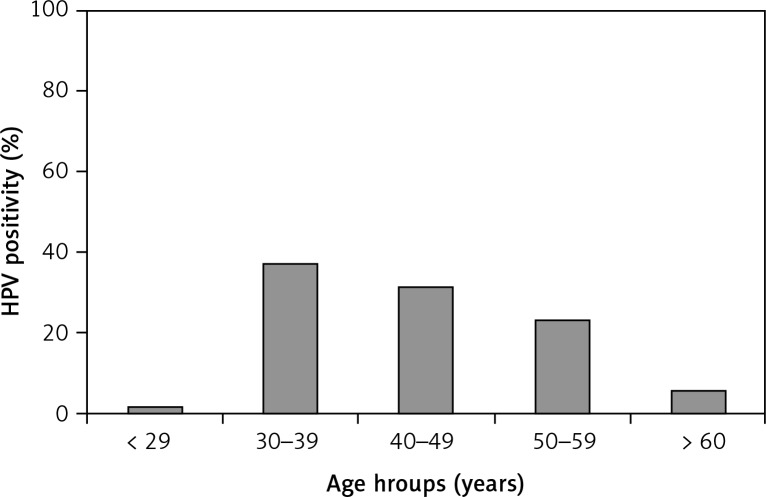
The incidence and prevalence of genital HPV infection may vary depending on variables such as sociocultural characteristics of the selected population, used methods, and quality of the sample taken for the study. In studies conducted in different countries, even in different regions of our country, HPV DNA positivity rates vary. Previous studies have reported that HPV prevalence ranges between 2 and 44% in women. The highest prevalence of HPV is observed in women aged 20–24 years and it decreases with age, advancing with the formation of immunity [7]. In this study, the vast majority of patients were between the 3rd and 5th decades, and the mean age of the patients was 44 ±9.12 (range 27–66) (Fig. 1). In a meta-analysis, among women with normal cytology the prevalence of HPV was found to be 10.4%. HPV prevalence was found to be higher in Africa (20–30%), Central America and Mexico but lower in North America, Europe and Asia (8–11%). The most common HPV types were HPV 16, HPV 18, HPV 31, HPV 58 and HPV 52 in the same study [2]. In the current study, the most common HPV types were as follows: HPV 16 (11.25%), HPV 31 (7.83%), HPV 51 (6.06%), and HPV 52 (3.16%). In a pathological evaluation of the cervical cancer specimens, Usubütün et al. [8] reported that HPV prevalence was 93.5%. The five most common HPV types identified as single types among HPV-positive cases were HPV 16 (64.7%), HPV 18 (9.9%), HPV 45 (9.9%), HPV 31 (3.0%) and HPV 33 (2.2%). Similar to our study, HPV 16 was the most common high-risk HPV in this study as well but not HPV 18.
Fig. 1.
Distribution of HPV positivity by age
HPV DNA isolation rates from cervical swab samples ranged from 10% to 66% in studies conducted in different parts of the world [9, 10]. The highest prevalence rates have been reported in the studies performed with PCR-based methods for HPV DNA detection. The possibility of detecting DNA may be reduced in the presence of certain inhibitor proteins such as haemoglobin. In addition, differences in the sensitivity of the methods studied may affect the results [10]. In a study by Domeh et al. in Ghana in 2002, the prevalence of HPV DNA was found to be 10.7% with the PCR test [12]. In another study conducted in 2686 patients in Indonesia, the HPV positivity rate was 11.4% and the prevalence of HPV in patients over 55 years of age was significantly lower [13]. In our study HPV DNA was detected in 325 (2.79%) samples from 11 624 cervical samples using the HC2 HPV DNA Test kit. In a study with the PCR test by Dursun et al. the prevalence of HPV was reported to be 25% [14]. In a study conducted with HC2, Leinonen and colleagues reported that the prevalence of high-risk HPV was 7.5% among 16 895 women [15]. In the current study, this rate was found to be 2.79%. The low HPV rate may be a consequence of hygiene, socio-cultural characteristics and widespread monogamy in our region’s women.
Today, it is known that the history of sexual life is related to HPV infection. If the number of sexual partners increases, the risk for high-risk HPV infection also increases. In a study investigating the prevalence of HPV in low-risk (general population) and high-risk women (sex workers) in Belgium, the prevalence of HPV was 14.3% and 34.4% respectively [16]. While it is not easy to question sexual life in our society, there was no statistical difference between the groups in terms of sexual partners and marriages. In some studies, HPV infection risk factors include premature marriage or low age at first sexual intercourse, but in some studies a significant relationship was not found between the age of first sexual intercourse and the development of HPV infection [17, 18]. The age of marriage was considered as the first age of sexual intercourse in our study group since the first sexual experience usually coincides with marriage in our country. In this study, HPV DNA was found to be higher in earlier marriages, but not statistically significantly (p > 0.05).
Castle and colleagues emphasized that smoking increases the risk of HPV infection. The study of Bahmanyar et al. also found a significant relationship between smoking and HPV infection [18, 19]. Forty-one point five percent of the patients were found to smoke in this study, but there was no statistically significant difference in HPV positivity among smokers and non-smokers (p > 0.05).
There were different results between parity and HPV DNA positivity in the studies performed. Tuncer et al. reported that they did not detect a correlation between HPV DNA positivity and parity, but in a study by Pereira et al., parity of three or more was reported to have an effect on the increase of HPV infection positivity in young women [20, 21]. Similarly, in our study, the number of births was found to be higher in HPV DNA positive patients, but the difference was not statistically significant (p > 0.05).
In a study by Sanjose and colleagues, the prevalence of HPV DNA was reported to be 6.7 times higher in divorced women. In another study by Stacy et al., the prevalence of high-risk HPV was found to be 3.6% in married women and 13.6% in divorced women [17, 22]. In our study, it was also found that 11.7% of the patients were divorced, but the difference was not statistically significant.
Age has been reported to have the most important effect on the prevalence of HPV in some studies. The lowest prevalence of HPV was found in women aged 14–19 years and the highest prevalence was found in women aged 20-24 years. Some studies have also reported a second peak in HPV prevalence in postmenopausal women. The prevalence of HPV increases every year between the ages of 14–24, and gradually decreases in later ages [11]. In a study conducted by De Sanjose and his colleagues in 2007, in which 346 000 women from 70 countries were assessed, the age-related prevalence of HPV was found to vary according to region and population. HPV prevalence was increasing around 25 years, but decreasing in older groups in most of the regions, and there has been an increase in elderly age due to newly acquired infection or reactivated latent infection in some regions [2]. Although there was no statistically significant difference in our study, HPV DNA positivity was found to be highest among women in the younger age group (30–39 years, 121 women, 37.2%), as in Smith’s study [2].
The limitations of the study include the fact that our study was conducted only in a case group of patients who attended gynaecology polyclinics of a 3rd stage health institution in the eastern Anatolian region of Turkey. For this reason, we believe that our results cannot be generalized to all of Turkey. It is necessary to perform a wider scale work across the country for HPV infection and vaccination. A second limitation of our study is that relatively few samples are examined and samples of low-level atypia such as ASC-US and LSIL often constitute the majority of the study group.
In conclusion, HPV diagnosis, which is regarded as a major factor in cervical cancer aetiology, is of great importance today. Cervical cancer differs from other cancer types as it is a “preventable” cancer type. For that reason, scanning, early diagnosis and treatment are important for HPV-related infections. Newly developed HPV vaccines are particularly effective against HPV 16 and HPV 18 from high-risk types. As in our study, HPV 18 was found less frequently in the global genotype-based prevalence studies of new-generation tests. Although our study group is small and the type spectrum of the method we use does not cover all types of HR-HPV, the most common HPV types in our study were as follows: HPV 16 (11.25%), HPV 31 (7.83%), HPV 51 (6.06%), and HPV 52 (3.16%). This may be explained by the fact that our case count is limited, and it may also be a reflection of the HPV prevalence and HPV type distribution showing geographical differences. Due to the high proportion of HR-HPV types except HPV 16 and HPV 18, we believe that the increased use of techniques for more specific identification of HPV types, type distribution and prevalence data may vary significantly, and that this may significantly affect the predicted efficacy of existing vaccines. We believe that a larger study is needed to determine the HPV prevalence and type distribution in the general population and cervical lesions in our country. This study confirmed the high prevalence of HPV infection in Turkey and highlighted regional differences according to risk genotypes. Moreover, this study provides an important database for future research studies due to its wide patient spectrum.
Footnotes
The authors declare no conflict of interest.
References
- 1.de Sanjose S, Quint WG, Alemany L, et al. Human papillomavirus genotype attribution in invasive cervical cancer: a retrospective cross-sectional worldwide study. Lancet Oncol. 2010;11:1048. doi: 10.1016/S1470-2045(10)70230-8. [DOI] [PubMed] [Google Scholar]
- 2.de Sanjosé S, Diaz M, Castellsagué X, et al. Worldwide prevalence and genotype distribution of cervical human papillomavirus DNA in women with normal cytology: a meta-analysis. Lancet Infect Dis. 2007;7:453. doi: 10.1016/S1473-3099(07)70158-5. [DOI] [PubMed] [Google Scholar]
- 3.Harro CD, Pand YY, Roden RB, et al. Safety and immunogenicity trial in adult volunteers of a human papillomavirus 16 L1 virus-like particle vaccine. J Natl Cancer Inst. 2001;93:284–92. doi: 10.1093/jnci/93.4.284. [DOI] [PubMed] [Google Scholar]
- 4.Clifford GM, Gallus S, Herrero R, et al. Worldwide distribution of human papillomavirus types in cytologically normal women in the International Agency for Research on Cancer HPV prevalence surveys: a pooled analysis. Lancet. 2005;366:991. doi: 10.1016/S0140-6736(05)67069-9. [DOI] [PubMed] [Google Scholar]
- 5.Banura C, Franceschi S, Doorn LJ, et al. Infection with human papillomavirus and HIV among young women in Kampala, Uganda. J Infect Dis. 2008;197:555. doi: 10.1086/526792. [DOI] [PubMed] [Google Scholar]
- 6.Evans MF, Adamson CS, Papillo JL, et al. Distribution of human papillomavirus types in thinprep papanicolaou tests classified according to the Bethesda 2001 terminology and correlations with patient age and biopsy outcomes. Cancer. 2006;106:1054–64. doi: 10.1002/cncr.21664. [DOI] [PubMed] [Google Scholar]
- 7.Kunze U, Böhm G. Public Health analysis – human papillomavirus data and facts for Austria. Wien Klin Wochenschr. 2010;122:655–9. doi: 10.1007/s00508-010-1496-9. [DOI] [PubMed] [Google Scholar]
- 8.Usubütün A, Alemany L, Küçükali T, et al. Human papillomavirus types in invasive cervical cancer specimens from Turkey. Int J Gynecol Pathol. 2009;28:541–8. doi: 10.1097/PGP.0b013e3181aaba0d. [DOI] [PubMed] [Google Scholar]
- 9.Hopman EH, Rozendaal L, Voorhorst FJ, Walboomers JM, Kenemans P, Helmerhorst TJ. High risk human papillomavirus in women with normal cervical cytology prior to the development of abnormal cytology and colposcopy. Br J Obstet Gynecol. 2000;107:600–4. doi: 10.1111/j.1471-0528.2000.tb13299.x. [DOI] [PubMed] [Google Scholar]
- 10.Adam E, Berkova Z, Daxnerova Z, Icenogle J, Reeves WC, Kaufman RH. Papillomavirus detection: demographic and behavioral characteristics influencing the identification of cervical disease. Am J Obstet Gynecol. 2000;182:257–64. doi: 10.1016/s0002-9378(00)70208-0. [DOI] [PubMed] [Google Scholar]
- 11.Erickson BK, Alvarez RD, Huh WK. Human papillomavirus: what every provider shhould know. Am J Obstet Gynecol. 2013;208:169–75. doi: 10.1016/j.ajog.2012.09.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Domfeh AB, Wiredu EK, Adjei AA, Ayeh-Kumi P, Adiku T, Tettey Y, Gyasi R, Armah H. Cervical human papillomavirus infection in Accra, Ghana. Ghana Med J. 2008;42:71–8. doi: 10.4314/gmj.v42i2.43596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Vet JN, de Boer MA, van den Akker BE, et al. Prevalence of human papillomavirus in Indonesia: a population-based study in three regions. Br J Cancer. 2008;99:214–8. doi: 10.1038/sj.bjc.6604417. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dursun P, Ayhan A, Mutlu L, et al. HPV Types in Turkey: Multicenter Hospital Based Evaluation of 6388 Patients in Turkish Gynecologic Oncology Group Centers. Turk Patoloji Derg. 2013;29:210–6. doi: 10.5146/tjpath.2013.01188. [DOI] [PubMed] [Google Scholar]
- 15.Leinonen M, Talonen LK, Anttila A, Dyba T, Tarkkanen J, Nieminen P. Prevalence of oncogenic human papillomavirus infection in an organised screening population in Finland. In J Cancer. 2008;123:1344–9. doi: 10.1002/ijc.23670. [DOI] [PubMed] [Google Scholar]
- 16.Ojiyi EC, Dike IE, Okeudo C, Ejikem C, Nzewuihe AC, Agbata A. Local risk factors in genital human papilloma virus infection in cervical smears. Ann Med Health Sci Res. 2013;3:529–35. doi: 10.4103/2141-9248.122082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.de Sanjose S, Almirall R, Lloveras B, et al. Cervical human papillomavirus infection in the female population in Barcelona, Spain. Sex Transm Dis. 2003;30:788–93. doi: 10.1097/01.OLQ.0000080177.82204.E0. [DOI] [PubMed] [Google Scholar]
- 18.Bahmanyar ER, Paavonen J, Naud P, Salmeron J, et al. Prevalence and risk factors for cervical HPV infection and abnormalities in young adult women at enrolment in the multinational PATRICIA trial. Gynecol Oncol. 2012;127:440–50. doi: 10.1016/j.ygyno.2012.08.033. [DOI] [PubMed] [Google Scholar]
- 19.Castle PE, Schiffman M, Herrero R, et al. A prospective study of age trends in cervical human papillomavirus acquisition and persistence in Guanacaste, Costa Rica. J Infect Dis. 2005;191:1808–16. doi: 10.1086/428779. [DOI] [PubMed] [Google Scholar]
- 20.Tuncer ZS, Başaran M, Ustaçelebi Ş, Mocan G. High-risk Human Papilloma Virus (HPV) İnfection determined by Hybrid Capture II assay in Turkish university hospital outpatient clinic. Gynecol Obstet Reprod Med. 2006;12:129–34. [Google Scholar]
- 21.Pereira CR, Rosa ML, Vasconcelos GA, Faria PC, Cavalcanti SM, Oliveira LH. Human papillomavirus prevalance and predictors for cervical cancer among high-risk women from Rio DE Janeiro, Brazil. Int J Gynecol Cancer. 2007;17:651–60. doi: 10.1111/j.1525-1438.2007.00844.x. [DOI] [PubMed] [Google Scholar]
- 22.Lindau ST, Melinda LD, Gaumer E, Surawska H, Jordan JA. Prevalance of high-risk human papillomavirus among older women. Obstet Gynecol. 2008;112:979–89. doi: 10.1097/AOG.0b013e31818b0df2. [DOI] [PMC free article] [PubMed] [Google Scholar]